Abstract
Introduction: In the quest for sustainable energy solutions and environmental protection, the management of end-of-life (EoL) batteries has emerged as a critical issue. Batteries, especially lithium-ion batteries (LIBs), power a wide range of devices and are central to modern life. As society’s reliance on batteries grows, there is an urgent need for sustainable battery recycling methods that can efficiently recover valuable materials, minimize environmental impact, and support the circular economy. Methods: A literature review was conducted to analyze the LIB market, the estimated return volumes and state-of-the-art sorting and recycling processes. Furthermore, a manual dismantling and input analysis was done for consumer LIB. Results: The current recycling processes operate for individual cathode active material input only. However, there is no sorting process or application in place to provide pre-sorted LIBs. This is why they need to be developed. X-ray transmission, X-ray fluorescence and optical sorting in theory can be applied to differentiate LIBs by their cathode active material. To support this hypothesis, further investigations need to be performed.
Keywords:
batteries; recycling; lithium ion; mechanical recycling; battery sorting; circular economy 1. Introduction
In the quest for sustainable energy solutions and the mitigation of environmental impact, the end-of-life (EoL) management of spent batteries has become an increasingly critical challenge. Batteries power a growing number of devices, from portable electronics to electrical vehicles (EVs), and their adoption is central to our modern way of life. The current and largest increase in demand for LIB to date is largely motivated by the mobility transition. One factor is the European Green Deal, which commits EU member states to achieving climate neutrality by 2050 [1]. To achieve these goals, many governments (such as the Federal Republic of Germany) are focusing on a shift from an internal combustion engine to an electric motor in the transportation sector. The production of LIBs requires materials such as cobalt, graphite, lithium, manganese and nickel, with China controlling most of the resources and European countries having limited mining capacity for these raw materials [2]. As a result, Europe’s lack of security of supply for key raw materials, such as lithium and cobalt, for the production of LIBs, and the environmental and social impacts of the mining of these raw materials has led to an ambitious update of the European battery legislation by the EU [3]. Moreover, with the growing reliance on batteries, comes the need for sustainable battery recycling processes that can recover valuable materials, reduce environmental impact and support the circular economy. Studies have shown that efficient recycling of LIBs and the use of recycled materials in LIB production can reduce carbon emissions up to 52% [4]. To incentivize recycling of LIBs, the updated EU Battery Directive 2023/1542 not only targets critical raw materials with increasing material recycling quotas but also sets standards for recycled material content in the production of new LIBs [3]. The recycling processes required to meet these revised recycling targets currently use only mono-batches of single, individual cathode active materials. However, there is currently no label for the cathode active material (CAM) of each individual LIB cell and sorting is only applied for individual battery types, such as LIB, Lead and Alkali Manganese. Further sorting by CAM is currently not applied. To determine necessary actions on EoL processes and develop strategies to close gaps in today’s EoL process chains, the actual construction and material composition of power tools, phones, laptops and light mobility battery packs have been assessed. This paper further reviews state-of-the-art recycling processes for consumer LIBs and discusses current research.
2. Legal Framework for the Recycling of Lithium-Ion Batteries in the EU
As part of the European Commission’s European Green Deal, a battery regulation was enacted on 17 August 2023, amending battery legislation. The European Battery Regulation regulates the sustainability, safety, labeling and information requirements for the marketing and use of batteries and will apply in all member states from 18 February 2024 [3]. It replaces the previous Battery Directive (2006/66/EC, last amended by Directive 2018/849). The current European Battery Directive includes six types of batteries:
- Portable batteries are sealed batteries that have a maximum weight of 5 kg and are not specifically designed for industrial purposes [3].
- General purpose portable batteries are batteries of the following types: 4.5 volt (3R12), button cell, D, C, AA, AAA, AAAA, A23 and 9 volt (PP3). Another feature of general purpose batteries is interoperability [3].
- A new type of battery according to the Battery Ordinance is batteries for Light Means of Transport (LMT), such as e-bikes or e-scooters [3]. These batteries are sealed and weigh no more than 25 kg. LMT batteries are designed for the traction of wheeled vehicles powered by an electric motor alone or by a muscle-motor combination.
- Starting, lighting and ignition batteries (SLI, Batteries for Starting, Lighting and Ignition) are designed to supply electrical energy for starting, lighting and ignition [3]. In practice, these are often lead-acid batteries.
- A new type of battery, according to the Battery Ordinance, is electric vehicle batteries (EV), which are used to supply electrical energy to the traction systems of hybrid electric and battery electric vehicles [3]. This applies to vehicle classes L according to Regulation (EU) No 168/2013 if the weight of the battery exceeds 25 kg and to vehicle classes M, N and O according to Regulation (EU) No 2018/858.
- Industrial batteries are batteries specially designed for industrial purposes. This includes all batteries weighing more than 5 kg that are not LMT, traction or automotive batteries [3].
The sustainability aspect of batteries is implemented through a number of measures, including:
- Indication of the carbon footprint of the manufacturing process;
- Minimum requirements for durability and performance;
- Specification and mandatory use of recycled materials (see Table 1);
 Table 1. Recommended Recyclate Content and Recycling Requirements. Adapted from Ref. [3].
Table 1. Recommended Recyclate Content and Recycling Requirements. Adapted from Ref. [3]. - Removability of batteries from electronic devices, such as mobile phones;
- Achieve recycling efficiencies for lithium-ion batteries from the current 50% to 65% (by 31 December 2025) and 70% (by 31 December 2030), as well as material recycling quotas for individual battery components.
The mandatory battery passport, which provides important information about material composition (including anode and cathode material), disassembly and spare parts suppliers, however, is only necessary to implement for batteries with a capacity of 2 kWh or more [3]. The capacity of power tool LIBs (PT-LIBs) is mostly up to 100 Wh, and for LMT-LIBs, it is up to 750 Wh. Therefore, it is not mandatory for all portable and general purpose portable batteries as well as most LMT batteries.
The calculation of recycling efficiency is carried out in accordance with European Regulation (EU) No. 493/2012 [5] and refers to the mass percentage recovered in relation to the mass of the battery cell. This takes into account recycled cell components that are no longer considered waste after their material recovery. In addition, the cell components from the slag may also be taken into account, provided that the slag is sent to a material recycling route other than landfill construction and backfilling. This regulation should continue to be applied, but the European Commission should be empowered to supplement this regulation [3].
In general, two kinds of batteries are available. Primary batteries cannot be recharged after being discharged when secondary batteries (or accumulators) can be recharged and discharged multiple times [6]. Furthermore, different battery technologies are currently available. Table 2 shows an overview of current battery technologies divided by battery type. Portable Batteries and General Purpose Portable Batteries can be summarized as consumer batteries or 3C-Batteries, which include batteries for computers, communication and consumers. These 3C-Batteries can be either primary or secondary. Since LMT-LIBs do not operate with capacities of more than 2 kWh, they can also be included in 3C-LIBs for this research. SLI batteries are mostly Pb batteries and are not further evaluated in this paper.

Table 2.
Different Battery Types. Adapted from Ref. [7].
3. Market Analysis Lithium-Ion Batteries
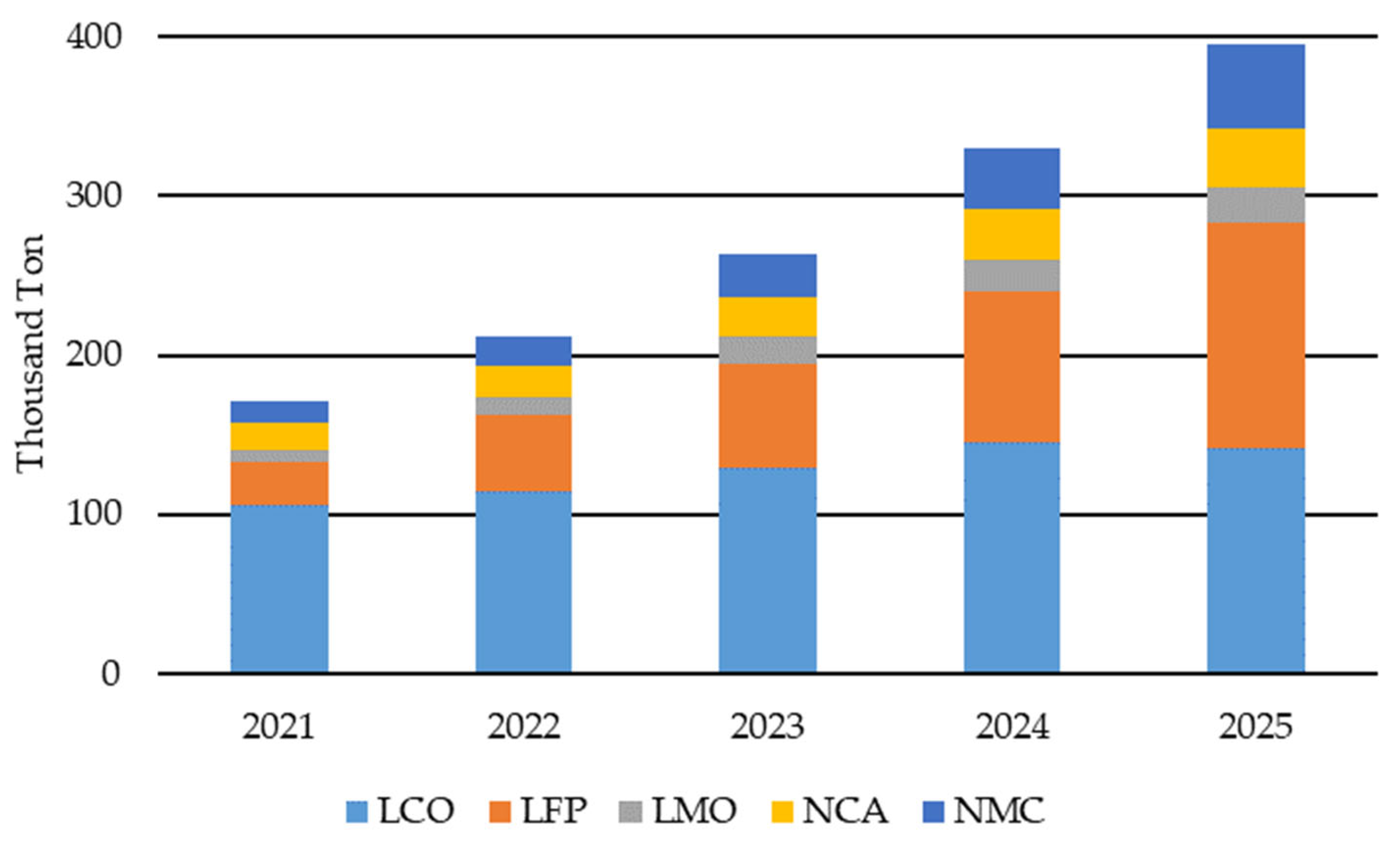
The demand for LIBs has grown steadily since their commercialization in the early 1990s. In the first phase, this was mainly driven by consumer electronics, such as digital cameras, mobile phones and laptops [8]. Subsequently, the electrification of motor vehicles, such as hybrid EVs and plug-in hybrid EVs, contributed to a further increase in demand [9]. In Germany alone, it is estimated that fifteen million private and commercial EVs (pEVs and cEVs) will be needed by 2030 to meet climate targets [10]. In addition to the large, expected increase in demand in the EV segment of between 25 and 50 percent per year, demand increases of up to 15 to 20 percent can also be expected from the sales of cordless household appliances, such as vacuum cleaners [11,12,13]. LIB represents half of the current battery production market [14]. Pb batteries have always been a close second in terms of annual revenue but still dominate the market with production capacity in MWh [15]. By 2030, however, the LIB will be responsible for double the market share of Pb batteries [14]. Other battery types, such as ZnMn, NiMH and NiCd batteries, only make up around 10%. A performance review of one of the German collection schemes shows that approximately 88% of all marketed power tool batteries in 2022 were LIBs, with the others mainly being AlMn, Zn and NiMH batteries [7]. Of the collected spent rechargeable batteries in 2022, 54% were LIBs, 29% NiCd batteries and 17% NiMH batteries. With the current market share of sold LIBs and the EU ban on NiCd batteries, it is expected that the market share of collected spent LIBs will extend significantly over the next few years. While during the introduction of LIBs, only lithium cobalt oxide (LCO) was used, various alternatives have been developed over the last 30 years. In particular, the two cathode active materials, lithium iron phosphate (LFP) and lithium nickel manganese cobalt oxide (NMC), have established themselves with a cumulative market share of about 60 to 65% [15]. In EV applications, lithium nickel cobalt aluminum oxide (NCA) is also used as a cathode active material [16]. Figure 1 shows the global amount of LIBs available for recycling for the years 2021 to 2025. It shows that LCO up until 2025 is still the leading CAM in EoL-LIBs, with LFP, NMC and NCA increasing their share. This can be explained by the increasing number of xEV.
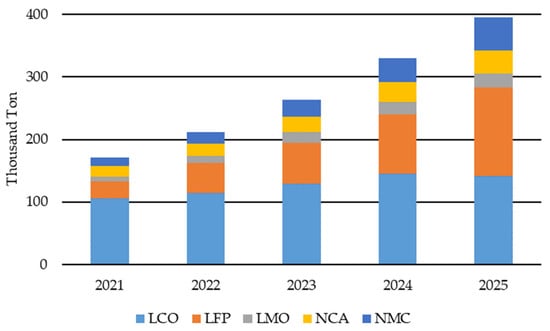
Figure 1.
Lithium-ion batteries (LIB) available for recycling globally: lithium cobalt oxide (LCO), lithium iron phosphate (LFP), lithium manganese oxide (LMO), lithium nickel cobalt aluminum oxide (NCA) and lithium nickel cobalt manganese oxide (NMC). Adapted from Ref. [17].
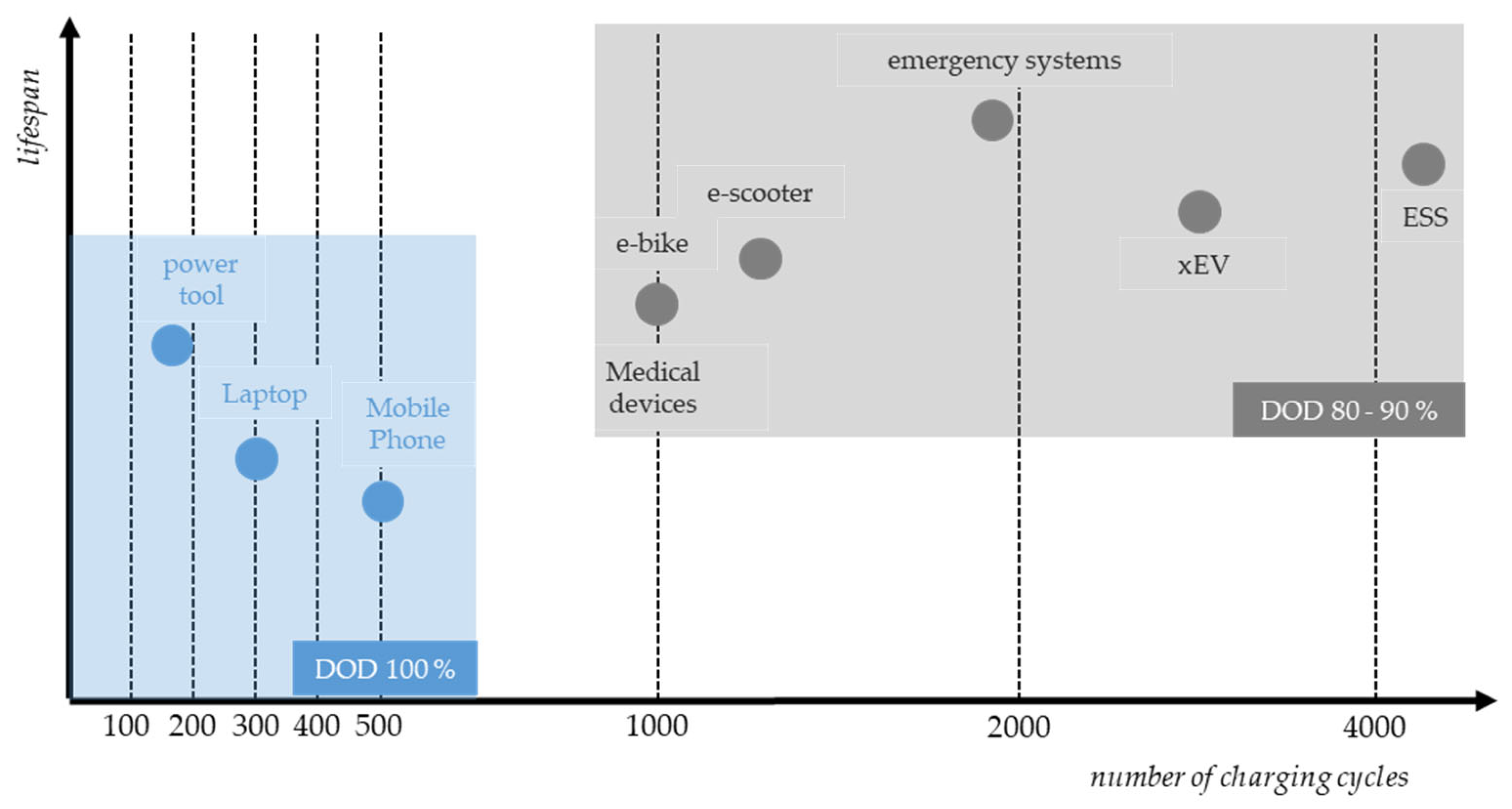
Leading LIB manufacturers are the Chinese companies CATL (approx. 37%) and BYD (approx. 16%), followed by the South Korean company LG Energy Solutions (approx. 14%) [18]. Other companies include Panasonic, SK On, CALB, Sony and Samsung SDI. CATL and BYD mostly produce EV-LIB [19], whereas LG, Panasonic, Sony and Samsung were the manufacturers found in the mass balance study of PT-LIB and phone/laptop LIB (HL-LIB). The evolution of demand is crucial for the evolution of the expected return volumes of LIBs in the EU. Studies by the Fraunhofer Society indicate an average expected lifetime of LIBs for LMT and 3C applications of about eight years and an average expected lifetime for EV applications of about thirteen years [12,13]. Based solely on the assumption of current demand trends, this would result in a 13-year delay in return volumes. However, LIB cells age differently and not every LIB cell has reached the end of its theoretical lifetime when being disposed. The lifetime of LIBs depends on the operating conditions, the materials used, the composition of the electrolyte and the quality of the respective manufacturing processes [20]. Figure 2 shows the average lifetime for some applications. Energy storage systems (ESS) are designed for the highest number of charging cycles and longevity, whereas the design of 3C-LIB is more focused on performance.
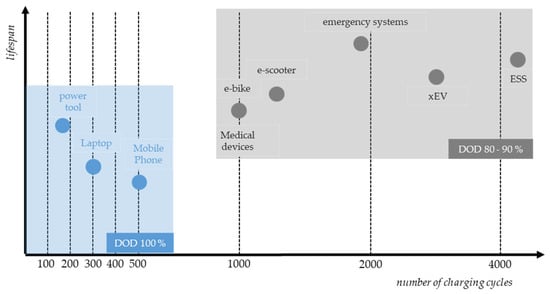
Figure 2.
Lifetime of LIBs in different applications, with and without limitations of the depth of discharge (DOD). Adapted from Ref. [21].
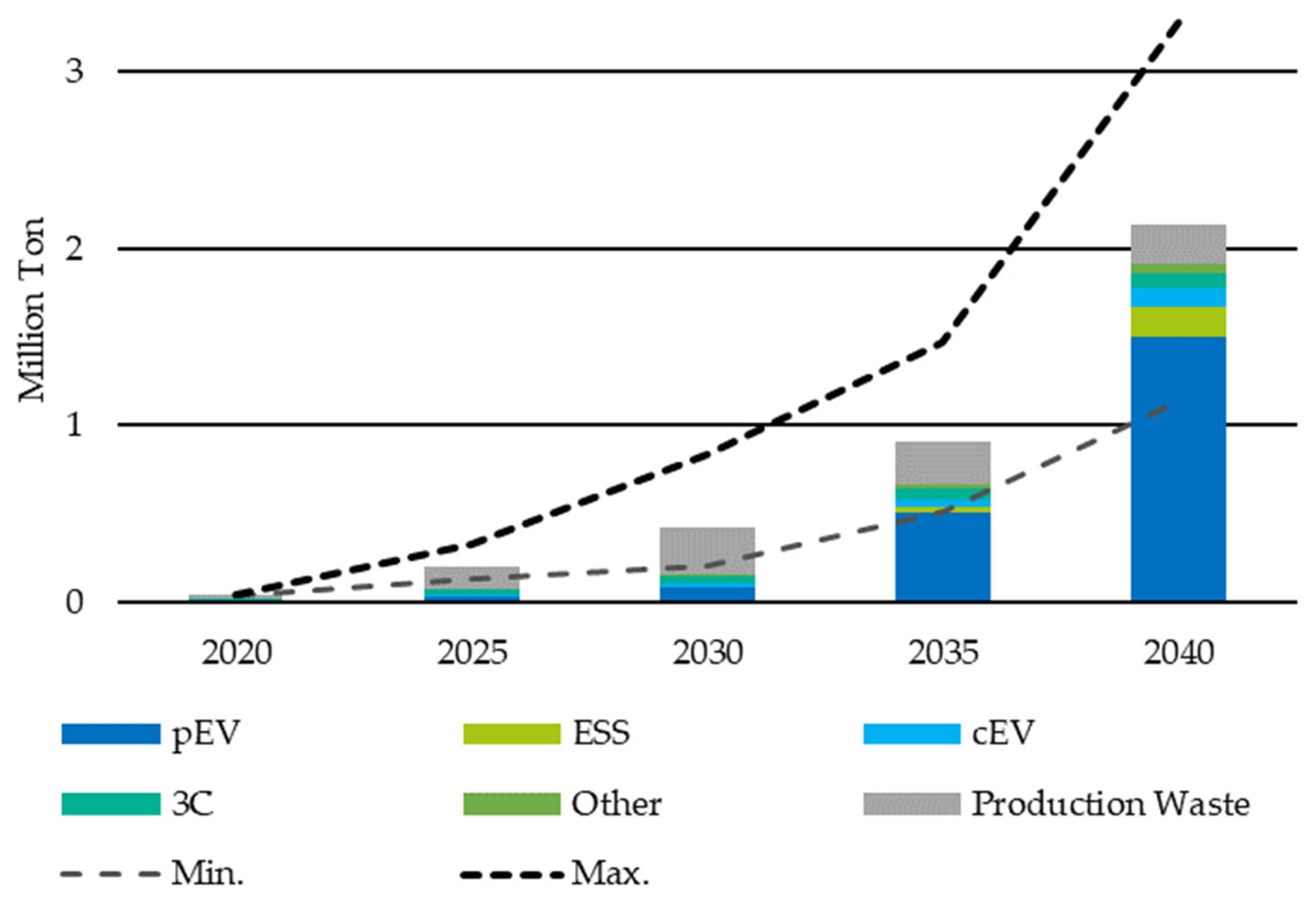
Schmaltz [11] incorporated the various factors into a prediction model for the expected return quantities of LIBs and calculated the quantities accordingly (see Figure 3).
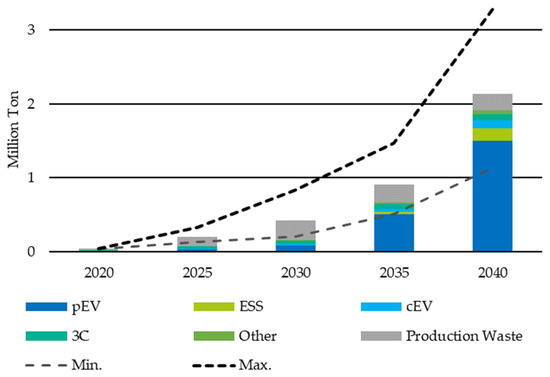
Figure 3.
Forecasted development of the return volume of LIBs in Europe by application: private and commercial Electric Vehicles (pEV, cEV), Energy Storage Systems (ESS), Communication, Computer and Consumer (3C). Reprinted with permission from Ref. [11]. 2023 Fraunhofer ISI.
In addition to the expected high volume of LIBs returned from the EV application, the high volume of production waste is the main factor to be observed. This decreases from 2035 but remains the second largest material stream. While the relative share of the 3C material flow is still about 50 percent of the total material flow in 2020, it decreases to less than 10 percent by 2040. However, the development of the absolute return quantities of 3C-LIB is also of considerable importance for the capacities of sorting and recycling facilities, with an increase of more than 100 percent in 2030 compared to 2020 (approx. 41,600 t to approx. 18,000 t). This indicates an increase in the return volume of 3C-LIBs by more than 200 percent. The current recycling capacities in Europe are around 120,000 t/a [11,22], which indicates the need for increasing capacity and innovative processes.
4. State-of-the-Art Recycling Processes for Lithium-Ion Batteries
The circular economy of LIBs consists of different phases, starting with the extraction of raw materials, through production and the use phase for disposal, reprocessing, recycling and the subsequent substitution of primary raw materials. In particular, the phases from disposal and collection to metallurgical processing into secondary raw materials play a central role in the recycling of the LIBs. In general, the end-of-life processes for LIBs contain most or all of the following process steps:
- Collection and sorting [23,24,25,26,27];
- Disassembly and discharge [28,29,30];
- Thermal deactivation [31,32,33];
- Mechanical processing [30,34,35,36];
- Metallurgical recycling [37,38].
This chapter presents the current state-of-the-art in sorting, processing and recycling LIBs. The dismantling and discharging steps occur mainly in the recycling of LIBs from electric mobility [28]. Depending on the recycling route, thermal deactivation of LIB cells is also required [31].
4.1. Basics of Lithium-Ion Batteries
LIB cells consist of two electrodes (anode and cathode), an electrolyte and a separator. The operation of an LIB cell is based on the principle of reversible lithium-ion intercalation and de-intercalation during charging and discharging [39]. Between the electrodes is an ion-conducting electrolyte containing a dissociated lithium-conducting salt, commonly used is LiPF6. Other components of the electrolyte are organic carbonates, such as diethyl carbonate, dimethyl carbonate and others [39]. A porous plastic film separates the two electrodes from each other and acts as a membrane for lithium ions [20]. The cathode of commercial LIB cells usually consists of a compound that can accept lithium ions, such as transition metal oxides or polyanion compounds [40]. The most commonly used transition metal oxides are the following [21,41,42,43,44,45,46]:
- Lithium cobalt oxide (LiCoO2, LCO),
- Lithium manganese oxide (LiMn2O4, LMO),
- Lithium nickel cobalt manganese oxide (LiNiXMnYCoZO2, NMC),
- Lithium nickel aluminum oxide (LiNi0.8Co0.15Al0.05O2, NCA).
Polyanion compounds include materials such as [21,44,47,48,49]:
- Lithium iron phosphate (LiFePO4, LFP),
- Lithium manganese phosphate (LiMnPO4, LMP),
- Lithium cobalt phosphate (LiCoPO4, LCP).
Aluminum foils are used as the current arrester for cathodes. In commercial use, most anodes use graphite as the anode active material [50,51,52] and a thin copper foil as the current arrester. Table 3 highlights some of the physical and electrochemical characteristics of the most common CAMs.

Table 3.
Different CAMs and Individual Characteristics. Adapted from Refs. [21,40,53].
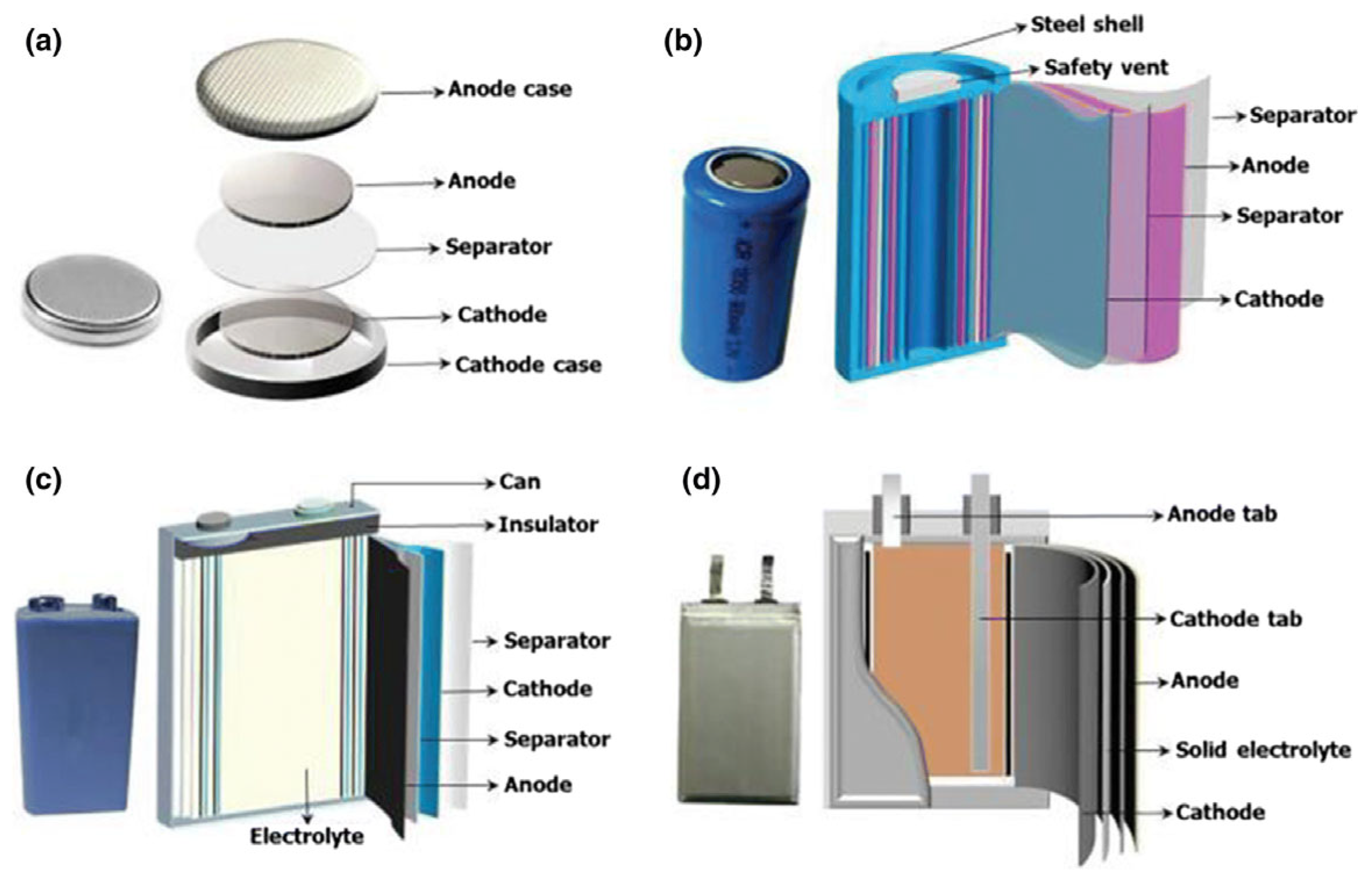
LIB are designed and built as individual cells. These cells are manufactured in various formats, and a distinction is made between button, cylindrical, prismatic and pouch cells [47,54]. Cylindrical cells are manufactured in different layers of anode, separator and cathode, which are rolled around a pin to a so-called jelly roll [55]. The jelly roll is further integrated into a steel shell, which is filled with the electrolyte, closed and welded [55]. Pouch cells are also manufactured with different layers of anode, separator and cathode sheets. These are stacked on top of each other, inserted into the pouch foil, a very thin aluminum-polymer compound, before being filled with the electrolyte and closed [55]. Figure 4 shows the different cell formats and how they are assembled. For cylindrical cells, the 18650 and 21700 cell formats, in particular, have become established [47]. The 4680 cell format is being discussed in particular by American car manufacturer Tesla and will be used there in the future [56,57]. The 18650 cell format is used for portable batteries in the consumer sector. The dimensions of this format are standardized by the American National Standards Institute in the ANSI C18.1M standard [58], while the dimensions of the prismatic and pouch cell formats for automotive applications are standardized by DIN SPEC 91252 [54]. Pouch cells used in the consumer sector for products such as laptops, tablets and phones are currently not subject to any standardization. Depending on the application, several cells can be connected in series and/or in parallel in a module [20]. Modules use a battery management system (BMS) to manage individual cells. The BMS determines the cell voltage and temperatures, monitors the current, and allows the battery system to be switched on and off [20]. For EV batteries, several modules are connected to a battery pack. Current trends also pursue the “cell-to-pack” approach, in which many cells are directly interconnected into a battery pack [59].
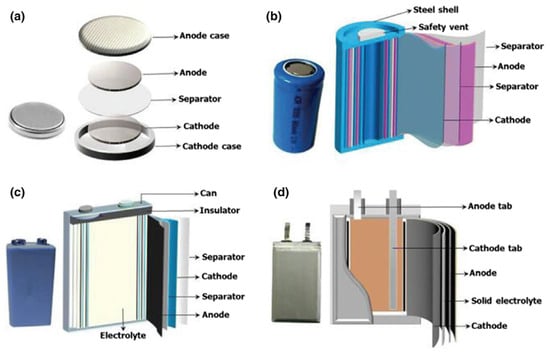
Figure 4.
Different LIB cell formats: (a) button cell, (b) cylindrical cell, (c) prismatic cell, and (d) pouch cell. Adapted from Refs. [60,61].
4.2. Collection and Sorting of Spent Batteries
Since the Battery Directive came into force in 1998, manufacturers and importers of batteries have been required to take back and recycle spent batteries and accumulators free of charge. The legislator provides so-called collection schemes for the return and proper recycling [62]. There are various collection schemes in Germany and other European countries, such as the following:
- Stiftung Gemeinsames Rücknahmesystem GRS-Batterien, today owned by Saubermacher AG [63];
- REBAT or REBAT+ [64];
- DS Entsorgungs- und Dienstleistung GmbH, a subsidiary of the Landbell Group [65];
- Stibat B.V. [66].
In Germany, spent batteries as well as waste electrical appliances must be taken back by every retailer according to § 9 BattG and § 17(1) 2. ElektroG2 [67,68]. For the individual cells and battery packs, there are disposal boxes in which the consumer can separate the used batteries into LIBs and others. Since a mixed waste battery fraction with different chemical compositions cannot be recycled [62,69] and a sorted return by the consumer cannot be guaranteed, sorting based on the chemical composition of the waste batteries is necessary.
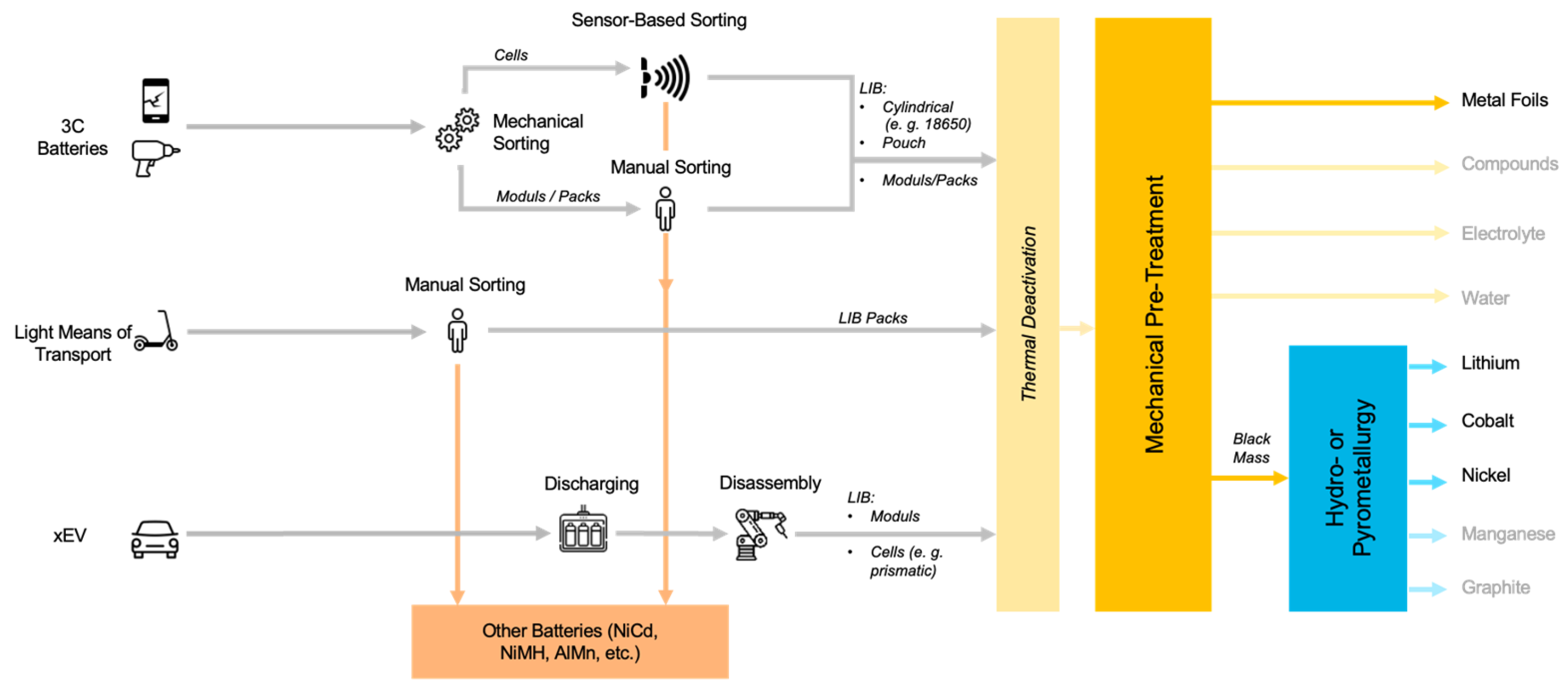
Sorting spent batteries according to their chemical composition can be done in different ways. A distinction is made between mechanical, automatic and manual sorting [62]. Mechanical sorting of individual, general purpose portable batteries is carried out, for example, by sieving, where the spent batteries are sorted according to their size [70]. In manual (or optical) sorting, batteries are sorted by humans based on visual characteristics, such as type designation or manufacturer. Typical sorting speeds are around 300 kg of batteries per person per hour [62]. Currently, every battery sorting facility in Europe performs (partially) the manual sorting of battery cells or battery packs. Automatic sorting of spent batteries according to their chemical composition (Li-ion, NiCd, NiMH, etc.) can be done by sensor-based sorting. In practice, XRT sorting [70], VIS sorting and sorting by weight sensors are used [71]. Currently, sorting is only done based on the battery type but not further by CAM. Figure 5 shows state-of-the-art sorting concepts for EoL batteries. In some research concepts, LIB cells have been further sorted into cobalt-rich and cobalt-poor fractions, but good data on the results have not been published [72].
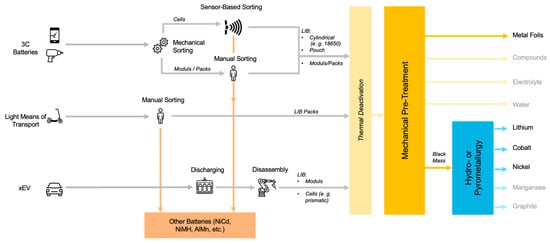
Figure 5.
State of the Art Battery Sorting Concepts (own illustration).
4.3. Mechanical Treatment of Spent Lithium-Ion Batteries
After collection and sorting, LIBs are pre-treated depending on the subsequent mechanical and metallurgical processing steps. These include the processes of deep discharging [73,74,75,76], dismantling [77] and thermal pretreatment [31]. Due to its mostly manual execution, the process of deep unloading is currently only carried out for EV-LIB [78]. 3C-LIBs are largely thermally deactivated [31] or crushed in a wet environment [79], in which case deep discharge is not necessary. Current developments in automated deep discharge are also carried out exclusively for EV applications [80]. LIBs in module, pack or cell format represent a composite material consisting of many different components. To separate them for subsequent material recycling, the various plug-in, welded and bonded joints must be separated from each other. Size reduction has been a proven method in other waste streams, including LIB recycling. Depending on the pre-treatment measures implemented for the LIB, the following forms of mechanical pulping are used:
- Size reduction after thermal deactivation [81];
- Comminution in an inert gas atmosphere [82,83];
- Wet grinding [79,83,84].
Depending on the pre-treatment and mechanical shredding, the (residual) electrolyte components are removed from the shredder output. In the case of thermal deactivation of the cells by means of pyrolysis, the electrolyte components are no longer present in the material stream prior to shredding; thus, electrolyte removal is not necessary in this case. Various processes have been investigated and developed for electrolyte removal after mechanical comminution, such as extraction by thermal drying [85], solvent extraction [86] and extraction by supercritical CO2 [87]. For further processing of the dry, non-organic material stream, the various materials need to be separated from each other. As in the processing of other waste streams, the aim is to sort the different materials as clean as possible. LIBs consist of many different components, some of which have very fine and complex structures and are therefore not easy to separate. An important sub-goal of material separation is the separation of the active materials for further metallurgical processing. Due to the small particle size of the active materials, screening is used [88]. This is typically used immediately after drying or (re)crushing. The separation and recovery of graphite from the active material mixture is performed by selective flotation [89]. This common process has been patented by Retriev Technologies [90] and the Warner Babcock Institute for Green Chemistry [91], among others. For the separation of ferromagnetic materials, a magnetic separator is usually used [92], as in other mechanical processing plants. The further separation of the conductor foils (consisting of aluminum and copper) from the less economically valuable separator foil, made from materials such as polyethylene or polypropylene, can be performed using different technologies. On the one hand, zig zag separators are used to exploit the density differences between the plastic and metal components [30]. In a two-stage separation process, the heavy metal components, such as housing parts, and then the light metal components, such as aluminum and copper foils, can be separated from the plastics. In wet mechanical processes, float-sink separation is another form of density separation to sort the metal and plastic components [79]. An alternative is separation with an eddy current separator. Here, the non-ferrous metals are induced by a rotating electromagnet and are thus separated from the non-magnetizable particles, such as the separator films [51].
4.4. Metallurgical Recycling
Due to the physical and chemical properties of the active materials, such as the small grain size, mechanical processes are not able to further separate the black mass by material components. Metallurgical processes can be used for these processing steps. Pyrometallurgy includes all processes aimed at recovering or refining metals at elevated temperatures [93]. In practice, either an electric arc furnace (EAF) or a shaft furnace (SF) are used to recycle EoL-LIB. These processes are part of extractive pyrometallurgy. Different pre-treatment processes are required for the pyrometallurgical processing of LIBs in EAF and SF. While only the black mass can be processed in EAF, whole LIB cells can also be recycled in SF [93]. Both processes use controlled reduction as the separation process so that the elements nickel, cobalt and copper end up in the metal phase and lithium, manganese, titanium, silicon, aluminum and iron in the slag phase. In this way, recycling efficiencies of around 60 percent can be achieved [93].
Hydrometallurgical recycling of LIBs primarily refers to the recovery of individual valuable metals from cathode active materials. This usually involves a combination of leaching and subsequent extraction processes. Leaching is a key step in the recovery process. The aim of leaching is to bring the metals of the cathode active materials into solution as ions. These can then be recovered via various extraction processes [94]. In hydrometallurgical recycling, a distinction is made between two feed streams depending on the pre-treatment. In the case of pyrometallurgical or thermal pre-treatment of the LIB or active mass, the alloy of copper, nickel, cobalt and iron is brought into solution by leaching. If no thermal pre-treatment is used, e.g., in the case of mechanical processing in an inert atmosphere [95], the metallic components are leached and the insoluble components such as graphite and, if applicable, binder are filtered off [96]. After leaching, the solution is first cleaned by hydroxide precipitation. In this process, copper and aluminum impurities are precipitated with the addition of, e.g., NaOH [97]. For the subsequent extraction of precious metals from the solution, either further precipitation steps or solvent extraction are used [98]. Chemical precipitation of precious metals results in the formation of insoluble compounds through the addition of suitable precipitants [99,100,101,102,103,104,105]. Solvent extraction is a process in which a two-phase system, usually consisting of an organic and an aqueous phase, is introduced. Here, separation can be achieved by the unequal distribution of the two phases, where solvent extraction agents with high selectivity are used after leaching to separate specific transition metals from the leach solution [105,106,107]. The lithium then remains in the solution and can be precipitated, e.g., as lithium carbonate, by adding sodium carbonate [97].
In addition to pyrometallurgy and hydrometallurgy, the direct recycling of the active material is a third alternative. This process has been developed for the reuse of cathode active material from LIB recycling in the production of new LIBs. The process basically consists of two process steps: the recovery of electrode material from LIBs and the subsequent rejuvenation of the recycled electrode material [107]. In this context, the process of re-lithiation by the hydrothermal method, the electrochemical method and the direct calcination method has been extensively researched [108,109]. Success on a laboratory scale has already been achieved in various studies, but industrial implementation has not yet taken place.
5. Discussion
Over the last few years, different recycling processes and strategies for LIBs have been developed. This literature review showed processes on both the industrial and laboratory levels. After reviewing the state-of-the-art technologies, the conclusion can be made that process transitions in particular still lack necessary solutions. Therefore, the following hypothesis for recycling strategies of spent PT-, HL- and LMT-LIBs can be enunciated.
Hypothesis 1.
For technically feasible and economically feasible recycling of cathode active materials, they must be separated and materially pure.
LFP and NMC cannot be processed together due to different precipitation limits [110]. Impurities, especially carbonates, copper and aluminum, have a negative impact on the purity of the resynthesized active material [111]. Hydrometallurgical processing of mixed cathode materials results in lower recoveries of individual elements, and in some cases, elements such as iron or phosphate cannot be recovered at all [94]. LFP can be recovered hydrometallurgically as the individual elements Fe, PO4 and Li, but this is not economically viable. Therefore, LFP should be recovered by direct recycling [112]. Direct recycling requires separate collection or sorting by cathode material.
Hypothesis 2.
Automated sorting is only possible by battery chemistry at the cell level; optical sorting of modules is only possible manually. Sorting by CAM does not currently take place.
Density-based sorting and separation by X-ray transmission (XRT) according to chemical composition is currently only possible by battery type and only in AA and AAA sizes. Current sorting concepts, such as the one used at Relux, require special mechanical pre-sorting of the spent batteries to be able to distinguish between NiCd/NiMH and LIB by XRT [70]. Current sorting systems for spent batteries, such as those from Relux Umwelt GmbH, Redux GmbH and Bebat N.V., manually sort the battery packs from power tools or light mobility equipment based on their battery chemistry [71]. Further processing steps in the optical sorting of battery packs are carried out exclusively mechanically [62]. In some cases, however, the type of designation on the packs is incorrect or may have been destroyed, making it impossible to assign them unambiguously. In a Danish research project, prompt gamma neutron activation analysis was used to sort modules into “cobalt-rich” and “cobalt-poor”, but no data on the sorting result or on an economic and ecological evaluation are available [72].
Hypothesis 3.
A sorting system to separate LIBs by CAM is necessary only for 3C- and LMT-LIB.
While the total return volume for EV-LIBs is significantly higher than the return volume for the 3C- and LMT-LIBs combined, the handling of the latter is a much more difficult challenge than the handling of EV-LIBs. The total return volume in 2030 of 100,000 tons EV-LIBs in 2030 will result in just around 340,000 pEV battery packs [11,113]. For the same year, the 42,000 tons PT-LIBs will result in around 105,000,000 R-PT-LIB battery packs in the EU alone (as shown in Figure 2) [11]. Due to the updated EU battery directive, for all EV- and ESS-LIBs, a battery passport, including the chemical composition of the CAM, will be necessary after 2026 [114]. This battery passport, however, will not be mandatory for all LIBs below a capacity of 2 kWh, which includes all 3C- and LMT-LIBs.
To develop the necessary sorting and recycling strategies for 3C- and LMT-LIBs, an input analysis of the different types has been conducted. This analysis aims especially towards possible automated sorting strategies by CAM.
6. Construction of Consumer Lithium-Ion Batteries
In general, LIBs are being divided into three different application classes: 3C-, industrial and EV-LIBs. 3C-LIBs are small battery cells, modules or packs being used, for example, in power tools, laptops or smartphones, and can be classified into portable batteries and general purpose batteries [114]. In this case, LMT-LIBs shall also be classified into consumer batteries. PT-LIBs are usually compact and operate in the voltage range up to 36 V. A distinction is made between applications for commercial use (up to 18 volts) and applications for professional use (up to 36 volts) [115]. Basically, PT-LIBs consist of a housing made of technical polymers (such as PA6), the cell pack, the screw connections, and the peripherals consisting of printed circuit board, cables, etc.
For a representative study of the assembly of PT-LIBs, 79 PT-LIBs were dismantled and balanced on three different days (19 June 2023, 27 June 2023, 13 July 2023, 29 November 2023) at the Löhne (NRW) and Kuppenheim (Baden-Württemberg) sites of Relux Umwelt GmbH and Circu Li-Ion GmbH. Figure 6 shows an exemplary, dismantled PT battery pack with different materials used. The PT-LIB has been manually dismantled and sorted into four categories:
- Casing (external and internal);
- Screws;
- Cells and;
- BMS.

Figure 6.
Structure of PT battery pack type TM 5INR19/65-2, voltage 18 V, capacity 4000 mAh, manufacturer: Robert Bosch Powertools GmbH, Leinfelden-Echterdingen, Germany (top left: overall view of the module, top right: dismantled PA66 housing halves, bottom left: cell pack 10 × 3.6 18650 LIB, bottom right: HDPE inner housing).
Figure 6.
Structure of PT battery pack type TM 5INR19/65-2, voltage 18 V, capacity 4000 mAh, manufacturer: Robert Bosch Powertools GmbH, Leinfelden-Echterdingen, Germany (top left: overall view of the module, top right: dismantled PA66 housing halves, bottom left: cell pack 10 × 3.6 18650 LIB, bottom right: HDPE inner housing).

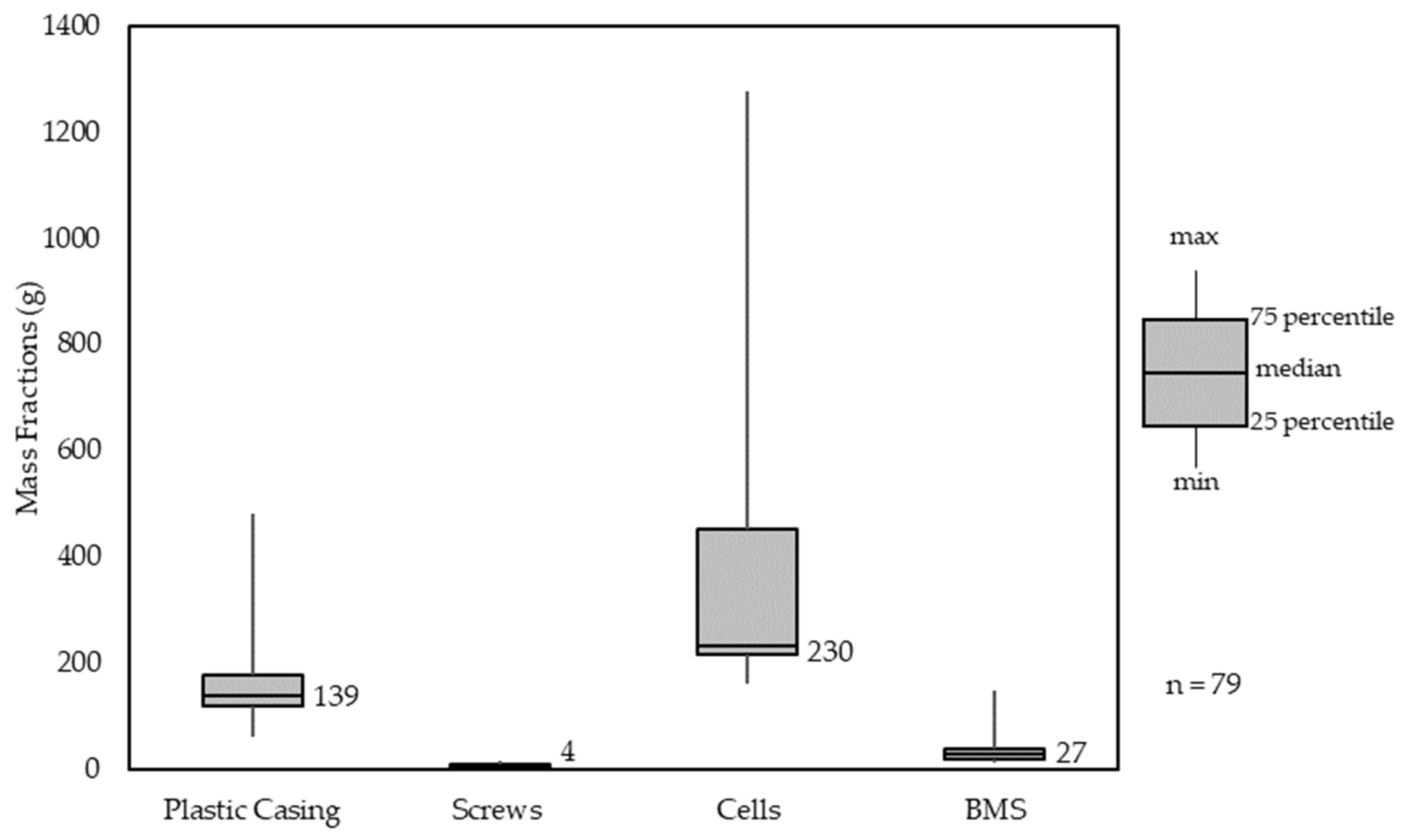
Of PT-LIBs, 82% contained either 4, 5, 8 or 10 individual 18650 cells with an average weight of 44.79 g per cell. The median PT-LIB included 230 g of 18650 cells, which resulted in 5.1 cells per battery pack (see Figure 7). The minimum number of cells in PT-LIBs was 4, with 50% containing either 4 or 5 cells. Overall, the number of cells used per pack varies a lot more than in other pack components. This needs to be taken into account for further sorting strategies. PT-LIBs are built with a polymer casing, most of the time also with inner polymer housing for cell stability while moving. The used polymers are all engineering polymers such as PA6, PA66, ABS, PC and HDPE. On average, there have been 4 steel screws used per battery pack with an individual weight of approximately 1 g. The residual material used is aluminum, copper cables, and printed circuit boards for the BMS. All, BMS, polymer casing and screws are relatively similar in mass content over all analyzed packs.
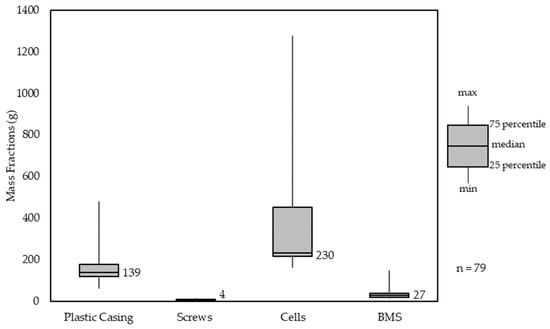
Figure 7.
Material Composition Analysis of power tool battery packs (PT-LIBs).
LMT-LIBs are interconnected as battery packs from different cells. In practice, packs of 18650 or 21700 LIB cells are the most common, but packs of interconnected prismatic cells are also found. Only pouch cells have not yet been used in light mobility applications. This may be due to high mechanical stress and limited space. Figure 8 shows an example of a battery pack using 18650 cells for an e-bike application. Five different LMT battery packs have been manually disassembled and classified. Those are from the manufacturers TIER, NINEBOT, BMZ and Derby Bike.

Figure 8.
Lithium-ion battery pack Bosch Powerpack Classic 300/400/500—36 V with 500 Wh. Adapted from Ref. [116].
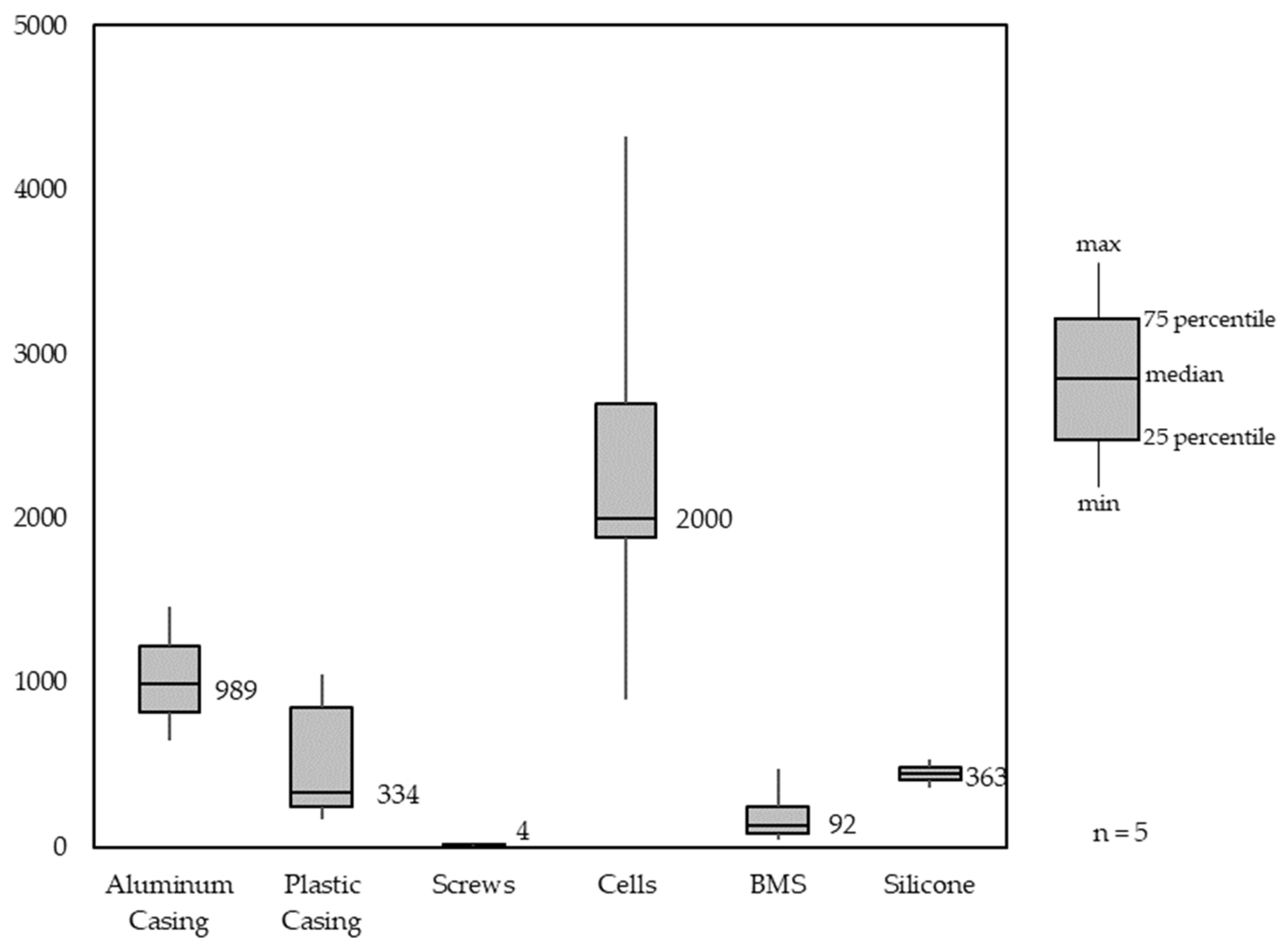
Figure 9 shows the material composition analysis for different LMT-LIBs. LMT-LIB battery packs, similar to PT-LIB battery packs, also contain two different casings, inner and outer housing. The difference here is that LMT-LIB housing halves are mostly made of aluminum, and only the inner housing is made of engineering polymers. For stability in some LMT-LIB battery packs, silicone is being used. The median number of cells being used in LMT-LIB battery packs is 40, with both cell formats, 21700 and 18650, being used.
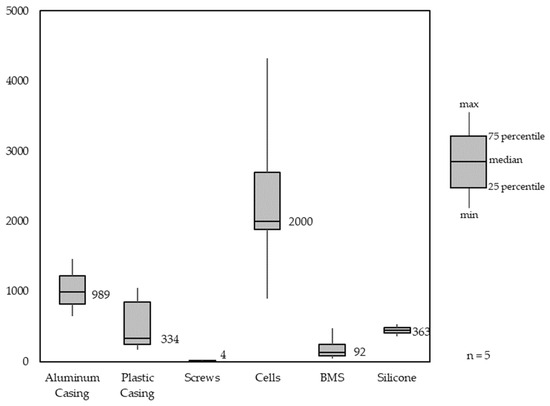
Figure 9.
Material Composition Analysis of light means of transport battery packs (LMT-LIB).
Similar to PT-LIB packs, LMT-LIB packs have the largest variety in the number of used cells per pack, which needs to be addressed when looking into different sorting strategies. Also, the casing material varies in different LMT packs. HL-LIBs not only have high performance requirements but also high geometric requirements due to their installation in mobile devices. There has been a particular evolution in the design of LIBs for laptops. When laptops were first introduced, LIBs were used in the form of modules containing 18650 cells (see Figure 10a), whereas more recent models mainly used thin pouch cells (see Figure 10b). LIBs for mobile phones and tablets have high geometric requirements, especially in terms of cell thickness. Due to the similar designs and sizes of today’s smartphones/cell phones, the declining mobile phone LIB has similar designs, whereas the laptop LIB has a high geometric variance. In contrast to PT-LIBs, HL-LIBs have lower power requirements and higher geometric requirements.

Figure 10.
Exemplary LIBs from smartphone, tablet and laptop usage: (a) spent laptop LIB cell type TM00741, Acer Inc. (New Taipei City, China), cylindrical cell, voltage 11.1 V, capacity 4400 mAh; (b) spent tablet LIB cell type SM-T230NW, Samsung (Suwon, Republic of Korea), pouch cell, voltage 3.65 V, capacity 3050 mAh.
7. State-of-the-Art Sensor-Based Sorting Technologies
After analyzing the different 3C-LIBs and their material composition, a literature review of different sensor-based sorting (SBS) technologies has been performed, to identify possible state-of-the-art online sorting technologies for LIBs. SBS is an automatic sorting process based on the evaluation of data collected by various sensors. In the circular economy, SBS is used in many waste streams, such as paper, plastic, metals and glass. The sensor sorting techniques relevant to battery sorting are explained below.
7.1. X-ray Transmission Technology
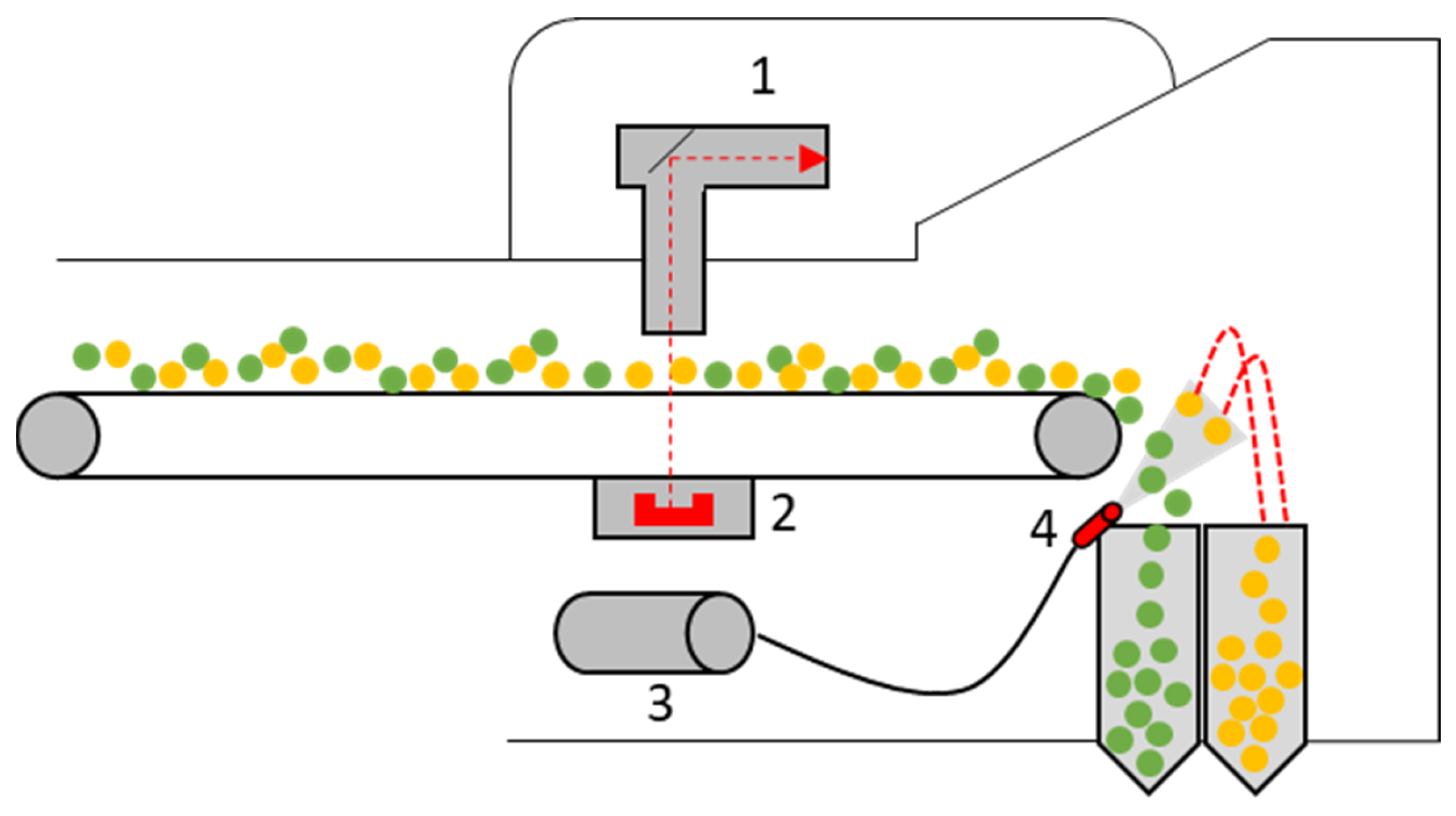
In X-ray transmission (XRT), X-rays are emitted at a defined wavelength by an X-ray source [117]. These interact with the particle mass of the object as they radiate through it [117]. Parts of the X-rays are absorbed by the particle mass. The degree of absorption depends on various factors, such as material composition and material density [117,118,119]. The transmitted X-rays are measured, for example, by a two-line scintillator and displayed as two-dimensional X-ray images on a gray scale [117,118,120,121]. In practice, XRT sorters are mostly used for sorting heavy and light metals, e.g., aluminum and copper [120,122]. Such a sorting device usually consists of the following individual parts: X-ray emitter; X-ray detector; hydraulic unit; and air pressure valve [119]. Figure 11 shows an example of such an XRT sorting device. When sorting using XRT, the material is transported via a conveyor belt or a vibrating feeder. The X-ray tube emits the radiation, and the object is scanned. The transmitted radiation is then absorbed by the X-ray receiver (usually below the conveyor belt) and output as a 2D density profile using an imaging processing algorithm. The object can be classified on the basis of the X-ray image and—if desired—removed from the material flow by a blast of compressed air.
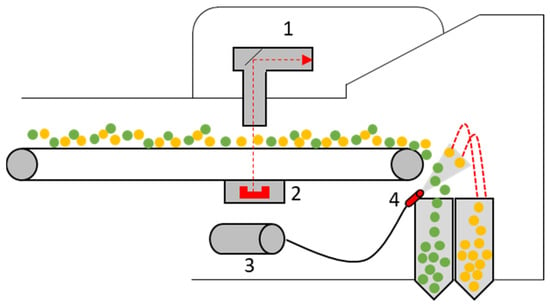
Figure 11.
Schematic structure of an automatic X-ray transmission (XRT) sorting device: 1—X-ray tube, 2—X-ray detector, 3—hydraulic unit, 4—air pressure valve. Green–Accept, Yellow–Reject. Adapted from Ref. [119].
Using XRT sorting, objects can be recognized and sorted based on the density, shape, size, contour and purity of the particles [121,123]. Limitations include the object size; modern XRT sorting systems can recognize and sort objects up to a particle size of approximately 5 mm with corresponding parameters [123]. Current state-of-the-art XRT sorting systems often use so-called “dual-energy systems” [119,123,124]. Here, X-rays are emitted at two different energy levels (high and low energy measurement) to enable sorting independent of the object density and exclusively according to atomic density [117,119,124]. Manufacturers of XRT sorting devices include the companies Tomra Sorting GmbH (Tomra X-Tract), Steinert GmbH (Steinert XSS) and Allgaier Process Technology GmbH (Msort X-Ray) [119,121,125,126,127].
7.2. X-ray Fluorescence Technology
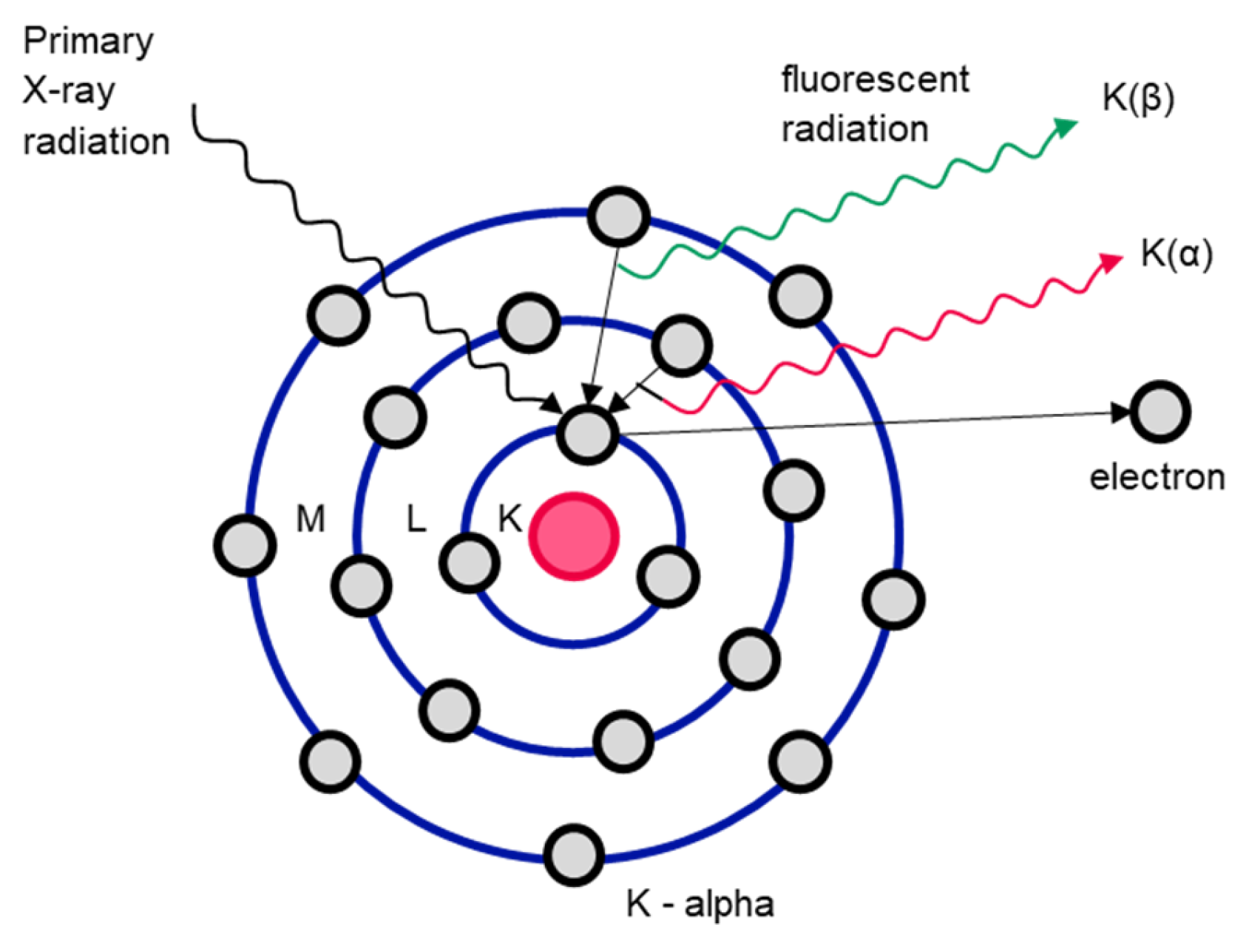
X-ray fluorescence analysis is used to characterize the elements of individual objects. In this process, an object is irradiated with X-rays. If the energy of the incident radiation is higher than the binding energy of the electrons on the orbital (ionization energy), electrons are emitted [128]. The vacated space on the orbital is then taken by an electron on one of the outer orbitals. The binding energy of the electrons on the outer orbitals is lower than that on the inner orbitals, which releases energy that is emitted as so-called X-ray fluorescence radiation (see Figure 12). This fluorescence radiation is characteristic for each element and for each individual element transition [119].
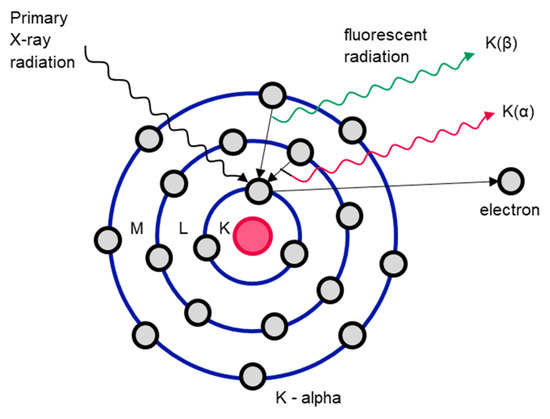
Figure 12.
Functional diagram of X-ray fluorescence analysis of the Bohr atomic model. Adapted from Ref. [129].
Element characterization based on X-ray fluorescence radiation can be carried out offline using X-ray fluorescence analysis (RFA) or online (XRF). XRF sorting is primarily used in glass sorting or the sorting of non-ferrous metals [128]. Similar to XRT sorting, XRF sorting is carried out by irradiating the objects with X-rays, whereby in XRF sorting, the wavelength of the fluorescence radiation is then measured, which provides information about the chemical composition of the objects [118]. The analysis based on fluorescence radiation is only carried out on the surface of the objects and is therefore only of limited use for objects made of material composites [118,130].
7.3. Optical Sorting—Visual and Near-Infrared
In addition to X-ray analysis, optical sorting is a core SBS technology for waste streams. Optical sorting uses the optical characteristics of the objects to be sorted, such as shape or color [130]. A distinction is made between the following types of optical SBS [119,121,130,131]:
- Detection and sorting of objects in the visible light spectrum according to shape and color (VIS);
- Irradiation, detection and analysis of reflected radiation in the near-infrared spectrum (NIR);
- Recognition and sorting of objects based on 3D shape models using 3D laser triangulation (3D-LT).
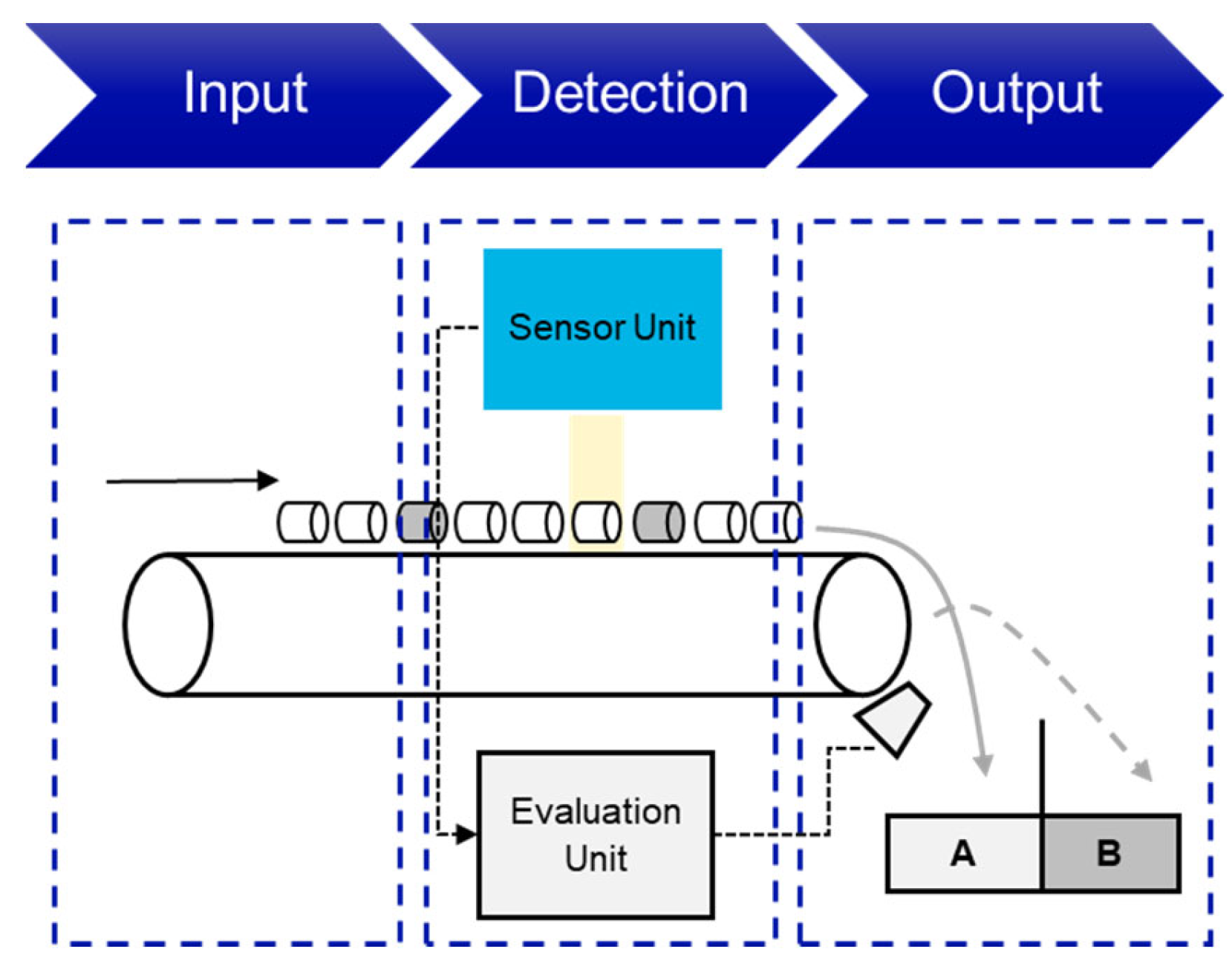
Figure 13 shows an example of the structure and mode of operation of an SBS system. Similar to XRT and XRF sorting, the objects are fed onto a conveyor belt, a vibrating feeder or similar. The objects are then detected by one of the above-mentioned sensors and analyzed by the evaluation unit. Depending on the settings and setup, a compressed air valve is then opened, for example, to sort out the desired object.
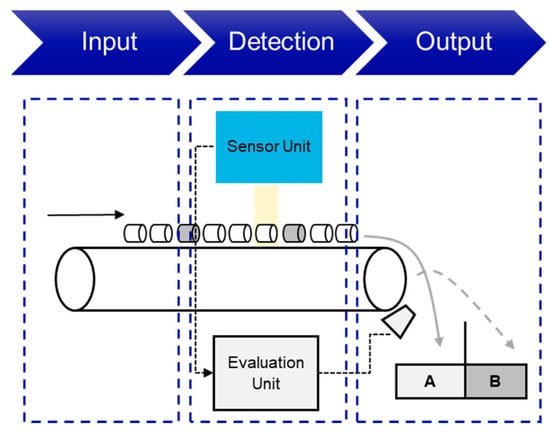
Figure 13.
Conceptual representation of an optical sensor-based sorting system (own illustration). A–Accept, B–Reject.
In optical color sorting, objects are differentiated based on their color, transparency and brightness in the visible light spectrum [130]. Color sorting is a surface-intensive analysis and sorting process, which is why coated objects (similar to XRF sorting) cannot be identified [121]. A high contrast between the objects and the background helps to optimize the detection and differentiation of the individual objects, e.g., black objects on a black conveyor belt are not detected [132]. However, visual camera systems (RGB) can only detect a 2D profile of the objects; 3D-LT can also be used to determine the surface of an object and thus its exact shape [133]. NIR sorting uses the near-infrared spectrum to identify the element-specific characteristics of the object surface [119]. Here, the objects are irradiated and the reflected radiation in the near-infrared spectrum is recorded via a detection unit and analyzed accordingly via an evaluation unit [130].
7.4. Prompt Gamma Neutron Activation Analysis
Prompt gamma neutron activation analysis (PGNAA) uses the neutron beam generated by a neutron source to irradiate the nucleus of various elements in a sample [119]. The irradiated element produces a neutron-receiving reaction, which radiates back as gamma radiation [119]. Californium-252 or americium beryllium are usually used as the neutron source [134]. The advantage of PGNAA is its high accuracy [119] and continuous measurement over the entire production cross-section [134]. In practice, PGNAA is mainly used for process control in the mineral sector [134].
8. Sorting Strategies for Spent Consumer Lithium-Ion Batteries by Cathode Active Material
This paper reviewed state-of-the-art sorting and recycling processes for LIBs. To achieve the high recovery targets within these processes, a pre-sorting system by CAM needs to be developed. A pre-sorting system by CAM needs only be developed for LIBs below a capacity of 2 kWh. For this size, different cells, modules and packs have been identified, especially 3C- and LMT-LIBs. LIBs can be differentiated by optical, physical or chemical features. Optical features can include brand and size. Physical and chemical features are not only the element composition but also atomic/material density and cell voltage (see Table 2). Based on the SBS described and reviewed in Section 7, a classification by CAM should be able for 3C-LIBs as follows:
Cylindrical cells, in particular 18650 cells, can be sorted optically via serial number and/or cell voltage. Often, one or both of these are printed on the cell casing or the sleeve around it. To have an online sorting system in operation, a database with necessary information needs to be in place and continuously updated. A challenge for the optical sorting of spent cylindrical cells is the condition of the cells. Cells in a bad condition or with missing serial number/cell voltage, may not be able to be identified via optical sensors. Cylindrical cells should be sorted via XRT based on their atomic density. Table 2 presents the different theoretical densities for each of the five major CAM. The cells were then analyzed and differentiated via a gray scale model, as explained in Section 7.1. Since the cell casing of cylindrical cells is made out of a thick layer of steel or aluminum, XRF technology is not applicable to identify the element composition of the CAM, it would only identify the casing’s elemental composition. An identification with PGNAA could be possible, however there is yet missing data to support the hypothesis.
Similar to individual cylindrical cells, PT-LIB can be sorted optically via serial number and/or cell voltage. Often, one or both of these are printed on the pack casing. To have an online sorting system in operation, a database with necessary information needs to be in place and continuously updated. A challenge for the optical sorting of spent PT-LIBs is the condition of the packs. Packs in bad condition or with missing serial number/cell voltage, may not be able to be identified via optical sensors. Furthermore, optical sorting is often carried out on a conveyor belt, where one side of the PT-LIB would be unable to be identified via camera systems. PT-LIBs should also be able to be sorted via XRT based on their atomic density. Since cylindrical cells are used in PT-LIBs, XRF technology is not applicable for identifying the element composition of CAM. An identification with PGNAA may not be possible due to the thick plastic casing around the individual cells.
HL-LIBs can also be sorted optically via serial number and/or cell voltage. Often, one or both of these are printed on the cell casing or sleeve around it. To have an online sorting system in operation, a database with necessary information needs to be in place and continuously updated. For optical sorting, similar challenges regarding the recognition of relevant information as in cylindrical cells and PT-LIBs can be expected. A density-based sorting system via XRT may also be applicable for HL-LIBs and pouch cells. Since pouch cells only use thin cell envelopes made of aluminum-polymer compounds, XRF technology should also be applicable. Here, the X-ray radiation can radiate through the thin layer of the aluminum-polymer compound and reflect the specific fluorescent radiation of the black mass. An identification with PGNAA may be possible; however, there are yet missing data to support the hypothesis.
A sorting system for LIBs by CAM is necessary and should be possible based on the literature reviewed. However, there are yet data to be collected to support the hypotheses. Further research needs to be done to develop classification and decision-making parameters to engineer a sorting system for LIB by CAM.
9. Summary and Outlook
Increasing demand for LIBs in Europe and worldwide will result in an exponential growth in returned spent LIBs over the next 15 to 25 years. The updated EU battery directive requires higher material recovery rates for materials, such as lithium, cobalt, nickel, manganese and copper. Also, mandatory recycling material content in production is introduced. State-of-the-Art EoL strategies for spent LIBs use collection systems to accumulate spent batteries and sort them by battery type (AlMn, NiMH, LIB, etc.). These sorting systems mostly use manual and semi-automatic processes. A mixed LIB waste stream, not separated by CAM, enters a thermal deactivation process before the mechanical pre-treatment processes. In this pre-treatment, different mechanical separation technologies (such as shredders, screens and zig zag separators) are used. The produced black mass is further hydro- or pyrometallurgically treated to recover valuable materials, such as cobalt, nickel and copper. To achieve the high recycling targets in the EU battery directive, a CAM pre-sorting system needs to be implemented. This paper reviewed state-of-the-art processes and three distinct hypotheses have been identified:
- For technically feasible and economically feasible recycling of cathode active materials, they must be separated and materially pure.
- Automated sorting is only possible by battery chemistry at cell level; optical sorting of modules is only possible manually. Sorting by CAM does not currently take place.
- A sorting system to separate LIBs by CAM is necessary only for 3C- and LMT-LIBs.
To develop sorting strategies for spent 3C- and LMT-LIBs, 79 PT- and 5 LMT-LIB packs have been manually disassembled and analyzed. Furthermore, state-of-the-art SBS technologies have been discussed and possible sorting strategies for each cell/pack type have been presented. XRT, XRF, PGNAA and optical sorting systems have been presented. The following SBS technologies have been identified:
- XRT for Cylindrical Cells, PT-LIB, LMT-LIB and Pouch Cells;
- XRF for Pouch Cells;
- VIS for Cylindrical Cells, PT-LIB and Pouch Cells.
To evaluate these possible sorting strategies, further research needs to be conducted. Next steps should include feasibility studies on whether, with the use of the described SBS technologies, a distinction between the different CAMs used can be made.
Author Contributions
Conceptualization, M.P. and S.F.; methodology, M.P.; formal analysis, M.P.; investigation, M.P.; resources, M.P.; data curation, M.P.; writing—original draft preparation, M.P.; writing—review and editing, M.P. and S.F.; visualization, M.P.; supervision, S.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge the kind support in form of material donation by Circu Li-Ion GmbH and Relux Umwelt GmbH.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- European Commission. Communication from the Commission: The European Green Deal; COM(2019) 640 Final; European Commission: Brussels, Belgium, 2019; Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:52019DC0640 (accessed on 7 December 2023).
- European Commission. RMIS—Raw Materials Information System: Battery Supply Chain Challanges. Available online: https://rmis.jrc.ec.europa.eu/analysis-of-supply-chain-challenges-49b749 (accessed on 15 January 2024).
- European Union. Regulation (EU) 2023/1542 of the European Parliament and of the Council of 12 July 2023 Concerning Batteries and Waste Batteries, Amending Directive 2008/98/EC and Regulation (EU) 2019/1020 and Repealing Directive 2006/66/EC: Regulation (EU) 2023/1542; European Union: Brussels, Belgium, 2023. [Google Scholar]
- Chen, Q.; Lai, X.; Gu, H.; Tang, X.; Gao, F.; Han, X.; Zheng, Y. Investigating carbon footprint and carbon reduction potential using a cradle-to-cradle LCA approach on lithium-ion batteries for electric vehicles in China. J. Clean. Prod. 2022, 369, 133342. [Google Scholar] [CrossRef]
- European Union. Commission Regulation (EU) No 493/2012 of 11 June 2012 Laying down, Pursuant to Directive 2006/66/EC of the European Parliament and of the Council, Detailed Rules Regarding the Calculation of Recycling Efficiencies of the Recycling Processes of Waste Batteries and Accumulators: Commission Regulation (EU) No 493/2012; European Union: Brussels, Belgium, 2012. [Google Scholar]
- Petrikowski, F.; Kohlmeyer, R.; Jung, M.; Steingrübner, E.; Leuthold, S. Batterien und Akkus: Ihre Fragen—Unsere Antworten zu Batterien, Akkus und Umwelt; Dessau, Germany. 2012. Available online: www.umweltbundesamt.de/sites/default/files/medien/publikation/long/4414.pdf (accessed on 22 November 2023).
- Hobohm, J. Erfolgskontrolle 2022 GRS Powertools: Gemäß § 15 (1) Batteriegesetz; GRS Batterien Service GmbH: Hamburg, Germany, 2023; Available online: https://www.grs-batterien.de/fileadmin/Downloads/Erfolgskontrollen/EK22_GRS_Powertools_N.pdf (accessed on 26 October 2023).
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 Years of Lithium-Ion Batteries. Adv. Mater. 2018, 30, e1800561. [Google Scholar] [CrossRef]
- Scrosati, B.; Garche, J. Lithium batteries: Status, prospects and future. J. Power Sources 2010, 195, 2419–2430. [Google Scholar] [CrossRef]
- Presse- und Informationsamt der Bundesregierung. Climate Action Programme 2030. Available online: https://www.bundesregierung.de/breg-en/issues/climate-action (accessed on 22 May 2023).
- Schmaltz, T. Recycling of Lithium-Ion Batteries will Increase Strongly in Europe: Quantity Scenarios of Lithium-Ion Batteries for Recycling and Their Origin. Available online: https://www.isi.fraunhofer.de/en/blog/themen/batterie-update/recycling-lithium-ionen-batterien-europa-starke-zunahme-2030-2040.html (accessed on 10 October 2023).
- Thielmann, A.; Sauer, A.; Wietschel, M. Gesamt-Roadmap Lithium-Ionen-Batterien 2030; Fraunhofer: Karlsruhe, Germany, 2015; Available online: https://www.isi.fraunhofer.de/content/dam/isi/dokumente/cct/lib/GRM-LIB.pdf (accessed on 18 April 2023).
- Paulsen, T.; Bürkin, B.; Latz, T.; Schenk, L.; Degen, F.; Diehl, M.; Krätzig, O.; Wessel, S.; Kampker, A.; Lackner, N.; et al. Umfeldbericht zum europäischen Innovationssystem Batterie 2022; Fraunhofer: Karlsruhe, Germany, 2022. [Google Scholar]
- Inkwood Research. Size of the Global Battery Market from 2018 to 2021, with a Forecast through 2030, by Technology (in Million U.S. Dollars). Available online: https://www.statista.com/statistics/1339880/global-battery-market-size-by-technology/ (accessed on 26 October 2023).
- Pillot, C. The Rechargeable Battery Market and Main Trends 2016–2025. Available online: http://cii-resource.com/cet/FBC-TUT8/Presentations/Pillot_Christophe.pdf (accessed on 15 October 2023).
- Lander, L.; Cleaver, T.; Rajaeifar, M.A.; Nguyen-Tien, V.; Elliott, R.J.R.; Heidrich, O.; Kendrick, E.; Edge, J.S.; Offer, G. Financial viability of electric vehicle lithium-ion battery recycling. iScience 2021, 24, 102787. [Google Scholar] [CrossRef] [PubMed]
- Melin, H.E. The Lithium-Ion Battery End-of-Life Market: A Baseline Study; World Economic Forum: Cologny, Switzerland, 2018; Available online: https://policycommons.net/artifacts/3375527/the-lithium-ion-battery-end-of-life-market/4174374/ (accessed on 13 January 2024).
- Venditti, B.; Lam, S. The Top 10 EV Battery Manufacturers in 2022. Available online: https://www.visualcapitalist.com/the-top-10-ev-battery-manufacturers-in-2022/ (accessed on 26 October 2023).
- CnEVPost. Global Market Distribution of Lithium-Ion Battery Makers between January and August 2023. Available online: https://www.statista.com/statistics/235323/lithium-batteries-top-manufacturers/ (accessed on 26 October 2023).
- Leuthner, S. Übersicht zu Lithium-Ionen-Batterien. In Handbuch Lithium-Ionen-Batterien, 1st ed.; Korthauer, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 13–19. ISBN 978-3-642-30652-5. [Google Scholar]
- Dorrmann, L.; Sann-Ferro, K.; Heininger, P.; Mähliß, J. Kompendium: Li-Ionen-Batterien: Grundlagen, Merkmale, Gesetze und Normen. Available online: https://www.dke.de/resource/blob/933404/fa7a24099c84ef613d8e7afd2c860a39/kompendium-li-ionen-batterien-data.pdf (accessed on 1 May 2023).
- Hartmann, S. Deutschland in Europa Vorreiter bei Recyclinganlagen für Lithium-Ionen-Batterien. Available online: https://www.euwid-recycling.de/news/wirtschaft/deutschland-in-europa-vorreiter-bei-recyclinganlagen-fuer-lithium-ionen-batterien-240522/ (accessed on 12 July 2022).
- Oliveira Neto, G.C.; Ruiz, M.S.; Correia, A.J.C.; Mendes, H.M.R. Environmental advantages of the reverse logistics: A case study in the batteries collection in Brazil. Production 2018, 28, e20170098. [Google Scholar] [CrossRef]
- Nigl, T.; Schwarz, T.E.; Walch, C.; Baldauf, M.; Rutrecht, B.; Pomberger, R. Characterisation and material flow analysis of end-of-life portable batteries and lithium-based batteries in different waste streams in Austria. Waste Manag. Res. 2020, 38, 649–659. [Google Scholar] [CrossRef]
- Ponce-Cueto, E.; Manteca, J.Á.G.; Carrasco-Gallego, R. Reverse Logistics for Used Portable Batteries in Spain: An Analytical Proposal for Collecting Batteries; Springer: Berlin/Heidelberg, Germany, 2011; pp. 593–604. [Google Scholar]
- Rogulski, Z.; Czerwiński, A. Used batteries collection and recycling in Poland. J. Power Sources 2006, 159, 454–458. [Google Scholar] [CrossRef]
- Terazono, A.; Oguchi, M.; Iino, S.; Mogi, S. Battery collection in municipal waste management in Japan: Challenges for hazardous substance control and safety. Waste Manag. 2015, 39, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Zorn, M.; Ionescu, C.; Klohs, D.; Zähl, K.; Kisseler, N.; Daldrup, A.; Hams, S.; Zheng, Y.; Offermanns, C.; Flamme, S.; et al. An Approach for Automated Disassembly of Lithium-Ion Battery Packs and High-Quality Recycling Using Computer Vision, Labeling, and Material Characterization. Recycling 2022, 7, 48. [Google Scholar] [CrossRef]
- Wegener, K.; Andrew, S.; Raatz, A.; Dröder, K.; Herrmann, C. Disassembly of Electric Vehicle Batteries Using the Example of the Audi Q5 Hybrid System. Procedia CIRP 2014, 23, 155–160. [Google Scholar] [CrossRef]
- Hanisch, C.; Loellhoeffel, T.; Diekmann, J.; Markley, K.J.; Haselrieder, W.; Kwade, A. Recycling of lithium-ion batteries: A novel method to separate coating and foil of electrodes. J. Clean. Prod. 2015, 108, 301–311. [Google Scholar] [CrossRef]
- Arnberger, A. Entwicklung Eines Ganzheitlichen Recyclingkonzeptes für Traktionsbatterien Basierend auf Lithium-Ionen-Batterien. Ph.D. Thesis, Montanuniversität Leoben, Leoben, Austria, 2016. [Google Scholar]
- Vest, M. Weiterentwicklung des Pyrometallurgischen IME Recyclingverfahrens für Li-Ionen Batterien von Elektrofahrzeugen. Ph.D. Thesis, RWTH Aachen University, Aachen, Germany, 2016. [Google Scholar]
- Georgi-Maschler, T. Entwicklung Eines Recyclingverfahrens für Portable Li-Ion-Gerätebatterien. Ph.D. Thesis, RWTH Aachen University, Aachen, Germany, 2011. [Google Scholar]
- Arnberger, A.; Coskun, E.; Rutrecht, B. Recycling von Lithium-Ionen-Batterien. In Recycling und Rohstoffe; Thiel, S., Thomé-Kozmiensky, E., Goldmann, D., Eds.; Thomé-Kozmiensky Verlag GmbH: Neuruppin, Germany, 2018; pp. 583–599. ISBN 978-3-944310-40-4. [Google Scholar]
- Zhang, T.; He, Y.; Ge, L.; Fu, R.; Zhang, X.; Huang, Y. Characteristics of wet and dry crushing methods in the recycling process of spent lithium-ion batteries. J. Power Sources 2013, 240, 766–771. [Google Scholar] [CrossRef]
- Diekmann, J.; Sander, S.; Sellin, G.; Petermann, M.; Kwade, A. Crushing of Battery Modules and Cells. In Recycling of Lithium-Ion Batteries: The LithoRec Way; Kwade, A., Diekmann, J., Eds.; Springer: Cham, Switzerland, 2018; pp. 127–138. ISBN 978-3-319-70571-2. [Google Scholar]
- Pagliaro, M.; Meneguzzo, F. Recycling of Lithium Batteries. In Sustainable Separation Engineering: Materials, Techniques and Process Development, 1st ed.; Szekely, G., Zhao, D., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2022; pp. 591–603. ISBN 978-1-119-74008-7. [Google Scholar]
- Ciez, R.E.; Whitacre, J.F. Examining different recycling processes for lithium-ion batteries. Nat. Sustain. 2019, 2, 148–156. [Google Scholar] [CrossRef]
- Korthauer, R. (Ed.) Handbuch Lithium-Ionen-Batterien, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 978-3-642-30652-5. [Google Scholar]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-Ion Battery Materials: Present and Future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Kalyani, P.; Kalaiselvi, N. Various Aspects of LiNiO2 Chemistry: A Review. Sci. Technol. Adv. Mater. 2005, 6, 689–703. [Google Scholar] [CrossRef]
- Gu, M.; Belharouak, I.; Zheng, J.; Wu, H.; Xiao, J.; Genc, A.; Amine, K.; Thevuthasan, S.; Baer, D.R.; Zhang, J.-G.; et al. Formation of the spinel phase in the layered composite cathode used in Li-ion batteries. ACS Nano 2013, 7, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Yabuuchi, N.; Ohzuku, T. Novel lithium insertion material of LiCo1/3Ni1/3Mn1/3O2 for advanced lithium-ion batteries. J. Power Sources 2003, 119–121, 171–174. [Google Scholar] [CrossRef]
- Doughty, D.H.; Roth, E.P. A General Discussion of Li Ion Battery Safety. Electrochem. Soc. Interface 2012, 21, 37–44. [Google Scholar] [CrossRef]
- Ding, Y.; Cano, Z.P.; Yu, A.; Lu, J.; Chen, Z. Automotive Li-Ion Batteries: Current Status and Future Perspectives. Electrochem. Energy Rev. 2019, 2, 1–28. [Google Scholar] [CrossRef]
- Li, W.; Lee, S.; Manthiram, A. High-Nickel NMA: A Cobalt-Free Alternative to NMC and NCA Cathodes for Lithium-Ion Batteries. Adv. Mater. 2020, 32, e2002718. [Google Scholar] [CrossRef]
- Link, S.; Neef, C.; Wicke, T.; Hettesheimer, T.; Diehl, M.; Krätzig, O.; Degen, F.; Klein, F.; Fanz, P.; Burgard, M.; et al. Development Perspectives for Lithium-Ion Battery Cell Formats; Fraunhofer: Karlsruhe, Germany, 2022; Available online: https://www.isi.fraunhofer.de/content/dam/isi/dokumente/cct/2022/Development_perspectives_for_lithium-ion_battery_cell_formats_Fraunhofer_2022.pdf (accessed on 20 April 2023).
- Song, J.; Li, B.; Chen, Y.; Zuo, Y.; Ning, F.; Shang, H.; Feng, G.; Liu, N.; Shen, C.; Ai, X.; et al. A High-Performance Li-Mn-O Li-rich Cathode Material with Rhombohedral Symmetry via Intralayer Li/Mn Disordering. Adv. Mater. 2020, 32, e2000190. [Google Scholar] [CrossRef]
- Zheng, J.; Myeong, S.; Cho, W.; Yan, P.; Xiao, J.; Wang, C.; Cho, J.; Zhang, J.-G. Li- and Mn-Rich Cathode Materials: Challenges to Commercialization. Adv. Energy Mater. 2017, 7, 1601284. [Google Scholar] [CrossRef]
- Wurm, C.; Öttinger, O.; Wittkämper, S.; Zauter, R.; Vuorilehto, K. Anodenmaterialien für Lithium-Ionen-Batterien. In Handbuch Lithium-Ionen-Batterien, 1st ed.; Korthauer, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 45–60. ISBN 978-3-642-30652-5. [Google Scholar]
- Zhang, H.; Yang, Y.; Ren, D.; Wang, L.; He, X. Graphite as anode materials: Fundamental mechanism, recent progress and advances. Energy Storage Mater. 2021, 36, 147–170. [Google Scholar] [CrossRef]
- Wu, Y. Modified natural graphite as anode material for lithium-ion batteries. J. Power Sources 2002, 111, 229–334. [Google Scholar] [CrossRef]
- Bettersize Instruments Ltd. Improving Lithium-Ion Batteries through Measuring Tapped Density. Available online: https://www.azom.com/article.aspx?ArticleID=21056 (accessed on 19 November 2023).
- Hettesmeier, T.; Thielmann, A.; Neef, C.; Möller, K.-C.; Wolter, M.; Lorentz, V.; Gepp, M.; Wenger, M.; Prill, T.; Zausch, J.; et al. Entwicklungsperspektiven für Zellformate von Lithium-Ionen-Batterien in der Elektromobilität; Fraunhofer: Pfinztal, Germany, 2017; Available online: https://publica.fraunhofer.de/entities/publication/0ca7ef53-3b51-4467-ad20-bdef36958520/details (accessed on 4 May 2023).
- Heimes, H.; Kampker, A.; Wennemar, S.; Plocher, L.; Bockey, G.; Michaelis, S.; Schütrumpf, J. Production Process of a Lithium-Ion Battery Cell. Available online: https://www.pem.rwth-aachen.de/global/show_document.asp?id=aaaaaaaabyilawq (accessed on 18 December 2023).
- Tesla. Battery Day Presentation Deck. 2020. Available online: https://tesla-share.thron.com/content/?id=96ea71cf-8fda-4648-a62c-753af436c3b6&pkey=S1dbei4 (accessed on 10 May 2023).
- Frank, A.; Sturm, J.; Steinhardt, M.; Rheinfeld, A.; Jossen, A. Impact of Current Collector Design and Cooling Topology on Fast Charging of Cylindrical Lithium-Ion Batteries. ECS Adv. 2022, 1, 40502. [Google Scholar] [CrossRef]
- American National Standards Institute. ANSI C18.1M; American National Standard for Portable Primary Cells and Batteries with Aqueous Electrolyte: General and Specifications. National Electrical Manufacturers Association: Rosslyn, VA, USA, 2021. Available online: https://www.nema.org/docs/default-source/standards-document-library/ansi-c18.1m-part-1-2021-contents-and-scope03ee42fa-4df1-4659-af17-1152feebb97f.pdf?sfvrsn=a1875742_3 (accessed on 21 December 2023).
- Gerlitz, E.; Botzem, D.; Weinmann, H.; Ruhland, J.; Fleischer, J. Cell-to-Pack-Technologie für Li-Ionen-Batterien. Z. Für Wirtsch. Fabr. 2021, 116, 689–694. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, C.-Z.; Yuan, H.; Chen, Y.; Zhang, W.; Huang, J.-Q.; Yu, D.; Liu, Y.; Titirici, M.-M.; Chueh, Y.-L.; et al. A review of rechargeable batteries for portable electronic devices. InfoMat 2019, 1, 6–32. [Google Scholar] [CrossRef]
- Chen, T.; Jin, Y.; Lv, H.; Yang, A.; Liu, M.; Chen, B.; Xie, Y.; Chen, Q. Applications of Lithium-Ion Batteries in Grid-Scale Energy Storage Systems. Trans. Tianjin Univ. 2020, 26, 208–217. [Google Scholar] [CrossRef]
- Sziegoleit, H. Sortierung von Gerätebatterien. In Recycling und Rohstoffe, 6th ed.; Thomé-Kozmiensky, K.J., Goldmann, D., Eds.; Thomé-Kozmiensky Verlag GmbH: Neuruppin, Germany, 2013; pp. 495–504. ISBN 978-3-935317-97-9. [Google Scholar]
- Recycling Magazin. GRS und Saubermacher Digitalisieren Rücknahmesysteme. Available online: https://www.recyclingmagazin.de/2021/12/22/grs-und-saubermacher-digitalisieren-ruecknahmesysteme/ (accessed on 22 May 2023).
- REBAT. Internetauftritt der Firma. Available online: https://rebat.de/ (accessed on 22 May 2023).
- Landbell Group. Internetauftritt der Firma. Available online: https://www.landbell.de/ds-entsorgung/ (accessed on 22 May 2023).
- Stibat, B.V. Internetauftritt der Firma. Available online: https://www.stibat.nl/ (accessed on 22 May 2023).
- Bundesministerium der Justiz. Gesetz über das Inverkehrbringen, die Rücknahme und die Umweltverträgliche Entsorgung von Batterien und Akkumulatoren: Batteriegesetz-BattG; Bundesministerium der Justiz: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Bundesministerium der Justiz. Gesetz über das Inverkehrbringen, die Rücknahme und die Umweltverträgliche Entsorgung von Elektro- und Elektronikgeräten: Elektro- und Elektronikgerätegesetz-ElektroG; Bundesministerium der Justiz: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Samarukha, I. Recycling strategies for End-of-Life Li-ion Batteries from Heavy Electric Vehicles. Master’s Thesis, Königliche Technische Hochschule, Stockholm, Sweden, 2020. [Google Scholar]
- Tomra Sorting GmbH. Relux Installiert Tomras X-Tract zur Erfolgreichen Batteriesortierung. Available online: https://languagesites.tomra.com/de-de/sorting/recycling/recycling-news/2021/relux-installs-tomra-xtract-successful-battery-sorting (accessed on 13 March 2023).
- Bebat, N.V. Bebat—Behind the Factory. Available online: https://www.youtube.com/watch?v=Hd2MBmurPJM (accessed on 13 March 2023).
- Sletsgaard, J.; Hald Pedersen, N. Øget Ressourcegenvinding ved Forbedret Karakterisering af Affaldsbatterier: Miljøteknologisk Udviklings- og Demonstrationsprogram 2012; Miljøprojekt: Kopenhagen, Denmark, 2014. [Google Scholar]
- Blank, T.; Badeda, J.; Kowal, J.; Sauer, D.U. Deep discharge behavior of lead-acid batteries and modeling of stationary battery energy storage systems. In Proceedings of the Intelec 2012 IEEE International Telecommunications Energy Conference, Scottsdale, AZ, USA, 30 September–4 October 2012; pp. 1–4, ISBN 978-1-4673-1000-0. [Google Scholar]
- Langner, T.; Sieber, T.; Acker, J. Studies on the deposition of copper in lithium-ion batteries during the deep discharge process. Sci. Rep. 2021, 11, 6316. [Google Scholar] [CrossRef]
- Ahrens, J. Rechargeable Battery Discharge Device for Discharging Rechargeable Batteries, and Method for Discharging a Plurality of Rechargeable Batteries. U.S. Patent Application No. 18/005,096, 12 July 2021. [Google Scholar]
- Hanisch, C.; Bußmann, T. Verfahren zum Wiederverwerten von Akkumulatoren und Akkumulator-Entladevorrichtung. DE102020118418A1, 18 March 2019. [Google Scholar]
- Gerbers, R.; Wegener, K.; Dietrich, F. Safe, Flexible and Productive Human-Robot-Collaboration for Disassembly of Lithium-Ion Batteries. In Recycling of Lithium-Ion Batteries: The LithoRec Way; Kwade, A., Diekmann, J., Eds.; Springer: Cham, Switzerland, 2018; pp. 99–126. ISBN 978-3-319-70571-2. [Google Scholar]
- Duesenfeld GmbH. Company Website. Available online: https://www.duesenfeld.com/ (accessed on 15 October 2023).
- Petzold, M. Nassmechanische Zerkleinerung und Aufbereitung von Lithium-Ionen-Batterien aus Elektrofahrzeugen zur optimierten Lithium-Rückgewinnung. In 12. Wissenschaftskongress Abfall- und Ressourcenwirtschaft am 9. und 10. März 2023 an der Technischen Universität Hamburg, Hamburg, 09.03.—10.03.2023; Deutsche Gesellschaft für Abfallwirtschaft, e.V., Ed.; Innsbruck University Press: Innsbruck, Austria, 2023; pp. 19–24. ISBN 978-3-99106-095-6. [Google Scholar]
- Christmann, D. Batterierecycling: Bosch Entwickelt Europas Erste vollautomatisierte Anlage zur Batterieentladung. Available online: https://www.bosch-presse.de/pressportal/de/de/batterierecycling-bosch-entwickelt-europas-erste-vollautomatisierte-anlage-zur-batteriedemontage-252928.html (accessed on 29 May 2023).
- Makuza, B.; Tian, Q.; Guo, X.; Chattopadhyay, K.; Yu, D. Pyrometallurgical options for recycling spent lithium-ion batteries: A comprehensive review. J. Power Sources 2021, 491, 229622. [Google Scholar] [CrossRef]
- Harper, G.; Sommerville, R.; Kendrick, E.; Driscoll, L.; Slater, P.; Stolkin, R.; Walton, A.; Christensen, P.; Heidrich, O.; Lambert, S.; et al. Recycling lithium-ion batteries from electric vehicles. Nature 2019, 575, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Velázquez-Martínez, O.; Valio, J.; Santasalo-Aarnio, A.; Reuter, M.; Serna-Guerrero, R. A Critical Review of Lithium-Ion Battery Recycling Processes from a Circular Economy Perspective. Batteries 2019, 5, 68. [Google Scholar] [CrossRef]
- Petzold, M.; Flamme, S.; Hams, S. Nassmechanische Aufbereitung von Lithium-Ionen Batterien. Müll und Abfall, 8 December 2023. [Google Scholar] [CrossRef]
- Haas, P.; Pfeifer, S.; Müller, J.; Bradtmöller, C.; Scholl, S. Separation of the Electrolyte—Solvent Extraction. In Recycling of Lithium-Ion Batteries: The LithoRec Way; Kwade, A., Diekmann, J., Eds.; Springer: Cham, Switzerland, 2018; pp. 155–176. ISBN 978-3-319-70571-2. [Google Scholar]
- Stehmann, F.; Bradtmöller, C.; Scholl, S. Separation of the Electrolyte—Thermal Drying. In Recycling of Lithium-Ion Batteries: The LithoRec Way; Kwade, A., Diekmann, J., Eds.; Springer: Cham, Switzerland, 2018; pp. 139–154. ISBN 978-3-319-70571-2. [Google Scholar]
- Rothermel, S.; Grützke, M.; Mönnighoff, X.; Winter, M.; Nowak, S. Electrolyte Extraction—Sub and Supercritical CO2. In Recycling of Lithium-Ion Batteries: The LithoRec Way; Kwade, A., Diekmann, J., Eds.; Springer: Cham, Switzerland, 2018; pp. 177–186. ISBN 978-3-319-70571-2. [Google Scholar]
- Pinegar, H.; Smith, Y.R. Recycling of End-of-Life Lithium-Ion Batteries, Part I: Commercial Processes. J. Sustain. Metall. 2019, 5, 402–416. [Google Scholar] [CrossRef]
- Larouche, F.; Tedjar, F.; Amouzegar, K.; Houlachi, G.; Bouchard, P.; Demopoulos, G.P.; Zaghib, K. Progress and Status of Hydrometallurgical and Direct Recycling of Li-Ion Batteries and Beyond. Materials 2020, 13, 801. [Google Scholar] [CrossRef] [PubMed]
- Novis Smith, W.; Swoffer, S. Recovery of Lithium-Ion Batteries. U.S. Patent 861475 B1, 18 June 2013. [Google Scholar]
- Poe, S.L.; Paradise, C.L.; Muollo, L.R.; Pal, R.; Warner, J.C.; Korzenski, M.B. Method for the Recovery of Lithium Cobalt Oxide from Lithium-Ion Batteries. U.S. Patent 9972830, 19 June 2012. [Google Scholar]
- Vieceli, N.; Nogueira, C.A.; Guimarães, C.; Pereira, M.F.C.; Durão, F.O.; Margarido, F. Hydrometallurgical recycling of lithium-ion batteries by reductive leaching with sodium metabisulphite. Waste Manag. 2018, 71, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Peters, L.; Friedrich, B. Proven Methods for Recovery of Lithium from Spent Batteries; DERA Workshop Lithium: Berlin, Germany, 2017. [Google Scholar]
- Yao, Y.; Zhu, M.; Zhao, Z.; Tong, B.; Fan, Y.; Hua, Z. Hydrometallurgical Processes for Recycling Spent Lithium-Ion Batteries: A Critical Review. ACS Sustain. Chem. Eng. 2018, 6, 13611–13627. [Google Scholar] [CrossRef]
- Diekmann, J.; Hanisch, C.; Froböse, L.; Schälicke, G.; Loellhoeffel, T.; Fölster, A.-S.; Kwade, A. Ecological Recycling of Lithium-Ion Batteries from Electric Vehicles with Focus on Mechanical Processes. J. Electrochem. Soc. 2017, 164, A6184–A6191. [Google Scholar] [CrossRef]
- Fraunhofer-Institut für System- und Innovationsforschung ISI. Recycling von Lithium-Ionen- Batterien: Chancen und Herausforderungen für den Maschinen- und Anlagenbau; Fraunhofer: Karlsruhe, Germany, 2021. [Google Scholar]
- Wang, H.; Vest, M.; Friedrich, B. Hydrometallurgical processing of Li-Ion battery scrap from electric vehicles. In Proceedings of the European Metallurgical Conference 2011, Düsseldorf, Germany, 22–24 June 2011; pp. 1033–1052. [Google Scholar] [CrossRef]
- Li, L.; Ge, J.; Wu, F.; Chen, R.; Chen, S.; Wu, B. Recovery of cobalt and lithium from spent lithium-ion batteries using organic citric acid as leachant. J. Hazard. Mater. 2010, 176, 288–293. [Google Scholar] [CrossRef]
- Gratz, E.; Sa, Q.; Apelian, D.; Wang, Y. A closed loop process for recycling spent lithium-ion batteries. J. Power Sources 2014, 262, 255–262. [Google Scholar] [CrossRef]
- Zhu, S.; He, W.; Li, G.; Zhou, X.; Zhang, X.; Huang, J. Recovery of Co and Li from spent lithium-ion batteries by combination method of acid leaching and chemical precipitation. Trans. Nonferrous Met. Soc. China 2012, 22, 2274–2281. [Google Scholar] [CrossRef]
- Contestabile, M.; Panero, S.; Scrosati, B. A laboratory-scale lithium-ion battery recycling process. J. Power Sources 2001, 92, 65–69. [Google Scholar] [CrossRef]
- Pegoretti, V.; Dixini, P.; Smecellato, P.C.; Biaggio, S.R.; Freitas, M. Thermal synthesis, characterization and electrochemical study of high-temperature (HT) LiCoO2 obtained from Co(OH)2 recycled of spent lithium-ion batteries. Mater. Res. Bull. 2017, 86, 5–9. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, X.; Cao, H.; Zhao, C.; Lin, X.; Ning, P.; Zhang, Y.; Jin, W.; Sun, Z. A Closed-Loop Process for Selective Metal Recovery from Spent Lithium Iron Phosphate Batteries through Mechanochemical Activation. ACS Sustain. Chem. Eng. 2017, 5, 9972–9980. [Google Scholar] [CrossRef]
- Chen, X.; Luo, C.; Zhang, J.; Kong, J.; Zhou, T. Sustainable Recovery of Metals from Spent Lithium-Ion Batteries: A Green Process. ACS Sustain. Chem. Eng. 2015, 3, 3104–3113. [Google Scholar] [CrossRef]
- Nan, J.; Han, D.; Zuo, X. Recovery of metal values from spent lithium-ion batteries with chemical deposition and solvent extraction. J. Power Sources 2005, 152, 278–284. [Google Scholar] [CrossRef]
- Swain, B.; Jeong, J.; Lee, J.; Lee, G.-H. Separation of cobalt and lithium from mixed sulphate solution using Na-Cyanex 272. Hydrometallurgy 2006, 84, 130–138. [Google Scholar] [CrossRef]
- Zhan, R.; Payne, T.; Leftwich, T.; Perrine, K.; Pan, L. De-agglomeration of cathode composites for direct recycling of Li-ion batteries. Waste Manag. 2020, 105, 39–48. [Google Scholar] [CrossRef]
- Zhang, X.; Xue, Q.; Li, L.; Fan, E.; Wu, F.; Chen, R. Sustainable Recycling and Regeneration of Cathode Scraps from Industrial Production of Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2016, 4, 7041–7049. [Google Scholar] [CrossRef]
- Ganter, M.J.; Landi, B.J.; Babbitt, C.W.; Anctil, A.; Gaustad, G. Cathode refunctionalization as a lithium ion battery recycling alternative. J. Power Sources 2014, 256, 274–280. [Google Scholar] [CrossRef]
- Kwade, A.; Diekmann, J. (Eds.) Recycling of Lithium-Ion Batteries: The LithoRec Way; Springer: Cham, Switzerland, 2018; ISBN 978-3-319-70571-2. [Google Scholar]
- Krüger, S.; Hanisch, C.; Kwade, A.; Winter, M.; Nowak, S. Effect of impurities caused by a recycling process on the electrochemical performance of Li[Ni0.33Co0.33Mn0.33]O2. J. Electroanal. Chem. 2014, 726, 91–96. [Google Scholar] [CrossRef]
- Jung, J.C.-Y.; Sui, P.-C.; Zhang, J. A review of recycling spent lithium-ion battery cathode materials using hydrometallurgical treatments. J. Energy Storage 2021, 35, 102217. [Google Scholar] [CrossRef]
- Jung, H.; Silva, R.; Han, M. Scaling Trends of Electric Vehicle Performance: Driving Range, Fuel Economy, Peak Power Output, and Temperature Effect. WEVJ 2018, 9, 46. [Google Scholar] [CrossRef]
- European Commission. Verordnung (EU) 2023/1542 des Europäischen Parlaments und des Rates vom 12 Juli 2023 über Batterien und Altbatterien, zur Änderung der Richtlinie 2008/98/EG und der Verordnung (EU) 2019/1020 und zur Aufhebung der Richtlinie 2006/66/EG: Verordnung (EU) 2023/1542; European Commission: Brussels, Belgium, 2023. [Google Scholar]
- Weydanz, W. Power Tools: Batteries. Available online: https://booksite.elsevier.com/brochures/ecps/PDFs/PowerTools_Batteries.pdf (accessed on 11 May 2023).
- HKM Akkutechnik. Akku-Tauschpack für Bosch Powerpack Classic. Available online: https://www.hkm-akkutechnik.de/Akku-Tauschpack-fuer-Bosch-Powerpack-Classic-300-400-500-36-V-mit-500-Wh?gclid=Cj0KCQjw6cKiBhD5ARIsAKXUdyZjypjTlN62avWkLlyDwP_nbMcAPFxlaHFN3-HByJ3iOJjS2e7jaY0aAuTMEALw_wcB (accessed on 9 May 2023).
- Neubert, K.; Wotruba, H. Investigations on the Detectability of Rare-Earth Minerals Using Dual-Energy X-ray Transmission Sorting. J. Sustain. Metall. 2017, 3, 3–12. [Google Scholar] [CrossRef]
- Sterkens, W.; Diaz-Romero, D.; Goedemé, T.; Dewulf, W.; Peeters, J.R. Detection and recognition of batteries on X-Ray images of waste electrical and electronic equipment using deep learning. Resour. Conserv. Recycl. 2021, 168, 105246. [Google Scholar] [CrossRef]
- Luo, X.; He, K.; Zhang, Y.; He, P.; Zhang, Y. A review of intelligent ore sorting technology and equipment development. Int. J. Min. Met. Mater. 2022, 29, 1647–1655. [Google Scholar] [CrossRef]
- Robben, C.; Condori, P.; Pinto, A.; Machaca, R.; Takala, A. X-ray-transmission based ore sorting at the San Rafael tin mine. Miner. Eng. 2020, 145, 105870. [Google Scholar] [CrossRef]
- Habich, U. Sensor-Based Sorting Systems in Waste Processing. In International Symposium MBT; Kühle-Wiedemeier, M., Ed.; Cuvillier Verlag: Göttingen, Germany, 2007; pp. 308–315. ISBN 9783867272360. [Google Scholar]
- Takezawa, T.; Uemoto, M.; Itoh, K. Combination of X-ray transmission and eddy-current testing for the closed-loop recycling of aluminum alloys. J. Mater. Cycles Waste Manag. 2015, 17, 84–90. [Google Scholar] [CrossRef]
- Mesina, M.B.; de Jong, T.; Dalmijn, W.L. Automatic sorting of scrap metals with a combined electromagnetic and dual energy X-ray transmission sensor. Int. J. Miner. Process. 2007, 82, 222–232. [Google Scholar] [CrossRef]
- Kern, M.; Tusa, L.; Leißner, T.; van den Boogaart, K.G.; Gutzmer, J. Optimal sensor selection for sensor-based sorting based on automated mineralogy data. J. Clean. Prod. 2019, 234, 1144–1152. [Google Scholar] [CrossRef]
- Steinert GmbH. Dichtesortierung mit dem Steinert XSS T EVO 5.0—Per Röntgentransmission Nach dem Dual-Energy-Prinzip. Available online: https://steinertglobal.com/de/magnete-sensorsortierer/sensorsortierung/roentgensortiersysteme/steinert-xss-t-evo-50/ (accessed on 16 May 2023).
- Tomra Sorting GmbH. X-Tract: Sekundärrohstoffe von höchster Qualität bei Metallanwendungen. Available online: https://languagesites.tomra.com/de-de/sorting/recycling/products/x-tract/ (accessed on 16 May 2023).
- Allgaier Process Technology GmbH. Sortierung Durch Röntgentransmission Nach Atomarer Dichte. Available online: https://www.allgaier-process-technology.com/de/maschine/MSort-X-Ray_113 (accessed on 16 May 2023).
- Weiss, M. Resource Recycling in Waste Management with X-ray Fluorescence. Diplomarbeit, Montanuniversität Leoben, Leoben, Austria, 2011. [Google Scholar]
- Stratmann, A. Edelsteinuntersuchung mit der Röntgenfluoreszenzanalyse. Available online: https://www.edelsteinlabor24.de/uncategorized/edelsteinuntersuchung-mit-der-roentgenfluoreszenzanalyse/ (accessed on 16 May 2023).
- Knapp, H.; Neubert, K.; Schropp, C.; Wotruba, H. Viable Applications of Sensor-Based Sorting for the Processing of Mineral Resources. ChemBioEng Rev. 2014, 1, 86–95. [Google Scholar] [CrossRef]
- Lukka, T.J.; Tossavainen, T.; Kujala, J.V.; Raiko, T. ZenRobotics Recycler—Robotic Sorting using Machine Learning. In Sensor-Based Sorting 2014; Sensor-Based Sorting, Aachen, 11.03.–13.03; Pretz, T., Wotruba, H., Eds.; GDMB: Clausthal-Zellerfeld, Germany, 2014; pp. 1–8. ISBN 978-3-940276-56-8. [Google Scholar]
- Kandlbauer, L.; Khodier, K.; Ninevski, D.; Sarc, R. Sensor-based Particle Size Determination of Shredded Mixed Commercial Waste based on two-dimensional Images. Waste Manag. 2021, 120, 784–794. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Jin, C.; Xu, J.; Yang, T. Measurement of Potato Volume with Laser Triangulation and Three-Dimensional Reconstruction. IEEE Access 2020, 8, 176565–176574. [Google Scholar] [CrossRef]
- Kurth, H. Anwendung von Echtzeit-Elementaranalyse auf Erzförderströme. AT Miner. Process. 2020, 10. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).