Effect of Kaolin/TiO2 Additions and Contact Temperature on the Interaction between DD6 Alloys and Al2O3 Shells
Abstract
:1. Introduction
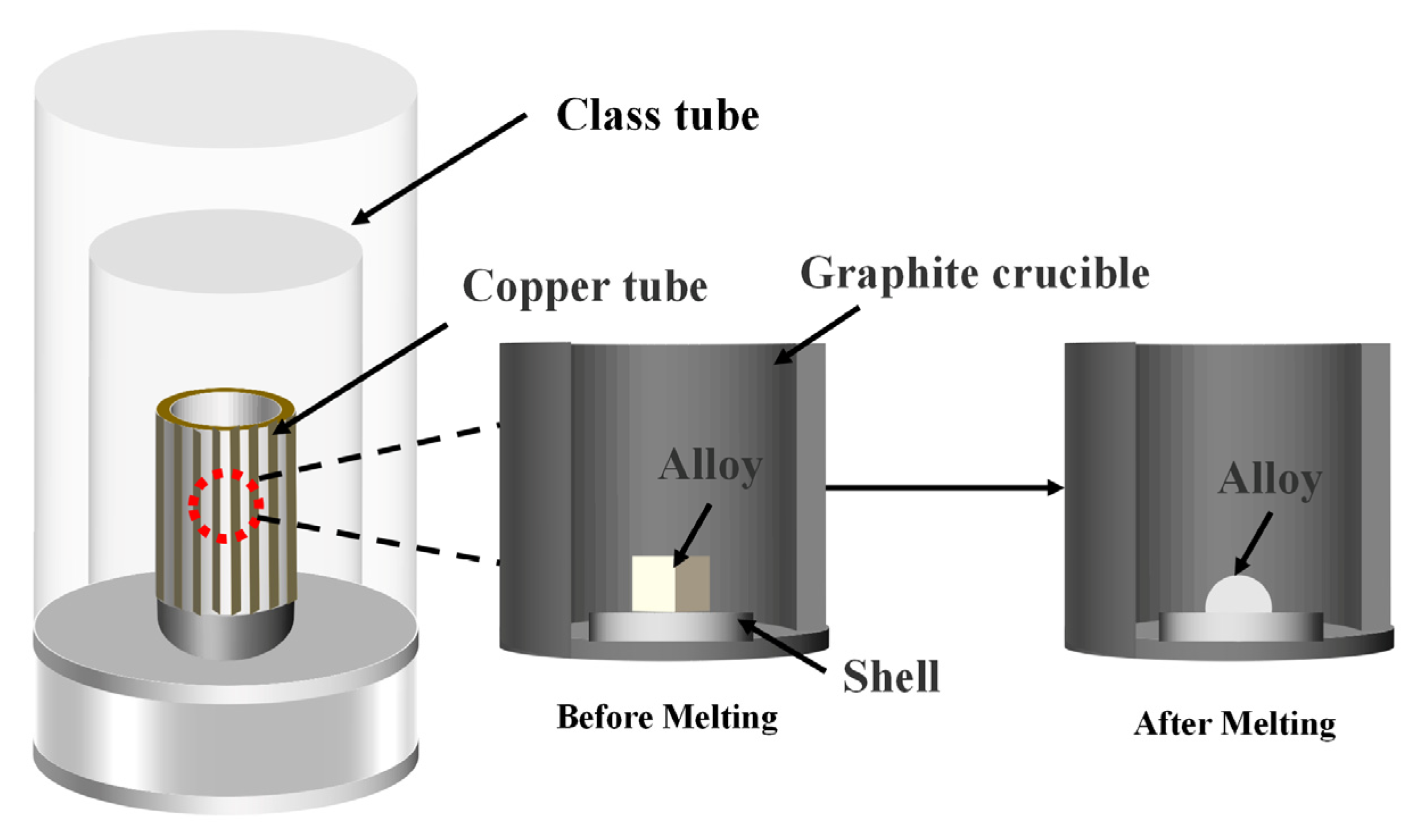
2. Experimental Procedure
3. Results and Discussion
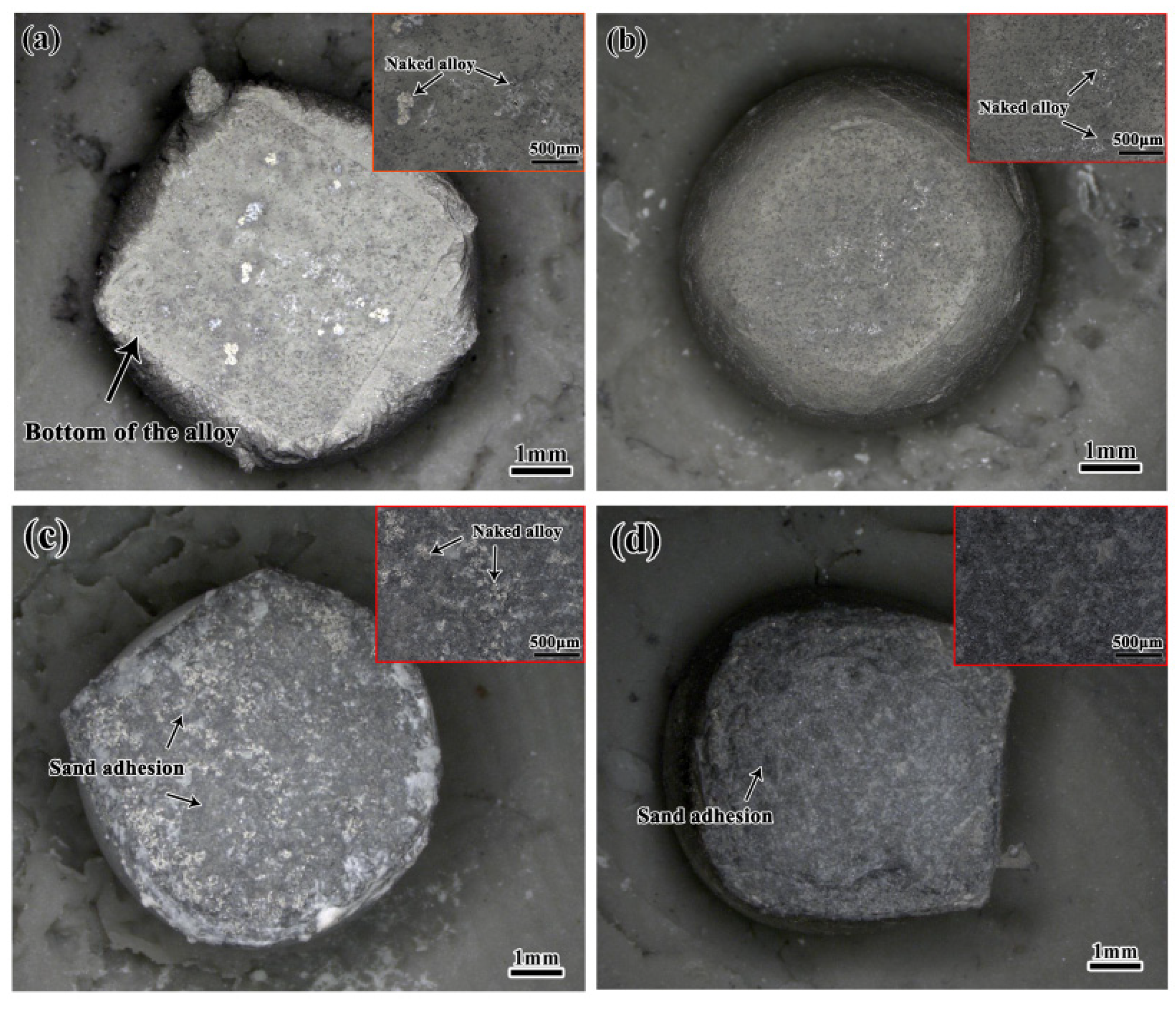
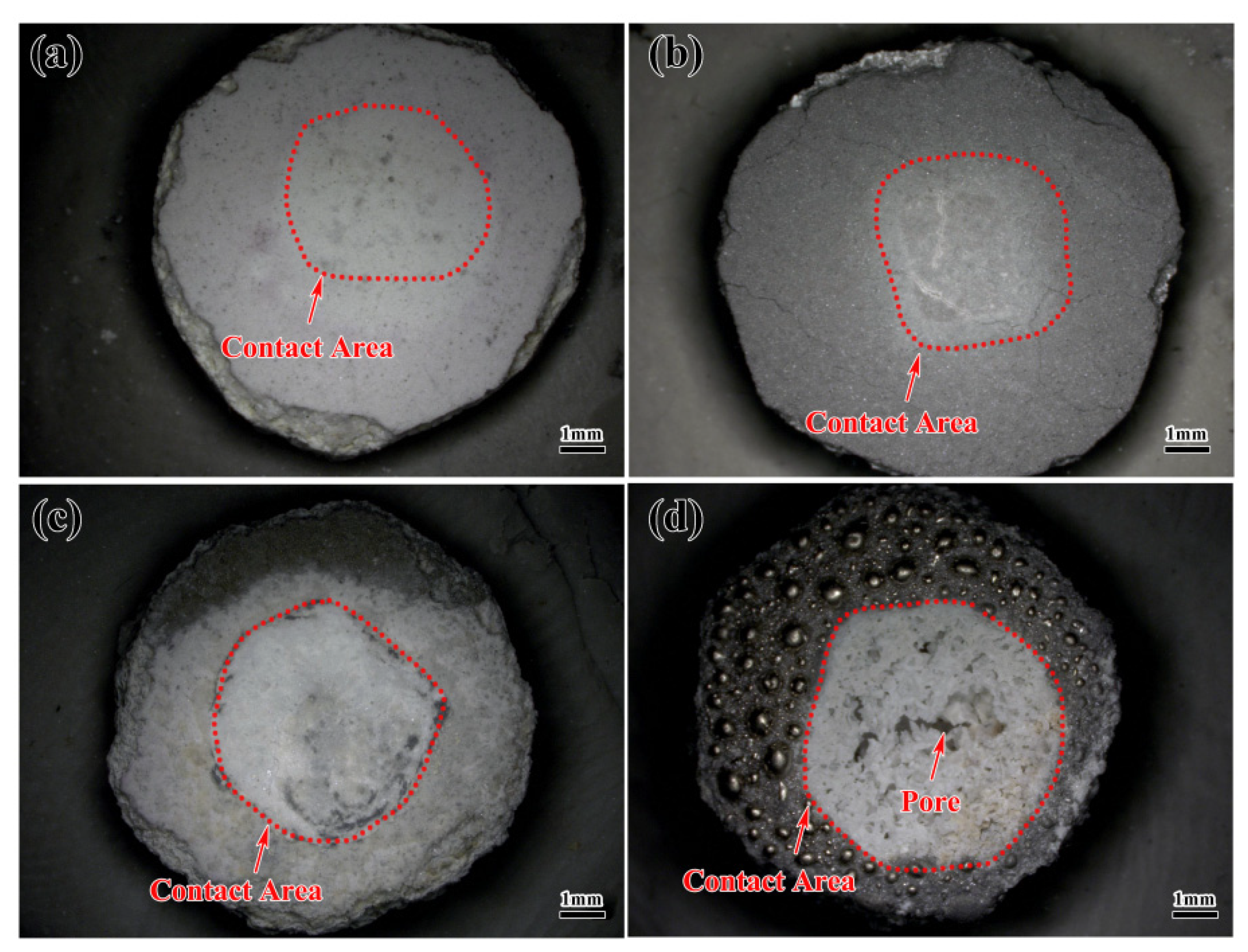
3.1. Macroscopic Morphology of the Alloys Contacted with Al2O3 Shells
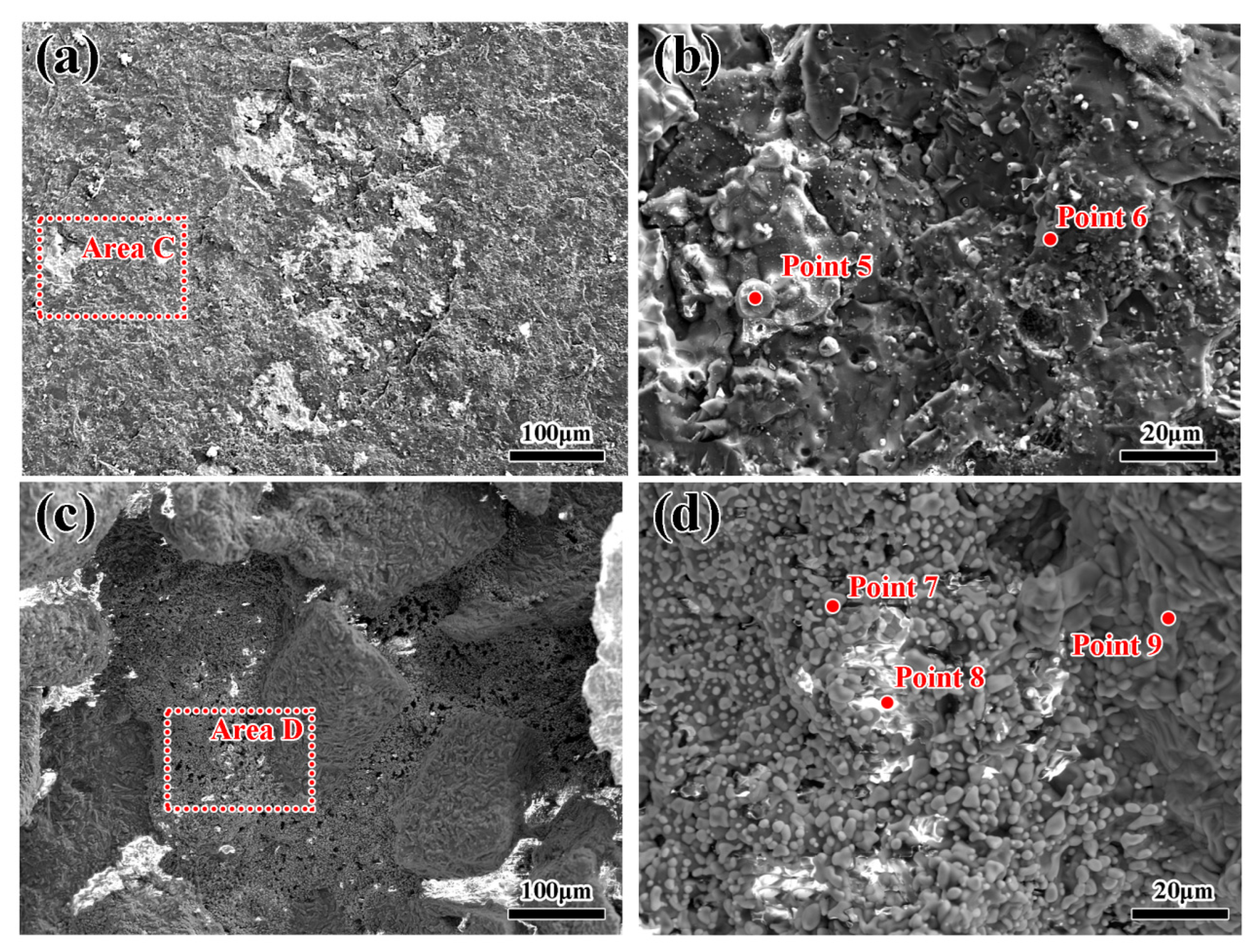
3.2. Microscopic Morphology of the Alloys Contacted with Al2O3 Shells
4. Conclusions
- (1)
- At a contact temperature of 1550 °C, both Al2O3 shells with kaolin and TiO2 additions exhibited good corrosion resistance to DD6 alloys, although a thin shell refractory layer appeared at the bottom of the alloys and no penetration of the shell refractory was observed in the interior of the alloys.
- (2)
- When the contact temperature was increased to 1600 °C, a significant interaction occurred between Al2O3 shells with kaolin and TiO2 additions. Both shells exhibited noticeable damage at the contact interface. This indicates that the increase in temperature could significantly intensify the interaction between the shell and the alloy.
- (3)
- Additionally, the thicknesses of the sand adhesions on the shells with kaolin and TiO2 additions were 120 μm and 220 μm, respectively. Although the temperature of 1600 °C was not suitable for utilizing Al2O3 shells in preparing the DD6 alloy. However, compared to adding TiO2, the addition of kaolin could also enhance the stability of the Al2O3 shell. This study provides theoretical guidance for the systematic investigation of the effect of an additive on the interaction between the shell and alloy. Additionally, it offers a reference for the preparation of high-quality Ni-based alloys.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Satyanarayana, D.V.V.; Eswara, P.N. Nickel-based superalloys. In Aerospace Materials and Material Technologies; Aerospace Materials: Sunshine West, Victoria, 2017; Volume 1, pp. 199–228. [Google Scholar] [CrossRef]
- Xia, W.; Zhao, X.; Yue, L.; Zhang, Z. Microstructural evolution and creep mechanisms in Ni-based single crystal superalloys: A review. J. Alloys Compd. 2020, 819, 152954. [Google Scholar] [CrossRef]
- Caron, P. High y’solvus new generation nickel-based superalloys for single crystal turbine blade applications. Superalloys 2000, 2000, 737–746. [Google Scholar]
- Pollock, T.M.; Tin, S. Nickel-based superalloys for advanced turbine engines: Chemistry, microstructure and properties. J. Propuls. Power 2006, 22, 361–374. [Google Scholar] [CrossRef]
- Venkat, Y.; Choudary, K.R.; Chatterjee, D.; Das, D.K.; Pandey, A.K.; Singh, S. Development of mullite-alumina ceramic shells for precision investment casting of single-crystal high-pressure turbine blades. Ceram. Int. 2022, 48, 28199–28206. [Google Scholar] [CrossRef]
- Yao, J.S.; Dong, L.P.; Wu, Z.Q.; Wang, L.L.; Shen, B.; Yang, X.W. Interfacial Reaction Mechanism between Ceramic Mould and Single Crystal Superalloy for Manufacturing Turbine Blade. Materials 2022, 15, 5514. [Google Scholar] [CrossRef] [PubMed]
- Liu, L. The progress of investment casting of nickel-based superalloys. Foundry 2012, 11, 1273–1285. [Google Scholar]
- Wang, L.L.; Li, J.R.; Tang, D.Z.; Tang, D.Z.; Gu, G.H.; Li, X.; Yao, J.S.; Fan, H.N.; Niu, S.X. Effect of directional solidification condition on interfacial reaction between DD6 single crystal superalloy and zirconia-silica ceramic core. Adv. Mater. Res. 2014, 926, 72–76. [Google Scholar] [CrossRef]
- Li, J.R.; Zhong, Z.G.; Tang, D.Z.; Liu, S.Z.; Wei, P.; Wei, P.Y.; Wu, Z.T.; Huang, D.; Han, M. A Low-cost second geneution single crystal superalloy DD6. Superalloys 2000, 777–783. [Google Scholar]
- Kanyo, J.E.; Schafföner, S.; Uwanyuze, R.S.; Leary, K.S. An overview of ceramic molds for investment casting of nickel superalloys. J. Eur. Ceram. Soc. 2020, 40, 4955–4973. [Google Scholar] [CrossRef]
- Li, F.; Ni, H.J.; Yang, L.X.; Jiang, Y.; Wang, D.H.; Sun, B.D. Investigation of fused alumina based-mold facecoats for DZ22B directionally solidified blades. Materials 2019, 12, 606. [Google Scholar] [CrossRef]
- Zhao, Y.D.; Wang, L.Z.; Chen, C.Y.; Li, J.Q.; Li, X. Effect of a MgO–CaO–ZrO2-based refractory on the cleanliness of a K4169 Ni-based superalloy. Ceram. Int. 2023, 49, 117–125. [Google Scholar] [CrossRef]
- Wang, H.; Shang, G.F.; Liao, J.F.; Yang, B.; Yuan, C. Experimental investigations and thermodynamic calculations of the interface reactions between ceramic moulds and Ni-based single-crystal superalloys: Role of solubility of Y in the LaAlO3 phase. Ceram. Int. 2018, 44, 7667–7673. [Google Scholar] [CrossRef]
- Zi, Y.; Meng, J.; Zhang, C.W.; Yang, Y.H.; Zhou, Y.Z.; Ding, Y.T. Effect of Y content on interface reaction and wettability between a nickel-base single crystal superalloy melt and ceramic mould. J. Alloys Compd. 2019, 789, 472–484. [Google Scholar] [CrossRef]
- Wang, H.W.; Yang, J.X.; Meng, J.; Yang, Y.H.; Zhou, Y.Z. Wettability and interfacial reactions of a low Hf-containing nickel-based superalloy on Al2O3-based, SiO2-based, ZrSiO4, and CoAl2O4 substrates. Ceram. Int. 2020, 46, 22057–22066. [Google Scholar] [CrossRef]
- Wiśniewski, P. Polymer Binders of Ceramic Nanoparticles for Precision Casting of Nickel-Based Superalloys. Nanomaterials 2021, 11, 1714. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Yuan, C. Advances in shell moulding for investment casting. J. Mater. Process. Technol. 2003, 135, 258–265. [Google Scholar] [CrossRef]
- Fan, J.X.; Fan, H.N.; Song, Z.; Guo, Y.J.; Li, M.M.; Li, X.; Qi, C.J.; Xu, X.Q. Thermal-shock stable Al2O3 crucible for superalloy smelting through slip casting with particle gradation. Ceram. Int. 2023, 49, 8762–8771. [Google Scholar] [CrossRef]
- Tamta, K.; Karunakar Benny, D. Enhancing mechanical properties and permeability of ceramic shell in investment casting process. Mater. Manuf. Process. 2018, 34, 612–623. [Google Scholar] [CrossRef]
- Zhu, L.; Dong, Y.C.; Li, L.L.; Liu, J.; You, S.J. Coal fly ash industrial waste recycling for fabrication of mullite-whisker-structured porous ceramic membrane supports. RSC Adv. 2015, 5, 11163–11174. [Google Scholar] [CrossRef]
- Zhu, W.Y.; Liu, Y.; Guan, K.; Peng, C.; Wu, J.Q. Preparation of ZrO2 fiber modified Al2O3 membrane supports with enhanced strength and permeability. J. Eur. Ceram. Soc. 2019, 39, 1712–1716. [Google Scholar] [CrossRef]
- Jansson, S.; Brabie, V.; Jönsson, P. Corrosion mechanism of commercial MgO–C refractories in contact with different gas atmospheres. ISIJ Int. 2008, 48, 760–767. [Google Scholar] [CrossRef]
- Liu, Y.S.; Gao, Y.Y.; Wang, E.H.; Chen, G.Y.; Xu, E.X.; Zhao, F.; Zhao, Y.S.; Li, C.H.; Hou, X.M. Interaction between CA6-MA crucible and molten wrought Ni-based superalloys. J. Eur. Ceram. Soc. 2023, 43, 1714–1722. [Google Scholar] [CrossRef]
- Zan, Y. Influence of TiO2 on Chemical Stability, High Temperature and Electrochemical Properties of Alumina Ceramics based on η-Al2O3. Int. J. Electrochem. Sci. 2021, 16, 210822. [Google Scholar] [CrossRef]
- Samal, S.; Blanco, I. Investigation of Dispersion, Interfacial Adhesion of Isotropic and Anisotropic Filler in Polymer Composite. Appl. Sci. 2021, 11, 8561. [Google Scholar] [CrossRef]
- Long, X.; Liu, L.; Yu, H.; Yang, W. Interfacial Reaction between DZ22B Superalloy and Al2O3 Ceramic Mold Shell for Investment Casting. Spec. Cast. Nonferrous Alloys 2018, 38, 525–528. [Google Scholar] [CrossRef]
- Dyamant, I.; Itzhak, D.; Hormadaly, J. Thermal properties and glass formation in the SiO2–B2O3–Bi2O3–ZnO quaternary system. J. Non-Cryst. Solids 2005, 351, 3503–3507. [Google Scholar] [CrossRef]





| Point | Element/at% | ||||
|---|---|---|---|---|---|
| Al | O | Si | Ni | Ti | |
| 1 | 20.15 | 12.32 | 15.77 | 57.87 | / |
| 2 | 62.10 | 37.79 | / | 00.11 | / |
| 3 | 21.32 | 07.90 | 12.70 | 58.08 | / |
| 4 | 49.63 | 50.18 | / | 00.18 | / |
| Point | Element/at% | |||||
|---|---|---|---|---|---|---|
| Al | O | Si | Ni | Ti | Zr | |
| 5 | 27.01 | 06.67 | 17.39 | 48.94 | / | / |
| 6 | 41.67 | 38.26 | 21.06 | / | / | / |
| 7 | / | 54.08 | / | / | / | 45.92 |
| 8 | / | 34.85 | / | / | / | 65.15 |
| 9 | 47.68 | 37.07 | 15.25 | / | / | / |
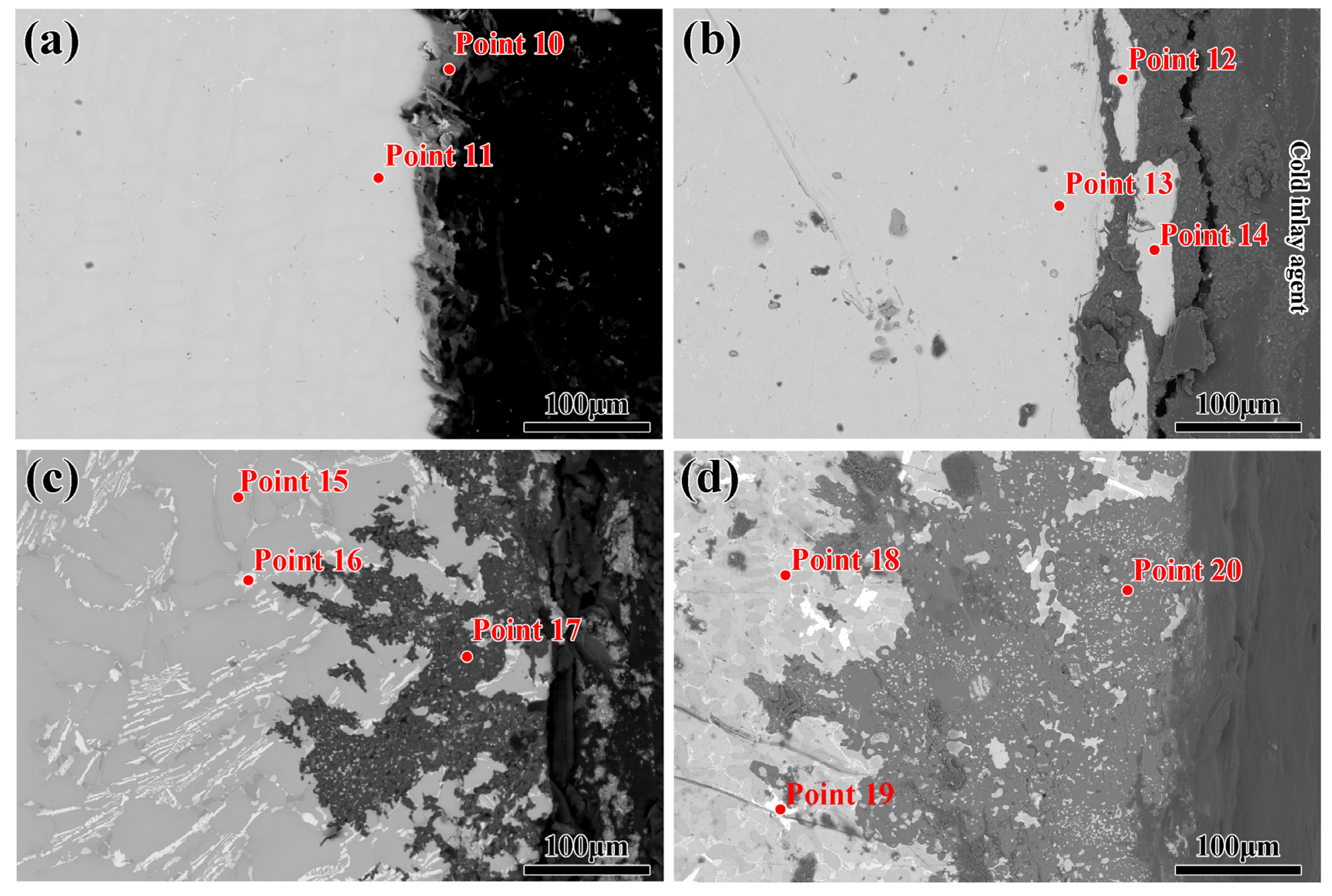
| Point | Element/at% | |||||
|---|---|---|---|---|---|---|
| Al | O | Si | Ni | Ti | Nb, Mo, Cr. | |
| 10 | 6.66 | / | 7.62 | 48.50 | / | Bal. |
| 11 | 14.65 | / | / | 65.12 | / | Bal. |
| 12 | 15.21 | / | / | 63.71 | 0.11 | Bal. |
| 13 | 13.55 | / | / | 64.80 | 0.13 | Bal. |
| 14 | 15.44 | / | / | 63.73 | 0.08 | Bal. |
| 15 | 12.70 | / | 0.32 | 44.03 | / | Bal. |
| 16 | / | / | / | / | Bal. | |
| 17 | 28.72 | 57.29 | 12.92 | 1.07 | / | Bal. |
| 18 | 28.45 | / | 2.40 | 61.12 | / | Bal. |
| 19 | 14.93 | / | 5.31 | 64.46 | / | Bal. |
| 20 | 33.94 | 55.44 | 10.61 | / | / | Bal. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, G.; Cai, Z.; Zhang, M.; Liu, Y.; Feng, Q.; Gao, P.; Hou, X.; Chen, X.; Li, F.; Li, C. Effect of Kaolin/TiO2 Additions and Contact Temperature on the Interaction between DD6 Alloys and Al2O3 Shells. Metals 2024, 14, 164. https://doi.org/10.3390/met14020164
Chen G, Cai Z, Zhang M, Liu Y, Feng Q, Gao P, Hou X, Chen X, Li F, Li C. Effect of Kaolin/TiO2 Additions and Contact Temperature on the Interaction between DD6 Alloys and Al2O3 Shells. Metals. 2024; 14(2):164. https://doi.org/10.3390/met14020164
Chicago/Turabian StyleChen, Guangyao, Zheyu Cai, Man Zhang, Yu Liu, Qisheng Feng, Pengyue Gao, Xinmei Hou, Xiaoyan Chen, Fei Li, and Chonghe Li. 2024. "Effect of Kaolin/TiO2 Additions and Contact Temperature on the Interaction between DD6 Alloys and Al2O3 Shells" Metals 14, no. 2: 164. https://doi.org/10.3390/met14020164
APA StyleChen, G., Cai, Z., Zhang, M., Liu, Y., Feng, Q., Gao, P., Hou, X., Chen, X., Li, F., & Li, C. (2024). Effect of Kaolin/TiO2 Additions and Contact Temperature on the Interaction between DD6 Alloys and Al2O3 Shells. Metals, 14(2), 164. https://doi.org/10.3390/met14020164










