Abstract
Laser powder bed fusion (LPBF) is a fusion-based additive manufacturing process. It has the advantage of allowing the manufacturing of metal matrix composites. This advantage arises from its small melting zone and rapid cooling rate, which minimize the risk of reinforcement segregation. In this work, 0.3 wt% and 1.0 wt% Y2O3 nanoparticles were added to 316L to fabricate oxide dispersion-strengthened (ODS) steels using the LPBF process. Notably, Y2O3 agglomerates were identified in the LPBF-fabricated 316L ODS steels, without inducing grain refinement, while the impact on tensile strength of Y2O3 addition proved negligible. Tensile elongation was decreased due to the poor bonding of the Y2O3 agglomerations to the matrix. The crucial role of the wettability of the reinforcement and the matrix in facilitating grain refinement and strength enhancement is discussed. The poor wettability of the Y2O3 particles and 316L emerged as the primary cause for Y2O3 agglomeration. This finding highlights the importance of addressing wettability issues to optimize the manufacturing process and enhance the overall performance of LPBF-fabricated metal matrix composites.
1. Introduction
The rise of additive manufacturing (AM) has captured significant attention, both within academic circles and across various industrial sectors. In comparison to traditional manufacturing processes, AM presents a host of advantages, ranging from enhanced performance to simplified fabrication. There are four prominent types of fusion-based metal AM processes, i.e., laser powder bed fusion (LPBF), electron beam powder bed fusion, laser-directed energy deposition, and wire arc additive manufacturing [1,2]. Among them, the LPBF process distinguishes itself by its exceptional precision in geometry because of its small laser spot size and highest cooling rate. Moreover, LPBF offers distinct advantages in manufacturing metal matrix composites. These advantages arise from its small melting zone and rapid cooling rate, which mitigate the risk of reinforcement segregation [3].
The first wall structural materials of fission and future fusion reactors are exposed to high temperatures, large time-varying stresses, chemically reactive surroundings, and intense neutron radiation [4,5,6]. Intense neutron irradiation is the primary cause of microstructural changes and degradation in the properties of materials used in fission and fusion reactors. In such environments, helium is generated through nuclear transmutation. Helium is insoluble in steels and tends to precipitate in the form of gas bubbles. He, with its low reactivity, can endure within materials for extended durations. This persistence can lead to the buildup of helium along grain boundaries, potentially causing grain swelling or inducing embrittlement [7]. Helium bubbles serve as sites for void formation, ultimately contributing to structural failure. To meet the extremely rigorous operational environment requirements, ODS steels have been designed for structural applications at elevated temperature in a high-energy neutron environment since the 1980s [8]. ODS steels are Fe-Cr-based materials containing a very high density of Y- or Y-Ti oxide nanoparticles that are uniformly embedded in the matrix.
A conventional approach to fabricating ODS steel involves powder metallurgy, where the powder undergoes mechanical alloying through high-energy ball milling for extended durations, often in the range of tens of hours. The ball-to-powder ratio typically falls within the range from 5:1 to 50:1 [9]. Then, consolidation is conducted via hot pressing, hot isostatic pressing, or spark plasma sintering. Mechanically alloyed ODS steels also typically contain small cavities caused by gas bubbles formed during milling at the matrix–precipitate interface and larger pores at the interface with the milled particles [10]. Therefore, consolidation is usually followed by hot rolling or hot extrusion to minimize these cavities [8,11,12]. The mechanical alloying (MA) process was developed to fabricate commercial ODS steels, such as PM2000 [13], MA956 [14], MA957 [14,15], etc.
However, the inherent limitation of this method lies in its restricted manufacturing freedom, resulting in the production of components with relatively simple shapes, such as tubes and bars. This limitation is primarily attributed to the intrinsic challenges associated with the mechanical alloying process. The fabrication of components with complex geometries using ODS steels remains a difficult task due to the inherent constraints of the traditional methods and the lack of effective welding processes. With the application of the AM process, which has the advantage of fabricating components with complex geometry due to its unique layer-by-layer building strategy, manufacturing the complex structure of ODS steel is possible.
Currently, there are limited investigations on the AM of ODS steel. Walker et al. [16] researched the application of LPBF to fabricate PM2000 ODS steel. However, the powder used was an irregular-shape mechanically alloyed powder with poor quality, unsuitable for the LPBF process. In addition, the maximum power of the laser was only 50 W, which is small. Boegelein et al. [17,18] used the same powder and LPBF system as Walker et al. to study the LPBF of ODS steel. In their paper, Y2O3 agglomerations and cracks were found in the LPBF-fabricated samples. Similarly, Hunt et al. [19] used the mechanically alloyed MA956 ODS powder for the LPBF process. Unsurprisingly, many pores were found in the samples.
Gao et al. [20] used pre-alloyed sphere Fe-18Cr-2W powder mixed with 0.5 wt% Ti and 0.3 wt% Y2O3 to investigate the electron beam melting (EBM) of ODS steel. However, they only researched the feasibility of fabricating ODS steel by LPBF without benchmarks. Gao et al. [21] also studied the 316L powder mixed with 0.4 wt% Y2O3 with a benchmark to research the effect of Y2O3 addition on the EBM of 316L steel. It should be noticed that the EBM benchmark property of 316L in ref [21] was quite poor when the yield strength was below 150 MPa. Thus, more studies on the AM of ODS steel need to be performed.
LPBF has been applied to fabricate various metal matrix composites with improved performance. Zhao et al. [22] studied the effect of TiC nanoparticles addition to 316L using the LPBF process. With the addition of 2 wt% 800 nm TiC particles, the tensile strength increased from 627.5 MPa to 737.8 MPa, but the elongation decreased significantly, from 40.1% to 20.8. The main cause for the decreased elongation was a low relative density of the LPBF-fabricated 316L–TiC composite. Tan et al. [23] reported a novel form of LPBF-fabricated AlSi10Mg with the addition of 0.5 wt% LaB6 nanoparticles. The grains were significantly refined, from about 50 µm to 2 µm. Although the strength did not change significantly, the elongation increased from 3% to 5%. Some other studies also reported similar results, e.g., for Inconel 718-TiC [24], Al-TiC [25], 15-5PH-WC [26], and 15-5PH-TiC [27]. Grain refinement was observed in these LPBF-fabricated metal matrix composites. However, in some cases, no significant grain refinement and strength enhancement were observed; this was the case, for example, for 316L-Y2O3 [28] and Al-Fe3O4 [29]. The underlying mechanisms and criteria for choosing refinement particles need to be further explored.
In this work, commercial 316L powder and Y2O3 nanoparticles were mixed using the low-energy ball milling process. The advantageous flowability characteristic of the 316L powder was successfully maintained even after the mixing process. The microstructure and tensile properties of LPBF-fabricated 316L ODS steels were studied. Furthermore, our research contributes to the field by proposing specific criteria for the selection of reinforcement materials aimed at achieving effective grain refinement in the context of fusion-based additive manufacturing processes. This framework serves as a valuable guideline for future endeavors seeking to optimize the mechanical properties of such composite materials.
2. Materials and Methods
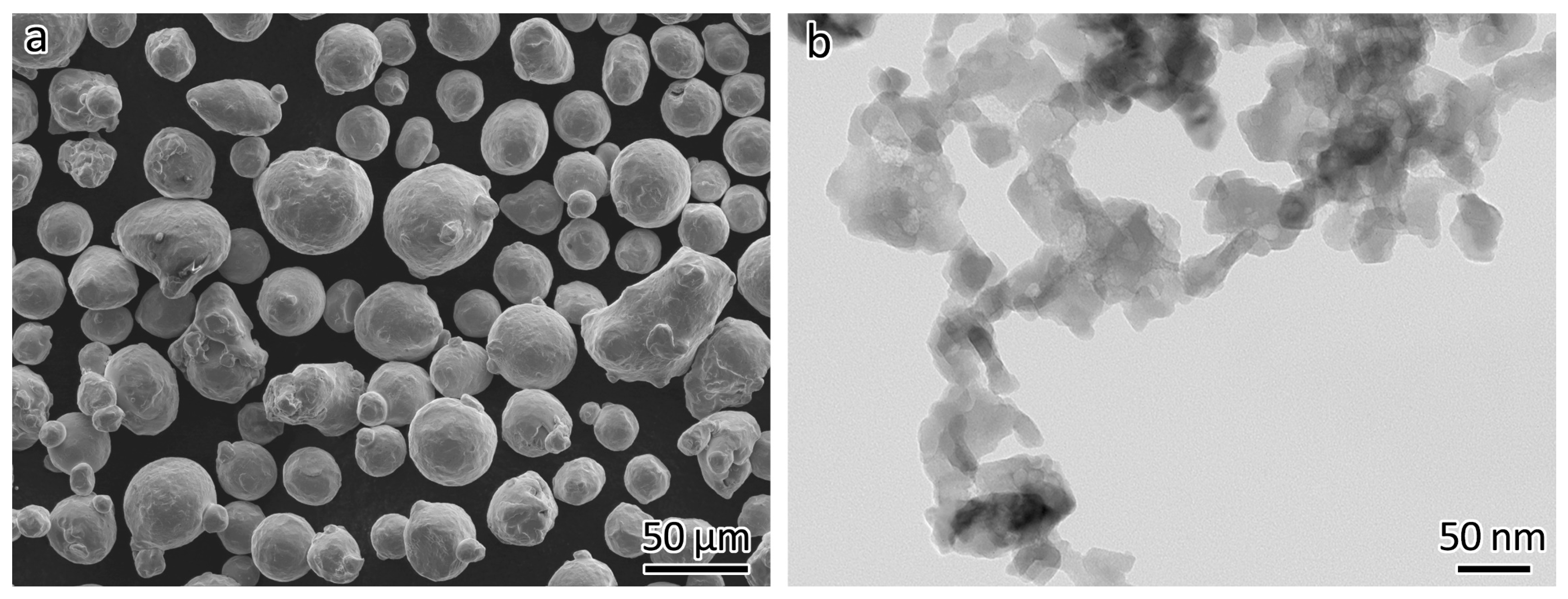
Commercial gas-atomized 316L powder (Höganäs AB, Höganäs, Sweden) was used as feedstock. The mean size was 42.3 µm (D10: 26.3 µm, D50: 39.8 µm, D90: 60.9 µm) as measured using a laser light-scattering size distribution analyzer. The chemical composition was as follows (wt%): Cr 16.8%, Ni 12.7%, Mo 2.5%, Mn 1.5%, Si 0.7%, and C 0.011%. The morphology of the 316L powder is shown in Figure 1a. The reinforcement consisted of Y2O3 nanoparticles (Nanostructured & Amorphous Materials, Inc., Houston, TX, USA). The vendor claimed that the size distribution was 30–45 nm. The actual size distribution was measured manually and resulted to be about 10–90 nm, with an average size of 42 nm. The morphology of the particles, as shown in Figure 1b, was obtained using transmission electron microscopy (JEOL 2010, JEOL Ltd., Tokyo, Japan).
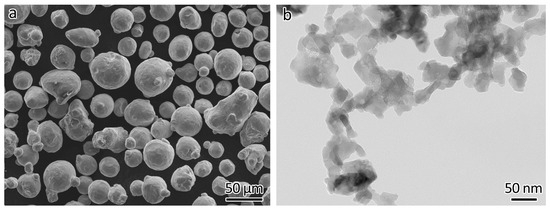
Figure 1.
Morphology of the feedstocks used. (a) 316L powder, (b) Y2O3 nanoparticles.
Traditionally, ODS steels contain a small amount of Y2O3 nanoparticles, from 0.3 to 1.0% [8,10,12,15]. Therefore, in this work, Y2O3 nanoparticles were added to the 316L powder to obtain the concentrations of 0.3 wt% and 1.0 wt%. The nanoparticles were mixed with the 316 L powder using the low-energy ball milling process. The details of the process of powder mixing were reported in our previous work [9]. The low-energy balling process allowed mixing the nanoparticles and the 316L powder uniformly without decreasing the flowability of the original powder.
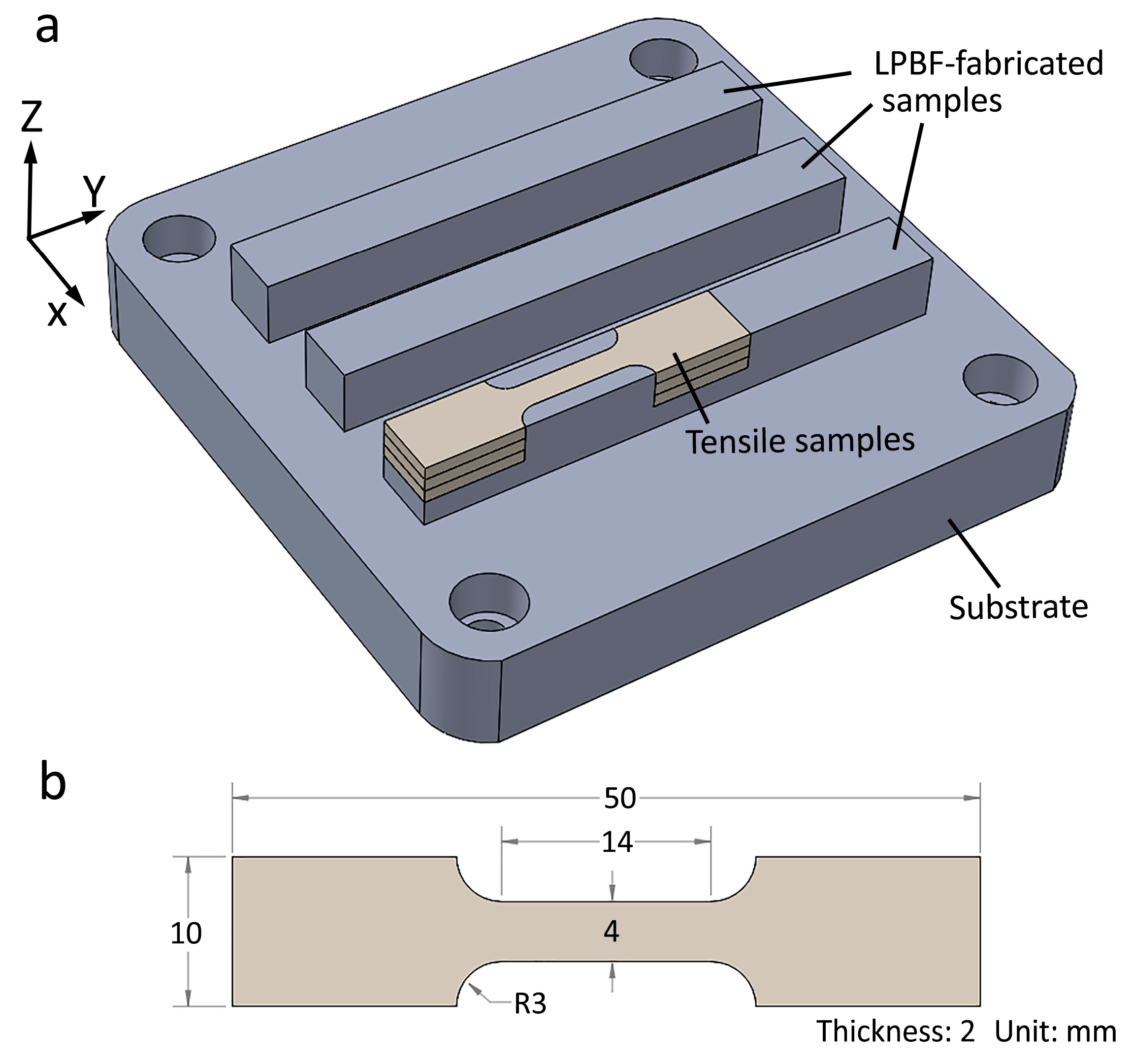
The commercial LPBF machine ProX DMP 300 (3D Systems, Rock Hill, CA, USA) was employed to print the samples. The machine was equipped with a fiber laser (wavelength at 1070 nm). The maximum laser power was 500 watts. The laser spot was 75 µm at its focal point. The substrate was 316L with the dimensions of 100 mm × 100 mm × 15 mm. The printing parameters were fixed as follows: laser power 250 W, scan speed 1200 mm/s, layer thickness 40 µm, and hatching space 55 µm. The laser scanning direction between two adjacent layers was rotated by 90°. Horizontal blocks with the size of 80 mm × 10 mm × 10 mm were printed. The LPBF-fabricated samples are shown in Figure 2a. Z is the building direction.
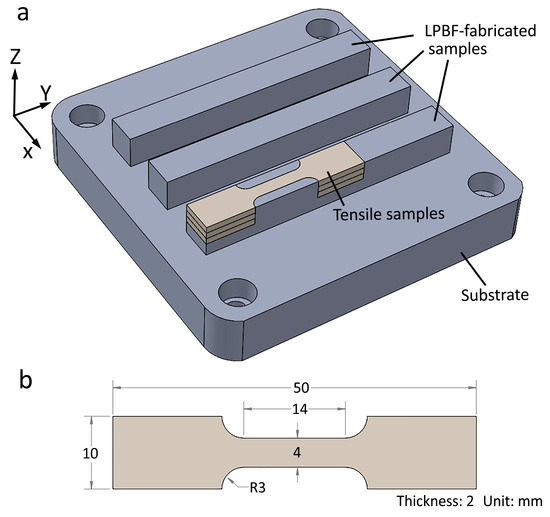
Figure 2.
(a) Illustration showing the LPBF-fabricated samples and the locations of the tensile samples, (b) dimensions of the tensile sample.
The microstructure of the printed samples was observed using an optical microscope (Zeiss Axioskop 2 MAT, Carl Zeiss, Oberkochen, Germany). Before the observation, the samples were polished to a mirror surface using a silicon oxide polishing suspension (OPS, Struers, Ballerup, Denmark) and an MD-Chem polishing plate (Struers, Denmark). An etching solution with the formula HF/HNO3/H2O = 1:6:12 (vol%) was used to etch the samples in order to observe the cellular structures using a scanning electron microscope (SEM, JEOL 7600F, Tokyo, Japan). Energy-dispersive X-ray spectroscopy (EDS, Oxford Instruments, Abingdon, UK) was also used to analyze the composition. Electron backscatter diffraction (EBSD, Oxford Instruments, UK) with a Nordlys detector was used to measure the grain size. The accelerated voltage of 10 kV was used for SEM observations, and a voltage of 20 kV was used for the EDS and EBSD measurements. A step size of 0.6 µm was used for the EBSD measurements.
The tensile samples were cut using electron discharge machining. Tensile tests were conducted at room temperature at the strain rate of 10−3 s−1 using an Instron 5982 (Instron Norwood, MA, US) universal tensile testing machine. An Instron AVE 2 video extensometer was used to measure the tensile strain. The dimensions of the tensile samples are shown in Figure 2b. Three tensile samples were tested for each condition. The locations of the tensile samples are shown in Figure 2.
3. Results
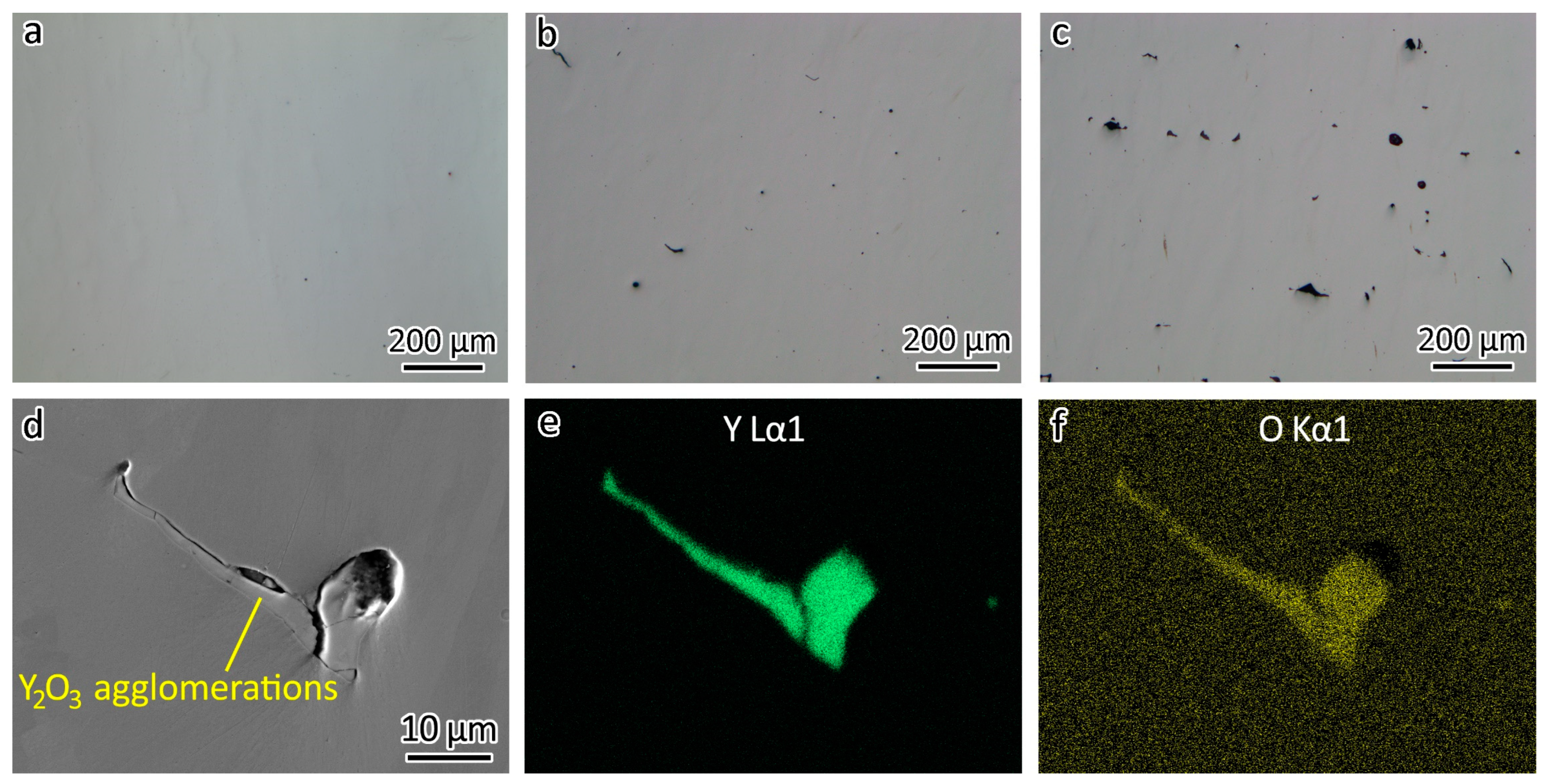
In Figure 3a, the optical image provides a clear view of the polished 316L without etching, revealing a surface devoid of any discernible defects such as lack of fusion or porosity, indicating a high relative density. However, with the addition of 0.3 wt% Y2O3 nanoparticles, defects appeared, as shown in Figure 3b. Increasing the addition of Y2O3 nanoparticles to 1.0 wt% led to more defects, as shown in Figure 3c. It is evident that an increase in Y2O3 concentration led to larger agglomerates.
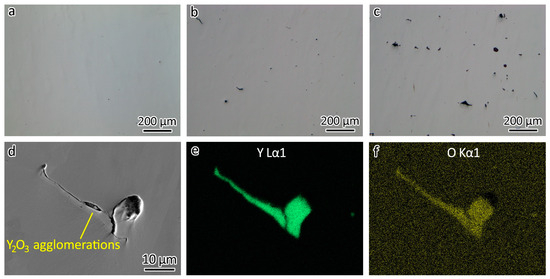
Figure 3.
Optical images showing defects in (a) 316L, (b) 316L-0.3Y2O3, and (c) 316L-1.0Y2O3. (d) SEM image showing an agglomeration of Y2O3 nanoparticles in 316L-1Y2O3. EDS map showing the distribution of (e) Y and (f) O in the agglomeration.
To gain a deeper understanding of these defects, a more detailed examination using SEM was conducted. Figure 3d shows the SEM image of a Y2O3 agglomeration. Figure 3e,f shows the distribution of Y and O detected using EDS. It is noteworthy that, despite the defects in the 316L-Y2O3 ODS steels resembling those caused by a lack of fusion, they were, in fact, Y2O3 agglomerations. These agglomerations arose during the LPBF process, when the Y2O3 nanoparticles underwent sintering, therefore forming the agglomerations. Gaps could be observed between the Y2O3 agglomerations and the 316L matrix, as shown in Figure 3d.
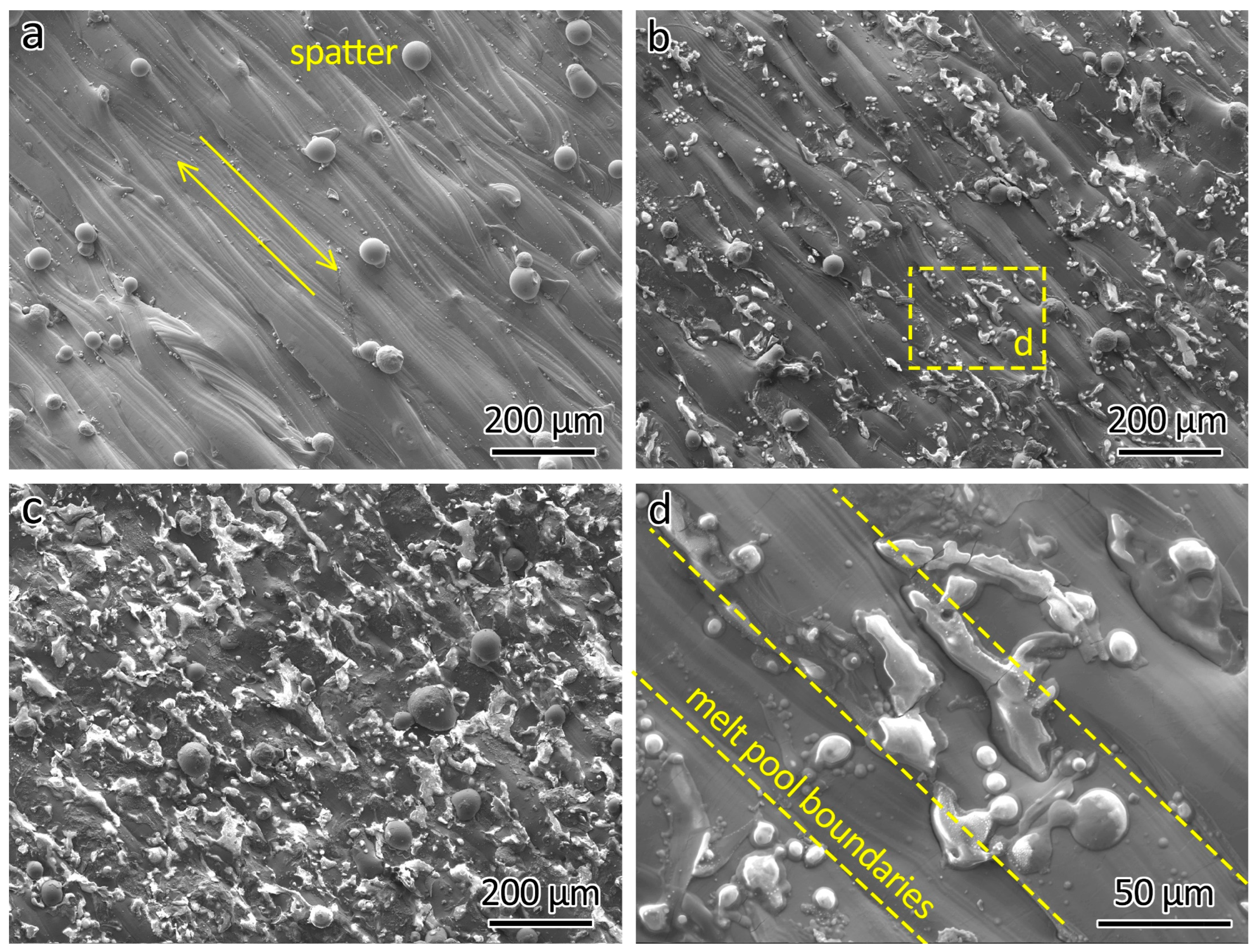
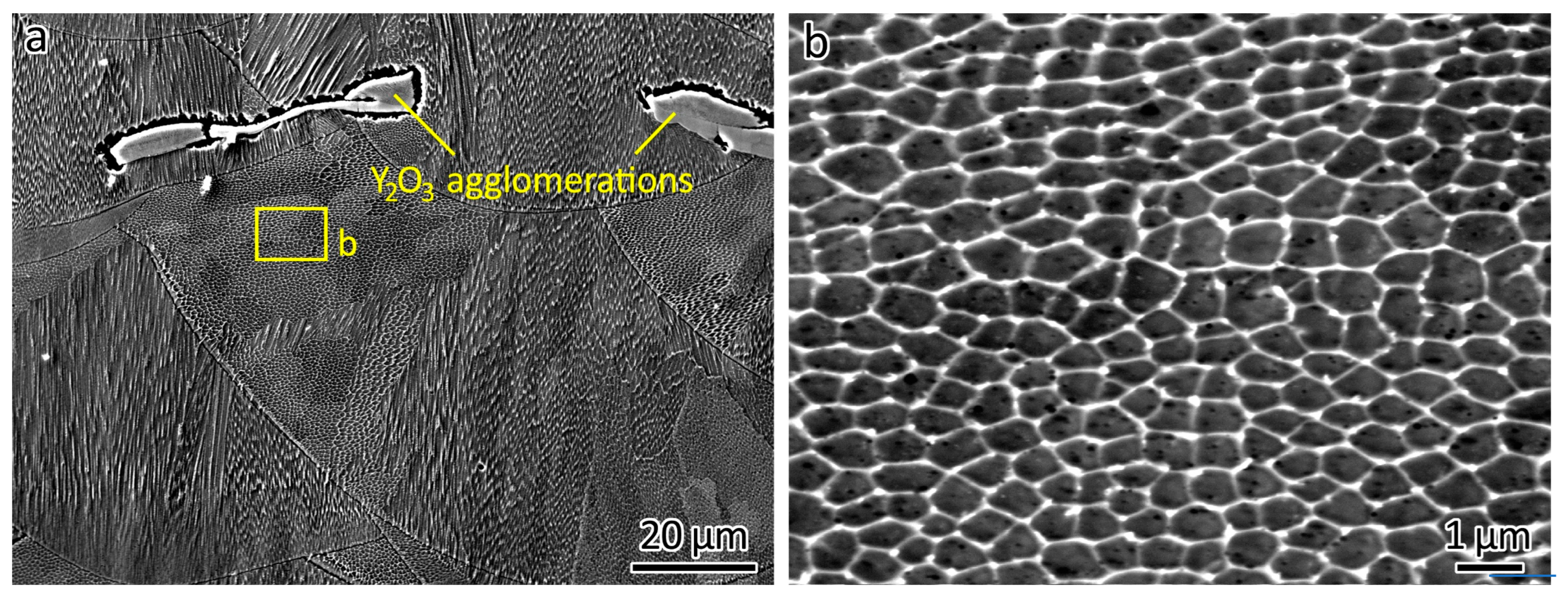
The SEM analysis of the top surfaces of the LPBF-printed ODS steels provided additional insights, as shown in Figure 4. Figure 4a presents the top surface of 316L, revealing powder adherent to the surface, which was produced by spatters generated during the LPBF process. The distinctly parallel laser-scanned tracks on the surface are the result of the track-by-track laser scanning strategy employed during fabrication.
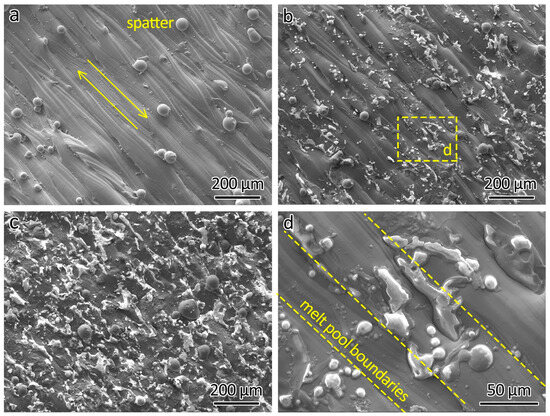
Figure 4.
SEM images showing the top surface of (a) 316L, (b) 316L-0.3Y2O3, and (c) 316L-1.0Y2O3 and (d) a close-up of 316L-0.3Y2O3 top surface showing Y2O3 agglomerations.
With the addition of 0.3 wt% Y2O3 nanoparticles, white agglomerations appeared on the top surface, as shown in Figure 4b. Further increasing the content of the Y2O3 nanoparticles to 1.0 wt% intensified the presence of these agglomerations on the top surface, as shown in Figure 4c. The agglomerations were sintered Y2O3 particles. A similar agglomeration phenomenon was found in LPBF-fabricated PM2000 (FeCrAl) ODS steel reported by Boegelein et al. [18].
A closer examination of the top surface of 316L-0.3Y2O3 is shown in Figure 4d. It can be seen that the locations of the Y2O3 agglomerations are independent of the melt pool boundaries.
The occurrence of cellular structures was frequently observed in LPBF-fabricated 316L [3,30]. The formation of these cellular structures can be attributed to the inherent rapid cooling rate (106–108 K/s) of the LPBF process. This phenomenon extended to LPBF-fabricated 316L-Y2O3 ODS steels, as shown in Figure 5. Figure 5a shows an overview of the microstructure of 316L-1.0Y2O3. The presence of cellular structures is notable.
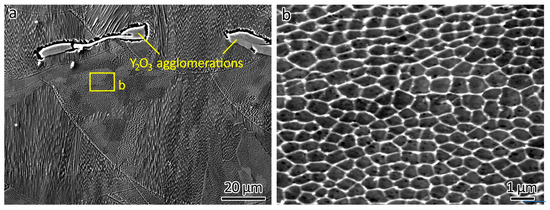
Figure 5.
SEM images showing the microstructure of (a) 316L-1Y2O3 and (b) a close-up displaying the cellular structures.
Gaps between the Y2O3 agglomerations and the 316L matrix can be observed due to their poor wettability. These observed gaps have significant implications for the bonding between the Y2O3 agglomerations and the 316L matrix.
A close-up of the cellular structures is shown in Figure 5b. The average size of the cellular structures was 0.49 µm for 316L, 0.45 µm for 316L-0.3Y2O3, and 0.57 µm for 316L-1.0Y2O3, as measured using the inter-section method. The cooling rate was estimated according to the empirical relationship λ = 80·T−0.33 [31], where λ is the cell spacing (µm), and T is the cooling rate (K/s). Due to the similar size of the cellular structures, the cooling rate for the three materials was similar. The formation of the cellular structure was due to the fast cooling rate of the LPBF process. The results from the atomic force microscopy study revealed that sub-grain walls extended beyond the surface [32], suggesting improved corrosion resistance in comparison to the cell interior. These sub-grain walls were identified as dislocated cells characterized by a higher concentration of Cr and Mo [30] (which exhibit greater corrosion resistance than Fe) compared to the cell interior.
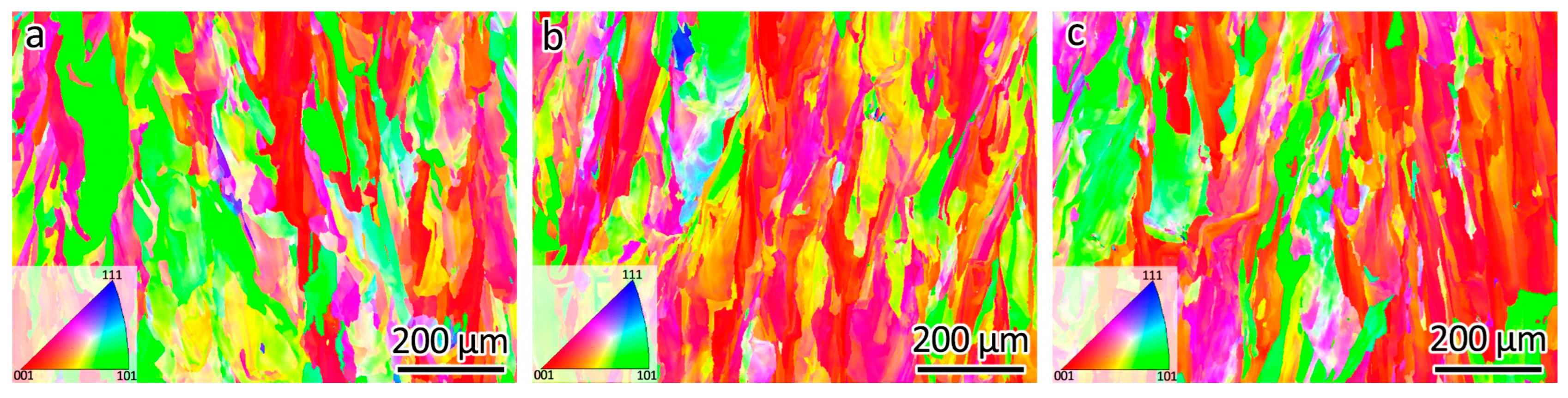
Figure 6 shows the EBSD orientation maps of the LPBF-fabricated 316L, 316L-0.3Y2O3, and 316L-1.0Y2O3 samples. 316L stainless steel fabricated using a forging process usually contains equiaxed grains with a high fraction of twin boundaries [3]. It can be seen that the grains of the LPBF samples under EBSD exhibited a stripe-like pattern. This pattern can be attributed to the epitaxial growth mode characteristic of the LPBF process. Notably, epitaxial grains within this sample demonstrated a <001> texture, showing a preferential alignment along the direction of the maximum temperature gradient, which corresponded to the building direction.

Figure 6.
EBSD orientation maps of (a) 316L, (b) 316L-0.3Y2O3, (c) 316L-1.0Y2O3.
The average grain size of the SLM 316L, 316L-0.3Y2O3, and 316L-1.0Y2O3 samples was calculated based on the grain boundary misorientation of 15°. The average grain size of 316L, 316L-0.3Y2O3, and 316L-1.0Y2O3 was 25.9 µm, 27.7 µm, and 21.6 µm, respectively. It can be seen that the effect of Y2O3 addition on the grain size was neglectable. The possible reason could be the poor wettability of 316L and Y2O3. The Y2O3 nanoparticles tended to agglomerate during the LPBF process, which prevented a significant grain refinement.
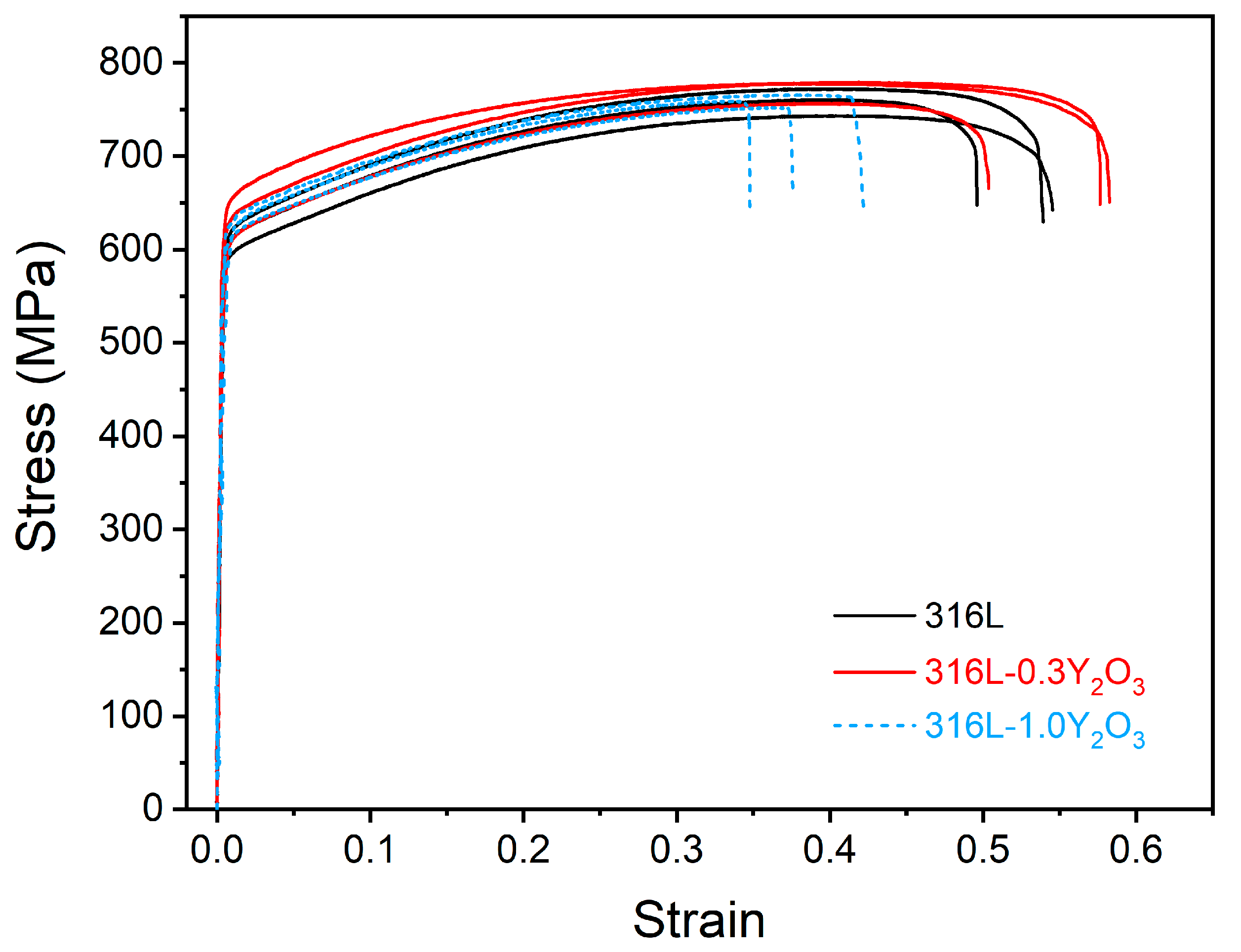
The stress–strain curves of the 316L, 316L-0.3Y2O3, and 316L-1.0Y2O3 steels are presented in Figure 7. The tensile results are compared in Table 1. Gao et al. [21] reported that 316L ODS steel fabricated using the spark plasma sintering process has a low UTS of 348 MPa with an elongation of 17.8%. Leo et al. [33] reported that 316L ODS steel has a yield strength of 458 MPa and a UTS of 723 MPa with an elongation of 46% when fabricated using the mechanical alloying process. The mechanical properties of the LPBF-fabricated 316L ODS steel appeared to be better than those of the mechanically alloyed one. According to the ASTM-A240 standard [34], for 316L, the minimum yield strength is 170 MPa, UTS is 485 Mpa, and elongation is 40%. The LPBF-fabricated 316L showed a good combination of high strength and ductility.
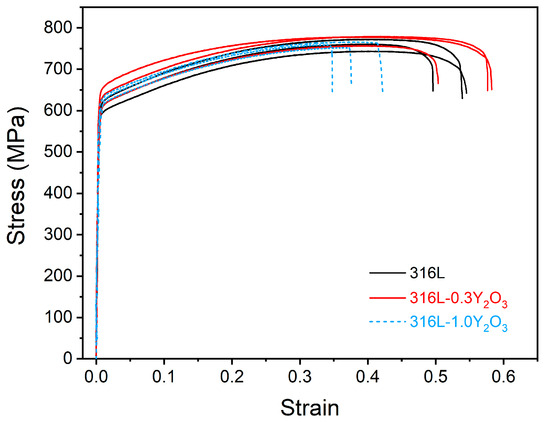
Figure 7.
Tensile stress–strain curves for 316L, 316L-0.3Y2O3, and 316L-1.0Y2O3 steels.

Table 1.
Tensile properties of LPBF-fabricated 316L, 316L-0.3Y2O3, and 316L-1.0Y2O3 steels.
It was noticed that the addition of 0.3% Y2O3 to 316L slightly improved the yield strength and the tensile strength without sacrificing ductility, although more defects appeared, including pores and defects caused by a lack of fusion in LPBF-fabricated 316L-0.3Y2O3 ODS steel. The increase in yield strength could be attributed to Orowan strengthening and was due to the fact that the added nanoparticles acted as barrier to dislocation movement [3,35]. However, with the 1.0% Y2O3 addition, the ductility decreased significantly. This was because of the severe agglomeration of the Y2O3 nanoparticles.
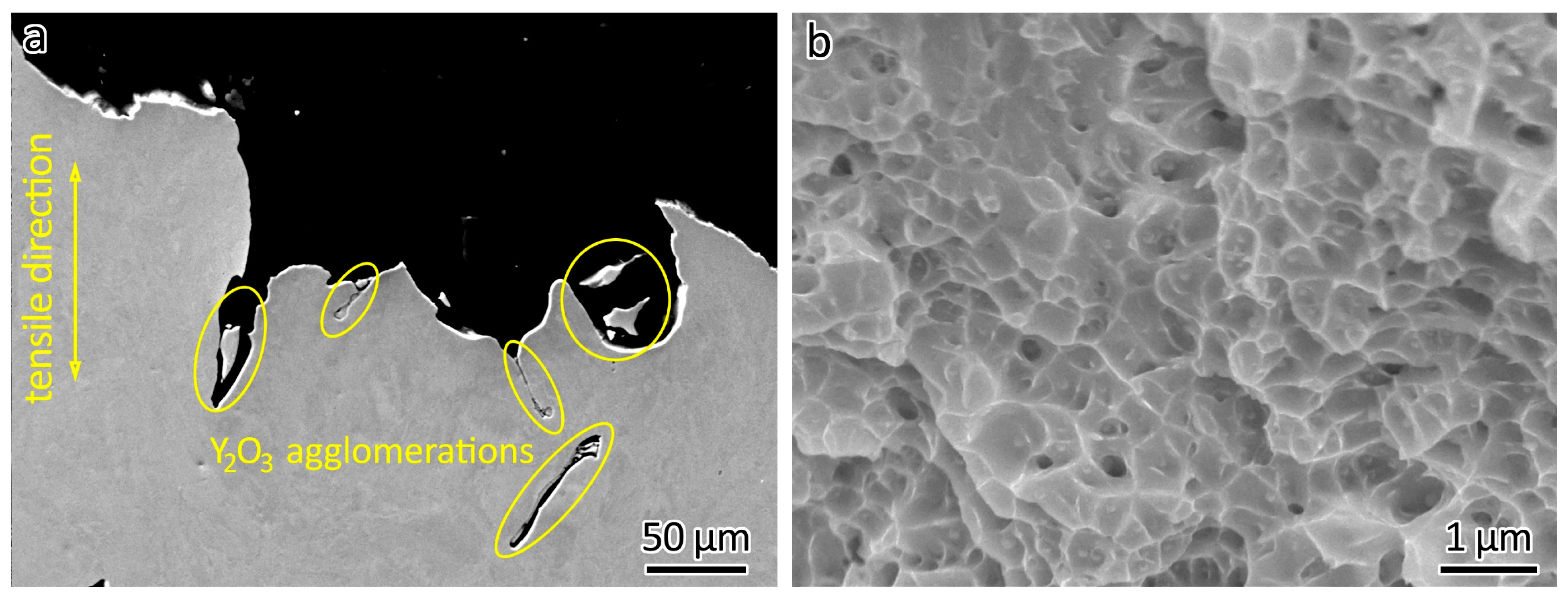
After the tensile tests, the fractographic features of the printed samples were further studied using SEM. Y2O3 agglomerations could be seen the fraction points, as shown in Figure 8a. The poor bonding between the Y2O3 agglomerations and the 316L matrix and the sharp corners of the agglomerations caused premature fractures during the tensile test, because fractures form easily in the presence of defects. Therefore, decreased elongation was obtained in the LPBF-fabricated 316L ODS steels.
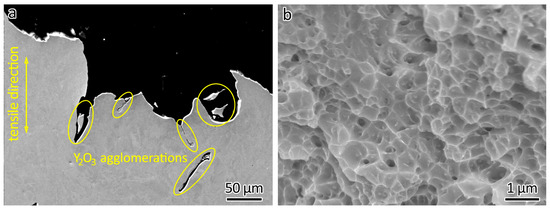
Figure 8.
(a) Y2O3 agglomerations and (b) dimples observed in 316L-1Y2O3 after fracturing.
Similar to LPBF-fabricated 316L without Y2O3 addition [3,36,37], dimples could also be observed in 316L ODS steels, as shown in Figure 8b. The formation of dimples in the fracture surface was the result of a microscopic void coalescence process. During tensile testing, the material underwent plastic deformation. Small voids formed and started to grow and elongate due to the deformation. Adjacent voids eventually coalesced, leading to the formation of larger voids.
4. Discussion
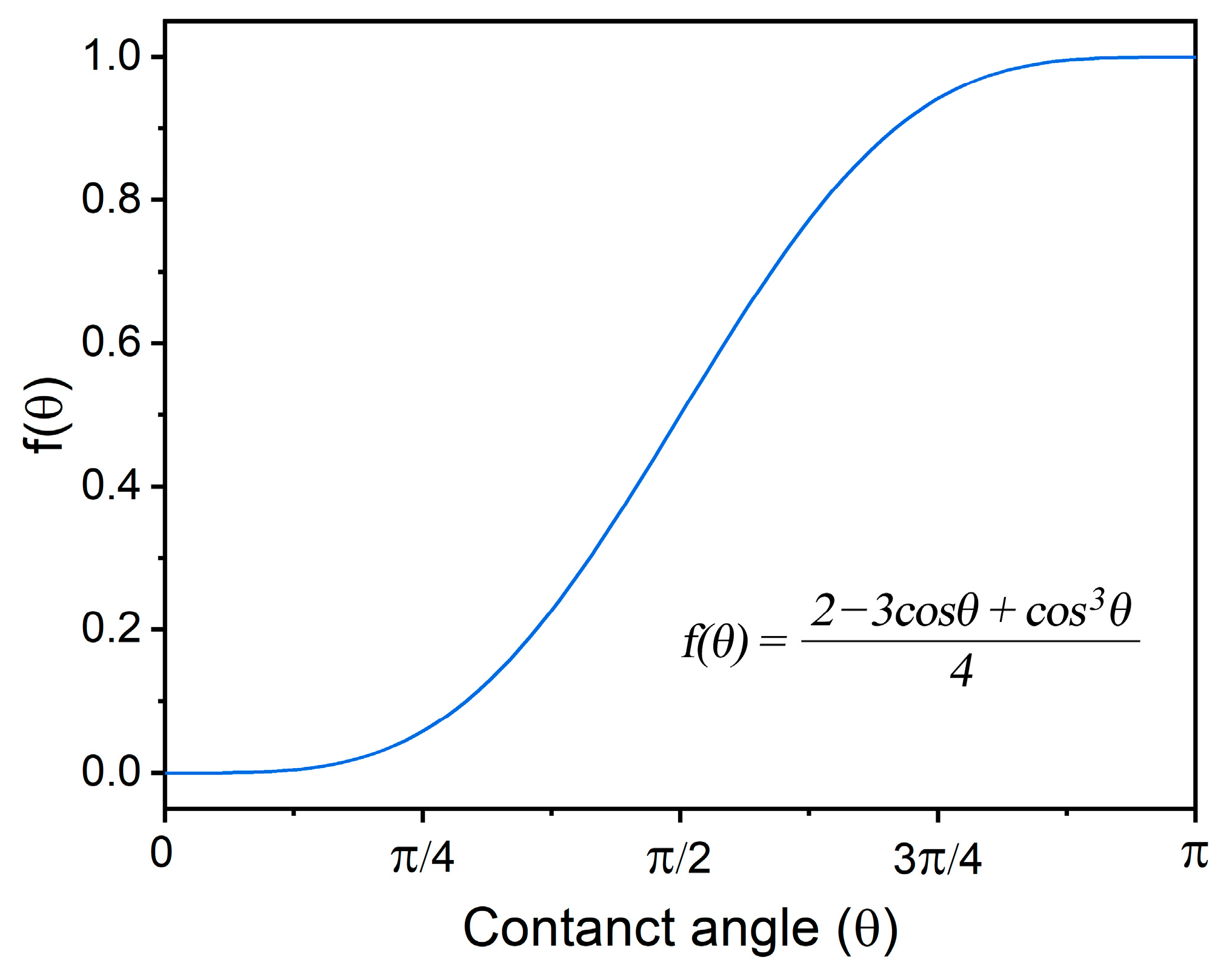
Examining the microstructures presented in Figure 3d and Figure 5a, it becomes evident that the existence of gaps between the Y2O3 agglomerations and the 316L matrix is indicative of poor wettability. This observation is crucial, as poor wettability hinders the promotion of heterogeneous nucleation, thereby impeding the intended grain refinement in the presence of Y2O3 nanoparticles, as shown in Figure 6. This phenomenon is related to the free energy required for heterogeneous nucleation, which is inherently a function of wettability (contact angle between particle surface and melt), as expressed below:
Here, is the free energy needed for heterogeneous nucleation; is the free energy needed for homogenous nucleation; and θ is the contact angle. The curve describing is shown in Figure 9. It can be seen that good wettability (small contact angle) is preferable for grain refinement. It is worthy to point out that there is no significant difference in grain refinement if the contact angle is smaller than 45°. When the contact angle is 90°, the value of is 0.5, which means that the free energy needed for heterogeneous nucleation is half of that required for homogenous nucleation. Consequently, the manifestation of poor wettability in this scenario significantly alters the dynamics of nucleation processes, leading to a lack of substantial grain refinement in the resultant microstructures.
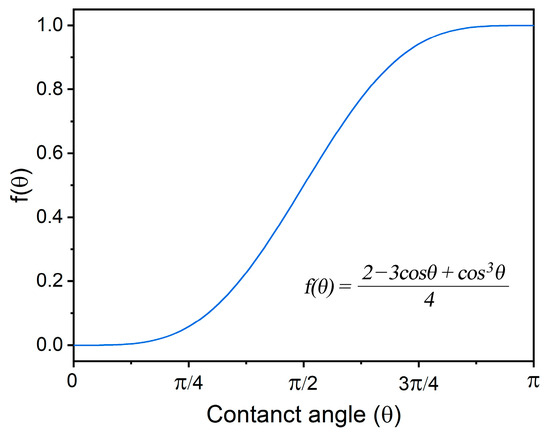
Figure 9.
The effect of wettability (contact angle) on the free energy needed for heterogeneous nucleation.
Y2O3 has a small density of 5 g/cm3, in contrast to 316L, which has a density of 8 g/cm3 for. In the 316L–Y2O3 melt, Y2O3 agglomerations were not embedded in the matrix; rather, their relatively lower density caused them to rise to the surface. Therefore, Y2O3 agglomerations were observed on the top surface (Figure 4). As noted, agglomerations formed in both ODS steels. A similar phenomenon was also observed in references [17,18,28]. Despite these observations, those studies reported the successful fabrication of ODS steel using the LPBF process, even though with unavoidable agglomerations. We concluded that the poor wettability of Y2O3 particles and 316L emerged as the primary cause for the formation of agglomerations. In this work, we highlight that the fusion-based metal additive manufacturing process produces no benefit concerning grain refinement and tensile properties.
TiC has a small density of 4.9 g/cm3; however, no TiC agglomerations were observed on the top surface of the steel in our previous studies [3,35,38]. The contact angle of TiC on stainless steel is small, being 30° [39], indicating a good wettability. Therefore, density is not a critical factor for the rising of the Y2O3 particles to the surface.
In our previous report, we studied the grain refinement achieved by the addition of micron-sized TiC particles [35,38] and TiC nanoparticles [3] to LPBF-processed 316L stainless steel. Significant grain refinement was observed due to the good wettability of TiC and stainless steel. The grains were refined from 25.9 µm to 2.7 µm [35]. Significant grain refinement was also observed in different alloy systems and ceramic particles fabricated using the LPBF process, such as AlSi10Mg-TiB2 [40], AlSi10Mg-LaB6 [23], 316L-TiN [41], and Ti-TiB/TiC [42].
The gaps between the Y2O3 agglomerations and the 316L matrix also indicated inadequacy of bonding in the composite material. Poor bonding contributed to the formation of stress concentration sites, particularly at the sharp corners of the agglomerations. During the tensile tests, these stress concentration sites became preferential locations for the initiation and propagation of fractures, ultimately leading to premature failure, as illustrated in Figure 8a. As a consequence, the incorporation of Y2O3 nanoparticles into the material resulted in a noticeable reduction in elongation, highlighting the detrimental impact of poor bonding on the mechanical properties of the composite.
It is hoped that this work will stimulate interest in studying the AM of ODS steels. Despite the poor wettability of Y2O3 and 316L, various potential strategies exist to enhance this crucial aspect. A noteworthy method involves the surface modification of the Y2O3 nanoparticles. If metallic elements are coated on the surface of the Y2O3 nanoparticles, the wettability can be improved. Research findings indicated that Ni-Y2O3 nanoparticles can be synthesized through a chemical reaction utilizing nickel nitrate and yttrium nitrate, followed by a sequence of stirring and calcination processes [43]. This could be a possible solution for the fabrication of ODS steels using fusion-based AM processes.
In summary, this study highlights the importance of wettability in the context of fusion-based additive manufacturing for achieving effective grain refinement and strength enhancement. The wettability of the reinforcement and the metal matrix emerged as a critical factor influencing the overall performance of the fabricated materials. In particular, optimal wettability enhances grain refinement processes, which, in turn, contribute to the overall structural integrity of the manufactured components. It is hoped that these results will stimulate interest in this field, leading to a comprehensive understanding of the role of wettability in LPBF-fabricated metal matrix composites. This will undoubtedly guide the development of improved materials and methodologies in fusion-based additive manufacturing, paving the way for enhanced performance and reliability in various engineering applications.
5. Conclusions
- Poor wettability of Y2O3 nanoparticles and 316L was observed;
- Agglomerations were observed in LPBF-fabricated 316L-0.3Y2O3 and 316L-1.0Y2O3;
- Poor wettability of 316L and Y2O3 was the main reason for the formation of the agglomerations;
- The addition of Y2O3 nanoparticles slightly increased yield strength and UTS. However, elongation significantly decreased due to the sharp corners of the agglomeration acting as stress concentration points.
Author Contributions
Conceptualization, W.Z. (Wei Zhou); methodology, W.Z. (Wengang Zhai), W.Z. (Wei Zhou) and S.M.L.N.; validation, W.Z. (Wengang Zhai), W.Z. (Wei Zhou) and S.M.L.N.; formal analysis, W.Z. (Wengang Zhai), W.Z. (Wei Zhou) and S.M.L.N.; investigation, W.Z. (Wengang Zhai), W.Z. (Wei Zhou) and S.M.L.N.; resources, W.Z. (Wei Zhou) and S.M.L.N.; data curation, W.Z. (Wengang Zhai); writing—original draft preparation, W.Z. (Wengang Zhai); writing—review and editing, W.Z. (Wengang Zhai), W.Z. (Wei Zhou) and S.M.L.N.; visualization, W.Z. (Wengang Zhai); supervision, W.Z. (Wei Zhou) and S.M.L.N.; project administration, W.Z. (Wei Zhou) and S.M.L.N.; funding acquisition, W.Z. (Wei Zhou) and S.M.L.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lewandowski, J.J.; Seifi, M. Metal Additive Manufacturing: A Review of Mechanical Properties. Annu. Rev. Mater. Res. 2016, 46, 151–186. [Google Scholar] [CrossRef]
- DebRoy, T.; Wei, H.L.; Zuback, J.S.; Mukherjee, T.; Elmer, J.W.; Milewski, J.O.; Beese, A.M.; Wilson-Heid, A.; De, A.; Zhang, W. Additive manufacturing of metallic components—Process, structure and properties. Prog. Mater. Sci. 2018, 92, 112–224. [Google Scholar] [CrossRef]
- Zhai, W.; Zhou, W.; Nai, S.M.L. Grain refinement and strengthening of 316L stainless steel through addition of TiC nanoparticles and selective laser melting. Mater. Sci. Eng. A 2022, 832, 142460. [Google Scholar] [CrossRef]
- Odette, G.R. On the status and prospects for nanostructured ferritic alloys for nuclear fission and fusion application with emphasis on the underlying science. Scr. Mater. 2018, 143, 142–148. [Google Scholar] [CrossRef]
- Hosemann, P.; Frazer, D.; Stergar, E.; Lambrinou, K. Twin boundary-accelerated ferritization of austenitic stainless steels in liquid lead–bismuth eutectic. Scr. Mater. 2016, 118, 37–40. [Google Scholar] [CrossRef]
- Hirata, A.; Fujita, T.; Wen, Y.R.; Schneibel, J.H.; Liu, C.T.; Chen, M.W. Atomic structure of nanoclusters in oxide-dispersion-strengthened steels. Nat. Mater. 2011, 10, 922–926. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.R.; Dudarev, S.L.; Nguyen-Manh, D.; Zheng, S.; Packer, L.W.; Sublet, J.C. Neutron-induced dpa, transmutations, gas production, and helium embrittlement of fusion materials. J. Nucl. Mater. 2013, 442, S755–S760. [Google Scholar] [CrossRef]
- Odette, G.R.; Alinger, M.J.; Wirth, B.D. Recent Developments in Irradiation-Resistant Steels. Annu. Rev. Mater. Res. 2008, 38, 471–503. [Google Scholar] [CrossRef]
- Zhai, W.; Zhou, W.; Nai, S.M.L.; Wei, J. Characterization of nanoparticle mixed 316 L powder for additive manufacturing. J. Mater. Sci. Technol. 2020, 47, 162–168. [Google Scholar] [CrossRef]
- Sakasegawa, H.; Ohtsuka, S.; Ukai, S.; Tanigawa, H.; Fujiwara, M.; Ogiwara, H.; Kohyama, A. Microstructural evolution during creep of 9Cr-ODS steels. Fusion Eng. Des. 2006, 81, 1013–1018. [Google Scholar] [CrossRef]
- Huang, J.; He, J.; Yu, X.; Li, C.; Fan, D. The study of mechanical strength for fusion-brazed butt joint between aluminum alloy and galvanized steel by arc-assisted laser welding. J. Manuf. Process. 2017, 25, 126–133. [Google Scholar] [CrossRef]
- Dadé, M.; Malaplate, J.; Garnier, J.; De Geuser, F.; Barcelo, F.; Wident, P.; Deschamps, A. Influence of microstructural parameters on the mechanical properties of oxide dispersion strengthened Fe-14Cr steels. Acta Mater. 2017, 127, 165–177. [Google Scholar] [CrossRef]
- Krautwasser, P.; Czyrska-Filemonowicz, A.; Widera, M.; Carsughi, F. Thermal stability of dispersoids in ferritic oxide-dispersionstrengthened alloys. Mater. Sci. Eng. A 1994, 177, 199–208. [Google Scholar] [CrossRef]
- Chou, T.S.; Bhadeshia, H.K.D.H. Crystallographic texture in mechanically alloyed oxide dispersion-strengthened MA956 and MA957 steels. Metall. Trans. A 1993, 24, 773–779. [Google Scholar] [CrossRef]
- Miller, M.K.; Hoelzer, D.T.; Kenik, E.A.; Russell, K.F. Nanometer scale precipitation in ferritic MA/ODS alloy MA957. J. Nucl. Mater. 2004, 329–333, 338–341. [Google Scholar] [CrossRef]
- Walker, J.C.; Berggreen, K.M.; Jones, A.R.; Sutcliffe, C.J. Fabrication of Fe-Cr-Al Oxide Dispersion Strengthened PM2000 Alloy Using Selective Laser Melting. Adv. Eng. Mater. 2009, 11, 541–546. [Google Scholar] [CrossRef]
- Boegelein, T.; Dryepondt, S.N.; Pandey, A.; Dawson, K.; Tatlock, G.J. Mechanical response and deformation mechanisms of ferritic oxide dispersion strengthened steel structures produced by selective laser melting. Acta Mater. 2015, 87, 201–215. [Google Scholar] [CrossRef]
- Boegelein, T.; Louvis, E.; Dawson, K.; Tatlock, G.J.; Jones, A.R. Characterisation of a complex thin walled structure fabricated by selective laser melting using a ferritic oxide dispersion strengthened steel. Mater. Charact. 2016, 112, 30–40. [Google Scholar] [CrossRef]
- Hunt, R.M.; Kramer, K.J.; El-Dasher, B. Selective laser sintering of MA956 oxide dispersion strengthened steel. J. Nucl. Mater. 2015, 464, 80–85. [Google Scholar] [CrossRef]
- Gao, R.; Zeng, L.; Ding, H.; Zhang, T.; Wang, X.; Fang, Q. Characterization of oxide dispersion strengthened ferritic steel fabricated by electron beam selective melting. Mater. Des. 2016, 89, 1171–1180. [Google Scholar] [CrossRef]
- Gao, R.; Ge, W.; Miao, S.; Zhang, T.; Wang, X.; Fang, Q. Hot rolling and annealing effects on the microstructure and mechanical properties of ODS austenitic steel fabricated by electron beam selective melting. Front. Mater. Sci. 2015, 10, 73–79. [Google Scholar] [CrossRef]
- Zhao, S.; Shen, X.; Yang, J.; Teng, W.; Wang, Y. Densification behavior and mechanical properties of nanocrystalline TiC reinforced 316L stainless steel composite parts fabricated by selective laser melting. Opt. Laser Technol. 2018, 103, 239–250. [Google Scholar] [CrossRef]
- Tan, Q.; Zhang, J.; Mo, N.; Fan, Z.; Yin, Y.; Bermingham, M.; Liu, Y.; Huang, H.; Zhang, M.-X. A novel method to 3D-print fine-grained AlSi10Mg alloy with isotropic properties via inoculation with LaB6 nanoparticles. Addit. Manuf. 2020, 32, 101034. [Google Scholar] [CrossRef]
- Luu, D.N.; Zhou, W.; Nai, S.M.L.; Yang, Y. Mitigation of solute segregation during solutionization of selective laser melted Inconel 718 through micron-TiC addition. J. Alloys Compd. 2022, 897, 163224. [Google Scholar] [CrossRef]
- Lin, T.C.; Cao, C.; Sokoluk, M.; Jiang, L.; Wang, X.; Schoenung, J.M.; Lavernia, E.J.; Li, X. Aluminum with dispersed nanoparticles by laser additive manufacturing. Nat. Commun. 2019, 10, 4124. [Google Scholar] [CrossRef]
- Chen, W.; Xu, L.; Hao, K.; Han, Y.; Zhao, L.; Jing, H. Additive manufacturing of 15-5PH/WC composites with the synergistic enhancement of strength and ductility. Mater. Sci. Eng. A 2022, 840, 142926. [Google Scholar] [CrossRef]
- Chen, W.; Xiao, B.; Xu, L.; Han, Y.; Zhao, L.; Jing, H. Additive manufacturing of martensitic stainless steel matrix composites with simultaneously enhanced strength-ductility and corrosion resistance. Compos. Part B Eng. 2022, 234, 109745. [Google Scholar] [CrossRef]
- Zhong, Y.; Liu, L.; Zou, J.; Li, X.; Cui, D.; Shen, Z. Oxide dispersion strengthened stainless steel 316L with superior strength and ductility by selective laser melting. J. Mater. Sci. Technol. 2020, 42, 97–105. [Google Scholar] [CrossRef]
- Ferreira, L.M.P.; Bayraktar, E.; Robert, M.H. Magnetic and electrical properties of aluminium matrix composite reinforced with magnetic nano iron oxide (Fe3O4). Adv. Mater. Process. Technol. 2016, 2, 165–173. [Google Scholar] [CrossRef]
- Liu, L.; Ding, Q.; Zhong, Y.; Zou, J.; Wu, J.; Chiu, Y.-L.; Li, J.; Zhang, Z.; Yu, Q.; Shen, Z. Dislocation network in additive manufactured steel breaks strength–ductility trade-off. Mater. Today 2018, 21, 354–361. [Google Scholar] [CrossRef]
- Elmer, J.W.; Allen, S.M.; Eagar, T.W. Microstructural development during solidification of stainless steel alloys. Metall. Trans. A 1989, 20, 2117–2131. [Google Scholar] [CrossRef]
- Birnbaum, A.J.; Steuben, J.C.; Barrick, E.J.; Iliopoulos, A.P.; Michopoulos, J.G. Intrinsic strain aging, Σ3 boundaries, and origins of cellular substructure in additively manufactured 316L. Addit. Manuf. 2019, 29, 100784. [Google Scholar] [CrossRef]
- Leo, J.R.O.; Barroso, S.P.; Fitzpatrick, M.E.; Wang, M.; Zhou, Z. Microstructure, tensile and creep properties of an austenitic ODS 316L steel. Mater. Sci. Eng. A 2019, 749, 158–165. [Google Scholar] [CrossRef]
- ASTM A240/A240M-19; Standard Specification for Chromium and Chromium-Nickel Stainless Steel Plate, Sheet, and Strip for Pressure Vessels and for General Applications. ASTM: West Conshohocken, PA, USA, 2019.
- Zhai, W.; Zhou, W.; Nai, S.M.L. In-situ formation of TiC nanoparticles in selective laser melting of 316L with addition of micronsized TiC particles. Mater. Sci. Eng. A 2022, 829, 142179. [Google Scholar] [CrossRef]
- Casati, R.; Lemke, J.; Vedani, M. Microstructure and Fracture Behavior of 316L Austenitic Stainless Steel Produced by Selective Laser Melting. J. Mater. Sci. Technol. 2016, 32, 738–744. [Google Scholar] [CrossRef]
- Gray, G.T.; Livescu, V.; Rigg, P.A.; Trujillo, C.P.; Cady, C.M.; Chen, S.R.; Carpenter, J.S.; Lienert, T.J.; Fensin, S.J. Structure/property (constitutive and spallation response) of additively manufactured 316L stainless steel. Acta Mater. 2017, 138, 140–149. [Google Scholar] [CrossRef]
- Zhai, W.; Zhu, Z.; Zhou, W.; Nai, S.M.L.; Wei, J. Selective laser melting of dispersed TiC particles strengthened 316L stainless steel. Compos. Part B Eng. 2020, 199, 108291. [Google Scholar] [CrossRef]
- Kiviö, M.; Holappa, L.; Louhenkilpi, S.; Nakamoto, M.; Tanaka, T. Studies on Interfacial Phenomena in Titanium Carbide/Liquid Steel Systems for Development of Functionally Graded Material. Metall. Mater. Trans. B 2016, 47, 2114–2122. [Google Scholar] [CrossRef]
- Xi, L.; Gu, D.; Guo, S.; Wang, R.; Ding, K.; Prashanth, K.G. Grain refinement in laser manufactured Al-based composites with TiB2 ceramic. J. Mater. Res. Technol. 2020, 9, 2611–2622. [Google Scholar] [CrossRef]
- Durga, A.; Pettersson, N.H.; Malladi, S.B.A.; Chen, Z.; Guo, S.; Nyborg, L.; Lindwall, G. Grain refinement in additively manufactured ferritic stainless steel by in situ inoculation using pre-alloyed powder. Scr. Mater. 2021, 194, 113690. [Google Scholar] [CrossRef]
- Xia, M.; Liu, A.; Wang, H.; Lin, Y.; Li, N.; Zhang, M.; Ding, H. Microstructure evolution and its effect on mechanical response of the multi-phase reinforced Ti-based composites by laser powder-bed fusion. J. Alloys Compd. 2019, 782, 506–515. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Gan, N.; Lim, Z.-Y.; Wu, C.; Peng, J.; Wang, W.G. Evaluation of Ni/Y2O3/Al2O3 catalysts for hydrogen production by autothermal reforming of methane. Int. J. Hydrog. Energy 2014, 39, 10971–10979. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).