Pilot Tests of Pre-Reduction in Chromium Raw Materials from Donskoy Ore Mining and Processing Plant and Melting of High-Carbon Ferrochromium
Abstract
:1. Introduction
- -
- The long heat treatment time can increase the overall process duration and potentially reduce production efficiency;
- -
- The high consumption of crucibles made of silicon carbide can lead to increased costs and potentially impact the economics of the process;
- -
- The process may require additional equipment or modifications to existing equipment to accommodate the application of ore fines without pelletizing;
- -
- The elimination of contact between the charge and oxidizing gases may require careful control and monitoring to ensure the desired reduction rate is achieved;
- -
- The opposite-flow of material and gases may require design considerations to optimize thermal efficiency and minimize heat loss;
- -
- The CO reheating process may require additional energy inputs and potentially increase operational costs.
2. Materials and Methods
- -
- Chromium concentrate of 0–3 mm fraction;
- -
- Chromium ore of 0–1 mm fraction, obtained by sieving from the 0–10 mm ore fraction;
- -
- Dust from the gas cleaning of the ore drying furnace.
- -
- Shubarkol coal of 0–10 mm fraction;
- -
- Shubarkol special coke of 0–5 mm fraction;
- -
- Anthracite of 0–15 mm fraction.
- -
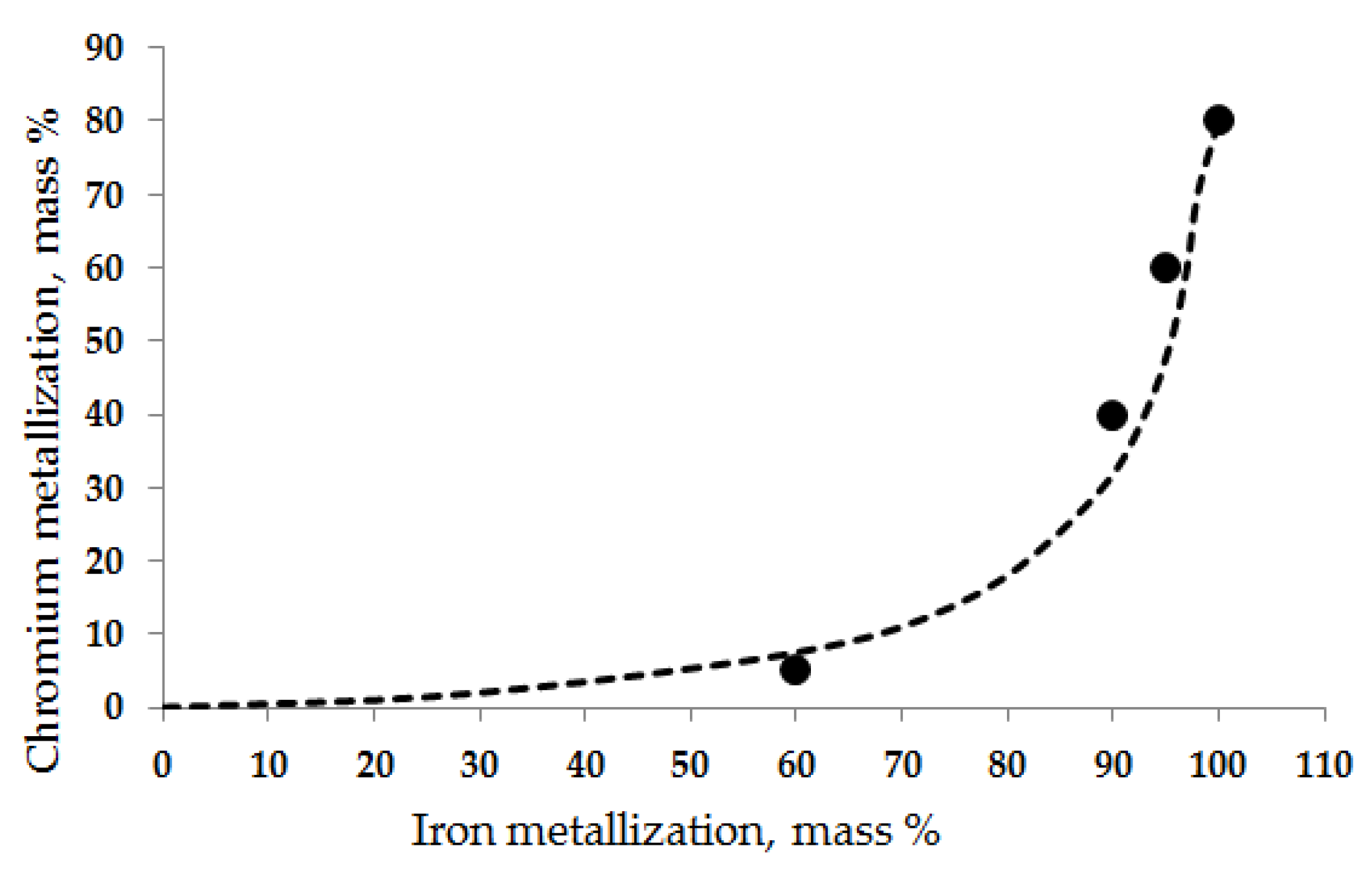
- The product pre-reduced by 65% has a lower metallic chromium content compared to the product with a pre-reduction degree of 65%. The assessment of the product pre-reduction degree is based on the ratio of metallic chromium content to the total chromium content in the charge. According to the research paper referenced, the pre-reduction degree is estimated as a fraction of the total reduction, which is the mass loss associated with the reduction in chromium and iron oxides to metal. It can also be assessed as a fraction of the oxygen removed from the ore related to chromium and iron;
- -
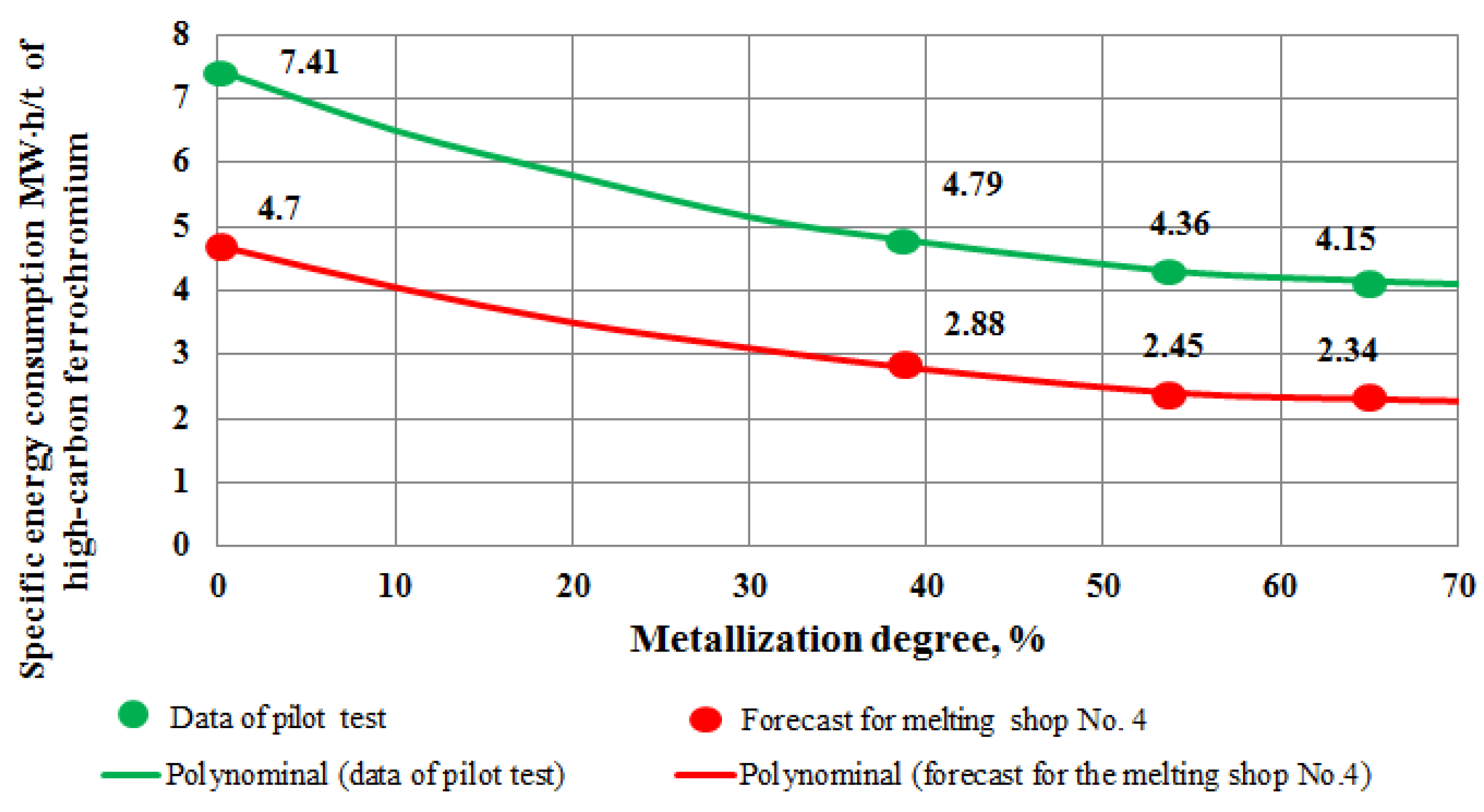
- The studies mentioned in the passage compare the specific energy consumption for melting high-carbon ferrochromium using different methods. The first method described is the melting of high-carbon ferrochromium in skull furnaces using Kazakhstan chromite. This method resulted in a specific energy consumption of 4.47 MW·h/t with a chromium oxide content of 59.2%.
3. Conclusions
- The scheme with pre-reduction has been proven to be more effective in reducing the total power consumption for melting high-carbon ferrochromium when compared to the schemes of heating and sintering the charge. This conclusion is based on the use of South African chromites with a chromium reduction degree of over 50% and Kazakhstan chromites with a chromium reduction degree of more than 60%.By implementing pre-reduction, the total power consumption for the melting process is reduced. Pre-reduction involves the reduction of chromite ore before it is charged into the furnace. This process helps to remove the excess oxygen from the ore, resulting in a more efficient melting process.The use of South African chromites with a chromium reduction degree of over 50% and Kazakhstan chromites with a chromium reduction degree of more than 60% further enhances the effectiveness of pre-reduction. These chromite ores have already undergone a significant reduction in chromium content, making them more suitable for the pre-reduction process.Overall, the scheme with pre-reduction offers a more energy-efficient solution for melting high-carbon ferrochromium compared to the alternative schemes of heating and sintering the charge. The combination of pre-reduction and the use of chromite ores with high chromium reduction degrees results in a reduction in total power consumption during the melting process;
- Results of the pilot tests have demonstrated the possibility of achieving a chromium reduction degree more than 60%. Thus, it provides the expediency of pre-reduction during the ferrochromium melting in the DC furnace;
- It is necessary to assess the real value of reduction in power consumption during remelting of the pre-melting product for the melting of the high-carbon ferrochromium in the ore-smelting furnace and DC furnace;
- The proposed pre-reduction technology can provide a high uniformity in the chromium reduction degree. Thus, it will have a good effect on the subsequent remelting process;
- It is necessary to refine upon solutions that will exclude the material sticking to the crucible walls and segregation of charge materials during the pre-reduction.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moyo, L.B.; Simate, G.S.; Hobane, N.; Dube, C. Characterization, kinetics and thermodynamic evaluation of struvite produced using ferrochrome slag as a magnesium source. S. Afr. J. Chem. Eng. 2024, 47, 83–90. [Google Scholar] [CrossRef]
- McCullough, S.; Hockaday, S.; Johnson, C.; Barcza, N.A. Pre-reduction and smelting characteristics of Kazakhstan ore samples. In Proceedings of the INFACON XII, Helsinki, Finland, 6–9 June 2010; pp. 249–262. (In English). [Google Scholar]
- Bilyalov, K.S.; Kaliakparov, A.G.; Panfilov, V.P.; Suindikov, D.B.; Baimagambetov, K.N. Technology Development for Chromium Ore Agglomeration Using Bentonite. Metallurgist 2023, 67, 462–468. [Google Scholar] [CrossRef]
- Kumar, P.H.; Srivastava, A.; Kumar, V.; Singh, V.K. Implementation of IndustrialWaste Ferrochrome Slag in Conventional and Low Cement Castables: Effect of Calcined Alumina. J. Asian Ceram. Soc. 2014, 2, 371–379. [Google Scholar] [CrossRef]
- Dehghanpour, H.; Doğan, F.; Subaşi, S.; Maraşli, M. Effects of single-walled carbon nanotubes and steel fiber on recycled ferro-hrome filled electrical conductive mortars. J. Sustain. Constr. Mater. Technol. 2022, 7, 250–265. [Google Scholar] [CrossRef]
- Gasik, M.I. Technology of Chromium and Its Ferroalloys. In Handbook of Ferroalloys: Theory and Technology; Gasik, M., Ed.; Butterworth-Heinemann Elsevier: Oxford, UK, 2013; pp. 299–316. ISBN 9780080977539. [Google Scholar]
- du Preez, S.P.; van Kaam, T.P.M.; Ringdalen, E.; Tangstad, M.; Morita, K.; Bessarabov, D.G.; van Zyl, P.G.; Beukes, J.P. An Oveview of Currently Applied Ferrochrome Production Processes and Their Waste Management Practices. Minerals 2023, 13, 809. [Google Scholar] [CrossRef]
- Tleugabulov, S.M.; Tleugabulov, B.S.; Koishina, G.M.; Altybaeva, D.K.; Tazhiev, E.B. Smelting Reduction of a Monocharge. Metallurgist 2016, 60, 31–37. (In English) [Google Scholar] [CrossRef]
- Kapure, G.; Tathavadkar, V.; Rao, C.B.; Rao, S.M.; Raju, K.S. Coal based direct reduction of preoxidized chromite ore at high temperature. In Proceedings of the Twelfth International Ferroalloys Congress, Helsinki, Finland, 6–9 June 2010; pp. 293–302. [Google Scholar]
- Campbell, K.; van Laar, J.H.; Booysen, W.; Kleingeld, M. Comparison of the prescribed emission quantification methods and potential carbon tax liability in the South African FeCr industry. Carbon Manag. 2020, 11, 213–229. [Google Scholar] [CrossRef]
- Basson, J.; Daavittila, J. High Carbon Ferrochrome Technology. In Handbook of Ferroalloys: Theory and Technology; Gasik, M., Ed.; Butterworth-Heinemann Elsevier: Oxford, UK, 2013; pp. 317–363. ISBN 9780080977539. [Google Scholar]
- Panda, C.R.; Mishra, K.K.; Nayak, B.D.; Rao, D.S.; Nayak, B.B. Release behaviour of chromium from ferrochrome slag. Int. J. Environ. Technol. Manag. 2012, 15, 261–274. [Google Scholar] [CrossRef]
- Motovilov, I.Y.; Luganov, V.A.; Chepushtanova, T.A.; Guseynova, G.D.; Itkulova, S.S. Processing of metallurgical wastes with obtaining iron oxides nanopowders. In WASTES—Solutions, Treatments and Opportunities II—Selected Papers from the 4th Edition of the International Conference Wastes: Solutions, Treatments and Opportunities, Porto, Portugal, 25–26 September 2017; CRC Press: Boca Raton, FL, USA, 2018; pp. 191–196. (In English) [Google Scholar]
- Shotanov, A.E.; Roshchin, A.V.; Panfilov, V.P.; Nurgali, N.Z. Prereduction of Chromite Raw Materials by the Höganäs Method. Metallurgist 2022, 66, 871–880. [Google Scholar] [CrossRef]
- Shotanov, A.E.; Nurgali, N.Z.; Roshchin, A.V.; Panfilov, V.P.; Baysanov, S.O.; Almagambetov, M.S. Smelting of High-Carbon Ferrochrome from Prereduced Chromite Raw Materials of the Donskoy Ore Mining and Processing Plant. Metallurgist 2023, 66, 1619–1624, (Russian Original Nos. 11–12, November–December, 2022). [Google Scholar] [CrossRef]
- Bologova, V.V. Improvement of Energy and Technological Efficiency of Coke-Chemical Production Based on Application of Natural Gas in Dry Coke Quenching Units: Abstract of Thesis of Candidate of Technical Sciences. Мaster’s Thesis, Federal State Budgetary Educational Institution of Higher Education National Research University MPEI, Moscow, Russian, 2016; 20p. (In Russian). [Google Scholar]
- Kleynhans, E.L.J.; Beukes, J.P.; van Zyl, P.G.; du Preez, S.P. Chemical beneficiation of chromite ore to improve the chromium-to-iron ratio for ferrochrome production. Miner. Eng. 2023, 201, 108196. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, C.; Ni, H.; Karasev, A.; Mu, W.; Jönsson, P.G.; Park, J.H. Effect of ferrochromium (FeCr) and ferroniobium (FeNb) alloys on inclusion and precipitate characteristics in austenitic stainless steels. J. Mater. Res. Technol. 2023, 25, 4989–5002. [Google Scholar] [CrossRef]
- Qu, N.; Han, S.; You, W.; Wang, Y. Study on Adaptive Parameter Internal Mode Control Method for Argon–Oxygen Refining Ferrochrome Alloy. Processes 2023, 11, 1461. [Google Scholar] [CrossRef]
- Niemelä, P.; Kauppi, M. Production, characteristics and use of ferrochromium slags. In Proceedings of the Innovations in Ferry Alloy Industry INFACON XI, New Delhi, India, 18–21 February 2007; pp. 171–179. [Google Scholar]
- Mukashev, N.Z.; Kosdauletov, N.Y.; Suleimen, B.T. Comparison of iron and chromium reduction from chrome ore concentrates by solid carbon and carbon monoxide. Solid State Phenom. 2020, 299, 1152–1157. (In English) [Google Scholar] [CrossRef]
- Moyo, L.B.; Simate, G.S.; Mamvura, T.A.; Danha, G. Recovering phosphorus as struvite from anaerobic digestate of pig manure with ferrochrome slag as a magnesium source. Heliyon 2023, 9, e15506. [Google Scholar] [CrossRef] [PubMed]
- Sahu, N.; Biswas, A.; Kapure, G.U. A Short Review on Utilization of Ferrochromium Slag. Miner. Process. Extr. Metall. Rev. 2016, 37, 211–219. [Google Scholar] [CrossRef]
- Yu, D.; Paktunc, D. Direct production of ferrochrome by segregation reduction of chromite in the presence of calcium chloride. Metals 2018, 8, 69. [Google Scholar] [CrossRef]
- Sager, D.; Grant, D.; Stadle, R.; Schreiter, T. Low cost ferroalloy extraction in DC-arc furnace at Middleburg Ferrochrome. J. South. Afr. Inst. Min. Metall. 2010, 110, 717–724. [Google Scholar]
- Suleimen, B.; Salikhov, S.P. Behavior of extrusion briquettes (Brex) and pellets from oolite iron ore in solid-phase metallization. AIP Conf. Proc. 2022, 2456, 020054. (In English) [Google Scholar]
- Dlamini, R.; von Blottnitz, H. Resource Intensity Trends in the South African Ferrochrome Industry from 2007 to 2020. Minerals 2023, 13, 44. [Google Scholar] [CrossRef]
- Acharya, P.K.; Patro, S.K. Evaluation of Functional, Microstructural, Environmental Impact, and Economic Performance of Concrete Utilizing Ferrochrome Ash and Slag. J. Sustain. Metall. 2022, 8, 1573–1589. [Google Scholar] [CrossRef]
- Sommerfeld, M.; Friedrich, B. Toward Green Ferroalloys: Replacement of Fossil Reductants in the Pre-reduction Process of Chromite by Bio-Based Alternatives. In REWAS 2022: Developing Tomorrow’s Technical Cycles; Lazou, A., Daehn, K., Fleuriault, C., Gökelma, M., Olivetti, E., Meskers, C., Eds.; The Minerals, Metals & Materials Series; Springer: Cham, Switzerland, 2022; Volume I. [Google Scholar] [CrossRef]
- Wei, W.; Samuelsson, P.B.; Jönsson, P.G.; Gyllenram, R.; Glaser, B. Energy Consumption and Greenhouse Gas Emissions of High-Carbon Ferrochrome Production. JOM 2023, 75, 1206–1220. [Google Scholar] [CrossRef]
- Ren, C.; Li, K.; Wang, Y.; Li, Y.; Tong, J.; Cai, J. Preparation and Hydration Mechanisms of Low Carbon Ferrochrome Slag-Granulated Blast Furnace Slag Composite Cementitious Materials. Materials 2023, 16, 2385. [Google Scholar] [CrossRef] [PubMed]
- Topaloglu, S.; Cetin, A.; Dayioglu, A.Y.; Aydilek, A.H. Laboratory Testing of Ferrochrome Slag as an Aggregate in Porous Pavements. Geotech. Test. J. 2024, 47, 197–214. [Google Scholar] [CrossRef]
- Erwee, M.; Swanepoel, S.; Reynolds, Q. The importance of controlling the chemistry of pre-oxidized chromite pellets for Submerged Arc Furnace FeCr smelting: A study on furnace Si control. In Proceedings of the 16th International Ferro-Alloys Congress (INFACON XVI), Virtual, 27–29 September 2021. [Google Scholar]
- Berryman, E.J.; Paktunc, D. Cr(VI) formation in ferrochrome-smelter dusts. J. Hazard. Mater. 2022, 422, 126873. [Google Scholar] [CrossRef] [PubMed]
- Suleimen, B.; Salikhov, S.P. Metallization of Oolitic Iron Ore after Oxidation Firing. Solid State Phenom. 2021, 316, 390–395. (In English) [Google Scholar] [CrossRef]
- Coetzee, J.J.; Bansal, N.; Chirwa, E.M.N. Chromium in Environment, Its Toxic Effect from Chromite-Mining and Ferrochrome Industries, and Its Possible Bioremediation. Expo. Health 2020, 12, 51–62. [Google Scholar] [CrossRef]
- Kaliakparov, A.G.; Baltabaev, S.R.; Strakhov, V.M.; Mukhtar, A.A. Manufacture of High-Carbon Ferrochrome Using Anthracite. Metallurgist 2018, 61, 765–769. [Google Scholar] [CrossRef]
- Makhambetov, Y.N.; Timirbayeva, N.R.; Baisanov, S.O.; Baisanov, A.S. Research of physical and chemical characteristics of the new complex calcium-containing ferroalloy. CIS Iron Steel Rev. 2020, 19, 18–22. [Google Scholar] [CrossRef]
- Makhambetov, E.N.; Baisanov, A.S.; Isagulov, A.Z.; Grigorovich, K.V.; Timirbayeva, N.R. Production of complex calcium-containing ferrous alloys of waste smelter slags and high-ash coals. Steel Transl. 2019, 49, 698–702. [Google Scholar] [CrossRef]
- Das, A.K.; Khaoash, S.; Das, S.P.; Mohapatra, B.K.; Dash, N.; Singh, S.K.; Mishra, P.; Mohanty, J. Processing of Low-Grade Chromite Ore for Ferroalloy Production: A Case Study from Ghutrigaon, Odisha, India. Trans. Indian Inst. Met. 2020, 73, 2309–2320. [Google Scholar] [CrossRef]
- Kumar, P.; Sahu, N.; Roshan, A.; Rout, B.N.; Tripathy, S.K. Influence of process parameters on impurity level in ferrochrome production-An industrial-scale analysis. Miner. Process. Extr. Metall. Rev. 2022, 43, 622–632. [Google Scholar] [CrossRef]
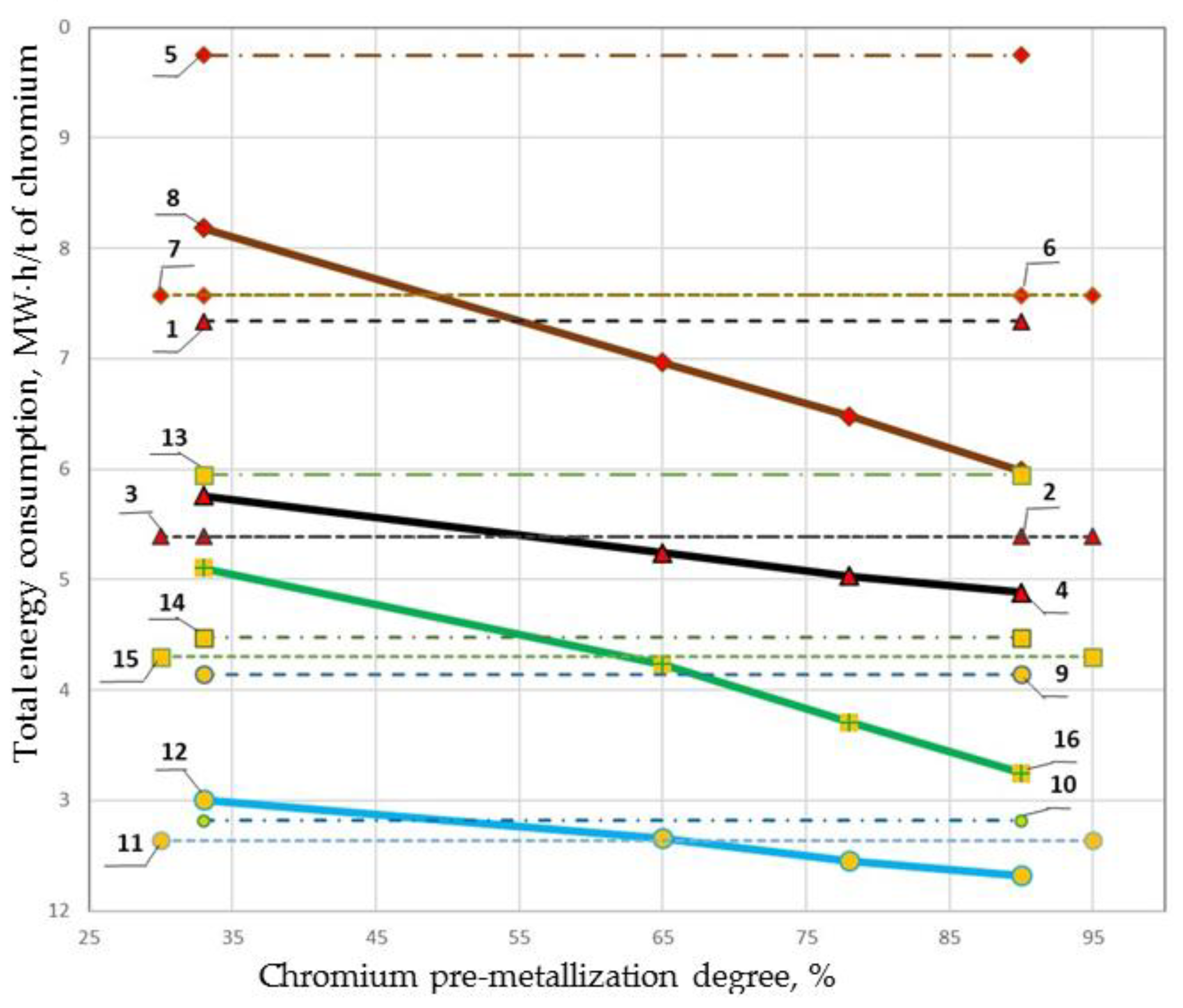

| No | Origin | Furnace Type | Process Scheme | Heating (by Waste, Gas) | Power Consumption, MW·h/t of Chromium | Total Power Consumption including a Coefficient of Efficiency from the Source, MW·h/t of Chromium | Total Power Consumption including a Coefficient of Efficiency from the Source, MW·h/t of Alloy | ||
|---|---|---|---|---|---|---|---|---|---|
| Pre-Reduction | Melting | Supplementary | |||||||
| 1 | South African chromites | Ore-smelting furnace | Standards | 26 °C | - | 6.58 | 0.84 | 17.3399 | 7.984 |
| 2 | With charge heating | T of carrier gas 850 °C | - | 6.02 | 0.945 | 16.02 | 8.05 | ||
| 3 | With sintering | - | - | 6.1 | 1005 | 16.2 | 8.1 | ||
| 4 | Pre-reduction by 32.98% | - | 3.31 | 4.98 | - | 15.76 | 7.66 | ||
| Pre-reduction by 64.8% | 5.49 | 3.90 | - | 15.24 | 7.34 | ||||
| Pre-reduction by 79.2% | 6.58 | 3.38 | - | 15.03 | 7.20 | ||||
| Pre-reduction by 90% | 7.81 | 2.83 | - | 14.89 | 7.09 | ||||
| 5 | DC furnace | Standard | 25 °C | - | 7.90 | - | 19.75 | 10.00 | |
| 6 | With charge heating | T of carrier gas 800 °C | - | 7.03 | - | 17.58 | 8.90 | ||
| 7 | With sintering | - | - | 7.03 | - | 17.58 | 8.90 | ||
| 8 | Pre-reduction by 32.98% | - | 3.31 | 5.95 | - | 18.19 | 8.84 | ||
| Pre-reduction by 64.8% | 5.49 | 4.59 | - | 16.97 | 8.17 | ||||
| Pre-reduction by 79.2% | 6.58 | 3.96 | - | 16.48 | 7.90 | ||||
| Pre-reduction by 90% | 7.81 | 3.27 | - | 15.99 | 7.59 | ||||
| 9 | Kazakhstan chromites | Ore-smelting furnace | Standard | 25 °C | - | 5.32 | 0.83915 | 14.14 | 9.48 |
| 10 | With charge heating | T of carrier gas 800 °C | - | 4.79 | 0.83915 | 12.81 | 8.59 | ||
| 11 | With sintering | - | - | 4.72 | 0.83915 | 12.64 | 8.47 | ||
| 12 | Pre-reduction by 32.98% | - | 2.23 | 4.31 | - | 13.01 | 8.72 | ||
| Pre-reduction by 64.8% | 4.16 | 3.40 | - | 12.66 | 8.40 | ||||
| Pre-reduction by 79.2% | 5.18 | 2.91 | - | 12.46 | 8.21 | ||||
| Pre-reduction by 90% | 6.37 | 2.38 | - | 12.32 | 8.06 | ||||
| 13 | DC furnace | Standard | 25 °C | - | 6.38 | - | 15.95 | 11.18 | |
| 14 | With charge heating | T of carrier gas 800 °C | - | 5.79 | - | 14.48 | 10.13 | ||
| 15 | With sintering | - | - | 5.72 | - | 14.30 | 10.00 | ||
| 16 | Pre-reduction by 32.98% | - | 2.23 | 5.15 | - | 15.11 | 10.12 | ||
| Pre-reduction by 64.8% | 4.16 | 4.03 | - | 14.24 | 9.43 | ||||
| Pre-reduction by 79.2% | 5.18 | 3.41 | - | 13.71 | 9.04 | ||||
| Pre-reduction by 90% | 6.37 | 2.75 | - | 13.25 | 8.66 | ||||
| Ore | Cr2O3 | FeO | SiO2 | MgO | Al2O3 | CaO | P | S | C.L.* | W |
|---|---|---|---|---|---|---|---|---|---|---|
| Concentrate | 51.78 | 13.3 | 6.35 | 19.22 | 7.51 | 0.011 | 0.002 | 0.087 | 1.49 | 0.25 |
| Ore | 52.1 | 12.48 | 6.53 | 19.73 | 6.73 | 0.11 | 0.0037 | 0.026 | 1.84 | 0.45 |
| Dust | 49.02 | 9.10 | 11.20 | 19.95 | 5.78 | 0.39 | 0.012 | 0.0175 | - | 0.60 |
| Materials * | S | P | W | A | V |
|---|---|---|---|---|---|
| Shubarkol coal | 0.38 | 0.0139 | 14.20 | 4.30 | 44.10 |
| Special coke | 0.40 | 0.0348 | 19.10 | 11.40 | 8.20 |
| Anthracite | 0.20 | 0.02 | 10.05 | 3.20 | 9.50 |
| Temperature of Holding Time | Holding Time, h | |
|---|---|---|
| 35 | 45 | |
| 1300 °C | 40 (+/−8)% | 50 (+/−10)% |
| 1350 °C | 50 (+/−10)% | 60 (+/−10)% |
| 1380 °C | 60 (+/−5)% | no test |
| Batch by Metallization Degree | Total Weight of Batch, kg | Weighted Average Metallization Degree, % |
|---|---|---|
| minimum degree | 4582 | 39.40 |
| medium degree | 5038 | 53.73 |
| maximum degree | 3816 | 65.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shabanov, Y.; Makhambetov, Y.; Saulebek, Z.; Toleukadyr, R.; Baisanov, S.; Nurgali, N.; Shotanov, A.; Dossekenov, M.; Zhumagaliyev, Y. Pilot Tests of Pre-Reduction in Chromium Raw Materials from Donskoy Ore Mining and Processing Plant and Melting of High-Carbon Ferrochromium. Metals 2024, 14, 202. https://doi.org/10.3390/met14020202
Shabanov Y, Makhambetov Y, Saulebek Z, Toleukadyr R, Baisanov S, Nurgali N, Shotanov A, Dossekenov M, Zhumagaliyev Y. Pilot Tests of Pre-Reduction in Chromium Raw Materials from Donskoy Ore Mining and Processing Plant and Melting of High-Carbon Ferrochromium. Metals. 2024; 14(2):202. https://doi.org/10.3390/met14020202
Chicago/Turabian StyleShabanov, Yerbol, Yerbolat Makhambetov, Zhalgas Saulebek, Ruslan Toleukadyr, Sailaubai Baisanov, Nurzhan Nurgali, Azamat Shotanov, Murat Dossekenov, and Yerlan Zhumagaliyev. 2024. "Pilot Tests of Pre-Reduction in Chromium Raw Materials from Donskoy Ore Mining and Processing Plant and Melting of High-Carbon Ferrochromium" Metals 14, no. 2: 202. https://doi.org/10.3390/met14020202
APA StyleShabanov, Y., Makhambetov, Y., Saulebek, Z., Toleukadyr, R., Baisanov, S., Nurgali, N., Shotanov, A., Dossekenov, M., & Zhumagaliyev, Y. (2024). Pilot Tests of Pre-Reduction in Chromium Raw Materials from Donskoy Ore Mining and Processing Plant and Melting of High-Carbon Ferrochromium. Metals, 14(2), 202. https://doi.org/10.3390/met14020202








