The Effect of Electroless Nickel–Polytetrafluoroethylene Coating on the Frictional Properties of Orthodontic Wires
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ni-PTFE Coating
2.2. Evaluation of Surface Properties
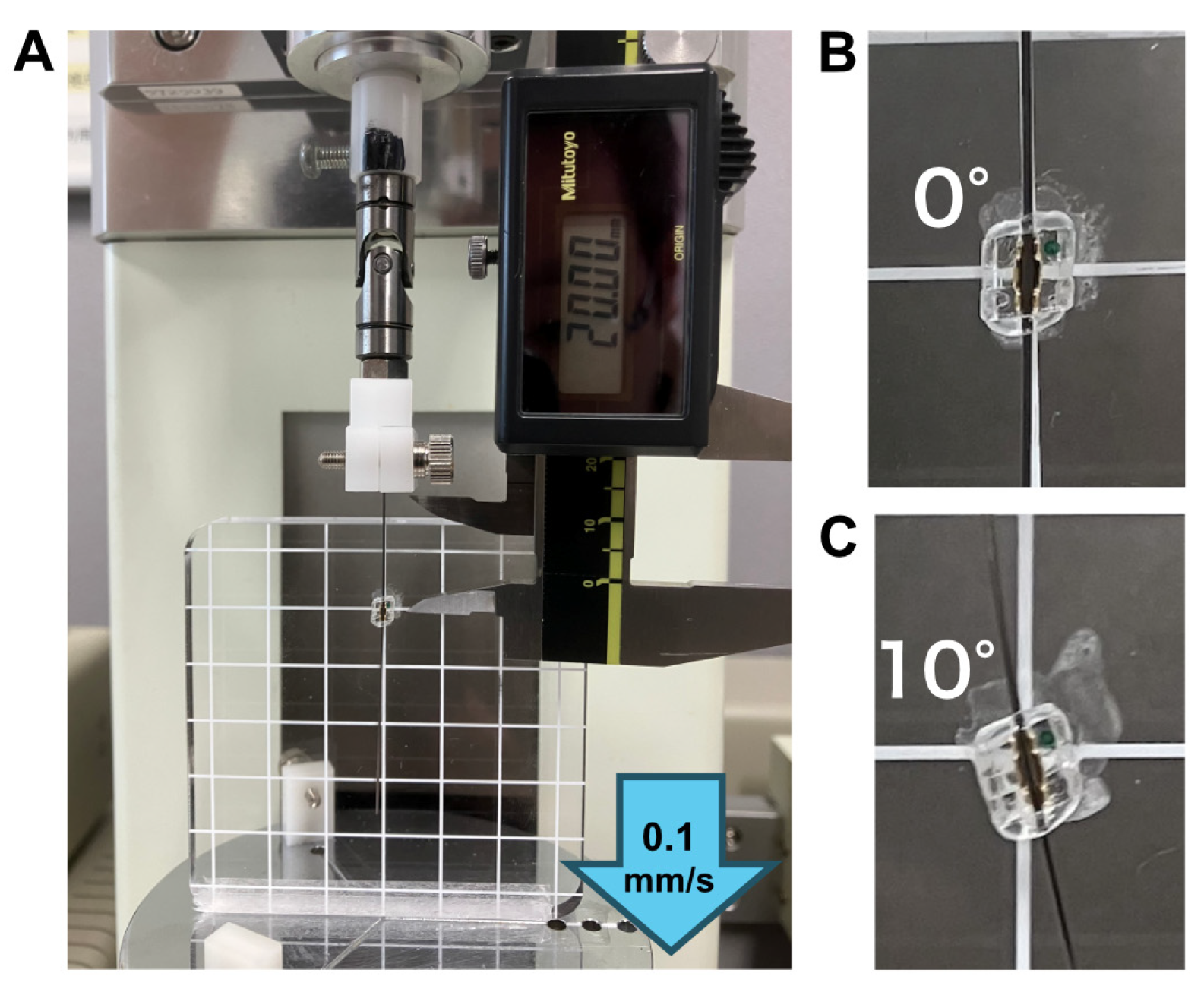
2.3. Friction Test
2.4. Nickel Dissolution Test
2.5. Statistical Analyses
3. Results
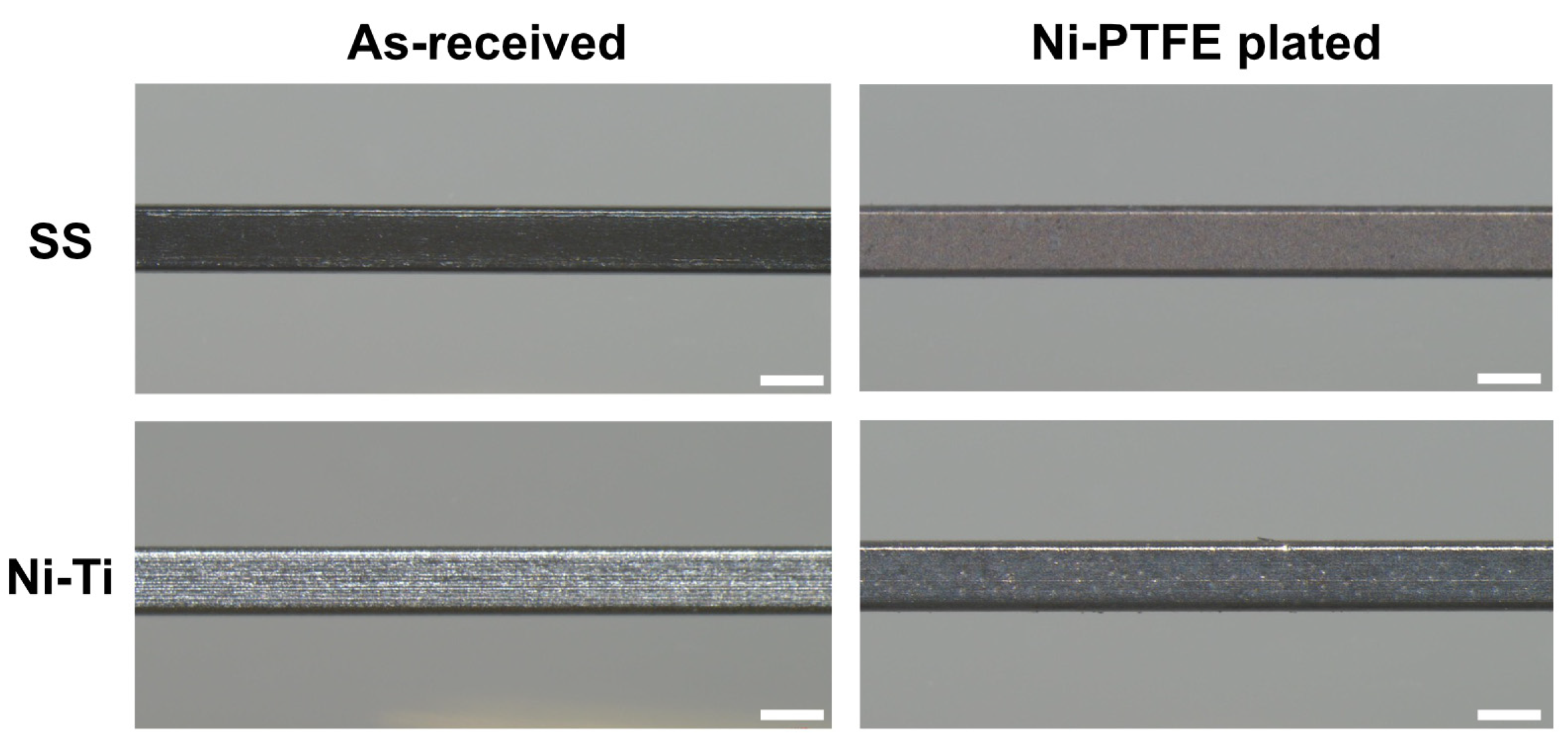
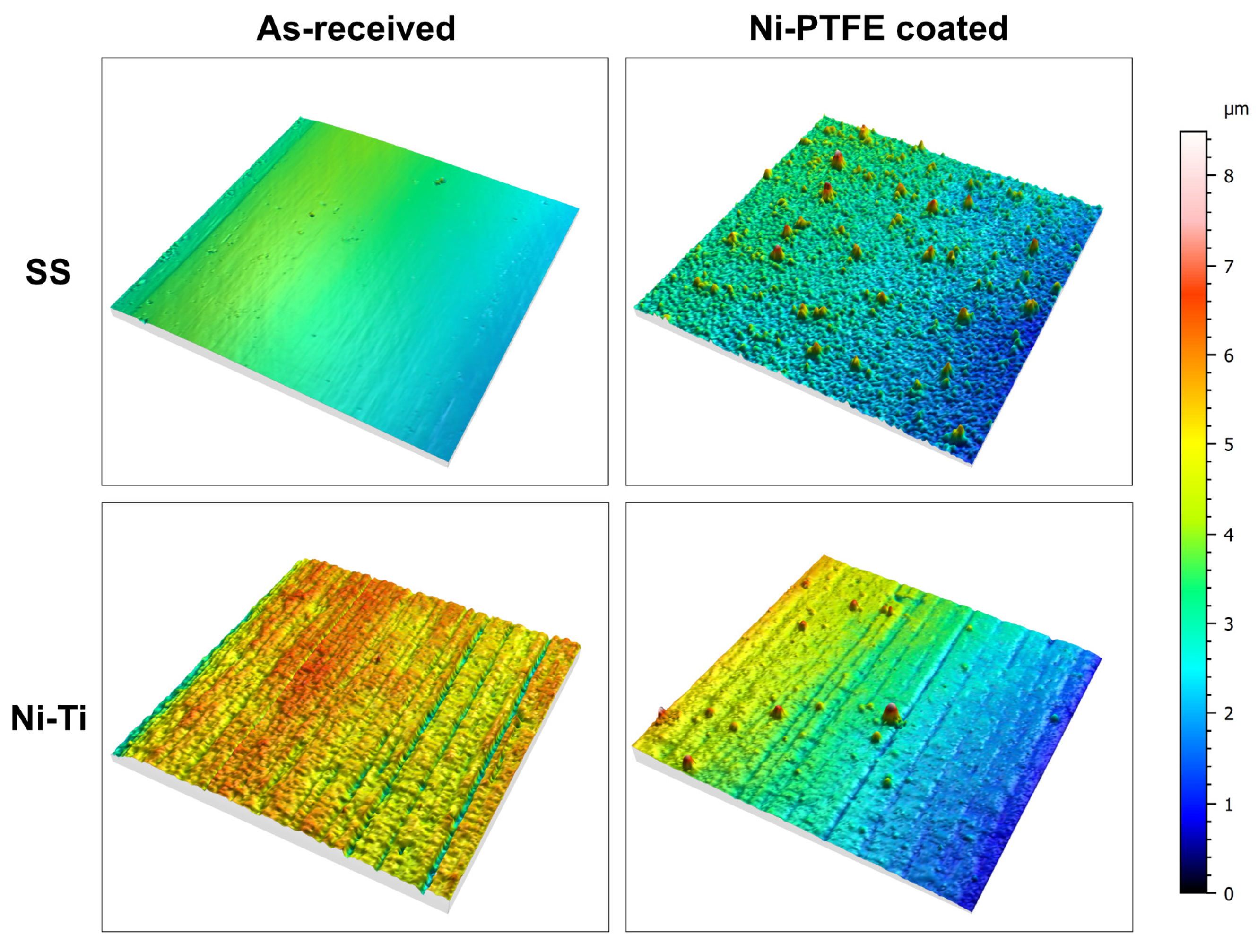
3.1. Surface Texture
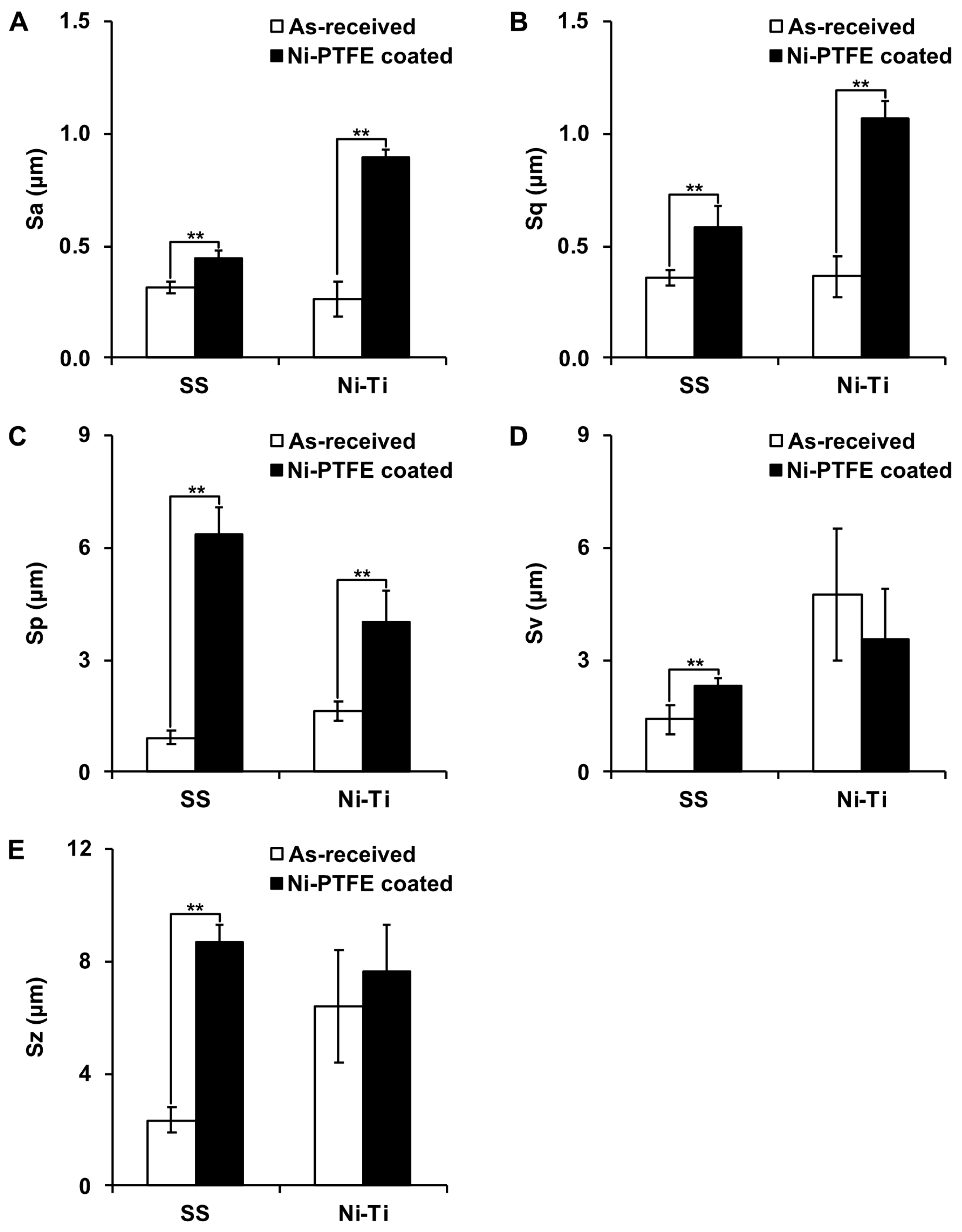
3.2. Surface Roughness
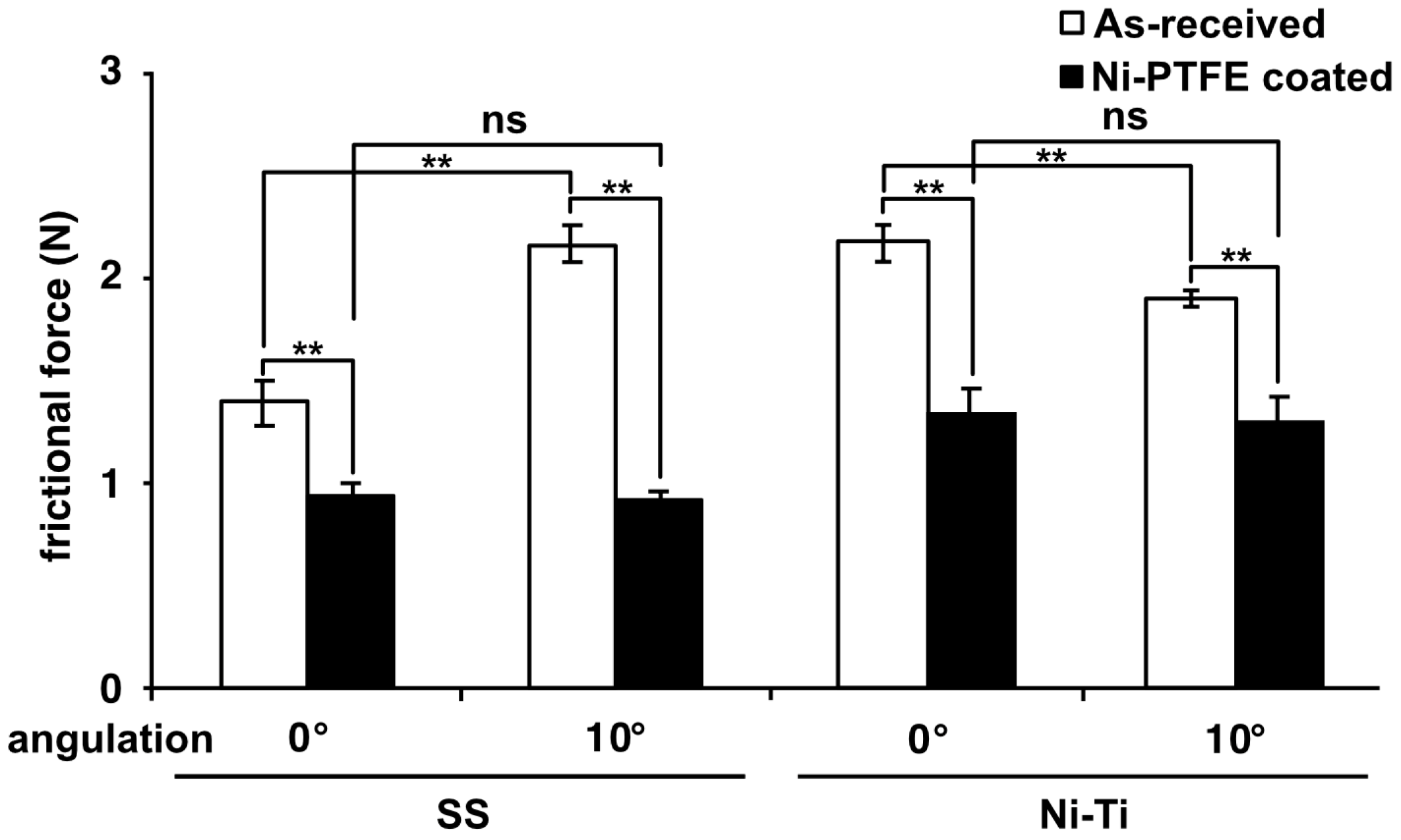
3.3. Frictional Force
3.4. Nickel Release
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Proffit, W.; Fields, H. Mechanical Principles in Orthodontic Force Control. In Contemporary Orthodontics, 6th ed.; Elsevier: Philadelphia, PA, USA, 2018; pp. 292–295. [Google Scholar]
- Burrow, S.J. Friction and resistance to sliding in orthodontics: A critical review. Am. J. Orthod. Dentofac. Orthop. 2009, 135, 442–447. [Google Scholar] [CrossRef]
- Araújo, R.C.; Bichara, L.M.; Araujo, A.M.; Normando, D. Debris and friction of self-ligating and conventional orthodontic brackets after clinical use. Angle Orthod. 2015, 85, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Khambay, B.; Millett, D.; McHugh, S. Evaluation of methods of archwire ligation on frictional resistance. Eur. J. Orthod. 2004, 26, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Dragomirescu, A.-O.; Bencze, M.-A.; Vasilache, A.; Teodorescu, E.; Albu, C.-C.; Popoviciu, N.O.; Ionescu, E. Reducing Friction in Orthodontic Brackets: A Matter of Material or Type of Ligation Selection? In-Vitro Comparative Study. Materials 2022, 15, 2640. [Google Scholar] [CrossRef] [PubMed]
- Thorstenson, G.A.; Kusy, R.P. Comparison of resistance to sliding between different self-ligating brackets with second-order angulation in the dry and saliva states. Am. J. Orthod. Dentofac. Orthop. 2002, 121, 472–482. [Google Scholar] [CrossRef]
- Sugisawa, H.; Kitaura, H.; Ueda, K.; Kimura, K.; Ishida, M.; Ochi, Y.; Kishikawa, A.; Ogawa, S.; Takano-Yamamoto, T. Corrosion resistance and mechanical properties of titanium nitride plating on orthodontic wires. Dent. Mater. J. 2018, 37, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Muguruma, T.; Iijima, M.; Brantley, W.A.; Mizoguchi, I. Effects of a diamond-like carbon coating on the frictional properties of orthodontic wires. Angle Orthod. 2011, 81, 141–148. [Google Scholar] [CrossRef]
- Kameda, T.; Sato, H.; Oka, S.; Miyazaki, A.; Ohkuma, K.; Terada, K. Low temperature polytetrafluoroethylene (PTFE) coating improves the appearance of orthodontic wires without changing their mechanical properties. Dent. Mater. J. 2020, 39, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Kameda, T.; Ohkuma, K.; Oka, S. Polytetrafluoroethylene (PTFE): A resin material for possible use in dental prostheses and devices. Dent. Mater. J. 2019, 38, 136–142. [Google Scholar] [CrossRef]
- Nishino, M.; Okazaki, Y.; Seto, Y.; Uehara, T.; Endo, K.; Yamamura, K.; Ohkubo, Y. Adhesive-Free Adhesion between Plasma-Treated Glass-Cloth-Containing Polytetrafluoroethylene (GC–PTFE) and Stainless Steel: Comparison between GC–PTFE and Pure PTFE. Polymers 2022, 14, 394. [Google Scholar] [CrossRef]
- Ramalho, A.; Miranda, J.C. Friction and wear of electroless NiP and NiP plus PTFE coatings. Wear 2005, 259, 828–834. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Liu, G. Friction and Wear of Electroless Ni-P-CS Composite Coating. Metals 2023, 13, 315. [Google Scholar] [CrossRef]
- Zhang, R.; Han, B.; Liu, X. Functional Surface Coatings on Orthodontic Appliances: Reviews of Friction Reduction, Antibacterial Properties, and Corrosion Resistance. Int. J. Mol. Sci. 2023, 24, 6919. [Google Scholar] [CrossRef] [PubMed]
- ISO 25178:2012; Geometrical Product Specifications (GPS)—Surface Texture: Area—Part 2: Terms, Definitions and Surface Texture Parameters. ISO: Geneva, Switzerland, 2012.
- Kusy, R.P.; Whitley, J.Q. Assessment of second-order clearances between orthodontic archwires and bracket slots via the critical contact angle for binding. Angle Orthod. 1999, 69, 71–80. [Google Scholar] [PubMed]
- Kuhta, M.; Pavlin, D.; Slaj, M.; Varga, S.; Lapter-Varga, M. Type of archwire and level of acidity: Effects on the release of metal ions from orthodontic appliances. Angle Orthod. 2009, 79, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Frank, C.A.; Nikolai, R.J. A comparative study of frictional resistances between orthodontic bracket and arch wire. Am. J. Orthod. Dentofac. Orthop. 1980, 78, 593–609. [Google Scholar] [CrossRef]
- Wang, Y.; Zhai, W.J.; Li, J.H.; Liu, H.T.; Li, C. Friction behavior of biodegradable electrospun polyester nanofibrous membranes. Tribol. Int. 2023, 188, 108891. [Google Scholar] [CrossRef]
- Yang, C.; Yin, C.H.; Wu, Y.Z.; Zhou, Q.; Liu, X.X. Atomic insights into the deformation mechanism of an amorphous wrapped nanolamellar heterostructure and its effect on self-lubrication. J. Mater. Res. Technol. 2023, 26, 4206–4218. [Google Scholar] [CrossRef]
- Sparnacci, K.; Antonioli, D.; Laus, M.; Zuccheri, G.; Boarino, L.; De Leo, N.; Comoretto, D. Preparation, properties and self-assembly behavior of PTFE-based core-shell nanospheres. In Proceedings of the 6th International Conference on Times of Polymers (TOP) and Composites, Ischia, Italy, 10–14 June 2012; Volume 1459, pp. 61–63. [Google Scholar] [CrossRef]
- Wang, Q.H.; Xue, Q.J.; Liu, W.M.; Chen, J.M. The friction and wear characteristics of nanometer SiC and polytetrafluoroethylene filled polyetheretherketone. Wear 2000, 243, 140–146. [Google Scholar] [CrossRef]
- Quirynen, M. The clinical meaning of the surface roughness and the surface free energy of intra-oral hard substrata on the microbiology of the supra- and subgingival plaque: Results of in vitro and in vivo experiments. J. Dent. 1994, 22 (Suppl. S1), S13–S16. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Shamohammadi, M.; Rastegaar, Z.; Skini, M.; Rakhshan, V. Effect of esthetic coating on surface roughness of orthodontic archwires. Int. Orthod. 2017, 15, 312–321. [Google Scholar] [CrossRef]
- Demling, A.; Elter, C.; Heidenblut, T.; Bach, F.W.; Hahn, A.; Schwestka-Polly, R.; Stiesch, M.; Heuer, W. Reduction of biofilm on orthodontic brackets with the use of a polytetrafluoroethylene coating. Eur. J. Orthod. 2010, 32, 414–418. [Google Scholar] [CrossRef]
- ISO 15841:2014; Dentistry—Wires for Use in Orthodontics. ISO: London, UK, 2014.
- ISO 9333:2022; Dentistry—Brazing Materials. ISO: Geneva, Switzerland, 2022.
- ISO 22674:2022; Dentistry—Metalilic Materials for Fixed and Removable Restorations and Appliances Appliances. ISO: Geneva, Switzerland, 2022.
- Imamura, T.; Kanno, Z.; Imai, H.; Sugiyama, T.; Wada, T.; Yoshida, M.; Sakama, M.; Ono, T.; Honda, E.; Uo, M. Infiltration of trace metal ions in the oral mucosa of a rat analyzed using SRXRF, XAFS, and ICP-MS. Dent. Mater. J. 2015, 34, 814–821. [Google Scholar] [CrossRef]
- Fróis, A.; Santos, A.C.; Louro, C.S. Corrosion of Fixed Orthodontic Appliances: Causes, Concerns, and Mitigation Strategies. Metals 2023, 13, 1955. [Google Scholar] [CrossRef]
- Ito, A.; Kitaura, H.; Sugisawa, H.; Noguchi, T.; Ohori, F.; Mizoguchi, I. Titanium Nitride Plating Reduces Nickel Ion Release from Orthodontic Wire. Appl. Sci. 2021, 11, 9745. [Google Scholar] [CrossRef]
- Lazić, M.M.; Mitić, D.; Radović, K.; Đorđević, I.; Majerič, P.; Rudolf, R.; Grgur, B.N. Corrosion Behavior of Nickel–Titanium Continuous-Casted Alloys. Metals 2024, 14, 88. [Google Scholar] [CrossRef]
- Katic, V.; Curkovic, H.O.; Semenski, D.; Barsic, G.; Marusic, K.; Spalj, S. Influence of surface layer on mechanical and corrosion properties of nickel-titanium orthodontic wires. Angle Orthod. 2014, 84, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Kitaura, H.; Noguchi, T.; Ohori, F.; Mizoguchi, I. Analysis of coating loss from coated stainless steel orthodontic wire. Appl. Sci. 2022, 12, 9497. [Google Scholar] [CrossRef]
| As-Received | Ni-PTFE Coated | |||
|---|---|---|---|---|
| Wire | Mean | SD | Mean | SD |
| SS | 0.051 | 0.003 | 152.345 | 12.475 |
| Ni-Ti | 0.047 | 0.005 | 239.852 | 5.374 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Numazaki, K.; Takahashi, M.; Ito, A.; Takada, Y.; Mizoguchi, I. The Effect of Electroless Nickel–Polytetrafluoroethylene Coating on the Frictional Properties of Orthodontic Wires. Metals 2024, 14, 213. https://doi.org/10.3390/met14020213
Numazaki K, Takahashi M, Ito A, Takada Y, Mizoguchi I. The Effect of Electroless Nickel–Polytetrafluoroethylene Coating on the Frictional Properties of Orthodontic Wires. Metals. 2024; 14(2):213. https://doi.org/10.3390/met14020213
Chicago/Turabian StyleNumazaki, Kento, Masatoshi Takahashi, Arata Ito, Yukyo Takada, and Itaru Mizoguchi. 2024. "The Effect of Electroless Nickel–Polytetrafluoroethylene Coating on the Frictional Properties of Orthodontic Wires" Metals 14, no. 2: 213. https://doi.org/10.3390/met14020213





