Atomistic Simulation of the Interaction between the Σ9[110](221) Shear-Coupled Grain Boundary Motion and the Cu-rich Precipitates in α-Iron
Abstract
1. Introduction
2. Computational Model
3. Results and Discussion
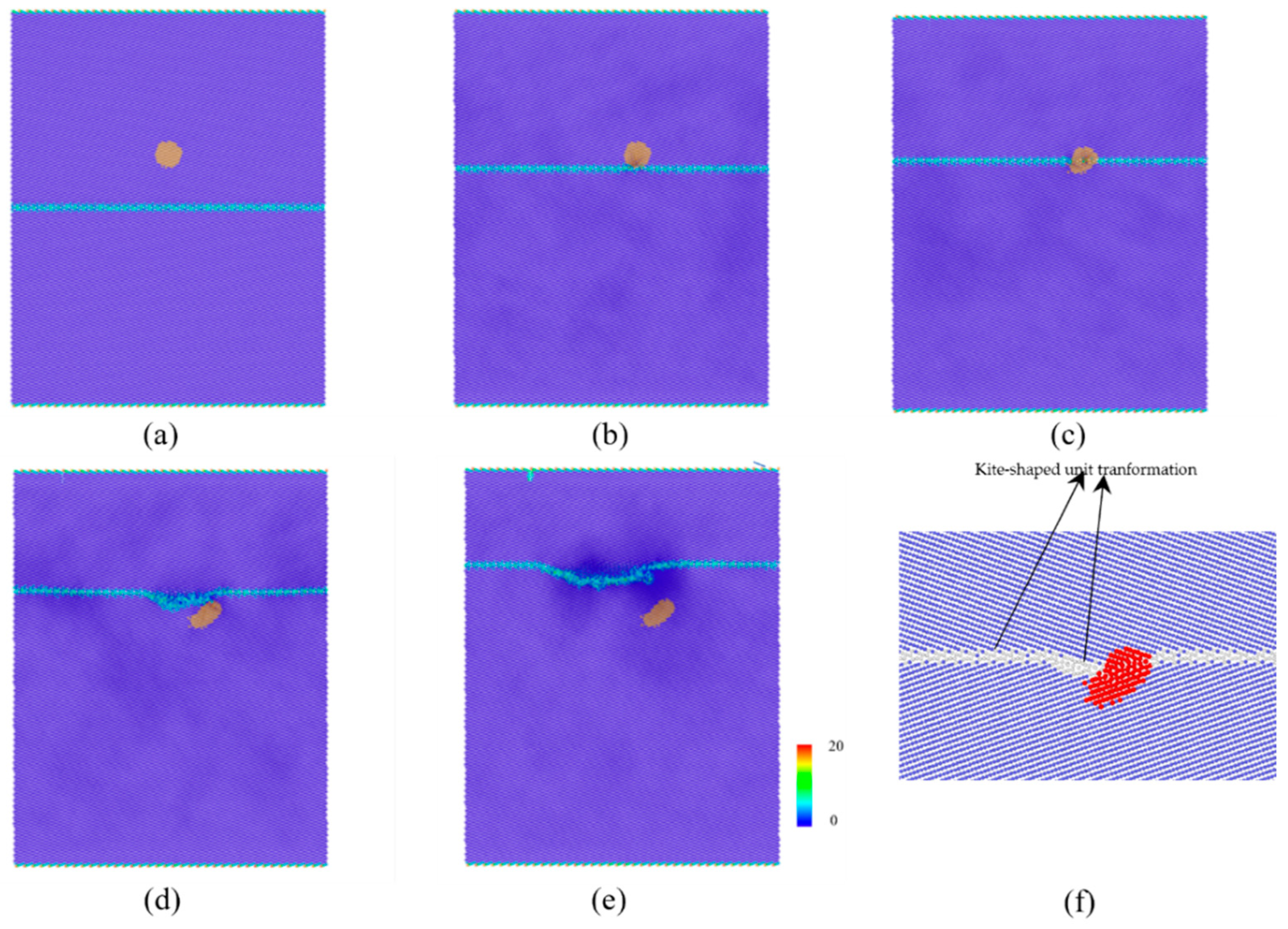
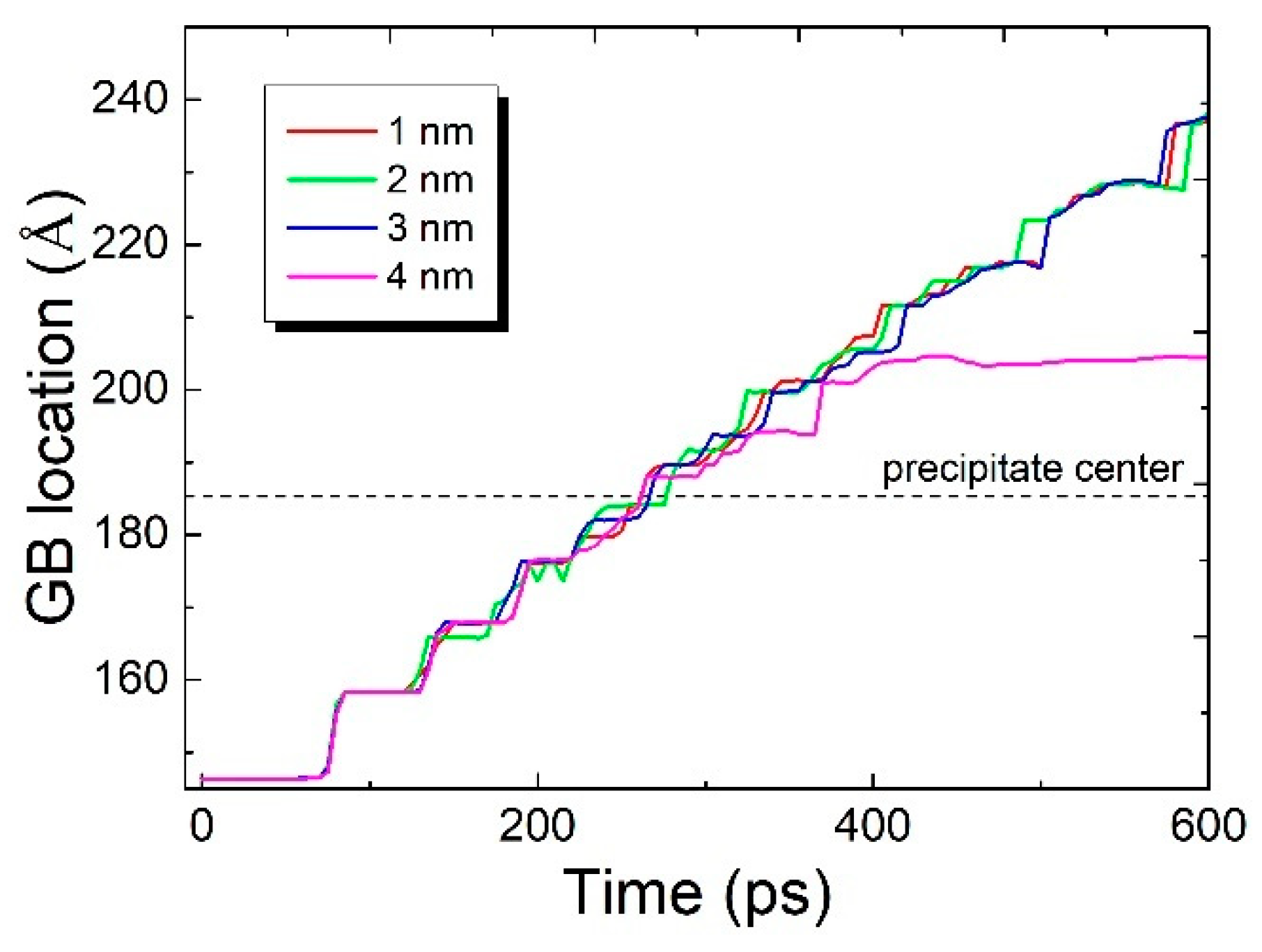
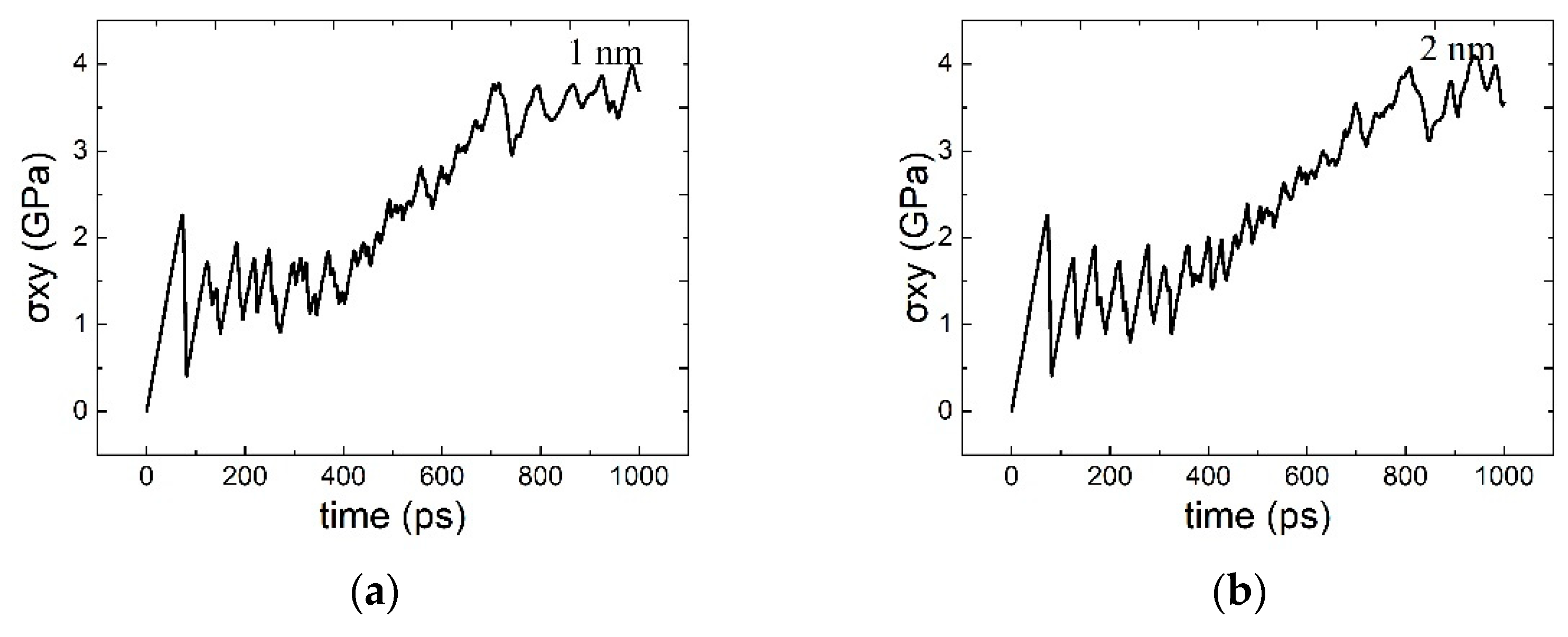
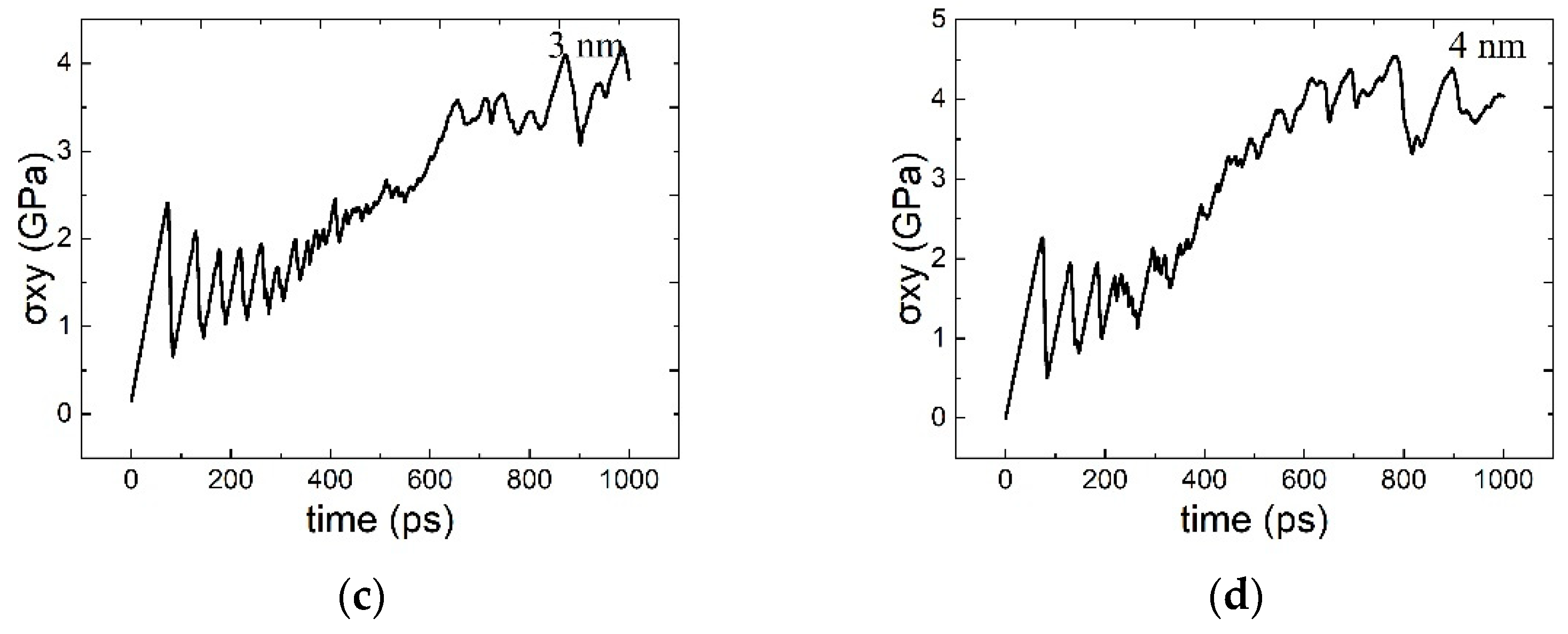
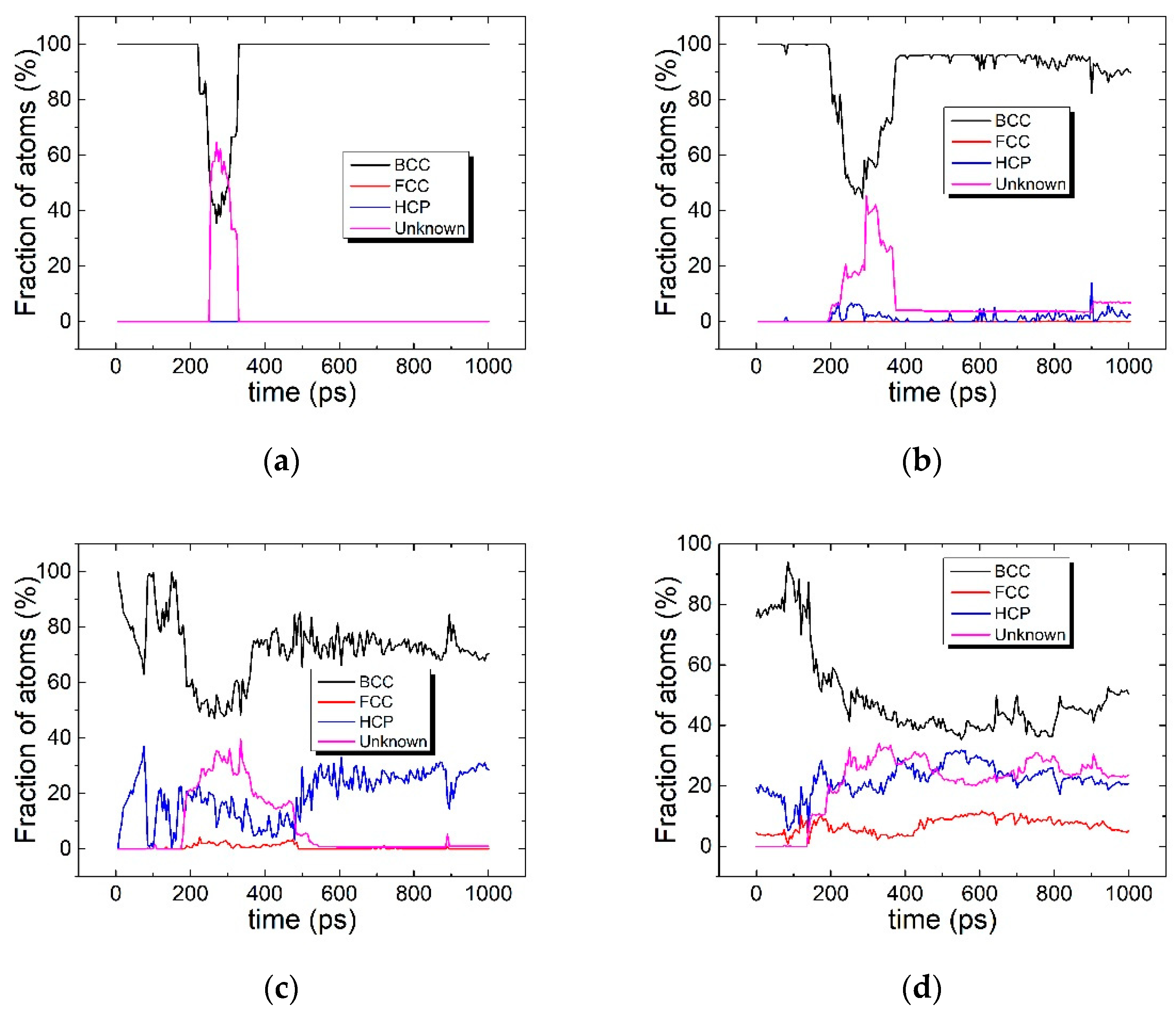
3.1. The Interaction between the SCGBM and Cu Precipitates at 10 K
3.2. The Structural Transformation of Cu Precipitates
3.3. The Interaction between the SCGBM and Cu Precipitates at 300 K
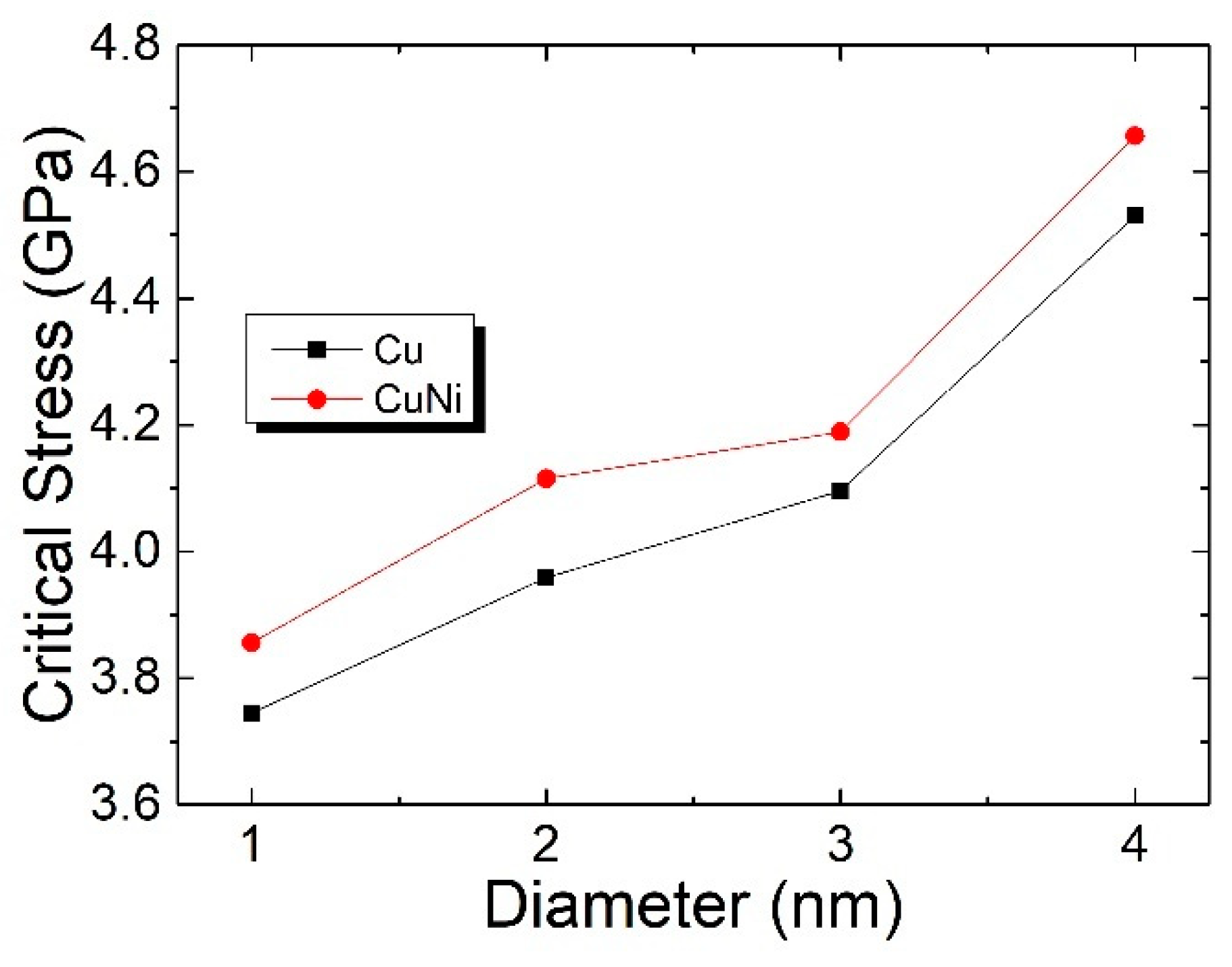
3.4. The Interaction between the SCGBM and CuNi Precipitates at 10 K
4. Summary and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cahn, J.W.; Mishin, Y.; Suzuki, A. Coupling grain boundary motion to shear deformation. Acta Mater. 2006, 54, 4953–4975. [Google Scholar] [CrossRef]
- Li, C.H.; Edwards, E.H.; Washburn, J.; Parker, E.R. Stress-induced movement of crystal boundaries. Acta Metall. 1953, 1, 223–229. [Google Scholar] [CrossRef]
- Wan, L.; Wang, S. Shear response of the Σ9⟨110⟩{221} symmetric tilt grain boundary in fcc metals studied by atomistic simulation methods. Phys. Rev. B 2010, 82, 214112. [Google Scholar] [CrossRef]
- Trautt, Z.T.; Adland, A.; Karma, A.; Mishin, Y. Coupled motion of asymmetrical tilt grain boundaries: Molecular dynamics and phase field crystal simulations. Acta Mater. 2012, 60, 6528–6546. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, C.; Tieu, K.; Zhao, X.; Pei, L. The shear response of copper bicrystals with Sigma11 symmetric and asymmetric tilt grain boundaries by molecular dynamics simulation. Nanoscale 2015, 7, 7224–7233. [Google Scholar] [CrossRef]
- Hoyt, J.J. Atomistic simulations of grain and interphase boundary mobility. Model. Simul. Mater. Sci. Eng. 2014, 22, 033001. [Google Scholar] [CrossRef]
- Fensin, S.J.; Asta, M.; Hoagland, R.G. Temperature dependence of the structure and shear response of a Σ11 asymmetric tilt grain boundary in copper from molecular-dynamics. Philos. Mag. 2012, 92, 4320–4333. [Google Scholar] [CrossRef]
- Niu, L.L.; Zhang, Y.; Shu, X.; Gao, F.; Jin, S.; Zhou, H.B.; Lu, G.H. Shear-coupled grain boundary migration assisted by unusual atomic shuffling. Sci. Rep. 2016, 6, 23602. [Google Scholar] [CrossRef]
- Niu, L.; Shu, X.; Zhang, Y.; Gao, F.; Jin, S.; Zhou, H.; Lu, G. Atomistic insights into shear-coupled grain boundary migration in bcc tungsten. Mater. Sci. Eng. A 2016, 677, 20–28. [Google Scholar] [CrossRef]
- Yin, J.; Wang, Y.; Yan, X.; Hou, H.; Wang, J.T. Atomistic simulation of shear-coupled motion of [1 1 0] symmetric tilt grain boundary in α-iron. Comput. Mater. Sci. 2018, 148, 141–148. [Google Scholar] [CrossRef]
- Frolov, T. Effect of interfacial structural phase transitions on the coupled motion of grain boundaries: A molecular dynamics study. Appl. Phys. Lett. 2014, 104, 211905. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, C.; Michal, G.; Tieu, K.; Zhao, X.; Deng, G. Influence of temperature and local structure on the shear-coupled grain boundary migration. Phys. Status Solidi (B) 2016, 254, 1600477. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Yu, Z.; Yin, J.; Lai, W. Revealing the influence of carbon on shear-coupled grain boundary migrationin alpha-iron via molecular dynamics simulations. Model. Simul. Mater. Sci. Eng. 2022, 30, 085001. [Google Scholar] [CrossRef]
- Bai, Z.; Yan, X.; Yin, J.; Hou, H. Influence of Chromium Atoms on the Shear-Coupled Motion of [110] Symmetric Tilt Grain Boundary in α-Iron: Atomic Simulation. Metals 2022, 12, 1451. [Google Scholar] [CrossRef]
- Aramfard, M.; Deng, C. Interaction of shear-coupled grain boundary motion with crack: Crack healing, grain boundary decohesion, and sub-grain formation. J. Appl. Phys. 2016, 119, 085308. [Google Scholar] [CrossRef]
- Xu, Y.; Geslin, P.A.; Karma, A. Elastically-mediated interactions between grain boundaries and precipitates in two-phase coherent solids. Phys. Rev. B 2016, 94, 144106. [Google Scholar] [CrossRef]
- Tan, F.; Fang, Q.; Li, J.; Wu, H. Interaction of precipitate with shear–coupled grain boundary migration. Acta Mech. 2020, 231, 237–250. [Google Scholar] [CrossRef]
- Phythian, W.J.; English, C.A. Microstrustural evolution in reactor pressure vessel steels. J. Nucl. Mater. 1993, 205, 162–177. [Google Scholar] [CrossRef]
- Odette, G.R.; Lucas, G.E. Recent progress in understanding reactor pressure vessel steel embrittlement. Radiat. Eff. Defects Solids 1998, 144, 189–231. [Google Scholar] [CrossRef]
- Bonny, G.; Terentyev, D.; Bakaev, A.; Zhurkin, E.E.; Hou, M.; Van Neck, D.; Malerba, L. On the thermal stability of late blooming phases in reactor pressure vessel steels: An atomistic study. J. Nucl. Mater. 2013, 442, 282–291. [Google Scholar] [CrossRef]
- Hu, S.Y.; Schmauder, S.; Chen, L.Q. Atomistic simulations of interactions between Cu precipitates and an edge dislocation in a b.c.c. Fe single crystal. Phys. Status Solidi B 2000, 220, 845–856. [Google Scholar] [CrossRef]
- Hu, S.Y.; Li, Y.L.; Watanabe, K. Calculation of internal stresses around Cu precipitates in the bcc Fe matrix by atomic simulation. Model. Simul. Mater. Sci. Eng. 1999, 7, 641–655. [Google Scholar] [CrossRef]
- Nedelcu, S.; Kizler, P.; Schmauder, S.; Moldovan, N. Atomic scale modelling of edge dislocation movement in the-Fe–Cu system. Model. Simul. Mater. Sci. Eng. 2000, 8, 181–191. [Google Scholar] [CrossRef]
- Shim, J.H.; Cho, Y.W.; Kwon, S.C.; Kim, W.W.; Wirth, B.D. Screw dislocation assisted martensitic transformation of a bcc Cu precipitate in bcc Fe. Appl. Phys. Lett. 2007, 90, 021906. [Google Scholar] [CrossRef]
- Harry, T.; Bacon, D.J. Computer simulation of the core structure of the <111> screw dislocation in α-iron containing copper precipitates II. Dislocation–precipitate interaction and the strengthening effect. Acta Mater. 2002, 50, 209–222. [Google Scholar]
- Harry, T.; Bacon, D.J. Computer simulation of the core structure of the <111> screw dislocation in α-iron containing copper precipitates I. Structure in the matrix and a precipitate. Acta Mater. 2002, 50, 195–208. [Google Scholar]
- Odette, G.R.; Wirth, B.D. A computational microscopy study of nanostructural evolution in irradiated pressure vessel steels. J. Nucl. Mater. 1997, 251, 157–171. [Google Scholar] [CrossRef]
- Fine, M.E.; Liu, J.Z.; Asta, M.D. An unsolved mystery: The composition of bcc Cu alloy precipitates in bcc Fe and steels. Mater. Sci. Eng. A 2007, 463, 271–274. [Google Scholar] [CrossRef]
- Schober, M.; Eidenberger, E.; Leitner, H.; Staron, P.; Reith, D.; Podloucky, R. A critical consideration of magnetism and composition of (bcc) Cu precipitates in (bcc) Fe. Appl. Phys. A 2010, 99, 697–704. [Google Scholar] [CrossRef]
- Zhang, C.; Enomoto, M. Study of the influence of alloying elements on Cu precipitation in steel by non-classical nucleation theory. Acta Mater. 2006, 54, 4183–4191. [Google Scholar] [CrossRef]
- Toyama, T.; Nagai, Y.; Tang, Z.; Hasegawa, M.; Almazouzi, A.; van Walle, E.; Gerard, R. Nanostructural evolution in surveillance test specimens of a commercial nuclear reactor pressure vessel studied by three-dimensional atom probe and positron annihilation. Acta Mater. 2007, 55, 6852–6860. [Google Scholar] [CrossRef]
- Al-Motasem, A.T.; Posselt, M.; Bergner, F. Nanoclusters in bcc-Fe containing vacancies, copper and nickel: Structure and energetics. J. Nucl. Mater. 2011, 418, 215–222. [Google Scholar] [CrossRef]
- Zhu, L.S.; Zhao, S.J. Influence of Ni on Cu precipitation in Fe–Cu–Ni ternary alloy by an atomic study. Chin. Phys. B 2014, 23, 063601. [Google Scholar] [CrossRef]
- Wagner, A.; Ulbricht, A.; Bergner, F.; Altstadt, E. Influence of the copper impurity level on the irradiation response of reactor pressure vessel steels investigated by SANS. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2012, 280, 98–102. [Google Scholar] [CrossRef]
- Fukuya, K.; Ohno, K.; Nakata, H.; Dumbill, S.; Hyde, J.M. Microstructural evolution in medium copper low alloy steels irradiated in a pressurized water reactor and a material test reactor. J. Nucl. Mater. 2003, 312, 163–173. [Google Scholar] [CrossRef]
- Lv, G.; Zhang, H.; He, X.; Yang, W.; Su, Y. Atomistic simulation of Cu–Ni precipitates hardening in α-iron. J. Phys. D Appl. Phys. 2015, 48, 115302. [Google Scholar] [CrossRef]
- Bonny, G.; Pasianot, R.C.; Castin, N.; Malerba, L. Ternary Fe-Cu-Ni many-body potential to model reactor pressure vessel steels: First validation by simulated thermal annealing. Philos. Mag. 2009, 89, 3531–3546. [Google Scholar] [CrossRef]
- Mendelev, M.I.; Han, S.; Srolovitz, D.J.; Ackland, G.J.; Sun, D.Y.; Asta, M. Development of new interatomic potentials appropriate for crystalline and liquid iron. Philos. Mag. 2003, 83, 3977–3994. [Google Scholar] [CrossRef]
- Mishin, Y.; Mehl, M.J.; Papaconstantopoulos, D.A.; Voter, A.F.; Kress, J.D. Structural stability and lattice defects in copper:Ab initio, tight-binding, and embedded-atom calculations. Phys. Rev. B 2001, 63, 224106. [Google Scholar] [CrossRef]
- Voter, A.F.; Chen, S.P. Accurate Interatomic Potentials for Ni, Al and Ni3Al. Mater. Res. Soc. Symp. Proc. 1987, 82, 175. [Google Scholar] [CrossRef]
- Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO-the Open Visualization Tool. Model. Simul. Mater. Sci. Eng. 2010, 18, 015012. [Google Scholar] [CrossRef]
- Tsuzuki, H.; Branicio, P.S.; Rino, J.P. Structural characterization of deformed crystals by analysis of common atomic neighborhood. Comput. Phys. Commun. 2007, 177, 518–523. [Google Scholar] [CrossRef]
- Kelchner, C.L.; Plimpton, S.J.; Hamilton, J.C. Dislocation nucleation and defect structure during surface indentation. Phys. Rev. B 1998, 58, 11085–11088. [Google Scholar] [CrossRef]
- Tschopp, M.A.; Coleman, S.P.; McDowell, D.L. Symmetric and asymmetric tilt grain boundary structure and energy in Cu and Al (and transferability to other fcc metals). Integr. Mater. Manuf. Innov. 2015, 4, 176–189. [Google Scholar] [CrossRef]
- Ahlawat, S.; Sarkar, S.K.; Sen, D.; Biswas, A. Revisiting temporal evolution of Cu-rich precipitates in Fe–Cu alloy: Correlative small angle neutron scattering and atom-probe tomography studies. Microsc. Microanal. 2019, 25, 840–848. [Google Scholar] [CrossRef]
- Yin, J.; Wang, Y.; Hou, H.; Wang, J.T.; Liu, X.; Xue, F. Atomistic simulation of interactions between an edge dislocation and Cu precipitates with different chemical compositions in α-Fe. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2019, 458, 39–43. [Google Scholar] [CrossRef]
- Osetsky, Y.N.; Bacon, D.; Mohles, V. Atomic modelling of strengthening mechanisms due to voids and copper precipitates in α-iron. Philos. Mag. 2003, 83, 3623–3641. [Google Scholar] [CrossRef]
- Jiao, Z.B.; Luan, J.H.; Zhang, Z.W.; Miller, M.K.; Ma, W.B.; Liu, C.T. Synergistic effects of Cu and Ni on nanoscale precipitation and mechanical properties of high-strength steels. Acta Mater. 2013, 61, 5996–6005. [Google Scholar] [CrossRef]
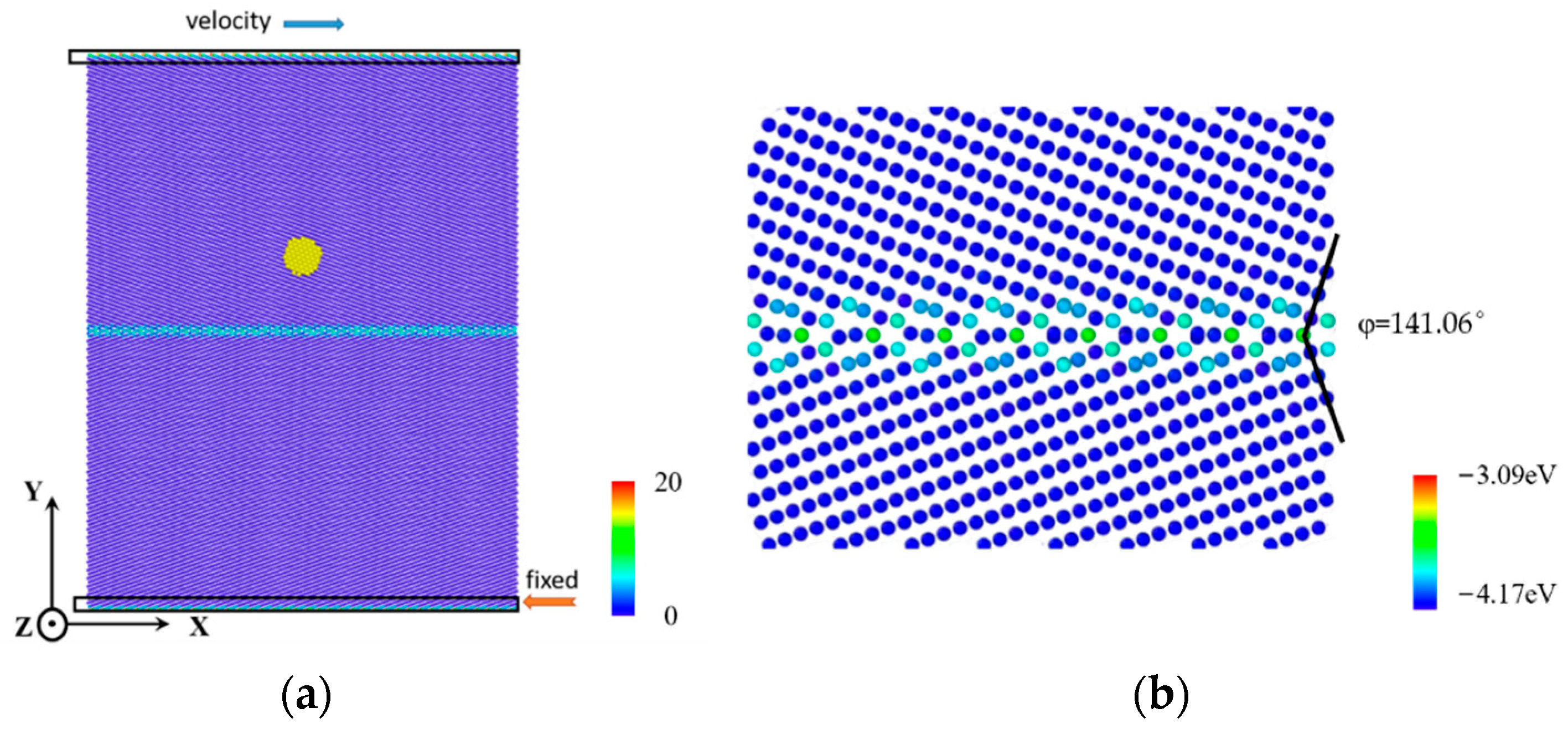

| Parameters | Value | Parameters | Value |
|---|---|---|---|
| LX (length in x direction) | 233.497 Å | T (temperature) | 10, 300 K |
| LY (length in y direction) | 291.021 Å | v (velocity) | 10 m/s |
| LZ (length in z direction) | 120.739 Å | c (Ni concentration) | 20% |
| D (precipitate diameter) | 1~4 nm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, J.; Hou, H.; Wang, J.-T.; Liu, X.; Xu, C.; Li, Y.; Qian, W.; Jin, X.; Wu, H.; Jia, W.; et al. Atomistic Simulation of the Interaction between the Σ9[110](221) Shear-Coupled Grain Boundary Motion and the Cu-rich Precipitates in α-Iron. Metals 2024, 14, 252. https://doi.org/10.3390/met14020252
Yin J, Hou H, Wang J-T, Liu X, Xu C, Li Y, Qian W, Jin X, Wu H, Jia W, et al. Atomistic Simulation of the Interaction between the Σ9[110](221) Shear-Coupled Grain Boundary Motion and the Cu-rich Precipitates in α-Iron. Metals. 2024; 14(2):252. https://doi.org/10.3390/met14020252
Chicago/Turabian StyleYin, Jian, Huaiyu Hou, Jing-Tao Wang, Xiangbing Liu, Chaoliang Xu, Yuanfei Li, Wangjie Qian, Xiao Jin, Huanchun Wu, Wenqing Jia, and et al. 2024. "Atomistic Simulation of the Interaction between the Σ9[110](221) Shear-Coupled Grain Boundary Motion and the Cu-rich Precipitates in α-Iron" Metals 14, no. 2: 252. https://doi.org/10.3390/met14020252
APA StyleYin, J., Hou, H., Wang, J.-T., Liu, X., Xu, C., Li, Y., Qian, W., Jin, X., Wu, H., Jia, W., & Quan, Q. (2024). Atomistic Simulation of the Interaction between the Σ9[110](221) Shear-Coupled Grain Boundary Motion and the Cu-rich Precipitates in α-Iron. Metals, 14(2), 252. https://doi.org/10.3390/met14020252






