Comments on the Intermediate-Temperature Embrittlement of Metals and Alloys: The Conditions for Transgranular and Intergranular Failure
Abstract
1. Introduction
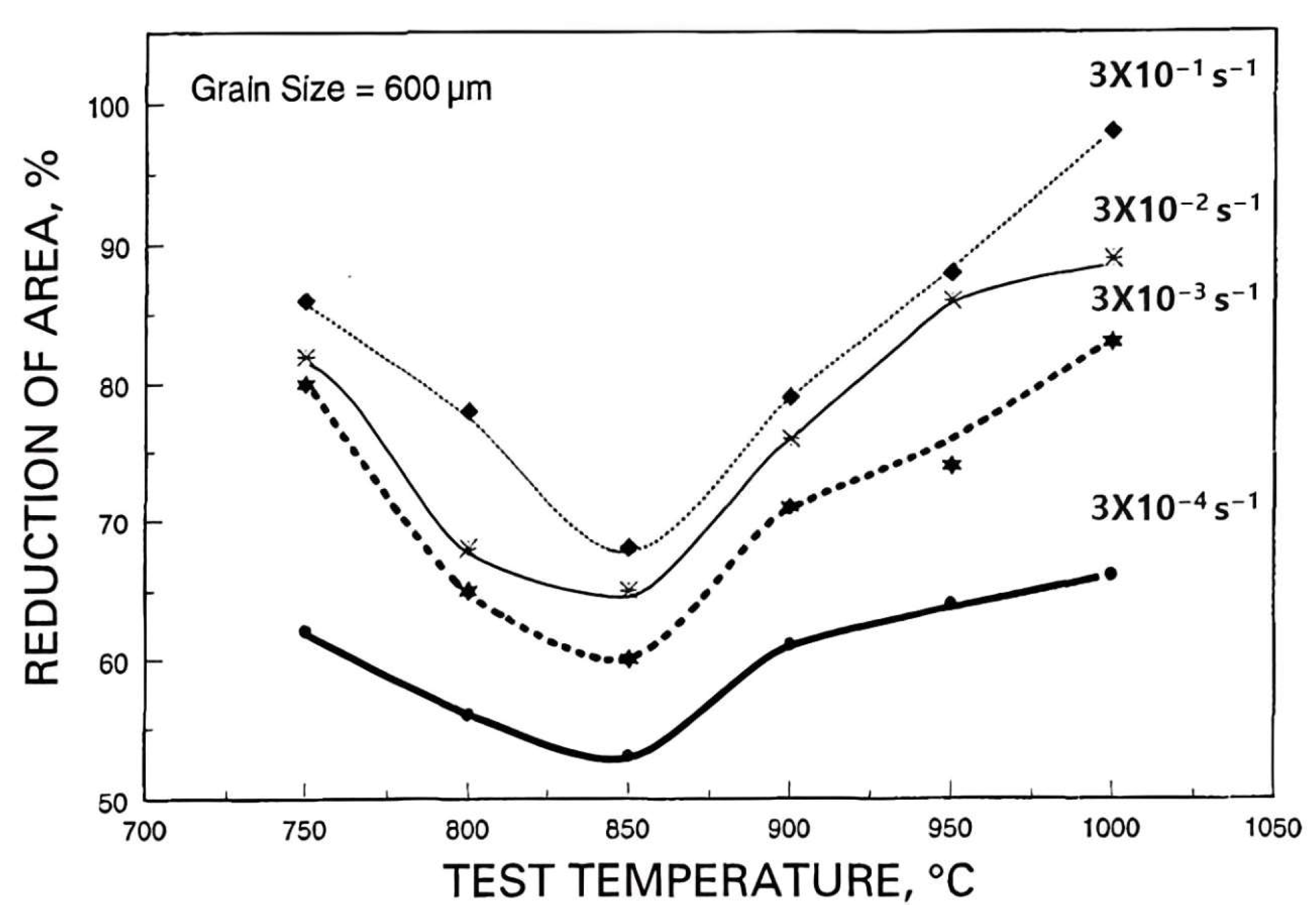
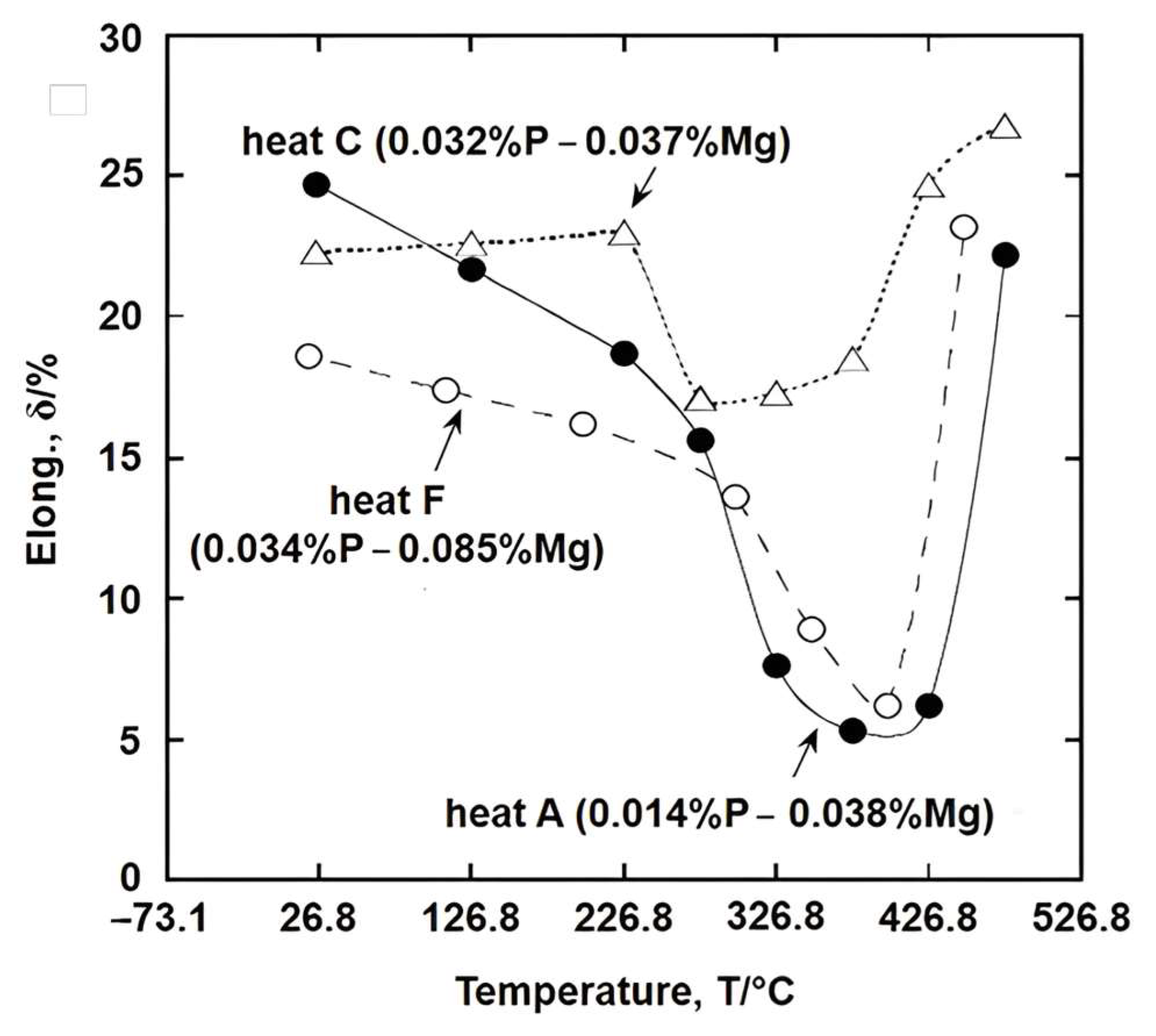
2. Hot Ductility of Steels
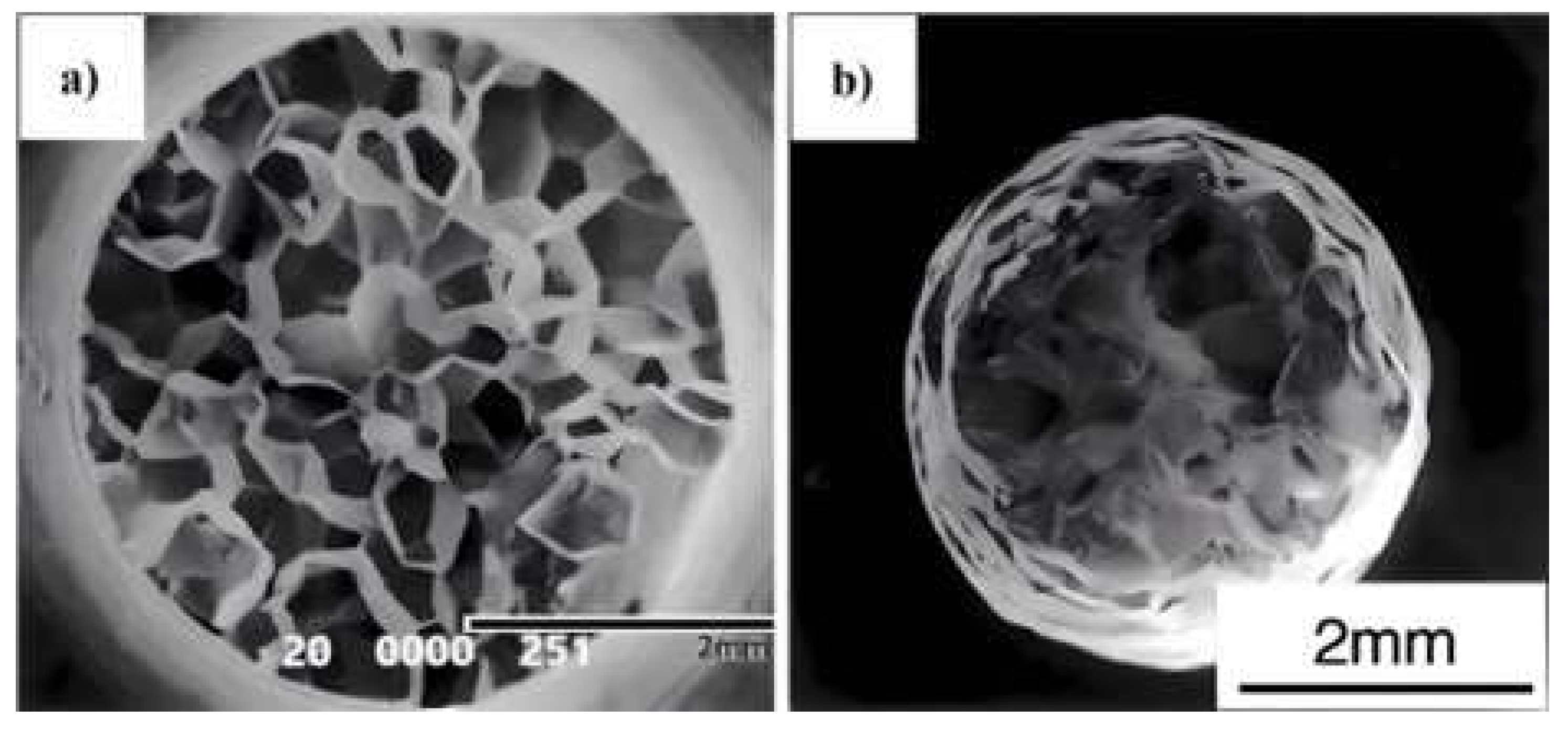
3. Troughs in Spheroidal Cast Irons
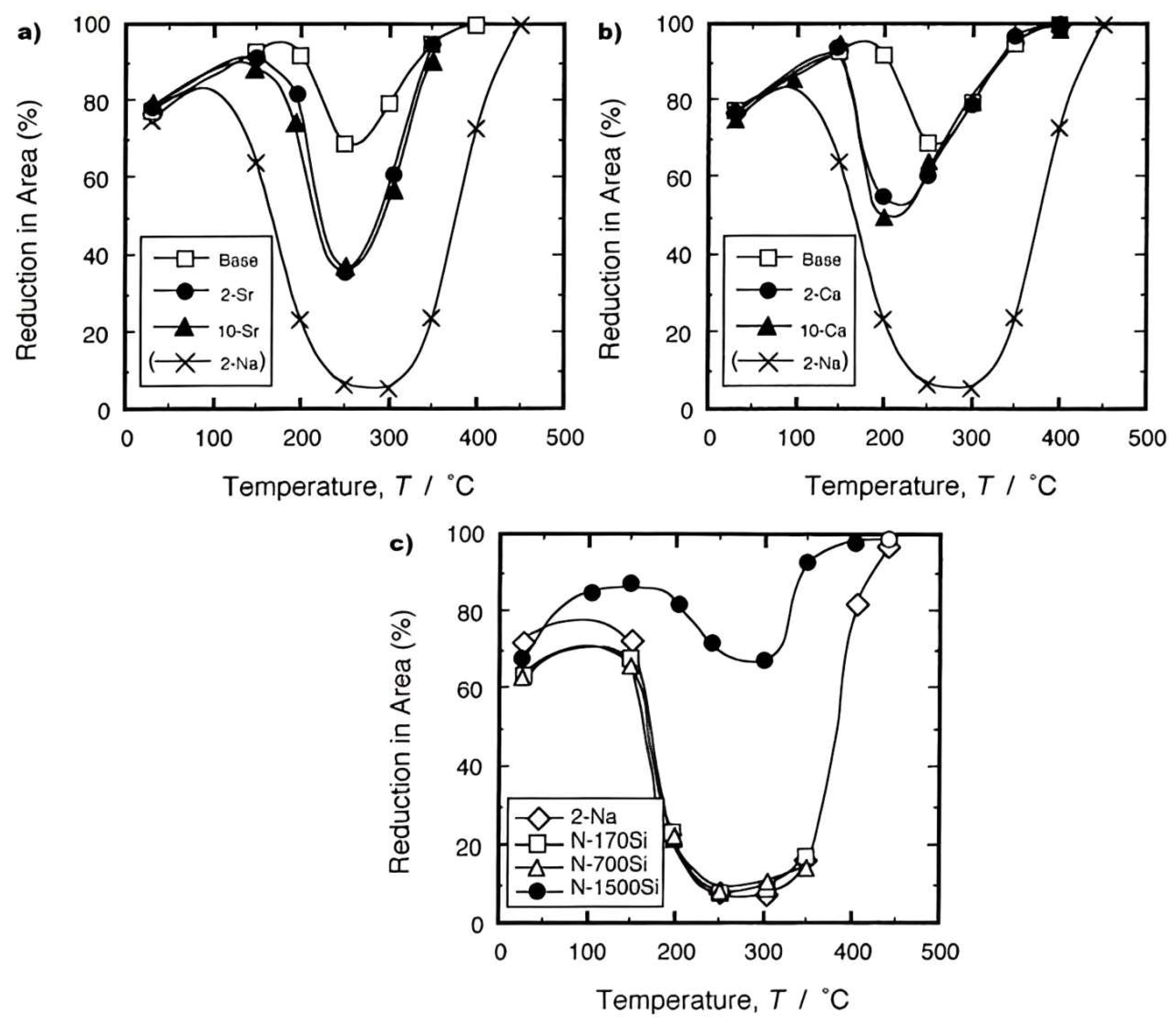
4. Troughs in Aluminium Alloys
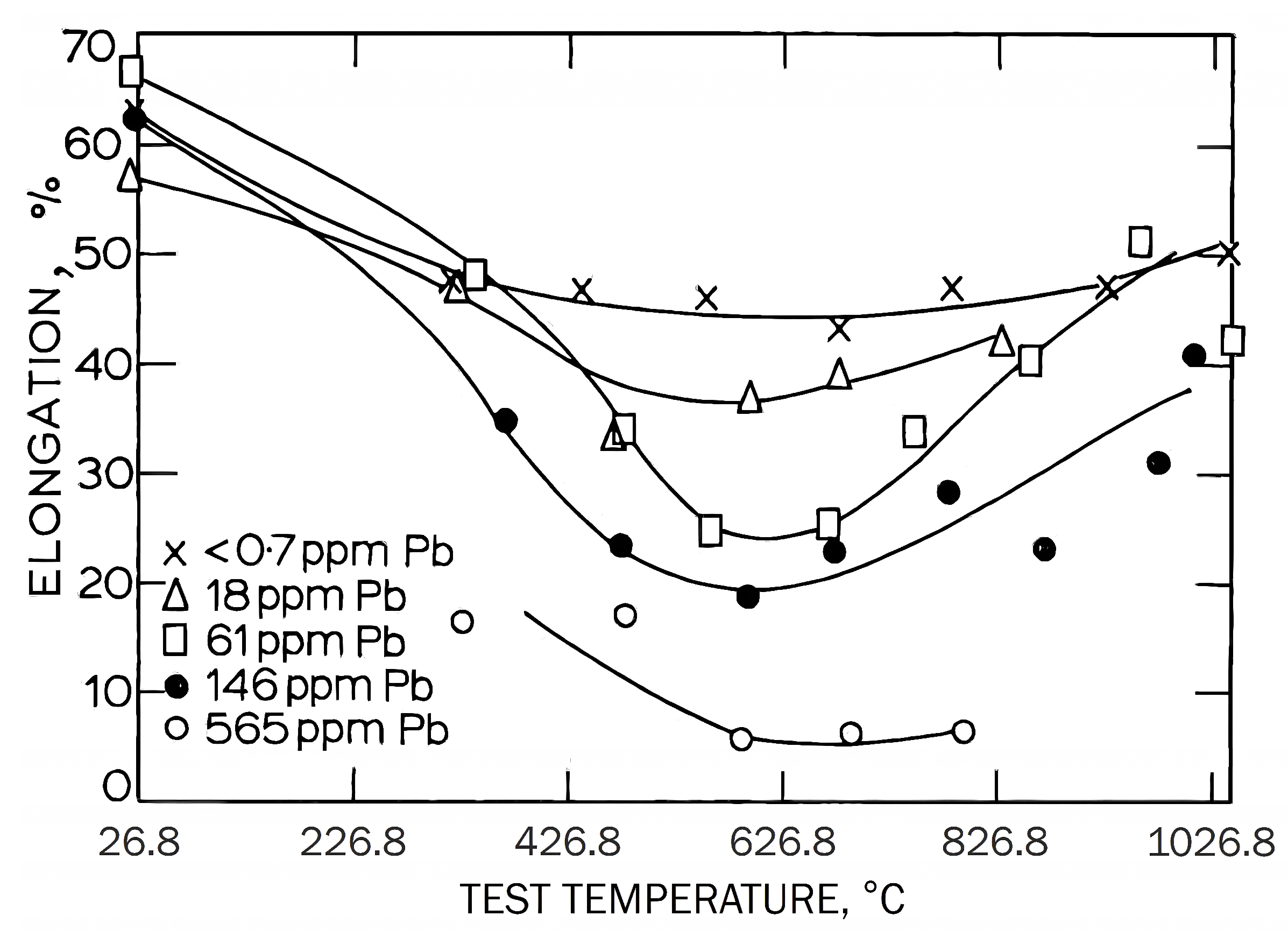
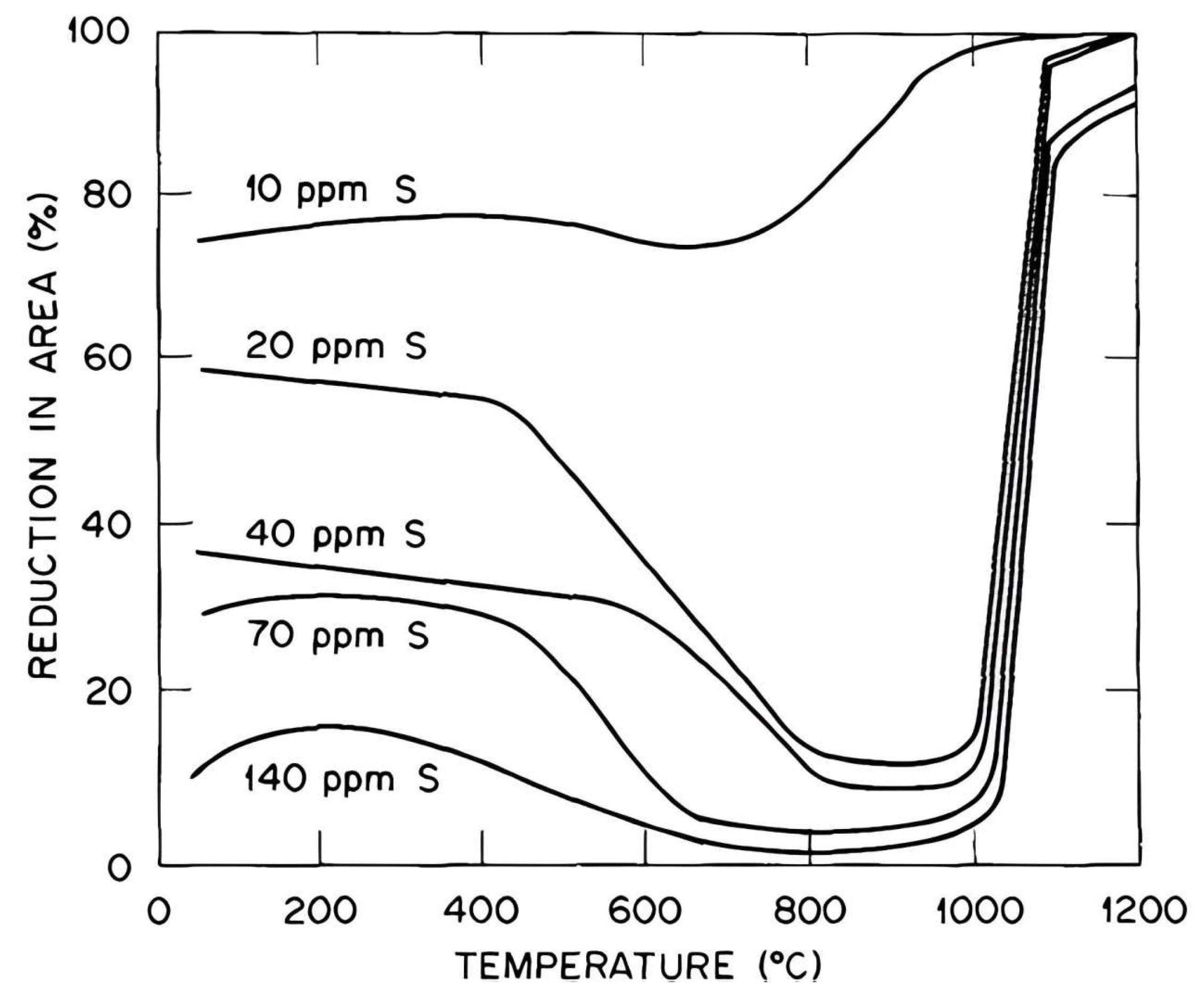
5. Troughs in Copper Alloys
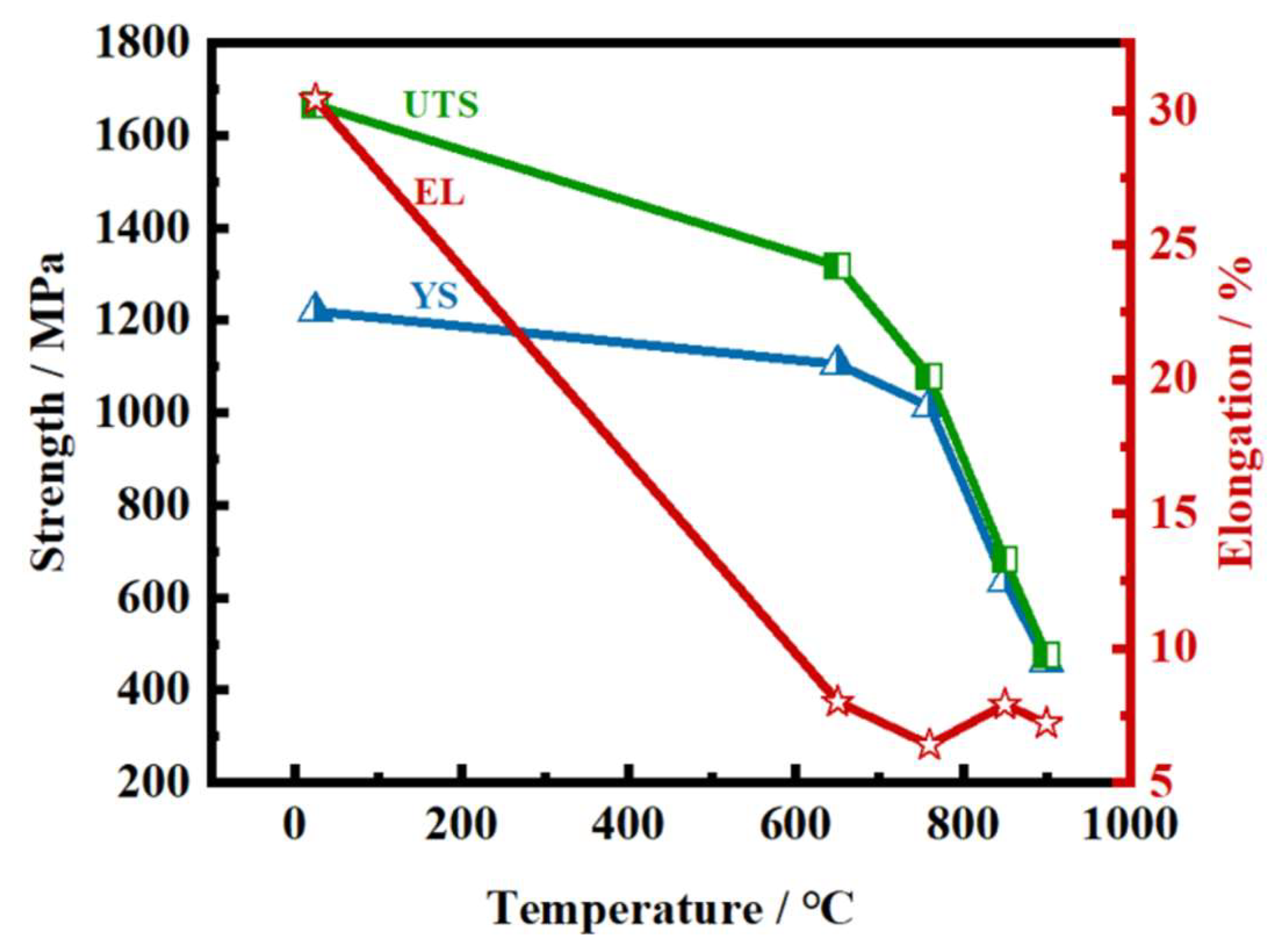
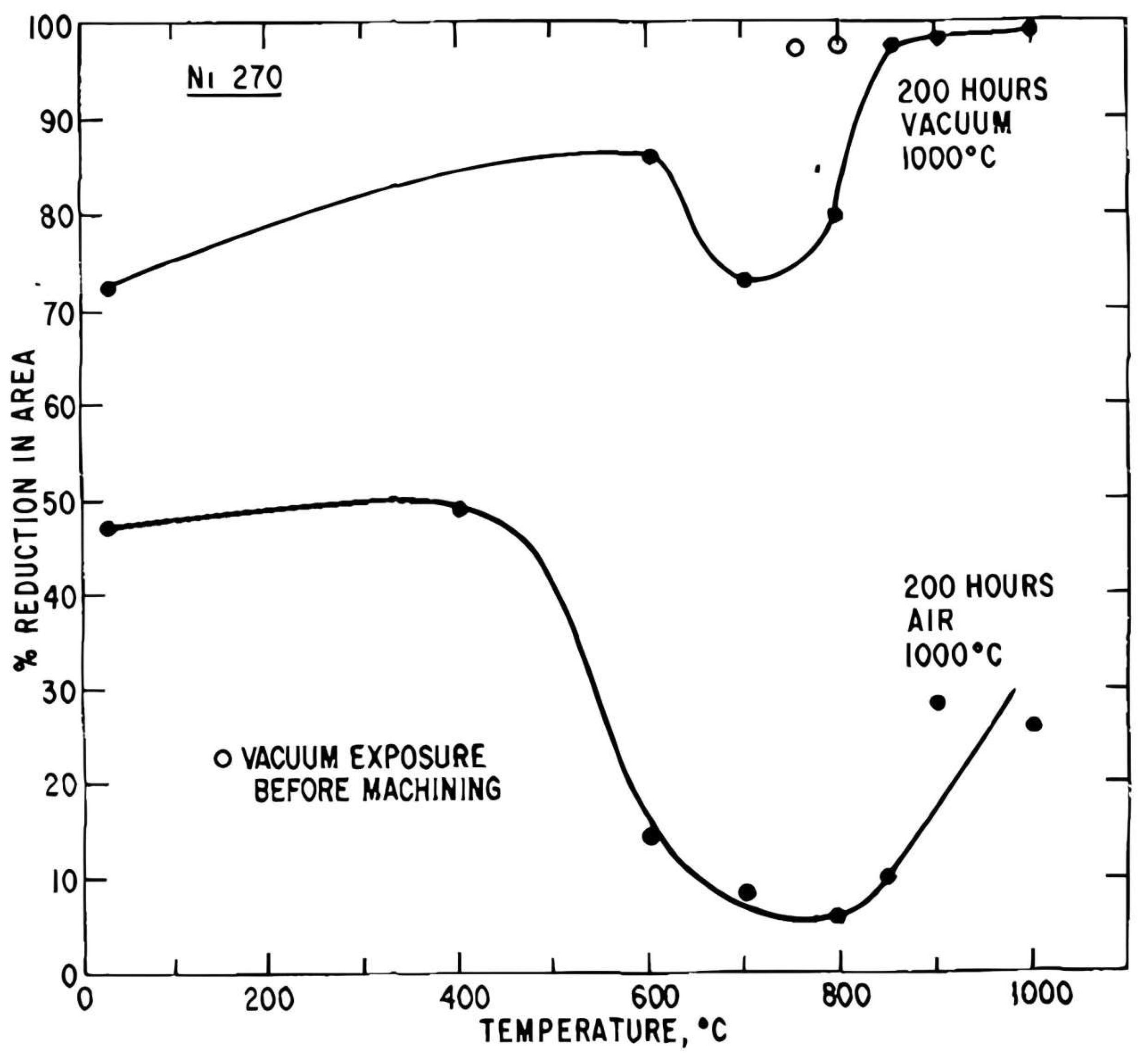
6. Ni and Nickel Alloys
7. Discussion
Origin of the Troughs and Intermediate-Temperature Embrittlement
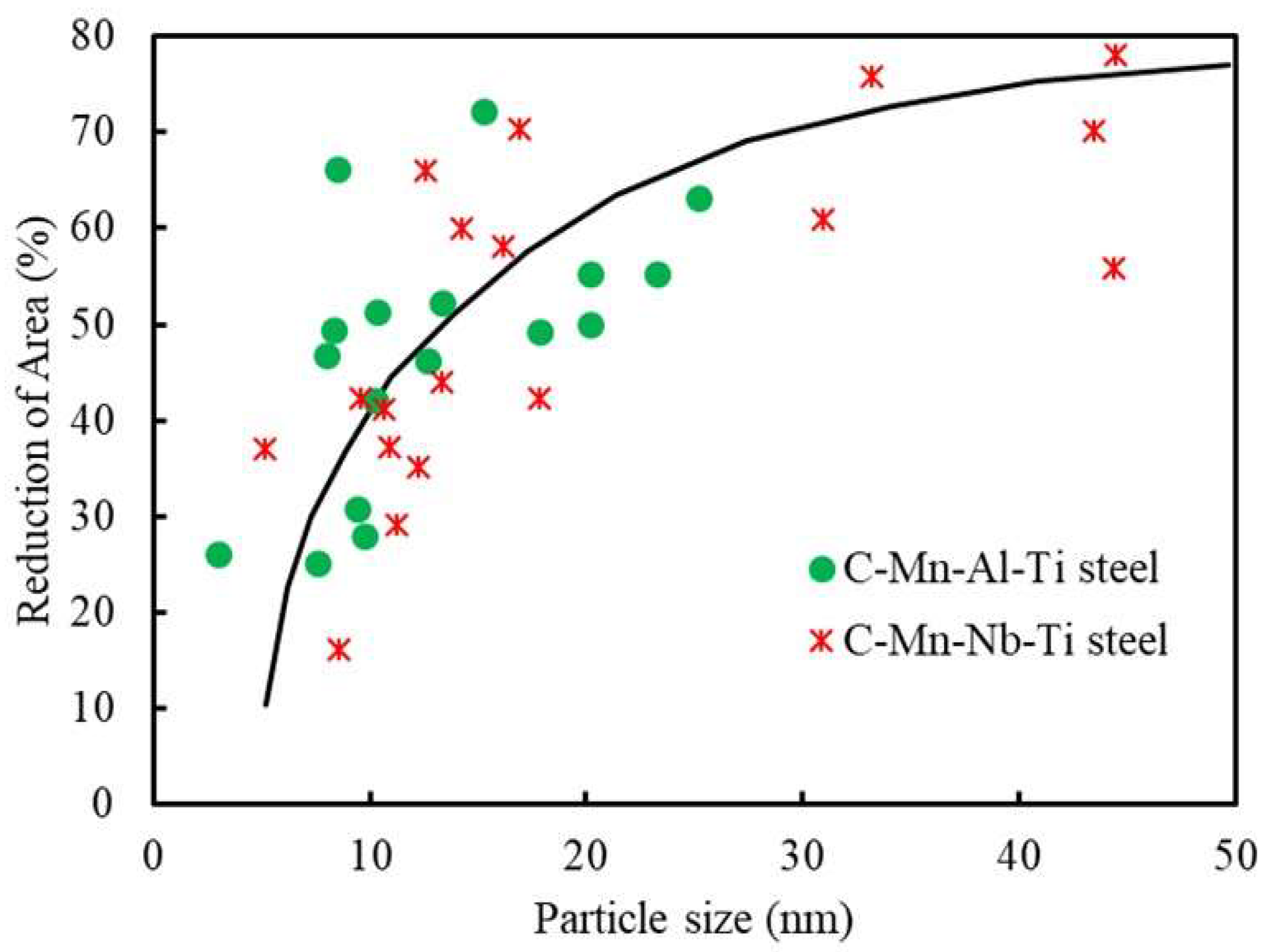
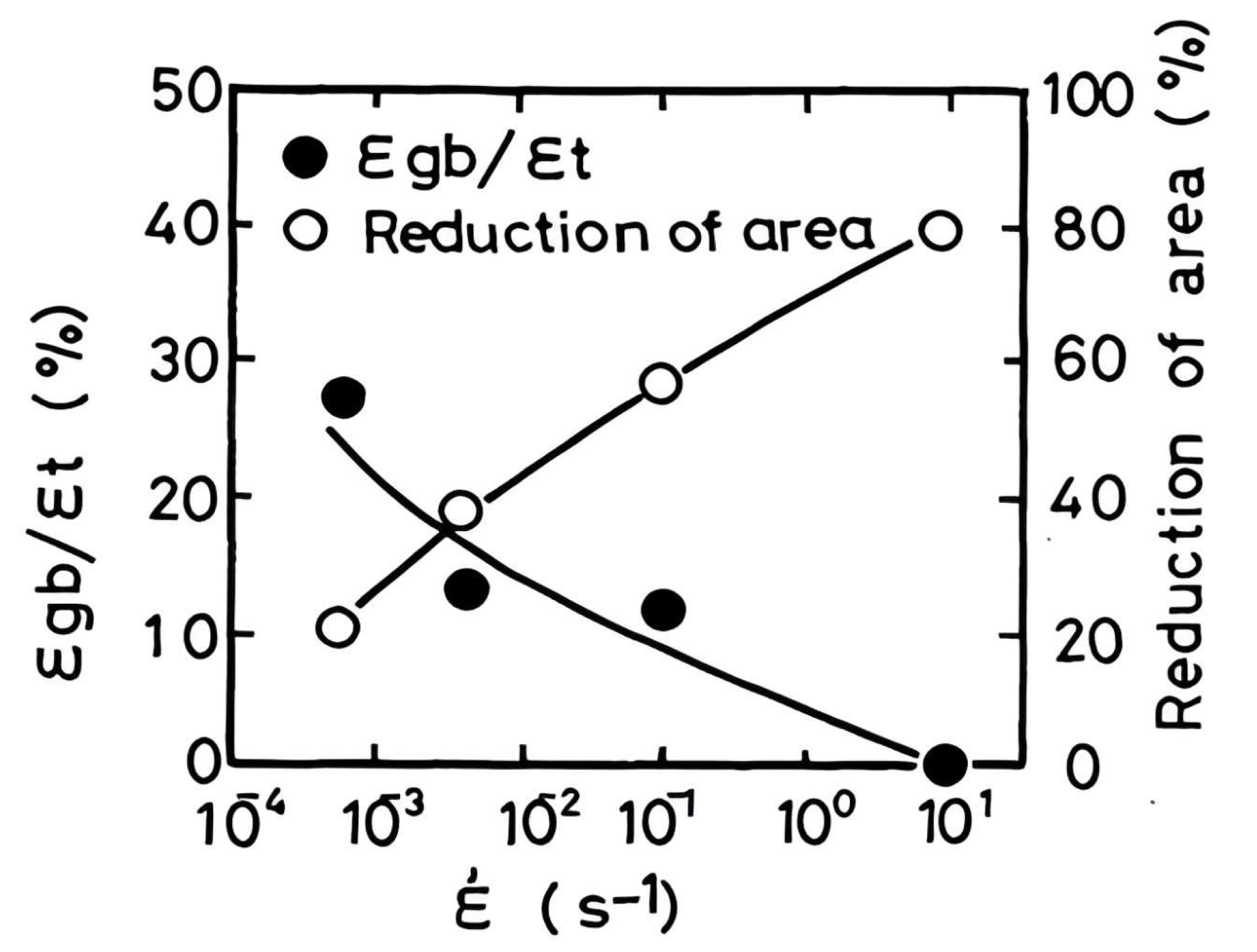
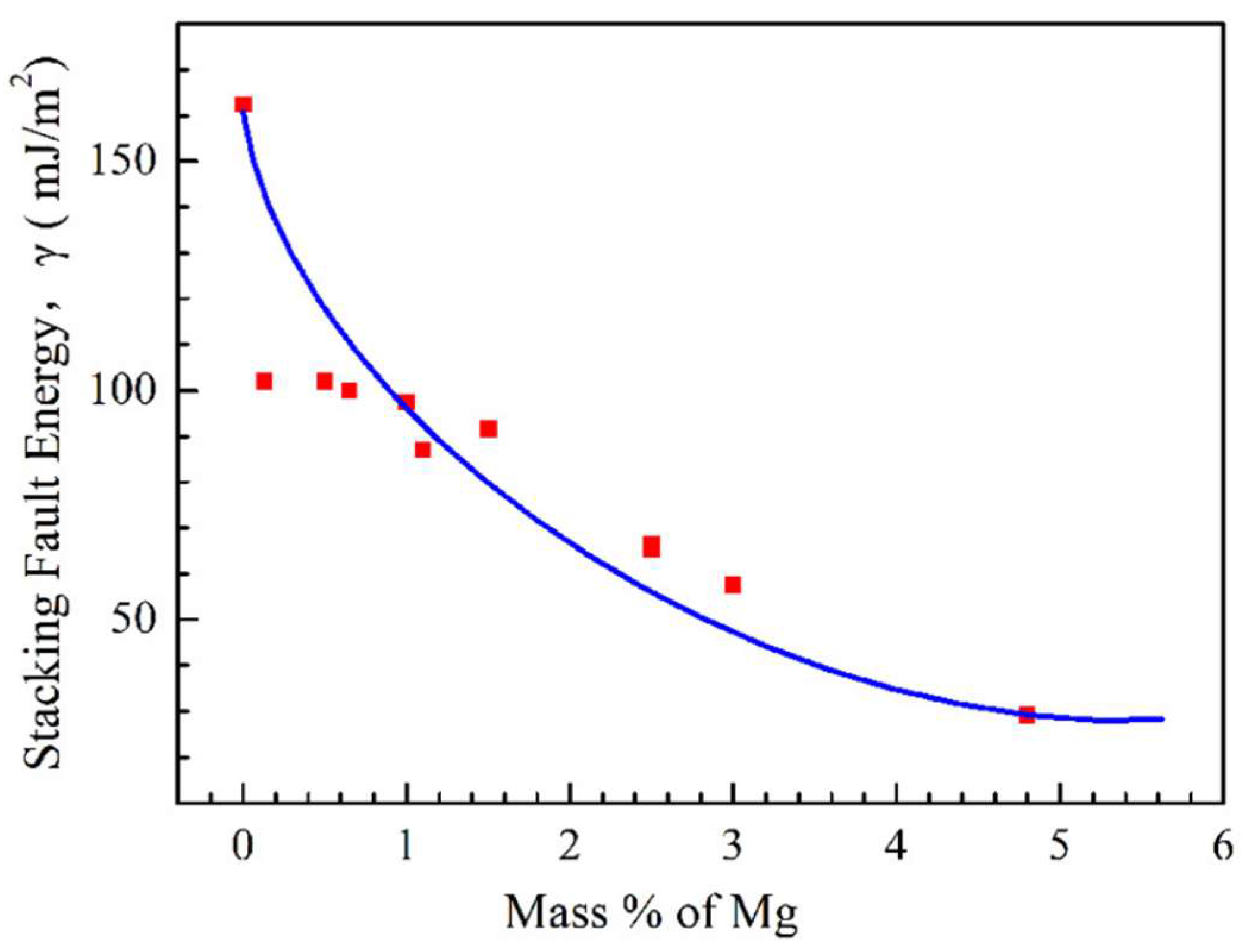
8. Influence of Composition, Grain Size, Strain Rate, and Precipitation on Hot Ductility
8.1. Grain Size
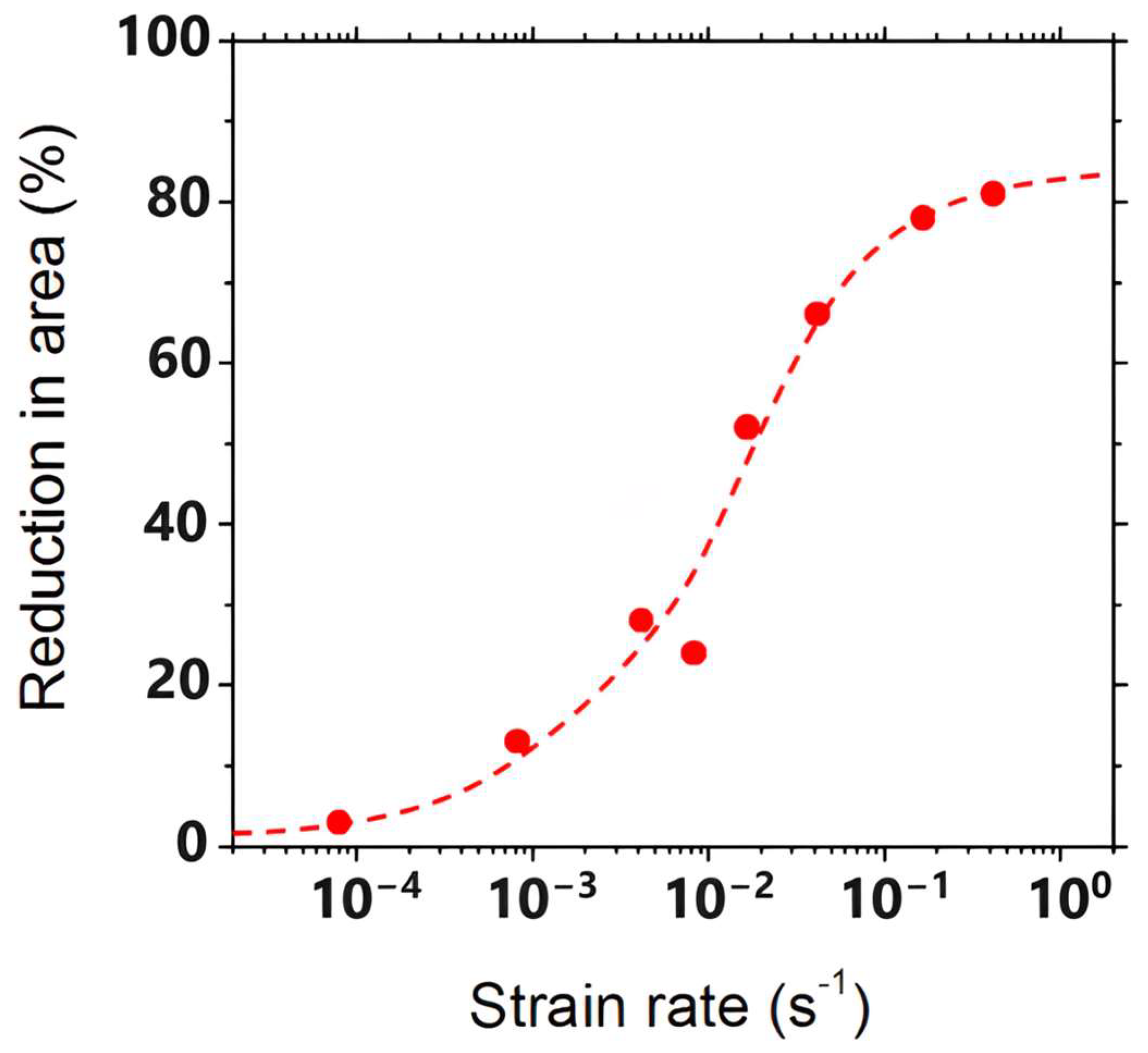
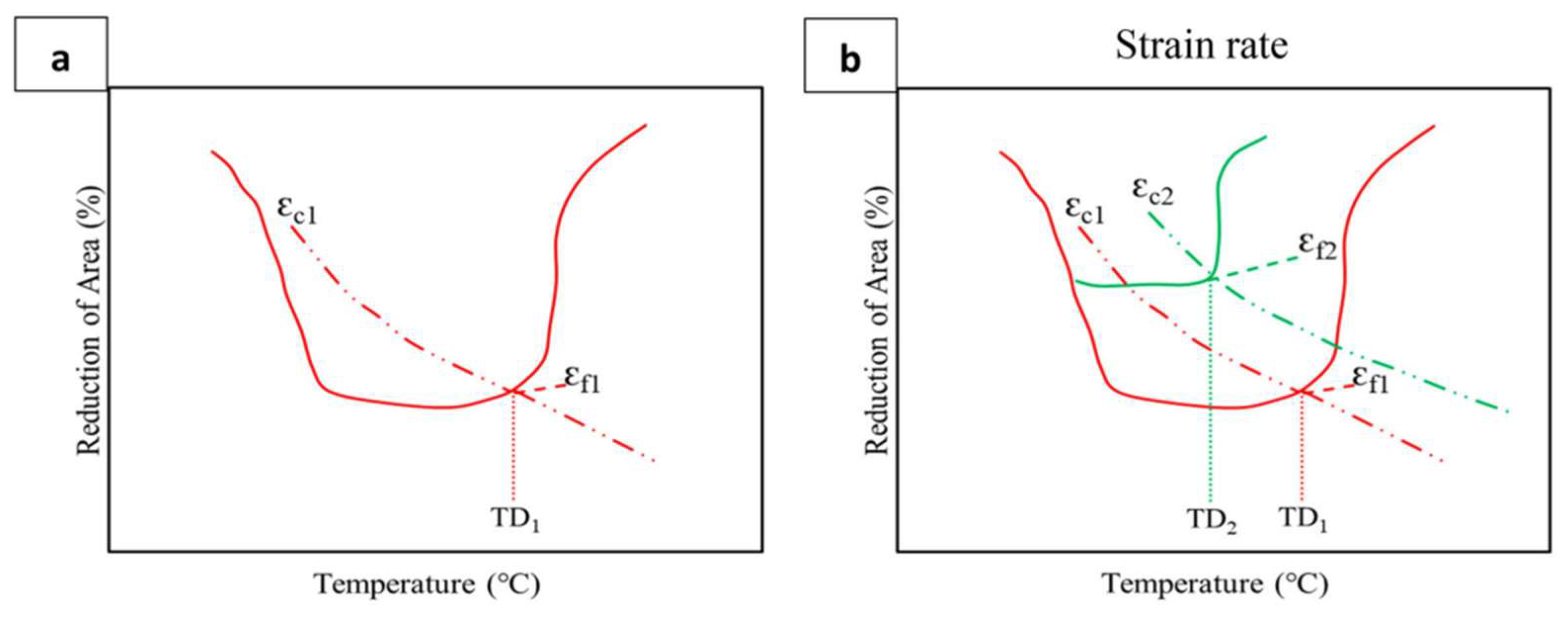
8.2. Strain Rate
| Metal or Alloy | Stacking Fault Energy (mJm−2) |
|---|---|
| Al | 180 |
| Ni | 75 |
| Cu | 75 |
| Brass | 25 |
| Austenitic stainless steel | 20 |
| Al-5% Mg | 20, Ref. [78] |
9. Importance of Segregation of Atoms and Ionic Bonding to the Cohesive Strength of Grain Boundaries
10. Summary and Conclusions
- (1)
- Low-ductility intergranular failures (intermediate-temperature embrittlement) can occur in both fcc and bcc alloys, but the causes are very diverse and, in many cases, not known with certainty because many of these explanations occur together and are synergistic.
- (2)
- In low- and medium-C steels, troughs can be caused by the thin film of ferrite, PFZs, segregation of deleterious elements to the boundaries influencing the bonding, and segregation to the boundaries leading to low-melting-point compounds. In other metals and alloys, the same origins apply, other than the presence of the ferrite film.
- (3)
- In steel and Ni alloys, creep failure is an important consideration as the unbending operation during continuous casting is so slow (10−3–10−4 s−1) and the temperature range is within the creep temperature range. The austenite phase in steel, at temperatures in the range 750–950 °C, is particularly prone to this low ductility due to the low strain rate pertaining to the bending operation. This does not take place in hot rolling when the strain rate is much higher.
- (4)
- Hence, increasing the strain rate, refining the grain size, and coarsening of the precipitates will all lead to an improvement in ductility of steel, nickel, and copper alloys.
- (5)
- Of all the ways of improving hot ductility, refining the as-cast grain size is the most effective.
- (6)
- The depth of the trough is very much influenced in low–medium-C steel by the presence of the ferrite film or, more generally, when the film is absent by the precipitates at the grain boundaries and/or atoms which segregate to the boundaries and alter the bonding.
- (7)
- The improvement in ductility at the high-temperature end of the trough is due to DRX in the case of fcc metals with low SFE or recovery in fcc metals with high SFE and bcc metals.
- (8)
- In Al alloys, intermediate intergranular embrittlement is a worry only in a few alloys in industry, as the main processing routes are cold or hot rolling, where the strain rates are high, and extrusion, where creep conditions do not normally apply. Failures can still be low-ductility intergranular if segregation takes place, leading to the boundaries forming liquid films, hydrogen and oxygen infiltration, or substantial weakening of the cohesive strength of the boundaries by foreign atoms. Although the magnitude of the effect is very variable, refining the grain size will always benefit hot ductility.
- (9)
- The review highlights the importance of grain boundary segregation, both equilibrium and nonequilibrium segregation, in controlling hot ductility, and it is felt that this is an area which has considerable potential in not only improving hot ductility but the room-temperature properties of metals and alloys, and further research should be focused in this direction. Although our understanding of segregation is still limited, techniques are available to clarify our understanding. Using grain boundary segregation to improve properties is also likely to prove very cost-effective, as strengthening the boundaries rather than the matrix will require very little solute.
Funding
Data Availability Statement
Conflicts of Interest
References
- Mintz, B. The influence of composition on the hot ductility of steels and to the problem of transverse cracking. ISIJ Int. 1999, 39, 833–855. [Google Scholar] [CrossRef]
- Mintz, B.; Crowther, D.N. Hot ductility of steels and its relationship to the problem of transverse cracking in continuous casting. Int. Mater. Rev. 2010, 55, 168–196. [Google Scholar] [CrossRef]
- Mintz, B.; Qaban, A. Understanding the high temperature side of the hot ductility curve for steels. Mater. Sci. Technol. 2021, 37, 237–249. [Google Scholar] [CrossRef]
- Zaitsev, A.; Arutyunyan, N.; Koldaev, A. Hot ductility, homogeneity of composition, structure and properties of high strength microalloyed steels: A critical review. Metals 2023, 13, 1066. [Google Scholar] [CrossRef]
- Mintz, B.; Qaban, A. The influence of precipitation, high levels of Al, Si, P and a small B addition on the hot ductility of TWIP and TRIP assisted steels: A critical review. Metals 2022, 12, 502. [Google Scholar] [CrossRef]
- Mintz, B.; Yue, S.; Jonas, J.J. Hot ductility of steels and its relationship to the problem of transverse cracking during continuous casting. Int. Mater. Rev. 1991, 36, 187–220. [Google Scholar] [CrossRef]
- Melford, D.A. The influence of residual and trace elements on hot shortness and high temperature embrittlement. Phil. Trans. R. Soc. Lond. A 1980, 295, 89–103. Available online: https://www.jstor.org/stable/36461 (accessed on 13 December 2023).
- Perrot-Simonetta, M.T.; Kobylanski, A. Influence of trace elements on hot ductility of an ultra high purity invar alloy. J. Phys. IV 1995, 5, C7-323–C7-334. [Google Scholar] [CrossRef][Green Version]
- Cardoso, G.; Mintz, B.; Yue, S. Hot ductility of Al and Ti containing steels with and without cyclic temperature oscillations. Ironmak. Steelmak. 1995, 22, 365–377. Available online: https://pascal-francis.inist.fr/vibad/index.php?action=getRecordDetail&idt=2928417 (accessed on 13 December 2023).
- Ouchi, C.; Matsumoto, K. Hot ductility in Nb-bearing High-strength low-alloy steels. Trans. ISIJ 1982, 22, 181–189. [Google Scholar] [CrossRef]
- Mintz, B.; Shaker, M.; Crowther, D.N. Hot ductility of an austenitic and a ferritic stainless steel. Mater. Sci. Technol. 1997, 13, 243–249. [Google Scholar] [CrossRef]
- Sakai, T.; Jonas, J.J. Recovery and Recrystallisation in Encyclopedia of Materials Science and Technology, 2011.
- McQueen, H.J.; Blum, W. Dynamic recovery: Sufficient mechanism in the hot deformation of Al (<99.99). Mater. Sci. Eng. A 2000, 290, 95–107. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, H.; Wang, K.; Xu, T. Nonequilibrium grain-boundary segregation mechanism of hot ductility loss for austenitic and ferritic stainless steels. J. Mater. Res. 2015, 30, 2117–2123. [Google Scholar] [CrossRef]
- Peng, H.-B.; Chen, W.-Q.; Chen, L.C.; Guo, D. Effect of tin, copper and boron on the hot ductility of 20CrMnTi steel between 650 °C and 1100 °C. High Temp. Mater. Proc. 2015, 34, 19–26. [Google Scholar] [CrossRef]
- Xu, T.; Zheng, L.; Wang, K.; Misra, R.D.K. Unified mechanism of intergranular embrittlement based on non-equilibrium grain boundary segregation. Inter. Mater. Rev. 2013, 58, 263–295. [Google Scholar] [CrossRef]
- Kang, M.H.; Lee, J.S.; Koo, Y.M.; Kim, S.-J.; Heo, N.H. Correlation between MnS precipitation, sulfur segregation kinetics and hot ductility in C-Mn steel. Metall. Mater. Trans. A 2014, 45, 5295–5299. [Google Scholar] [CrossRef]
- Laha, K.; Kyono, J.; Kishimoto, S.; Shinya, N. Beneficial effect of B segregation on creep cavitation in a type 347 austenitic stainless steel. Scr. Mater. 2005, 52, 675–678. [Google Scholar] [CrossRef]
- Heo, N.H.; Shin, H.S.; Kim, S.-J. Role of power ratio on ductility-dip cracking of Ni-Cr-Fe weld. Met. Mater. Int. 2014, 20, 129–133. [Google Scholar] [CrossRef]
- Mintz, B.; Cowley, A.; Talian, C.; Crowther, D.N.; Abushosha, R. Influence of P on the hot ductility of high C, Al, and Nb containing steels. Mater. Sci. Technol. 2003, 19, 184–188. [Google Scholar] [CrossRef]
- Comineli, O.; Qaban, A.; Mintz, B. Influence of Cu and Ni on the hot ductility of low C steels with respect to the straightening operation when continuous casting. Metals 2022, 12, 1671. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, X.M.; Song, S.H.; Shangguan, Y.J. Phosphorus-induced hot ductility enhancement of 1Cr-0.5Mo low alloy steel. Mater. Sci. Eng. A 2013, 574, 46–53. [Google Scholar] [CrossRef]
- Prasad, R. Influences of the formation of cast iron with nodular graphite using magnesium treatment processes. Inter. Res. J. Eng. Technol. 2022, 9, 489–493. Available online: https://www.irjet.net/archives/V9/i5/IRJET-V9I594.pdf (accessed on 13 December 2023).
- Wright, R.N.; Farrell, T.R. Elevated temperature brittleness of ferritic ductile iron. Trans. Am. Foundrymen’s Soc. 1985, 93, 853–866. [Google Scholar]
- González-Martínez, R.; Sertucha, J.; Lacaze, J. The mechanism of intermediate temperature embrittlement of cast irons by magnesium. Mater. Today Commun. 2023, 35, 106128. [Google Scholar] [CrossRef]
- Kobayashi, T.; Nishino, K.; Kimoto, J.; Awano, Y.; Hibino, Y.; Ueno, H. 673K embrittlement of ferritic spheroidal graphite cast iron by magnesium. J. Jpn. Foundry Eng. Soc. 1998, 70, 273–278. [Google Scholar]
- Iwabuchi, Y.; Kobayashi, I. Suppression of elevated temperature brittleness in spheroidal graphite cast iron by increasing phosphorus content. Key Eng. Mater. 2011, 457, 428–432. [Google Scholar] [CrossRef]
- Chen, S.F.; Lui, T.S.; Chen, L.H. The effect of phosphorus segregation on the intermediate-temperature embrittlement of ferritic, spheroidal graphite cast iron. Metall. Mater. Trans. A 1994, 25, 557–561. Available online: https://link.springer.com/article/10.1007/BF02651597 (accessed on 13 December 2023). [CrossRef]
- Yanagisawa, O.; Ishii, H.; Matsugi, K.; Hatayama, T. 673K embrittlement of ferritic spheroidal graphite cast iron with low residual magnesium content. J. Jpn. Foundry Eng. Soc. 2000, 72, 604–609. [Google Scholar] [CrossRef]
- Yanisawa, O.; Lui, T.S.; Lin, H. Influence of structure on the 673 K embrittlement of ferritic ductile iron. Trans. Jpn. Inst. Metals 1983, 24, 858–867. [Google Scholar] [CrossRef]
- Oikawa, H. Lattice diffusion in iron—A review. Tetsu Hagané 1982, 68, 1489–1497. [Google Scholar] [CrossRef] [PubMed]
- Seah, M.P. Adsorption-induced interface decohesion. Acta Metall. 1980, 28, 955–962. [Google Scholar] [CrossRef]
- Okada, H.; Kanno, M. Hot ductility of Al-Mg and Al-Mg-Y alloys impaired by trace sodium. Scripta Mater. 1997, 37, 781–786. [Google Scholar] [CrossRef]
- Horikawa, K.; Kuramoto, S.; Kanno, M. High temperature embrittlement caused by traces of calcium or strontium in an Al-5.5mol%Mg alloy. Scr. Mater. 1998, 37, 861–866. [Google Scholar] [CrossRef]
- Horikawa, K.; Kuramoto, S.; Kanno, M. Sources of a trace amount of sodium, and its effect on hot ductility of an Al-5 mass%Mg alloy. Light Metals Rev. 2000, 7, 18–23. [Google Scholar]
- Aluminum: Properties and Physical Metallurgy; Hatch, J.E., Ed.; American Society for Metals: Metals Park, OH, USA, 1984. [Google Scholar]
- Turner, M.; Yeomans, S.; Ahmed, N. Forum on “Aluminium in Ships”; Aluminium Development Council (Manuka, Australia): Melbourne, Australia, 1995. [Google Scholar]
- Sampath, D.; Moldenhaus, S.; Schipper, H.R.; Schrijvers, A.J.; Haszler, A.; Weber, G.; Mechsner, K.; Tack, L. Development of advanced ship building materials. In Proceedings of the 6th ICAA, Toyohashi, Japan, 5–10 July 1998; Japan Institute of Light Metals: Tokyo, Japan, 2009. [Google Scholar]
- Godlewski, L.A.; Su, X.; Pollock, T.; Allison, J.E. The effect of aging on the relaxation of residual stress in cast aluminum. Met. Mat. Trans. A 2013, 44, 4809–4818. Available online: https://link.springer.com/article/10.1007/s11661-013-1800-1 (accessed on 13 December 2023). [CrossRef]
- Zhang, S.; Han, Q.; Liu, Z.-K. Fundamental understanding of Na-induced high temperature embrittlement in Al-Mg alloys. Phil. Mag. 2007, 87, 147–157. [Google Scholar] [CrossRef]
- Deschamps, A.; Péron, S.; Bréchet, Y.; Ehrström, J.-C.; Poizat, L. High Temperature, high strain rate embrittlement of Al-Mg-Mn alloy: Evidence of cleavage of an fcc alloy. Mater. Sci. Technol. 2002, 18, 1085–1091. [Google Scholar] [CrossRef]
- Ramsley, C.E.; Talbot, D.E.J. The embrittlement of aluminium-magnesium alloys by sodium. J. Inst. Metals 1959, 88, 150–158. [Google Scholar]
- Scamans, G.M.; Alani, R.; Swann, P.R. Pre-exposure embrittlement and stress corrosion failure in Al-Zn-Mg alloys. Corros. Sci. 1976, 16, 443–459. [Google Scholar] [CrossRef]
- Tuck, C.D.S. The embrittlement of Al-Zn-Mg and Al-Mg alloys by water vapor. Metall. Trans. A 1985, 16, 1503–1514. Available online: https://link.springer.com/article/10.1007/BF02658682 (accessed on 13 December 2023). [CrossRef]
- Horikawa, K.; Kuramoto, S.; Kanno, M. Intergranular fracture caused by trace impurities in an Al-5.5 mol% Mg alloy. Acta Mater. 2001, 49, 3981–3989. [Google Scholar] [CrossRef]
- Kim, S.-J.; Ryu, K.M.; Oh, M.-S. Addition of cerium and yttrium to ferritic steel weld metal to improve hydrogen trapping efficiency. Int. J. Miner. Metall. Mater. 2017, 24, 415–422. Available online: https://link.springer.com/article/10.1007/s12613-017-1422-5 (accessed on 13 December 2023). [CrossRef]
- Talbot, D.E.J.; Granger, D.A. Effects of sodium and bismuth in aluminum-magnesium alloys. JOM 1995, 47, 44–46. Available online: https://link.springer.com/article/10.1007/BF03221407 (accessed on 13 December 2023). [CrossRef]
- Ueda, K.; Horikawa, K.; Kanno, M. Suppression of high temperature embrittlement of Al-5%Mg alloys containing a trace of sodium caused by antimony addition. Scr. Mater. 1997, 37, 1105–1110. [Google Scholar] [CrossRef]
- Lynch, S.P. Comments on “Intergranular fracture caused by trace impurities in an Al-5.5 mol% Mg alloy. Scr. Mater. 2002, 47, 125–129. [Google Scholar] [CrossRef]
- Suzuki, S.; Lejeck, P.; Hofmann, S. Effect of metallurgical factors on grain boundary segregation of solute atoms in iron. Mater. Trans. JIM 1999, 40, 463–473. [Google Scholar] [CrossRef][Green Version]
- Hammad, A.-H.M.; Ramadan, K.K. Mechanical properties of Al-Mg alloys at elevated temperatures. Int. J. Mater. Res. 1989, 80, 178–185. [Google Scholar] [CrossRef]
- Lin, Y.C.; Dong, W.Y.; Zhu, X.H.; Wu, Q.; He, Y.J. Deformation behavior and precipitation features in a stretched Al-Cu alloy at intermediate temperatures. Materials 2020, 13, 2495. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wu, Y.; Gong, H.; Li, S.; Ahmad, A.S. A physically based constitutive model and continuous dynamic recrystallisation behavior analysis of 2219 aluminum alloy during hot deformation process. Materials 2018, 11, 1443. [Google Scholar] [CrossRef] [PubMed]
- Lui, L.; Wu, Y.; Gong, H.; Dong, F.; Ahmad, A.S. Modified kinetics model for describing continuous dynamic recrystallisation behavior of Al 2219 alloy during hot deformation process. J. Alloys Compd. 2020, 817, 153301. [Google Scholar] [CrossRef]
- Otsuka, M.; Horiuchi, R. Ductility loss of Al-Mg alloys at high temperature. J. Jpn. Inst. Met. 1981, 48, 688–693. [Google Scholar] [CrossRef][Green Version]
- Gavin, S.A.; Billingham, J.; Chubb, J.P.; Hancock, P. Effect of trace impurities on hot ductility of as-cast cupronickel alloys. Metals Technol. 1978, 5, 397–401. [Google Scholar] [CrossRef]
- Chubb, J.P.; Billingham, J. Effect of Ni on hot ductility of binary copper-nickel alloys. Metals Technol. 1978, 5, 100–103. [Google Scholar] [CrossRef]
- White, C.L.; Schneibel, J.H.; Padgett, R.A. High temperature embrittlement of Ni and Ni-Cr alloys by trace elements. Metall. Trans. A 1983, 14, 595–610. Available online: https://link.springer.com/article/10.1007/BF02643776 (accessed on 13 December 2023). [CrossRef]
- Zheng, L.; Chellali, R.; Schlesiger, R.; Baither, D.; Schmitz, G. Intermediate temperature embrittlement in high-purity Ni and binary Ni(Bi) alloy. Scr. Mater. 2011, 65, 428–431. [Google Scholar] [CrossRef]
- Hu, R.; Zhan, J.; Yang, C.; Du, J.; Luo, X.; Bi, Z.; Gan, B. Temperature effects on the deformation mechanisms in a Ni-Co-based superalloys. Crystals 2022, 12, 1409. [Google Scholar] [CrossRef]
- Zheng, L.; Schmitz, G.; Meng, Y.; Chellali, R.; Schlesiger, R. Mechanism of Intermediate Temperature Embrittlement of Ni and Ni-based Superalloys. Crit. Rev. Solid State Mater. Sci. 2012, 37, 181–214. [Google Scholar] [CrossRef]
- Lozinskiy, M.G.; Volkogon, G.M.; Pertsovskiy, N.Z. Investigation of the influence of zirconium additions on the ductility and deformation structure of nickel over wide temperature range. Russ. Met. 1967, 5, 65–72. Available online: https://jglobal.jst.go.jp/en/detail?JGLOBAL_ID=201602015193930837 (accessed on 13 December 2023).
- Christien, F. Role of impurity sulphur in the ductility trough of austenitic iron-nickel alloys. Materials 2020, 13, 539. [Google Scholar] [CrossRef] [PubMed]
- Bricknell, R.H.; Woodford, D.A. The embrittlement of nickel following high temperature air exposure. Metal. Trans. A 1981, 12, 425–433. Available online: https://link.springer.com/article/10.1007/BF02648539 (accessed on 13 December 2023). [CrossRef]
- Lu, X.; Ma, Y.; Wang, D. On the hydrogen embrittlement behavior of nickel-based alloys: Alloys 718 and 725. Mater. Sci. Eng. A 2020, 792, 139785. [Google Scholar] [CrossRef]
- Iwasaki, H.; Kariya, R.; Mabuchi, M.; Tagata, T.; Higashi, K. Effects of temperature and strain rate on elongation at elevated temperature in Al - 4.5Mg alloy. Mater. Trans. 2001, 42, 1771–1776. [Google Scholar] [CrossRef]
- Ebner, A.S.; Jakob, S.; Clemens, H.; Pippan, R.; Maier-Kiener, V.; He, S.; Ecker, W.; Scheiber, D.; Razumovskiy, V.I. Grain boundary segregation in Ni-base alloys: A combined atom probe tomography and first principles study. Acta Mater. 2021, 221, 117354. [Google Scholar] [CrossRef]
- Saha, M. Grain boundary segregation in steels: Towards engineering the design of internal interfaces. arXiv, 2022; arXiv:arXiv:2022020096. [Google Scholar] [CrossRef]
- Rajagopalan, M.; Tschopp, M.A.; Solanki, K.N. Grain boundary segregation of interstitial and substitutional impurity atoms in alpha-iron. JOM 2014, 66, 129–138. [Google Scholar] [CrossRef]
- Lejcek, P. Grain Boundary Segregation in Metals; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Lejcek, P.; Sob, M.; Paidar, V. Interfacial segregation and grain boundary embrittlement: An overview and critical assessment of experimental data and calculated results. Prog. Mater. Sci. 2017, 87, 83–139. [Google Scholar] [CrossRef]
- He, T.; Qi, Y.; Ji, Y.; Feng, M. Grain boundary segregation-induced strengthening-weakening transition and its ideal maximum strength in nanopolycrystalline FeNiCrCoCu high-entropy alloys. Int. J. Mech. Sci. 2023, 238, 107828. [Google Scholar] [CrossRef]
- Han, L.; Xu, X.; Li, Z.; Liu, B.; Liu, C.T.; Liu, Y. A novel equiaxed eutectic high-entropy alloy with excellent mechanical properties at elevated temperatures. Mater. Res. Lett. 2020, 8, 373–382. [Google Scholar] [CrossRef]
- Feng, C.; Wang, X.; Yang, L.; Guo, Y.; Wang, Y. High hardness and wear resistance in AlCrFeNiV high-entropy alloy induced by dual-phase body-centered cubic coupling effects. Materials 2022, 15, 6896. [Google Scholar] [CrossRef]
- Mintz, B.; Lewis, J.; Jonas, J.J. Importance of deformation induced ferrite and factors which control its formation. Mater. Sci. Technol. 1997, 13, 379–388. [Google Scholar] [CrossRef]
- Smallman, R.E.; Dillamore, I.L.; Dobson, P.S. The measurement of stacking fault energy. J. Phys. Colloques 1966, 27, C3-86–C3-93. Available online: https://hal.science/jpa-00213120/document (accessed on 13 December 2023). [CrossRef]
- Ratanaphan, S.; Olmsted, D.L.; Bulatov, V.V.; Holm, E.A.; Rollett, A.D.; Rohrer, G.S. Grain boundary energies in body-centred cubic metals. Acta Mater. 2015, 88, 346–354. [Google Scholar] [CrossRef]
- Lui, Y.; Liu, M.; Chen, X.; Cao, Y.; Roven, H.J.; Murashkin, M.; Valiev, R.Z.; Zhou, H. Effect of Mg on microstructure and mechanical properties of Al-Mg alloys produced by high pressure torsion. Scr. Mater. 2019, 159, 137–141. [Google Scholar] [CrossRef]
- Muzyk, M.; Pakiela, Z.; Kurzydlowski, K.J. Ab initio calculations of the generalized stacking fault energy in aluminum alloys. Scr. Mater. 2011, 64, 916–918. [Google Scholar] [CrossRef]
- Morishige, T.; Hirata, T.; Uesugi, T.; Takigawa, Y.; Tsujikawa, M.; Higashi, K. Effect of Mg content on the minimum grain size of Al-Mg alloys obtained by friction stir processing. Scr. Mater. 2011, 64, 355–358. [Google Scholar] [CrossRef]
- Schulthess, T.C.; Turchi, P.E.A.; Gonis, A.; Nieh, T.-G. Systematic study of stacking fault energies of random Al-based alloys. Acta Mater. 1998, 46, 2215–2221. [Google Scholar] [CrossRef]
- Igwemezie, V.C.; Ugwuegbu, C.C.; Mark, U. Physical metallurgy of modern creep-resistant steel for steam power plants: Microstructure and phase transformations. J. Metall. 2016, 2016, 5468292. [Google Scholar] [CrossRef]
- Li, Z.; Li, Z.; Tian, W. Strengthening effect of Nb on ferrite grain boundary in X70 pipeline steel. Materials 2021, 14, 61. [Google Scholar] [CrossRef] [PubMed]
- Muller, D.A.; Subramanian, S.; Sass, S.L.; Silcox, J.; Batson, P.E. Local electronic structure and cohesion of grain boundaries in Ni3Al. MRS Online Proc. Lib. 1994, 364, 743–748. [Google Scholar] [CrossRef]
- Özerinç, S.; Tai, K.; Vo, N.Q.; Bellon, P.; Averback, R.S.; King, W.P. Grain boundary doping strengthens nanocrystalline copper alloys. Scr. Mater. 2012, 67, 720–723. [Google Scholar] [CrossRef]
- Briant, C.L. Solid solubility and grain boundary segregation. Phil. Mag. Lett. 1996, 73, 345–350. [Google Scholar] [CrossRef]
- Messmer, R.P.; Briant, C.L. The role of chemical bonding in grain boundary embrittlement. Acta Metall. 1982, 30, 457–467. [Google Scholar] [CrossRef]
- Nako, H.; Taniguchi, G.; Zhu, S.-Q.; Ringer, S.P. Influence of grain boundary segregation on temper embrittlement of Cr-Mo heat resistant steel weld metal and quantitative analysis of the amount of segregated atoms. In Proceedings of the 5th International Symposium on Steel Science (ISSS 2017), Kyoto, Japan, 13–16 November 2017; Available online: https://www.isij.or.jp/publication/ISSS2017/data/isss2017-13.pdf (accessed on 13 December 2023).
- Briant, C.L.; Banerji, S.K. Intergranular failure in steel: The role of grain boundary composition. Int. Met. Rev. 1978, 23, 164–199. [Google Scholar] [CrossRef]
- Gittins, A.; Tegart, W.J.M. From Creep to Working—Reflections on Fracture Modes. Metals Forum. 1981, 4, 57–62. [Google Scholar]
- Roesler, J.; Harders, H.; Baeker, M. Mechanical Behaviour of Engineering Materials: Metals, Ceramics and Composites; Springer: Berlin, Germany, 2007; Available online: https://link.springer.com/book/10.1007/978-3-540-73448-2 (accessed on 13 December 2023).
- Olmsted, D.L.; Foiles, S.M.; Holm, E.A. Survey of computed grain boundary properties in face-centered cubic metals: I. Grain boundary energy. Acta Mater. 2009, 57, 3694–3703. [Google Scholar] [CrossRef]
- Gault, B.; Breen, A.J.; Chang, Y.; He, J.; Jägle, E.A.; Kontis, P.; Kürnsteiner, P.; Da Silva, A.K.; Makineni, S.K.; Mouton, I.; et al. Interfaces and defect composition at the near-atomic scale through atom probe tomography investigations. J. Mater. Res. 2018, 33, 4018–4030. [Google Scholar] [CrossRef]
- Pun, C.P.; Wang, W.; Khalajhedayati, A.; Schuler, T.D.; Trelewicz, J.R.; Rupert, T.J. Nanocrystalline Al-Mg with extreme strength due to grain boundary doping. Mater. Sci. Eng. A 2017, 696, 404–406. [Google Scholar] [CrossRef]
- Da Rosa, G.; Maugis, P.; Portavoce, A.; Drillet, J.; Valle, N.; Lentzen, E.; Hoummada, K. Grain-boundary segregation of boron in high-strength steel studied by nano-SIMS and atom probe tomography. Acta Mater. 2020, 182, 226–234. [Google Scholar] [CrossRef]
- Stoklosa, A.; Laskowska, B. Cohesion energy, metallic radius and bonding energy of electrons in the ionic core of metal atoms. Arch. Metall. Mater. 2005, 50, 783–801. Available online: http://yadda.icm.edu.pl/baztech/element/bwmeta1.element.baztech-article-BSW3-0017-0019 (accessed on 13 December 2023).
- Huang, Z.; Wang, P.; Chen, F.; Shen, Q.; Zhang, L. Understanding solute effect on grain boundary strength based on atomic size and electronic attraction. Sci. Rep. 2020, 10, 16856. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, F.; Shen, Q.; Zhang, L.; Rupert, T.J. Combined effects of nonmetallic impurities and planned metallic dopants on grain boundary energy and strength. Acta Mater. 2019, 166, 113–125. [Google Scholar] [CrossRef]
- Yu, J.; McMahon, C.J., Jr. The effects of composition and carbide precipitation on the temper embrittlement of 2.25 Cr-1 Mo steel: Part II. Effects of Mn and Si. Metall. Trans. A 1980, 11, 291–300. [Google Scholar] [CrossRef]
- Mechanical Metallurgy; Dieter, G.E., Ed.; McGraw-Hill Book Company: Singapore, 1988. [Google Scholar]
- Mulford, R.A.; McMahon, C.J.; Pope, D.P.; Feng, H.C. Temper Embrittlement of Ni-Cr steels by phosphorus. Met. Trans. A 1976, 7, 1183–1195. [Google Scholar] [CrossRef]
- Chandra, K.; Kain, V.; Srinivasan, N.; Samajdar, I.; Balasubrahmanian, A.K. Temper embrittlement and corrosion behaviour of martensitic stainless steel 420. Adv. Mater. Res. 2013, 794, 757–765. [Google Scholar] [CrossRef]
- Mintz, B.; Abushosha, R.; Jonas, J.J. Influence of dynamic recrystallization on the tensile ductility of steels in the temperature range 700 to 1150 °C. ISIJ Int. 1992, 32, 241–249. [Google Scholar] [CrossRef]





| Metal or Alloy | Melting Point (Tm) °C | Temp. Centre Hot-Ductility Trough °C | Ratio: Temperature at the Centre of Hot-Ductility Trough HD/Tm |
|---|---|---|---|
| Steel | 1540 | 900 | 0.6 |
| Nickel | 1455 | 800 | 0.5 |
| Copper | 1085 | 700 | 0.6 |
| Cast iron | 1050 | 350–500 | 0.4 |
| Aluminium | 660 | 250 | 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salas-Reyes, A.E.; Qaban, A.; Mintz, B. Comments on the Intermediate-Temperature Embrittlement of Metals and Alloys: The Conditions for Transgranular and Intergranular Failure. Metals 2024, 14, 270. https://doi.org/10.3390/met14030270
Salas-Reyes AE, Qaban A, Mintz B. Comments on the Intermediate-Temperature Embrittlement of Metals and Alloys: The Conditions for Transgranular and Intergranular Failure. Metals. 2024; 14(3):270. https://doi.org/10.3390/met14030270
Chicago/Turabian StyleSalas-Reyes, Antonio Enrique, Abdullah Qaban, and Barrie Mintz. 2024. "Comments on the Intermediate-Temperature Embrittlement of Metals and Alloys: The Conditions for Transgranular and Intergranular Failure" Metals 14, no. 3: 270. https://doi.org/10.3390/met14030270
APA StyleSalas-Reyes, A. E., Qaban, A., & Mintz, B. (2024). Comments on the Intermediate-Temperature Embrittlement of Metals and Alloys: The Conditions for Transgranular and Intergranular Failure. Metals, 14(3), 270. https://doi.org/10.3390/met14030270







