Phase Equilibrium Study of Rare Earth Oxide–Fluoride Salt System: A Review
Abstract
:1. Introduction
2. Methodology for the Phase Equilibrium Study of the RE2O3-REF3-LiF System
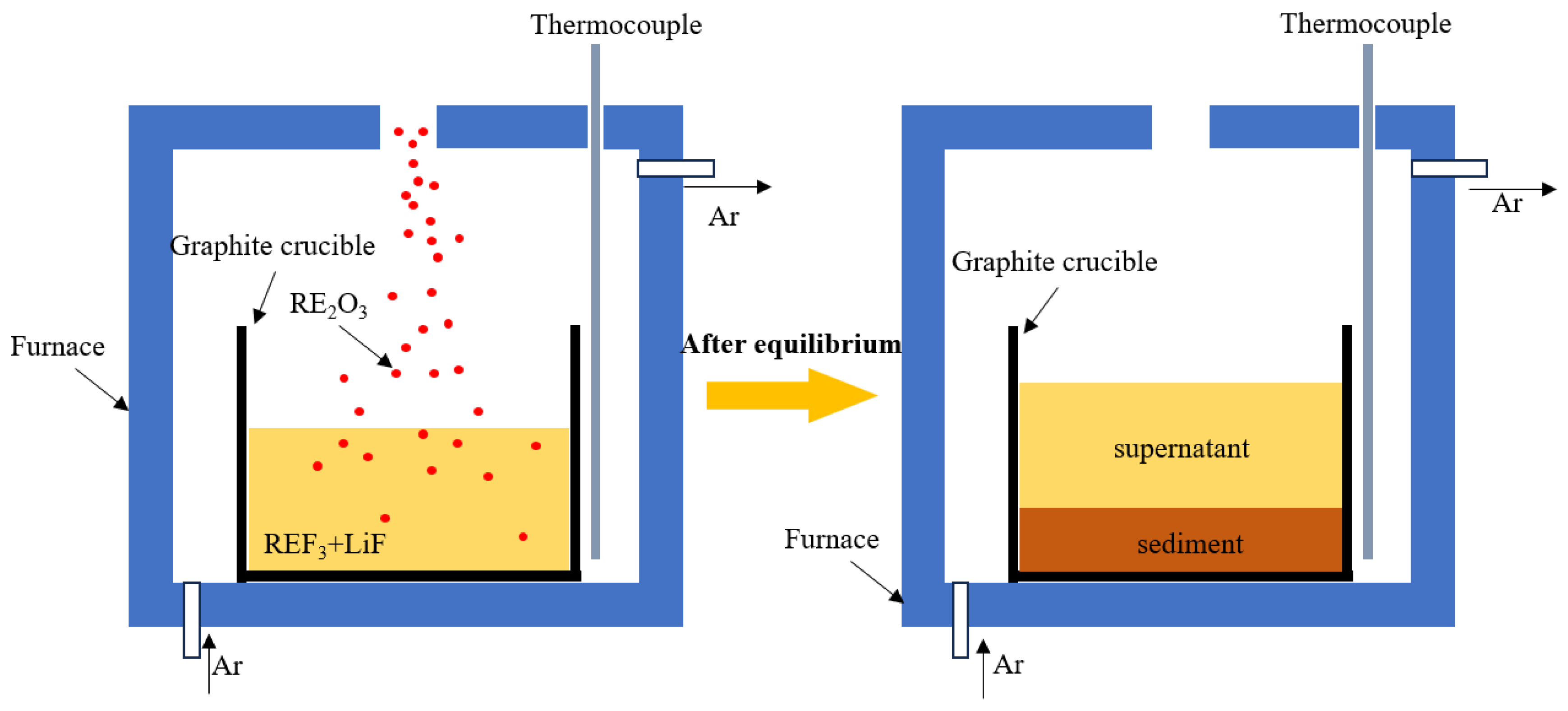
2.1. Isothermal Saturation Method
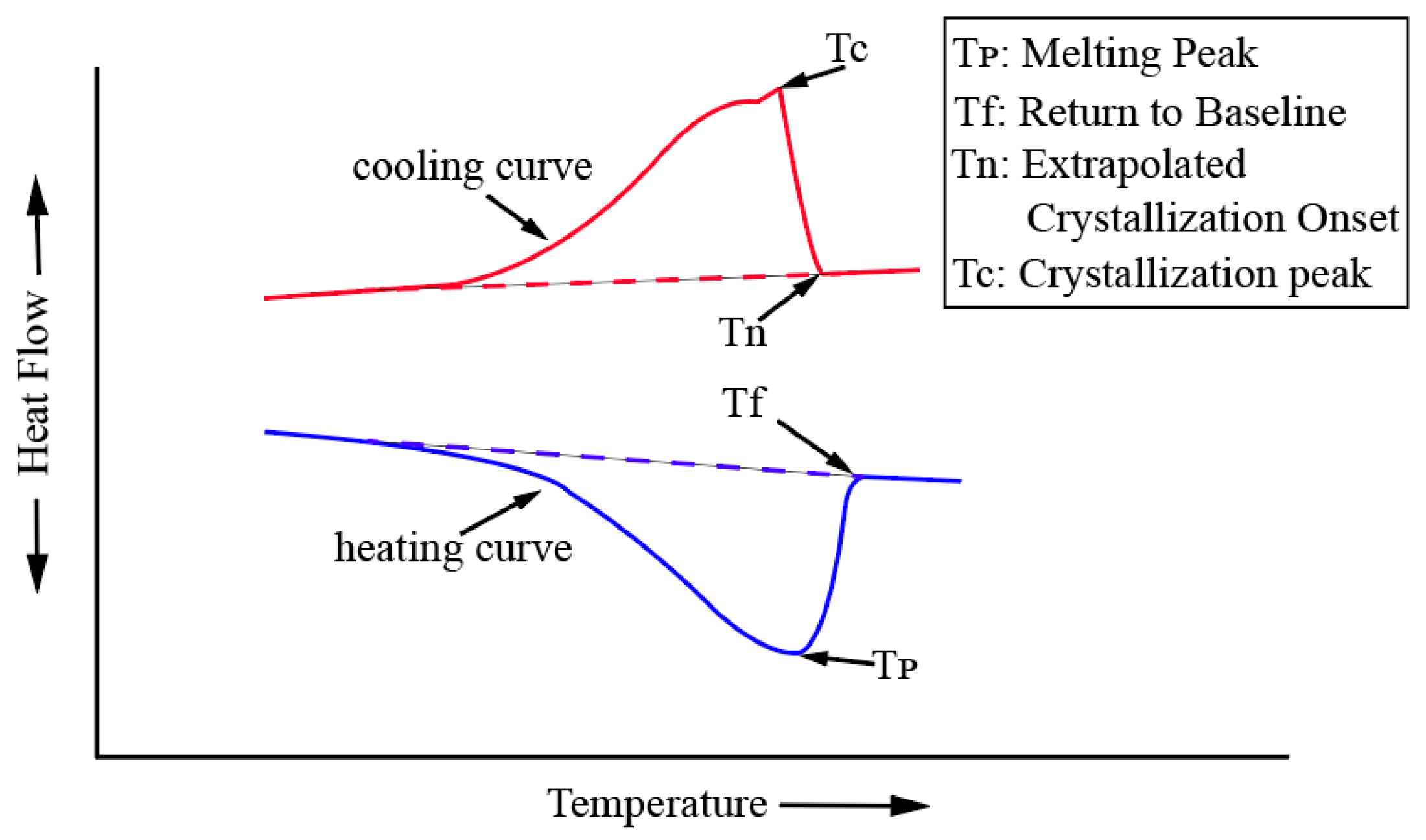
2.2. Primary Crystallization Temperature Method
2.3. Square Wave Voltammetry Method
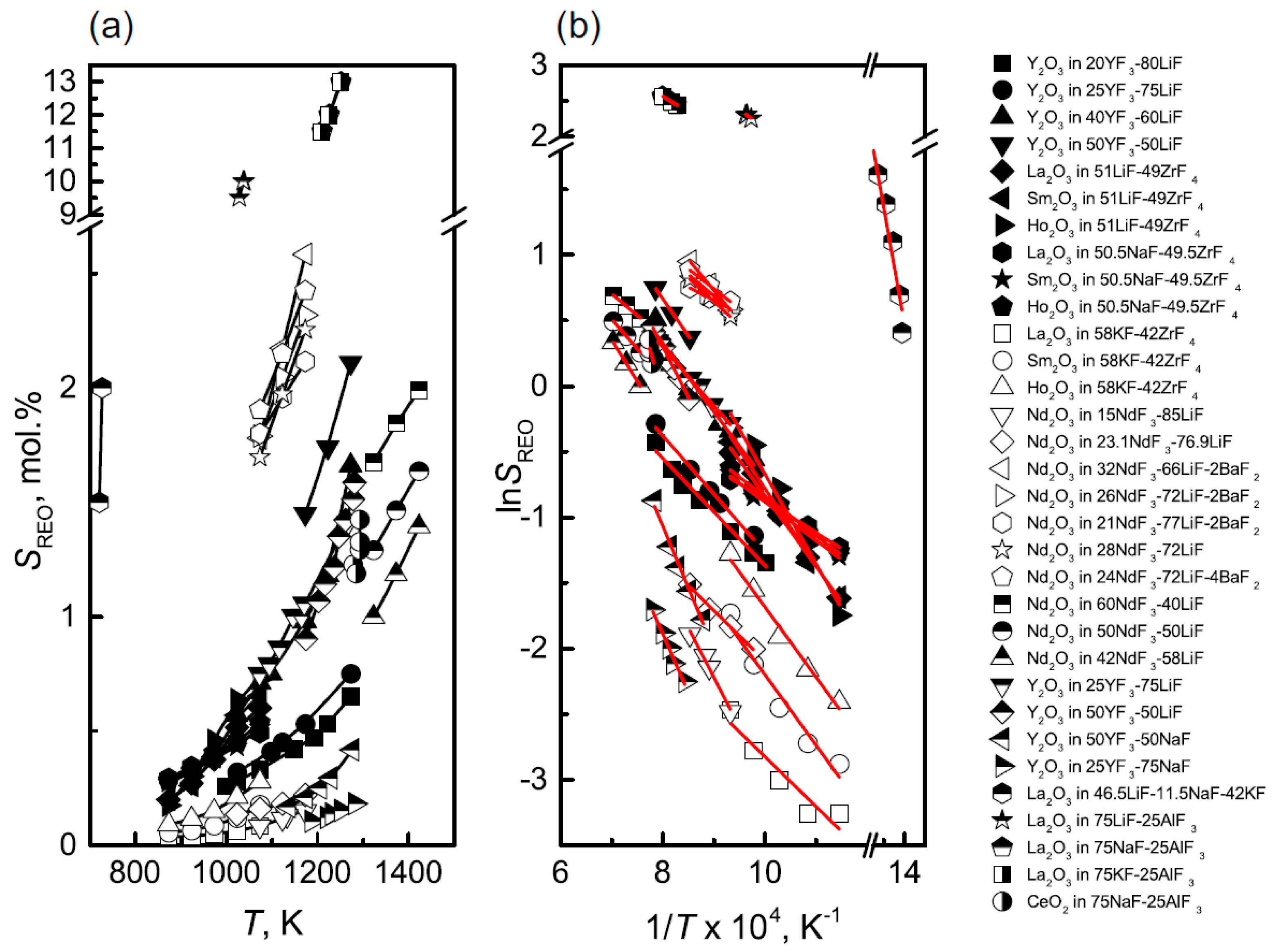
3. Phase Equilibrium Studies in the RE2O3-REF3-LiF System
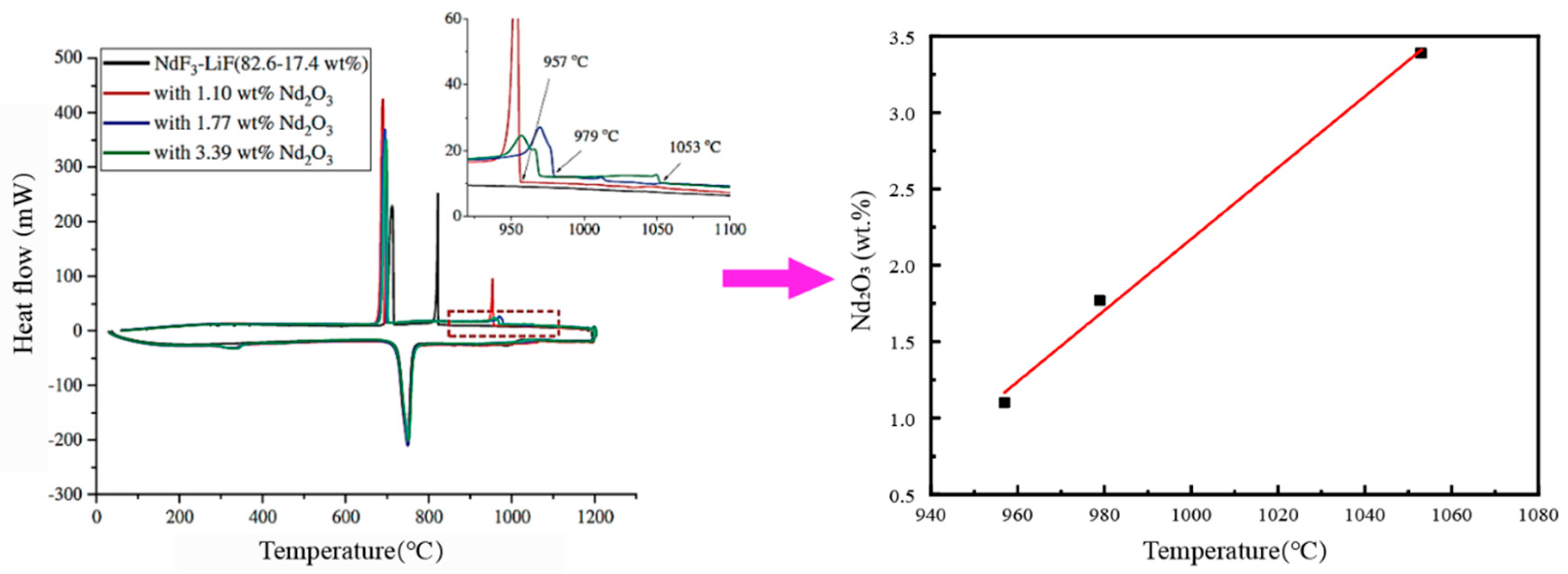
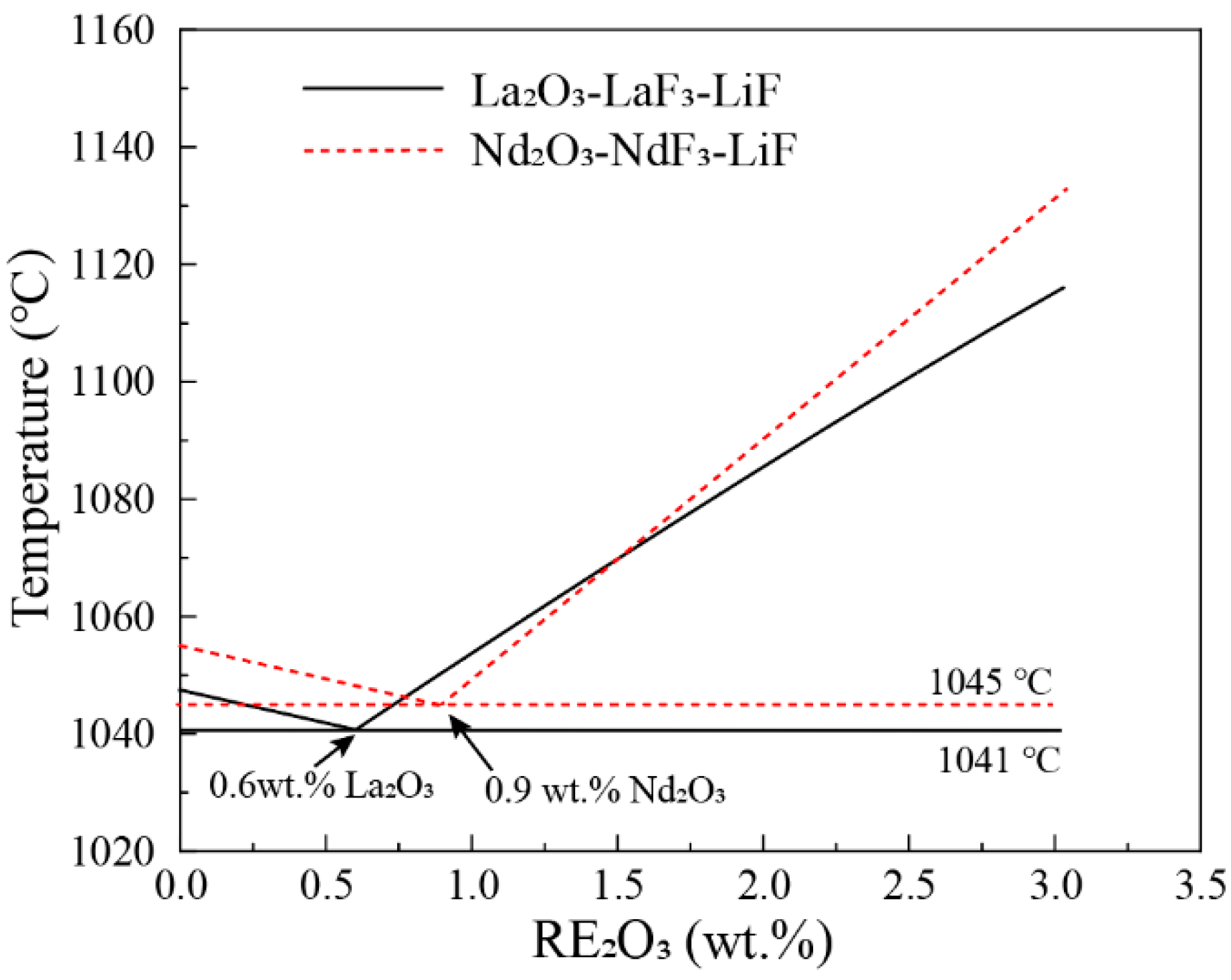
3.1. Nd2O3-NdF-LiF System
3.2. La2O3-LaF-LiF System
3.3. Y2O3-YF3-LiF System
3.4. Solubility Model of RE2O3-REF3-LiF System
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Balaram, V. Rare earth elements: A review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geosci. Front. 2019, 10, 1285. [Google Scholar] [CrossRef]
- Huang, X.W.; Li, H.W.; Wang, C.F.; Wang, G.Z.; Xue, X.X.; Zhang, G.C. Development Status and Research Progress in Rare Earth Industry in China. Chin. J. Rare Met. 2007, 31, 279–288. [Google Scholar]
- Baranovskaya, B.V.; Karpov, Y.A.; Korotkova, N.A. Actual trends in the application of rare-earth metals and their compounds in the production of magnetic and lumi-nescent materials: A review. Russ. J. Non-Ferr. Met. 2021, 62, 10. [Google Scholar] [CrossRef]
- Cheisson, T.; Schelter, E.J. Rare earth elements: Mendeleev’s bane, modern marvels. Science 2019, 363, 489–493. [Google Scholar] [CrossRef]
- Lima, I.B.D. Chapter 19—Rare Earths Industry and Eco-management: A Critical Review of Recycling and Substitutes. In Rare Earths Industry; Elsevier: Amsterdam, The Netherlands, 2015; pp. 293–304. [Google Scholar]
- Lima, I.B.D.; Filho, W.L. Highlights on Rare Earths. In Rare Earths Industry; Elsevier: Amsterdam, The Netherlands, 2016; pp. 395–424. [Google Scholar]
- Fernandez, V. Rare-earth elements market: A historical and financial perspective. Resour. Policy 2017, 53, 26–45. [Google Scholar] [CrossRef]
- Zepf, V. An Overview of the Usefulness and Strategic Value of Rare Earth Metals. In Rare Earths Industry; Elsevier: Amsterdam, The Netherlands, 2015; pp. 3–17. [Google Scholar]
- Balachandran, G. Extraction of Rare Earths for Advanced Applications, 3, Treatise on Process Metallurgy; Elsevier: Washington, DC, USA, 2014; p. 1291. [Google Scholar]
- Liu, T.C.; Chen, J. Extraction and separation of heavy rare earth elements: A review. May Separ. Purif. Technol. 2021, 276, 119263. [Google Scholar] [CrossRef]
- Traore, M.; Gong, A.; Wang, Y.; Qiu, L.; Bai, Y.; Zhao, W.; Liu, Y.; Chen, Y.; Liu, Y.; Wu, H.; et al. Research progress of rare earth separation methods and technologies. J. Rare Earths 2023, 41, 182–189. [Google Scholar] [CrossRef]
- Wang, W.; Liu, L.; Liu, H.Z.; Zhang, B.; Cao, Y.H.; Wang, H.L. Progress and trend of rare earth extraction technology. Conserv. Util. Miner. Resour. 2020, 40, 32. [Google Scholar]
- Yin, T.Q.; Xue, Y.; Yan, Y.D.; Ma, Z.C.; Ma, F.Q.; Zhang, M.L.; Wang, G.L.; Qiu, M. Recovery and separation of rare earth elements by molten salt electrolysis. Int. J. Miner. Metall. Mater. 2021, 28, 899–913. [Google Scholar] [CrossRef]
- Chen, Y.X. Research Progress of Preparation of Rare Earth Metals by Electrolysis in Fluoride Salt System. Chin. Rare Earth 2014, 35, 99–107. [Google Scholar]
- Zhu, H.M. Rare Earth Metal Production by Molten Salt Electrolysis. In Encyclopedia of Applied Electrochemistry; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1765–1772. [Google Scholar]
- Han, W.; Li, M.; Zhang, M.L.; Yan, Y.D. Progress in preparation of rare earth metals and alloys by electrodeposition in molten salts. Rare Met. 2016, 35, 811–825. [Google Scholar] [CrossRef]
- Sarfo, P.; Das, A.; Young, C. Extraction and optimization of neodymium from molten fluoride electrolysis. Sep. Purif. Technol. 2021, 256, 117770. [Google Scholar] [CrossRef]
- Pang, S.; Yan, S.; Li, Z.; Chen, D.; Xu, L.; Zhao, B. Development on Molten Salt Electrolytic Methods and Technology for Preparing Rare Earth Metals and Alloys in China. Chin. J. Rare Met. 2011, 35, 440–450. [Google Scholar]
- Liu, H.; Zhang, Y.; Luan, Y.; Yu, H.; Li, D. Research Progress on Preparation of Rare Earth Metals by Molten Salt Electrolysis. Chin. Rare Earths 2021, 42, 133–143. [Google Scholar]
- Liu, Y.B.; Chen, G.H.; Yu, B.; Chen, X. Research Progress of Tapping Technology for Molten Salt Electrolytic Preparation of Rare Earth Metals. Chin. Rare Earths 2018, 39, 134–140. [Google Scholar]
- Wu, Y.F.; Ma, S.Y.; Wang, Z.F.; Bian, X.; Liu, Z.X.; Liu, Y.L. Formation Mechanism and countermeasures of nodules at furnace bottom of 15kA rare earth molten salt electrilysis cell. China Nonferrous Metall. 2023, 52, 81–87. [Google Scholar]
- Ji, Y.Z.; Xiao, F.X.; Sun, S.C.; Chen, J.Q.; Tu, G.F. Research Progress on Model Cell of High Current Rare Earth Reduction Cell. J. Chin. Soc. Rare Earth 2022, 40, 38–45. [Google Scholar]
- Guo, X.L.; Sun, Z.; Sietsma, J.; Blanpain, B.; Guo, M.X.; Yang, Y. Quantitative Study on Dissolution Behavior of Nd2O3 in Fluoride Melts. Ind. Eng. Chem. Res. 2018, 57, 1380–1388. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.L.; Sietsma, J.; Yang, Y.X. Chapter 15-A Critical Evaluation of Solubility of Rare Earth Oxides in Molten Fluorides. In Rare Earths Industry; Elsevier: Amsterdam, The Netherlands, 2015; pp. 223–234. [Google Scholar]
- Guo, X.L.; Sun, Z.; Sietsma, J.; Yang, Y.X. Semiempirical Model for the Solubility of Rare Earth Oxides in Molten Fluorides. Ind. Eng. Chem. Res. 2016, 55, 4773–4781. [Google Scholar] [CrossRef]
- Zhao, J.C. (Ed.) Methods for Phase Diagram Determination; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Thoma, R.E. Phase Diagrams of Binary and Ternary Fluoride Systems. Adv. Molten Salt Chem. 1975, 3, 275–455. [Google Scholar]
- Xue, J.Q.; Liu, Z.F.; Zhang, J.; Tang, C.B.; Bi, Q. Determination and Calculation of NdF3-LiF Binary System. Chin. J. Rare Met. 2014, 38, 832–838. [Google Scholar]
- Baek, S.; Jung, I.H. Phase diagram study and thermodynamic assessment of the Y2O3-YF3 system. J. Eur. Ceram. Soc. 2022, 42, 5079–5092. [Google Scholar] [CrossRef]
- Baek, S.; Jung, I.H. Phase diagram study and thermodynamic modeling of the MgO-Y2O3-MgF2-YF3 system. J. Eur. Ceram. Soc. 2023, 43, 1723–1734. [Google Scholar] [CrossRef]
- Kang, Z.; He, M.; Lu, G. Thermodynamic evaluation and optimization of (LiF + ReF3)(Re = La, Nd, Gd, Yb, Y, Lu) binary systems. Calphad 2023, 83, 102612. [Google Scholar] [CrossRef]
- Liao, C.; Fu, Z.; Que, L.; Tang, H.; Wang, X. Thermodynamic Analysis and Phase Diagram Calculation of LiF–NdF3 Based on Factsage. Soc. Sci. Res. 2022, preprints. [Google Scholar] [CrossRef]
- Kang, Z.; Robelin, C.; He, M.; Chartrand, P. Thermodynamic evaluation and optimization of the (KF + YF3), (KCl + YCl3) and (YF3 + YbF3) binary systems. J. Chem. Thermodyn. 2016, 98, 242–253. [Google Scholar] [CrossRef]
- Wu, W.Y.; Sun, J.Z.; Hai, L.; Gao, H.J. Solubility of Nd2O3 in Fluorine Salt System. Chin. Rare Rare Earths 1991, 3, 34–37. [Google Scholar]
- Stefanidaki, E.; Photiadis, G.M.; Kontoyannis, C.G.; Vik, A.F.; Østvold, T. Oxide solubility and Raman spectra of NdF3-LiF-KF-MgF2-Nd2O3 melts. J. Chem. Soc. Dalton Trans. 2002, 11, 2302–2307. [Google Scholar] [CrossRef]
- Zhu, X.P.; Sun, S.C.; Liu, C.; Tu, G.F. Solubility of RE2O3 (RE = La and Nd) in light rare earth fluoride molten salts. J. Rare Earths 2018, 36, 765–771. [Google Scholar] [CrossRef]
- Zhang, L.J. Study on Determination Method of Rare Earth Oxide Solubility in Rare Earth Molten Salt. Ph.D. Thesis, Northeastern University, Shenyang, China, 2018. [Google Scholar]
- Liu, K.R. Study on Molten Salt Properties and Electrode Process of Neodymium Electrolysis. Ph.D. Thesis, Northeastern University, Shenyang, China, 2001. [Google Scholar]
- Hu, X.W. Research and Application of Molten Salt Structure of NdF3-LiF-Nd2O3 Series. Ph.D. Thesis, Northeastern University, Shenyang, China, 2009. [Google Scholar]
- Liang, X.; Chen, S.; Hong, K.; Chen, D.; Li, Z.; Lai, Y.; Xu, J. Solubility of La2O3 in LaF3-LiF molten salt system. J. Chin. Soc. Rare Earths 2021, 39, 594–600. [Google Scholar]
- Xu, M.X. Study on Physicochemical Properties of Molten Salts of LaF3-LiF-La2O3 Series. Ph.D. Thesis, Northeastern University, Boston, MA, USA, 2014. [Google Scholar]
- Zhu, X.P. Research of Physical and Chemical Properties of LaF3-LiF-La2O3 Molten Salt System. Master’s Thesis, Northeastern University, Shenyang, China, 2012. [Google Scholar]
- Cai, B.Q. Preparation of Yb-Ni Master Alloy by Electrolysis of LiF-YbF3-Yb2O3 Molten Salt System and Its Mechanism. Ph.D. Thesis, Jiangxi University of Science and Technology, Ganzhou, China, 2022. [Google Scholar]
- Murphy, J.E.; Dysinger, D.K.; Chambers, M.F. Electrowinning Neodymium Metal from Chloride and Oxide–Fluoride Electrolytes. In Proceedings of the Technical Sessions Presented by the TMS Light Metals Committee at the 123rd TMS Annual Meeting, San Francisco, CA, USA, 1 January 1994; p. 137. [Google Scholar]
- Chen, J.S. Electrolytic Molten Salt Properties and Electrode Process of Rare Earth Oxides in Cheap Fluorine Salt System. Ph.D. Thesis, Northeastern University, Shenyang, China, 1999. [Google Scholar]
- Xiong, Z.W.; Liu, A.M.; Liu, Y.B. Dissolution Behavior of La2O3 in LiF-NaF Molten Salt System. J. Mater. Metall. 2022, 21, 167–171, 183. [Google Scholar]
- Wang, X.; Zeng, J.Y.; Cai, B.Q. Solubility of Y2O3 in NaF-AlF3-LiF molten salt system. Rare Met. Cem. Carbides 2020, 48, 29–32. [Google Scholar]
- Reddy, R.G.; Kumar, S.G. Solubility and thermodynamic properties of Y2O3 in LiF-YF3 melts. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 1994, 25, 91–96. [Google Scholar] [CrossRef]
- Liao, C.F.; Sun, Q.C.; Wang, X. Study on Solubility of Yb2O3 in LiF-CaF2 Molten Salt. J. Chin. Soc. Rare Earths 2019, 37, 99–104. [Google Scholar]
- Du, S.L.; Wu, M.H.; Du, F.Y.; Liu, Y.M. Solubility of Rare Earth Oxides in Alkali Metal and Alkaline Earth Metal Fluoride Molten Salts. Chin. Rare Earths 1987, 2, 59–62. [Google Scholar]
- Porter, B.; Brown, E.A. Determination of Oxide Solubility in Molten Fluorides; U. S. Dept. of the Interior; Bureau of Mines: Washington, DC, USA, 1961.
- Wu, W.Y.; Zhang, J.S. Solubility of Rare Earth Oxide in LiF-BaF2-REF3 System. Non-Ferr. Min. Metall. 2000, 6, 34–36. [Google Scholar]
- Pshenichny, R.N.; Omelchuk, A.A. Interaction of rare-earth oxides with binary molten mixtures of zirconium and alkali metal fluorides. Russ. J. Inorg. Chem. 2012, 57, 115–119. [Google Scholar] [CrossRef]
- Ambrová, M.; Jurišová, J.; Danielik, V.; Gabčová, J. On the solubility of lanthanum oxide in molten alkali fluorides. J. Therm. Anal. Calorim. 2008, 91, 569–573. [Google Scholar] [CrossRef]
- Liu, F.Y. Fundamental Electrochemical Study on Neodymium Molten Salt Electrolysis in Fluoride Bath. Ph.D. Thesis, The Faculty and the Board of Trustees of the Colorado School of Mines, St. Golden, CO, USA, 2019. [Google Scholar]
- Zhu, X.P. The study on Physicochemical Properties of REF3-LiF-RE2O3 Molten Salt. Ph.D. Thesis, Northeastern University, Shenyang, China, 2012. [Google Scholar]
- Chen, L.Y.; Liu, S.Z.; Li, B. Study on Solubility and Dissolution Rate of Nd2O3 in NdF3-LiF Molten Salt by Square Wave Voltammetry. Chin. J. Rare Met. 2016, 40, 225–243. [Google Scholar]
- Keller, R.; Larimer, K.T. Electrolysis of Neodymium Oxide. Final Report for the Period August 19, 1991 through February 28, 1997; EMEC Consultants: Export, PA, USA, 1997. [Google Scholar]
- Abbasalizadeh, A.; Teng, L.; Seetharaman, S.; Sietsma, J.; Yang, Y. Rare Earth Extraction from NdFeB Magnets and Rare Earth Oxides Using Aluminum Chloride/Fluoride Molten Salts. Rare Earths Ind. Technol. Econ. Environ. Implic. 2016, 357–373. [Google Scholar]
- Yang, Y.; Walton, A.; Sheridan, R.; Güth, K.; Gauß, R.; Gutfleisch, O.; Buchert, M.; Steenari, B.M.; Gerven, V.T.; Jones, P.T.; et al. REE Recovery from End-of-Life NdFeB Permanent Magnet Scrap: A Critical Review. J. Sustain. Metall. 2017, 3, 122–149. [Google Scholar] [CrossRef]
- Remazeilles, C.; Gibilaro, M.; Massot, L.; Chiron, C.; Martinez, A.M.; Chamelot, P. In-situ electrochemical oxide monitoring in LiF-NdF3-Nd2O3: Application to Nd2O3 solubility determination. J. Electroanal. Chem. 2021, 893, 115334. [Google Scholar] [CrossRef]
- Takeda, O.; Nakano, K.; Kobayashi, F.; Lu, X.; Sato, Y.; Zhu, H. Solubilities of RE2O3 in REF3-LiF (RE = Nd, Dy) at 1473 K. J. Sustain. Metall. 2022, 8, 1498–1508. [Google Scholar] [CrossRef]
- Bratland, D. On the Possible Electrowinning of Yt-Al Alloys. The Solubility of Yttria and of Alumina in Molten Mixtures of Yttrium Fluoride and Lithium Fluoride. Light Met. 1976, 1, 183–201. [Google Scholar]
- Jung, I.V.; Ende, M. Computational thermodynamic calculations: FactSage from CALPHAD thermodynamic database to virtual process simulation. Metall. Mater. Trans. B 2020, 51, 1851–1874. [Google Scholar] [CrossRef]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.; Eriksson, G.; Gheribi, A.; Hack, K.; Jung, I.H.; Kang, Y.B.; Melançon, J.; et al. Reprint of: FactSage thermochemical software and databases, 2010–2016. Calphad 2016, 55, 1–19. [Google Scholar] [CrossRef]
- Davies, R.; Dinsdale, A.; Gisby, J.; Robinson, J.; Martin, S. MTDATA-thermodynamic and phase equilibrium software from the national physical laboratory. Calphad 2002, 26, 229–271. [Google Scholar] [CrossRef]
- Andersson, J.; Helander, T.; Höglund, L.; Shi, P.; Sundman, B. Thermo-Calc & DICTRA, computational tools for materials science. Calphad 2002, 26, 273–312. [Google Scholar]


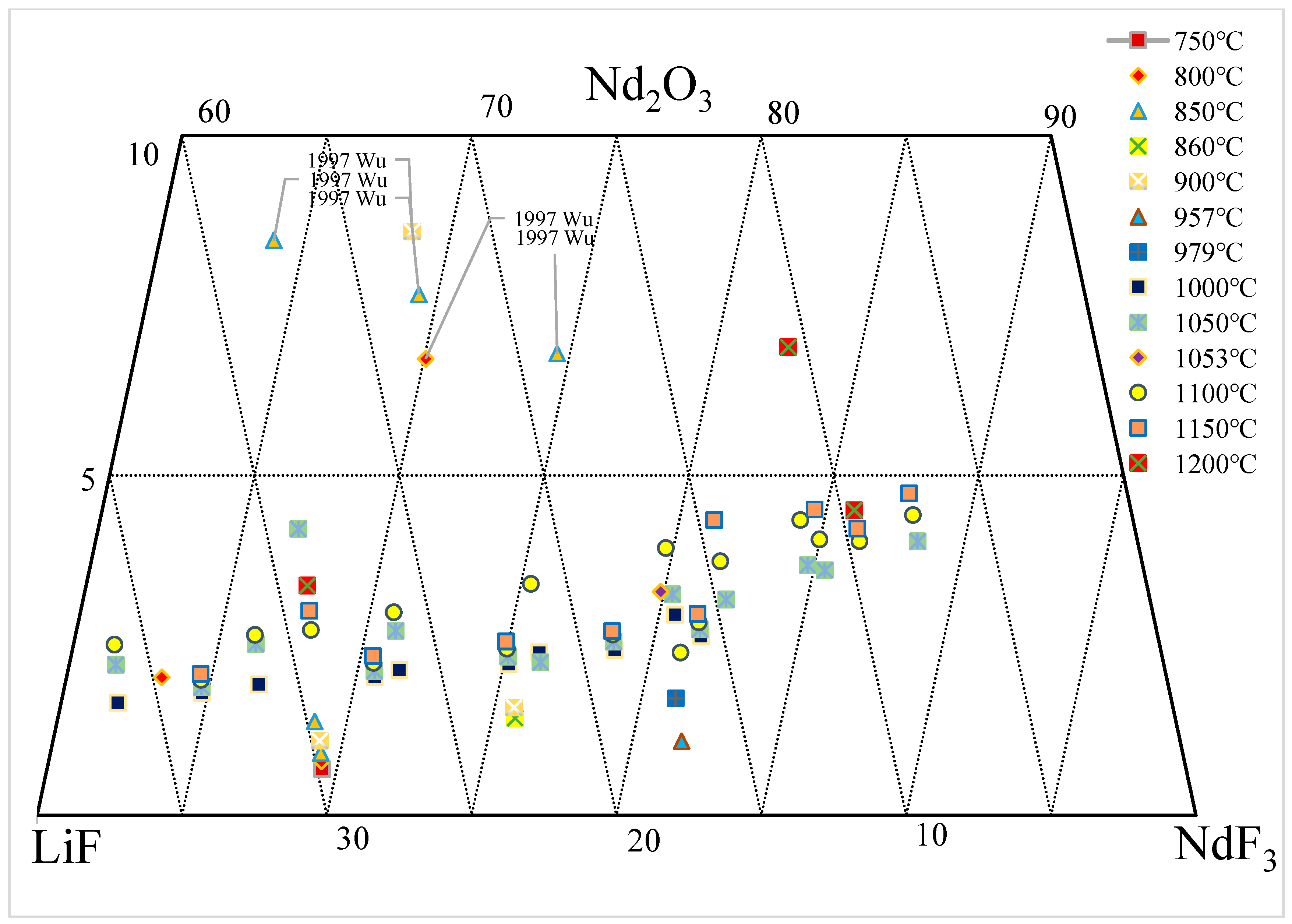

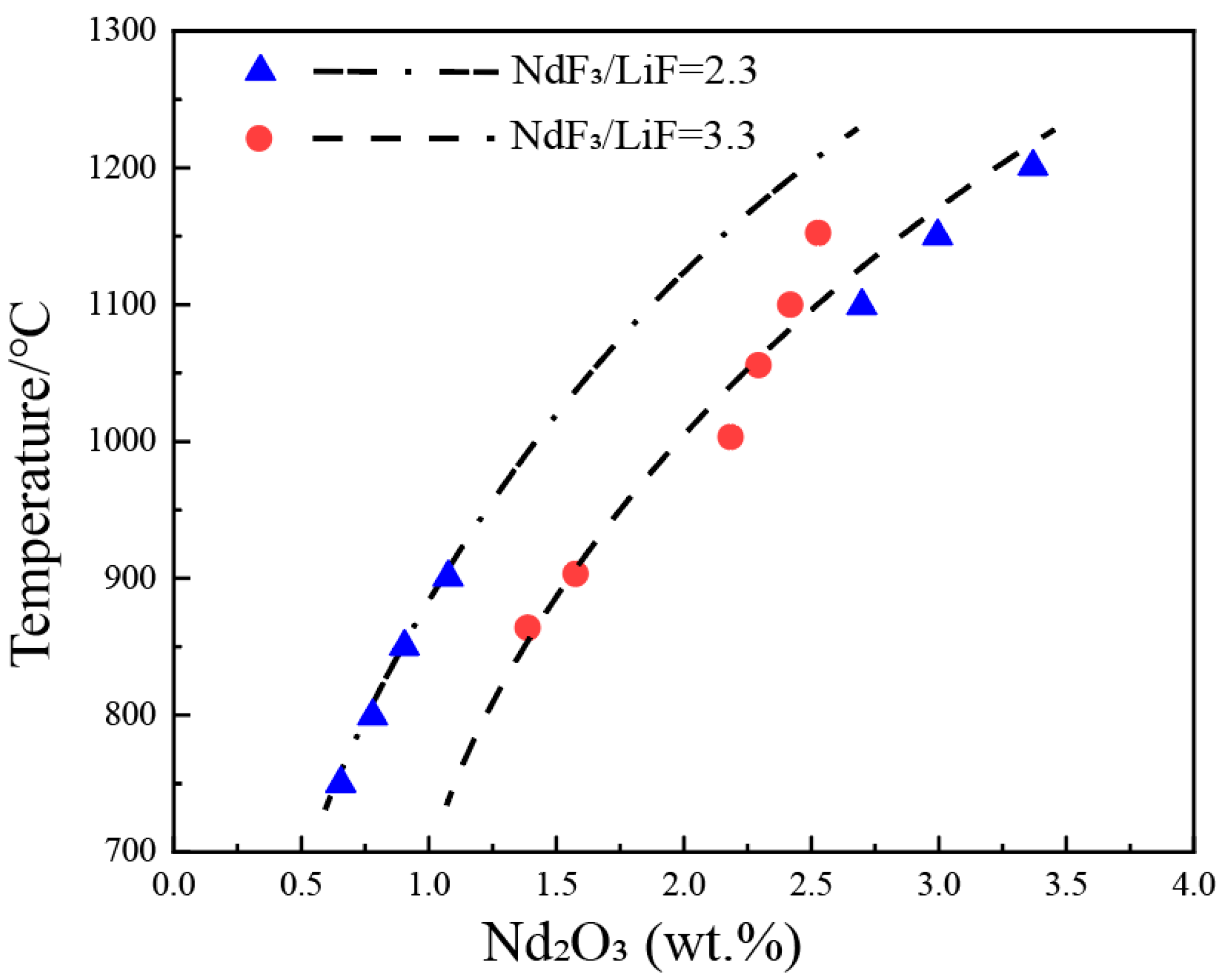
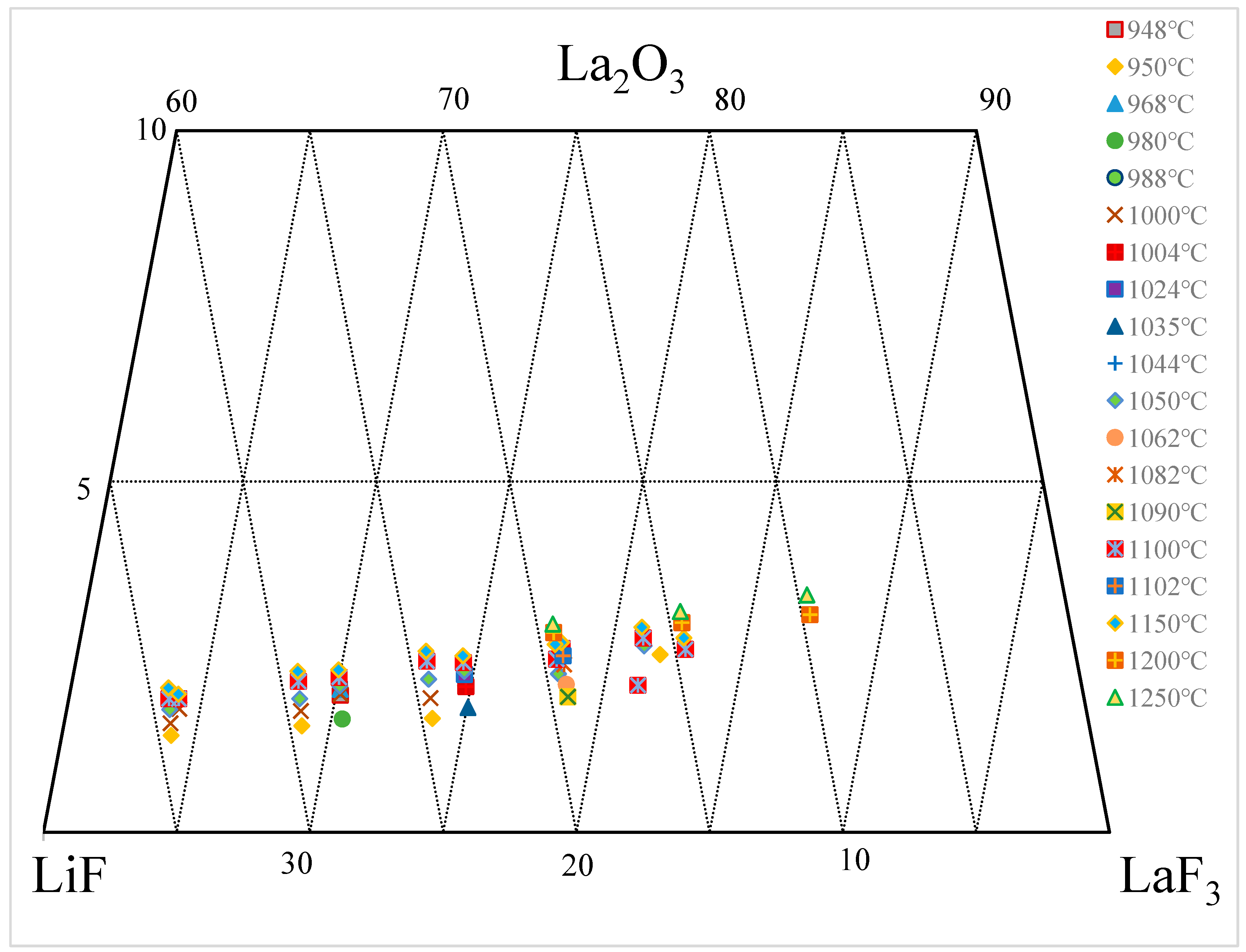

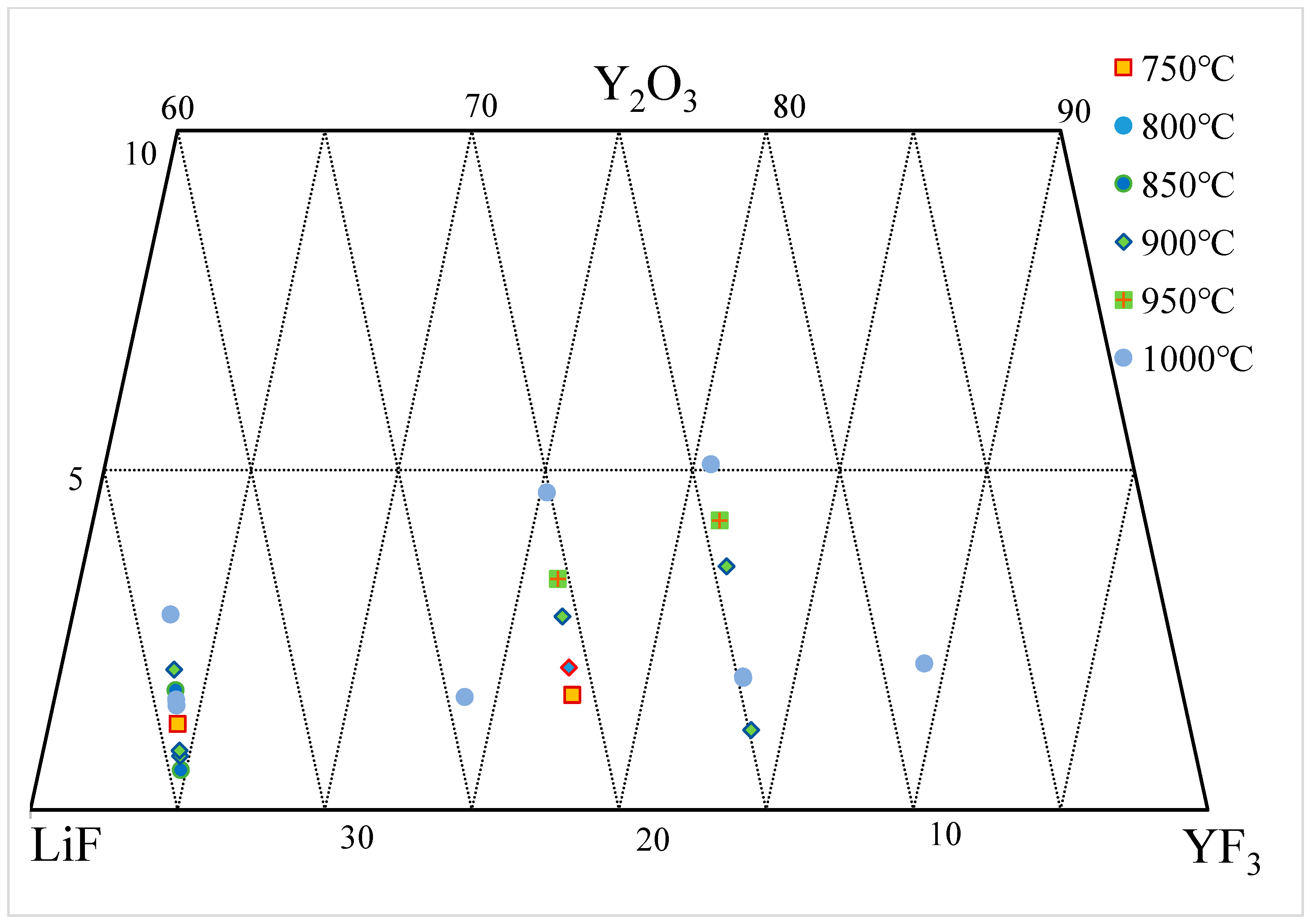



| wt.% | mol% | Normalization (wt.%) | T. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NdF3 | LiF | Nd2O3 | NdF3 | LiF | Nd2O3 | NdF3 | LiF | Nd2O3 | (°C) | Ref. |
| 58 | 42 | 0.54 | 15 | 85 | 0.08 | 57 | 42 | 0.54 | 800 | [35] |
| 58 | 42 | 0.75 | 15 | 85 | 0.12 | 57 | 42 | 0.74 | 850 | [35] |
| 58 | 42 | 0.82 | 15 | 85 | 0.13 | 57 | 42 | 0.81 | 860 | [35] |
| 58 | 42 | 0.96 | 15 | 85 | 0.15 | 57 | 42 | 0.95 | 900 | [35] |
| 63 | 37 | 1.68 | 18 | 82 | 0.29 | 62 | 36 | 1.65 | 1000 | [38] |
| 63 | 37 | 2.27 | 18 | 82 | 0.40 | 62 | 36 | 2.21 | 1050 | [38] |
| 63 | 37 | 2.57 | 18 | 82 | 0.45 | 61 | 36 | 2.51 | 1100 | [38] |
| 65 | 35 | 2.07 | 19 | 81 | 0.37 | 63 | 35 | 2.03 | 800 | [58] |
| 66 | 34 | 1.83 | 20 | 80 | 0.34 | 65 | 33 | 1.8 | 1000 | [36] |
| 66 | 34 | 1.92 | 20 | 80 | 0.35 | 65 | 33 | 1.88 | 1050 | [36] |
| 66 | 34 | 2.03 | 20 | 80 | 0.37 | 65 | 33 | 1.99 | 1100 | [36] |
| 66 | 34 | 2.12 | 20 | 80 | 0.39 | 65 | 33 | 2.08 | 1150 | [36] |
| 68 | 32 | 1.96 | 22 | 78 | 0.38 | 67 | 31 | 1.92 | 1000 | [35] |
| 68 | 32 | 2.59 | 22 | 78 | 0.50 | 66 | 31 | 2.52 | 1050 | [35] |
| 68 | 32 | 2.73 | 22 | 78 | 0.53 | 66 | 31 | 2.65 | 1100 | [35] |
| 70 | 30 | 9.24 | 23 | 77 | 1.96 | 64 | 28 | 8.46 | 850 | [34] |
| 70 | 30 | 1.4 | 23 | 77 | 0.28 | 69 | 30 | 1.38 | 850 | [61] |
| 70 | 30 | 4.4 | 23 | 77 | 0.90 | 67 | 29 | 4.21 | 1050 | [61] |
| 70 | 30 | 0.68 | 23 | 77 | 0.14 | 69 | 30 | 0.68 | 750 | [38] |
| 70 | 30 | 0.8 | 23 | 77 | 0.16 | 69 | 30 | 0.79 | 800 | [38] |
| 70 | 30 | 0.92 | 23 | 77 | 0.18 | 69 | 30 | 0.91 | 850 | [38] |
| 70 | 30 | 1.11 | 23 | 77 | 0.22 | 69 | 30 | 1.1 | 900 | [38] |
| 70 | 30 | 2.8 | 23 | 77 | 0.57 | 68 | 29 | 2.72 | 1100 | [57] |
| 70 | 30 | 3.1 | 23 | 77 | 0.63 | 68 | 29 | 3.01 | 1150 | [57] |
| 70 | 30 | 3.5 | 23 | 77 | 0.71 | 68 | 29 | 3.38 | 1200 | [57] |
| 72 | 28 | 2.07 | 25 | 75 | 0.44 | 71 | 27 | 2.03 | 1000 | [36] |
| 72 | 28 | 2.17 | 25 | 75 | 0.46 | 71 | 27 | 2.12 | 1050 | [36] |
| 72 | 28 | 2.29 | 25 | 75 | 0.48 | 70 | 27 | 2.24 | 1100 | [36] |
| 72 | 28 | 2.4 | 25 | 75 | 0.51 | 70 | 27 | 2.34 | 1150 | [36] |
| 73 | 27 | 2.18 | 26 | 74 | 0.47 | 71 | 26 | 2.14 | 1000 | [38] |
| 73 | 27 | 2.79 | 26 | 74 | 0.60 | 71 | 26 | 2.71 | 1050 | [38] |
| 73 | 27 | 3.08 | 26 | 74 | 0.67 | 71 | 26 | 2.99 | 1100 | [38] |
| 75 | 25 | 7.2 | 28 | 72 | 1.69 | 70 | 23 | 6.72 | 800 | [34] |
| 75 | 25 | 8.3 | 28 | 72 | 1.96 | 69 | 23 | 7.66 | 850 | [34] |
| 75 | 25 | 9.4 | 28 | 72 | 2.24 | 69 | 23 | 8.59 | 900 | [34] |
| 77 | 23 | 1.45 | 30 | 70 | 0.34 | 76 | 23 | 1.43 | 860 | [35] |
| 77 | 23 | 1.61 | 30 | 70 | 0.38 | 76 | 23 | 1.58 | 900 | [35] |
| 77 | 23 | 2.27 | 30 | 70 | 0.54 | 75 | 23 | 2.22 | 1000 | [36] |
| 77 | 23 | 2.39 | 30 | 70 | 0.57 | 75 | 23 | 2.33 | 1050 | [36] |
| 77 | 23 | 2.51 | 30 | 70 | 0.60 | 75 | 23 | 2.45 | 1100 | [36] |
| 77 | 23 | 2.62 | 30 | 70 | 0.63 | 75 | 23 | 2.55 | 1150 | [36] |
| 78 | 22 | 2.45 | 31 | 69 | 0.60 | 76 | 21 | 2.39 | 1000 | [38] |
| 78 | 22 | 2.3 | 31 | 69 | 0.56 | 76 | 22 | 2.25 | 1050 | [38] |
| 78 | 22 | 3.52 | 31 | 69 | 0.87 | 75 | 21 | 3.4 | 1100 | [38] |
| 80 | 20 | 7.29 | 34 | 66 | 1.95 | 75 | 19 | 6.79 | 850 | [34] |
| 81 | 19 | 2.49 | 35 | 65 | 0.66 | 79 | 19 | 2.43 | 1000 | [36] |
| 81 | 19 | 2.62 | 35 | 65 | 0.70 | 79 | 19 | 2.55 | 1050 | [36] |
| 81 | 19 | 2.73 | 35 | 65 | 0.73 | 79 | 19 | 2.66 | 1100 | [36] |
| 81 | 19 | 2.78 | 35 | 65 | 0.74 | 79 | 19 | 2.7 | 1150 | [36] |
| 83 | 17 | 1.1 | 39 | 61 | 0.31 | 82 | 17 | 1.09 | 957 | [55] |
| 83 | 17 | 1.75 | 39 | 61 | 0.49 | 81 | 17 | 1.72 | 979 | [55] |
| 83 | 17 | 3.4 | 39 | 61 | 0.97 | 80 | 17 | 3.29 | 1053 | [55] |
| 83 | 17 | 3.04 | 39 | 61 | 0.86 | 81 | 16 | 2.95 | 1000 | [38] |
| 83 | 17 | 3.36 | 39 | 61 | 0.96 | 80 | 16 | 3.25 | 1050 | [38] |
| 83 | 17 | 2.45 | 39 | 61 | 0.69 | 81 | 17 | 2.39 | 1100 | [37] |
| 83 | 17 | 4.09 | 39 | 61 | 1.17 | 80 | 16 | 3.93 | 1100 | [38] |
| 84 | 16 | 2.7 | 40 | 60 | 0.79 | 82 | 16 | 2.63 | 1000 | [36] |
| 84 | 16 | 2.81 | 40 | 60 | 0.82 | 82 | 16 | 2.73 | 1050 | [36] |
| 84 | 16 | 2.92 | 40 | 60 | 0.86 | 81 | 16 | 2.84 | 1100 | [36] |
| 84 | 16 | 3.05 | 40 | 60 | 0.90 | 81 | 16 | 2.96 | 1150 | [36] |
| 85 | 15 | 3.28 | 42 | 58 | 1.00 | 82 | 15 | 3.18 | 1050 | [39] |
| 85 | 15 | 3.88 | 42 | 58 | 1.18 | 82 | 15 | 3.74 | 1100 | [39] |
| 85 | 15 | 4.54 | 42 | 58 | 1.39 | 81 | 14 | 4.34 | 1150 | [39] |
| 88 | 12 | 3.82 | 49 | 51 | 1.29 | 85 | 12 | 3.68 | 1050 | [38] |
| 88 | 12 | 4.54 | 49 | 51 | 1.54 | 84 | 11 | 4.34 | 1100 | [38] |
| 88.5 | 11.5 | 3.74 | 50 | 50 | 1.29 | 85 | 11 | 3.61 | 1050 | [39] |
| 88.5 | 11.5 | 4.23 | 50 | 50 | 1.46 | 85 | 11 | 4.06 | 1100 | [39] |
| 88.5 | 11.5 | 4.71 | 50 | 50 | 1.63 | 85 | 11 | 4.5 | 1150 | [39] |
| 88.5 | 11.5 | 7.4 | 51 | 49 | 2.66 | 82 | 11 | 6.89 | 1200 | [62] |
| 90 | 10 | 4.2 | 54 | 46 | 1.54 | 86 | 10 | 4.03 | 1100 | [57] |
| 90 | 10 | 4.4 | 54 | 46 | 1.61 | 86 | 10 | 4.21 | 1150 | [57] |
| 90 | 10 | 4.7 | 54 | 46 | 1.73 | 86 | 10 | 4.49 | 1200 | [57] |
| 92 | 8 | 4.2 | 60 | 40 | 1.67 | 88 | 8 | 4.03 | 1050 | [39] |
| 92 | 8 | 4.62 | 60 | 40 | 1.84 | 88 | 8 | 4.42 | 1100 | [39] |
| 92 | 8 | 4.97 | 60 | 40 | 1.99 | 88 | 8 | 4.73 | 1150 | [39] |
| wt.% | mol% | Normalization (wt.%) | T. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LaF3 | LiF | La2O3 | LaF3 | LiF | La2O3 | LaF3 | LiF | La2O3 | (°C) | Ref. |
| 65 | 35 | 1.4 | 20 | 80 | 0.26 | 64 | 35 | 1.38 | 950 | [40] |
| 65 | 35 | 1.58 | 20 | 80 | 0.29 | 64 | 34 | S | 1000 | [40] |
| 65 | 35 | 1.78 | 20 | 80 | 0.33 | 64 | 34 | 1.75 | 1050 | [40] |
| 65 | 35 | 1.94 | 20 | 80 | 0.36 | 64 | 34 | 1.9 | 1100 | [40] |
| 65 | 35 | 2.1 | 20 | 80 | 0.39 | 64 | 34 | 2.06 | 1150 | [40] |
| 65 | 35 | 1.79 | 20 | 80 | 0.33 | 64 | 34 | 1.76 | 1000 | [36] |
| 65 | 35 | 1.87 | 20 | 80 | 0.35 | 64 | 34 | 1.84 | 1050 | [36] |
| 65 | 35 | 1.94 | 20 | 80 | 0.36 | 64 | 34 | 1.9 | 1100 | [36] |
| 65 | 35 | 2.01 | 20 | 80 | 0.37 | 64 | 34 | 1.97 | 1150 | [36] |
| 70 | 30 | 1.54 | 24 | 76 | 0.32 | 69 | 30 | 1.52 | 950 | [40] |
| 70 | 30 | 1.76 | 24 | 76 | 0.36 | 69 | 29 | 1.73 | 1000 | [40] |
| 70 | 30 | 1.94 | 24 | 76 | 0.40 | 69 | 29 | 1.9 | 1050 | [40] |
| 70 | 30 | 2.2 | 24 | 76 | 0.45 | 68 | 29 | 2.15 | 1100 | [40] |
| 70 | 30 | 2.35 | 24 | 76 | 0.49 | 68 | 29 | 2.3 | 1150 | [40] |
| 72 | 28 | 1.99 | 25 | 75 | 0.43 | 70 | 28 | 1.95 | 948 | [41] |
| 72 | 28 | 2.08 | 25 | 75 | 0.45 | 70 | 28 | 2.04 | 968 | [41] |
| 72 | 28 | 1.64 | 25 | 75 | 0.35 | 70 | 28 | 1.61 | 980 | [42] |
| 72 | 28 | 2.17 | 25 | 75 | 0.47 | 70 | 28 | 2.12 | 988 | [41] |
| 72 | 28 | 2.01 | 25 | 75 | 0.43 | 70 | 28 | 1.97 | 1000 | [36] |
| 72 | 28 | 2.13 | 25 | 75 | 0.46 | 70 | 28 | 2.09 | 1050 | [36] |
| 72 | 28 | 2.26 | 25 | 75 | 0.49 | 70 | 28 | 2.21 | 1100 | [36] |
| 72 | 28 | 2.37 | 25 | 75 | 0.51 | 70 | 28 | 2.32 | 1150 | [36] |
| 75 | 25 | 1.65 | 28 | 72 | 0.38 | 74 | 25 | 1.62 | 950 | [40] |
| 75 | 25 | 1.95 | 28 | 72 | 0.45 | 74 | 25 | 1.91 | 1000 | [40] |
| 75 | 25 | 2.23 | 28 | 72 | 0.52 | 73 | 24 | 2.18 | 1050 | [40] |
| 75 | 25 | 2.5 | 28 | 72 | 0.58 | 73 | 24 | 2.44 | 1100 | [40] |
| 75 | 25 | 2.65 | 28 | 72 | 0.62 | 73 | 24 | 2.58 | 1150 | [40] |
| 76 | 24 | 2.23 | 30 | 70 | 0.53 | 75 | 23 | 2.18 | 1000 | [36] |
| 76 | 24 | 2.12 | 30 | 70 | 0.50 | 75 | 23 | 2.08 | 1004 | [41] |
| 76 | 24 | 2.3 | 30 | 70 | 0.55 | 75 | 23 | 2.25 | 1024 | [41] |
| 76 | 24 | 1.81 | 30 | 70 | 0.43 | 75 | 23 | 1.78 | 1035 | [42] |
| 76 | 24 | 2.36 | 30 | 70 | 0.56 | 75 | 23 | 2.31 | 1044 | [41] |
| 76 | 24 | 2.35 | 30 | 70 | 0.56 | 75 | 23 | 2.3 | 1050 | [36] |
| 76 | 24 | 2.48 | 30 | 70 | 0.59 | 75 | 23 | 2.42 | 1100 | [36] |
| 76 | 24 | 2.58 | 30 | 70 | 0.62 | 74 | 23 | 2.52 | 1150 | [36] |
| 80 | 20 | 2.31 | 35 | 65 | 0.61 | 78 | 20 | 2.26 | 1050 | [40] |
| 80 | 20 | 2.53 | 35 | 65 | 0.67 | 78 | 20 | 2.47 | 1100 | [40] |
| 80 | 20 | 2.75 | 35 | 65 | 0.73 | 78 | 19 | 2.68 | 1150 | [40] |
| 80 | 20 | 2.93 | 35 | 65 | 0.78 | 78 | 19 | 2.85 | 1200 | [40] |
| 80 | 20 | 3.06 | 35 | 65 | 0.82 | 78 | 19 | 2.97 | 1250 | [40] |
| 80 | 20 | 2.56 | 35 | 65 | 0.68 | 78 | 19 | 2.5 | 1050 | [36] |
| 80 | 20 | 2.15 | 35 | 65 | 0.57 | 79 | 19 | 2.1 | 1062 | [41] |
| 80 | 20 | 2.45 | 35 | 65 | 0.65 | 78 | 19 | 2.39 | 1082 | [41] |
| 80 | 20 | 1.97 | 35 | 65 | 0.52 | 79 | 19 | 1.93 | 1090 | [36] |
| 80 | 20 | 2.69 | 35 | 65 | 0.71 | 78 | 19 | 2.62 | 1100 | [36] |
| 80 | 20 | 2.58 | 35 | 65 | 0.69 | 78 | 19 | 2.52 | 1102 | [41] |
| 80 | 20 | 2.78 | 35 | 65 | 0.74 | 78 | 19 | 2.7 | 1150 | [36] |
| 83 | 17 | 2.14 | 39 | 61 | 0.62 | 81 | 17 | 2.1 | 1100 | [37] |
| 83 | 17 | 2.73 | 39 | 61 | 0.79 | 81 | 16 | 2.66 | 1050 | [36] |
| 83 | 17 | 2.85 | 39 | 61 | 0.83 | 81 | 16 | 2.77 | 1100 | [36] |
| 83 | 17 | 3.01 | 39 | 61 | 0.88 | 81 | 16 | 2.92 | 1150 | [36] |
| 85 | 15 | 2.68 | 43 | 57 | 0.83 | 83 | 15 | 2.61 | 1100 | [40] |
| 85 | 15 | 2.85 | 43 | 57 | 0.88 | 83 | 15 | 2.77 | 1150 | [40] |
| 85 | 15 | 3.08 | 43 | 57 | 0.96 | 82 | 15 | 2.99 | 1200 | [40] |
| 85 | 15 | 3.25 | 43 | 57 | 1.01 | 82 | 15 | 3.15 | 1250 | [40] |
| 90 | 10 | 3.2 | 54 | 46 | 1.19 | 87 | 10 | 3.1 | 1200 | [40] |
| 90 | 10 | 3.5 | 54 | 46 | 1.30 | 87 | 10 | 3.38 | 1250 | [40] |
| wt.% | mol% | Normalization (wt.%) | T. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| YF3 | LiF | Y2O3 | YF3 | LiF | Y2O3 | YF3 | LiF | Y2O3 | (°C) | Ref. |
| 58 | 42 | 1.17 | 20 | 80 | 0.26 | 58 | 41 | 1.16 | 725 | [48] |
| 58 | 42 | 1.25 | 20 | 80 | 0.28 | 58 | 41 | 1.23 | 750 | [48] |
| 58 | 42 | 1.48 | 20 | 80 | 0.33 | 58 | 41 | 1.46 | 800 | [48] |
| 58 | 42 | 1.87 | 20 | 80 | 0.42 | 57 | 41 | 1.84 | 875 | [48] |
| 58 | 42 | 2.09 | 20 | 80 | 0.47 | 57 | 41 | 2.05 | 920 | [48] |
| 58 | 42 | 2.35 | 20 | 80 | 0.53 | 57 | 41 | 2.3 | 950 | [48] |
| 58 | 42 | 2.87 | 20 | 80 | 0.65 | 57 | 40 | 2.79 | 1000 | [48] |
| 65 | 35 | 1.28 | 25 | 75 | 0.32 | 64 | 34 | 1.26 | 750 | [48] |
| 65 | 35 | 0.45 | 25 | 75 | 0.11 | 65 | 35 | 0.45 | 810 | [63] |
| 65 | 35 | 1.63 | 25 | 75 | 0.41 | 64 | 34 | 1.6 | 825 | [48] |
| 65 | 35 | 0.5 | 25 | 75 | 0.12 | 65 | 35 | 0.5 | 831 | [63] |
| 65 | 35 | 1.79 | 25 | 75 | 0.45 | 64 | 34 | 1.76 | 850 | [48] |
| 65 | 35 | 0.59 | 25 | 75 | 0.15 | 65 | 35 | 0.59 | 850 | [63] |
| 65 | 35 | 0.8 | 25 | 75 | 0.20 | 65 | 35 | 0.79 | 882 | [63] |
| 65 | 35 | 0.8 | 25 | 75 | 0.20 | 65 | 35 | 0.79 | 900 | [63] |
| 65 | 35 | 2.11 | 25 | 75 | 0.53 | 64 | 34 | 2.07 | 900 | [48] |
| 65 | 35 | 0.88 | 25 | 75 | 0.22 | 65 | 34 | 0.87 | 900 | [63] |
| 65 | 35 | 1.65 | 25 | 75 | 0.41 | 64 | 34 | 1.62 | 1000 | [63] |
| 65 | 35 | 2.96 | 25 | 75 | 0.75 | 63 | 34 | 2.87 | 1000 | [48] |
| 65 | 35 | 1.65 | 25 | 75 | 0.41 | 64 | 34 | 1.62 | 1000 | [63] |
| 75 | 25 | 1.69 | 35 | 65 | 0.51 | 74 | 24 | 1.66 | 1000 | [63] |
| 79 | 21 | 1.72 | 40 | 60 | 0.57 | 78 | 21 | 1.69 | 750 | [48] |
| 79 | 21 | 2.14 | 40 | 60 | 0.71 | 77 | 21 | 2.1 | 800 | [48] |
| 79 | 21 | 2.26 | 40 | 60 | 0.75 | 77 | 21 | 2.21 | 825 | [48] |
| 79 | 21 | 2.93 | 40 | 60 | 0.98 | 77 | 20 | 2.85 | 900 | [48] |
| 79 | 21 | 3.52 | 40 | 60 | 1.18 | 76 | 20 | 3.4 | 950 | [48] |
| 79 | 21 | 4.9 | 40 | 60 | 1.66 | 75 | 20 | 4.67 | 1000 | [48] |
| 85 | 15 | 3.72 | 50 | 50 | 1.45 | 82 | 15 | 3.59 | 900 | [48] |
| 85 | 15 | 1.19 | 50 | 50 | 0.46 | 84 | 15 | 1.18 | 900 | [63] |
| 85 | 15 | 1.41 | 50 | 50 | 0.54 | 84 | 15 | 1.39 | 929 | [63] |
| 85 | 15 | 1.51 | 50 | 50 | 0.58 | 84 | 15 | 1.49 | 943 | [63] |
| 85 | 15 | 4.45 | 50 | 50 | 1.75 | 81 | 14 | 4.26 | 950 | [48] |
| 85 | 15 | 1.61 | 50 | 50 | 0.62 | 84 | 15 | 1.58 | 964 | [63] |
| 85 | 15 | 1.78 | 50 | 50 | 0.69 | 83 | 15 | 1.75 | 974 | [63] |
| 85 | 15 | 1.88 | 50 | 50 | 0.73 | 83 | 15 | 1.85 | 988 | [63] |
| 85 | 15 | 5.36 | 50 | 50 | 2.11 | 81 | 14 | 5.09 | 1000 | [48] |
| 85 | 15 | 1.98 | 50 | 50 | 0.77 | 83 | 15 | 1.94 | 1000 | [63] |
| 85 | 15 | 2 | 50 | 50 | 0.77 | 83 | 15 | 1.96 | 1000 | [63] |
| 85 | 15 | 2.09 | 50 | 50 | 0.81 | 83 | 15 | 2.05 | 1009 | [63] |
| 91 | 9 | 2.2 | 64 | 36 | 1.02 | 89 | 9 | 2.15 | 1000 | [63] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Q.; Liao, J.; Liao, C.; Zhao, B. Phase Equilibrium Study of Rare Earth Oxide–Fluoride Salt System: A Review. Metals 2024, 14, 314. https://doi.org/10.3390/met14030314
Zhou Q, Liao J, Liao C, Zhao B. Phase Equilibrium Study of Rare Earth Oxide–Fluoride Salt System: A Review. Metals. 2024; 14(3):314. https://doi.org/10.3390/met14030314
Chicago/Turabian StyleZhou, Quan, Jinfa Liao, Chunfa Liao, and Baojun Zhao. 2024. "Phase Equilibrium Study of Rare Earth Oxide–Fluoride Salt System: A Review" Metals 14, no. 3: 314. https://doi.org/10.3390/met14030314







