Effects of Different CaO/Al2O3 Ratios on the Phase Composition and Desulfurization Ability of CaO-Based Desulfurizers in Hot Metal
Abstract
1. Introduction
2. Materials and Methods
2.1. Production of the Desulfurization Agent
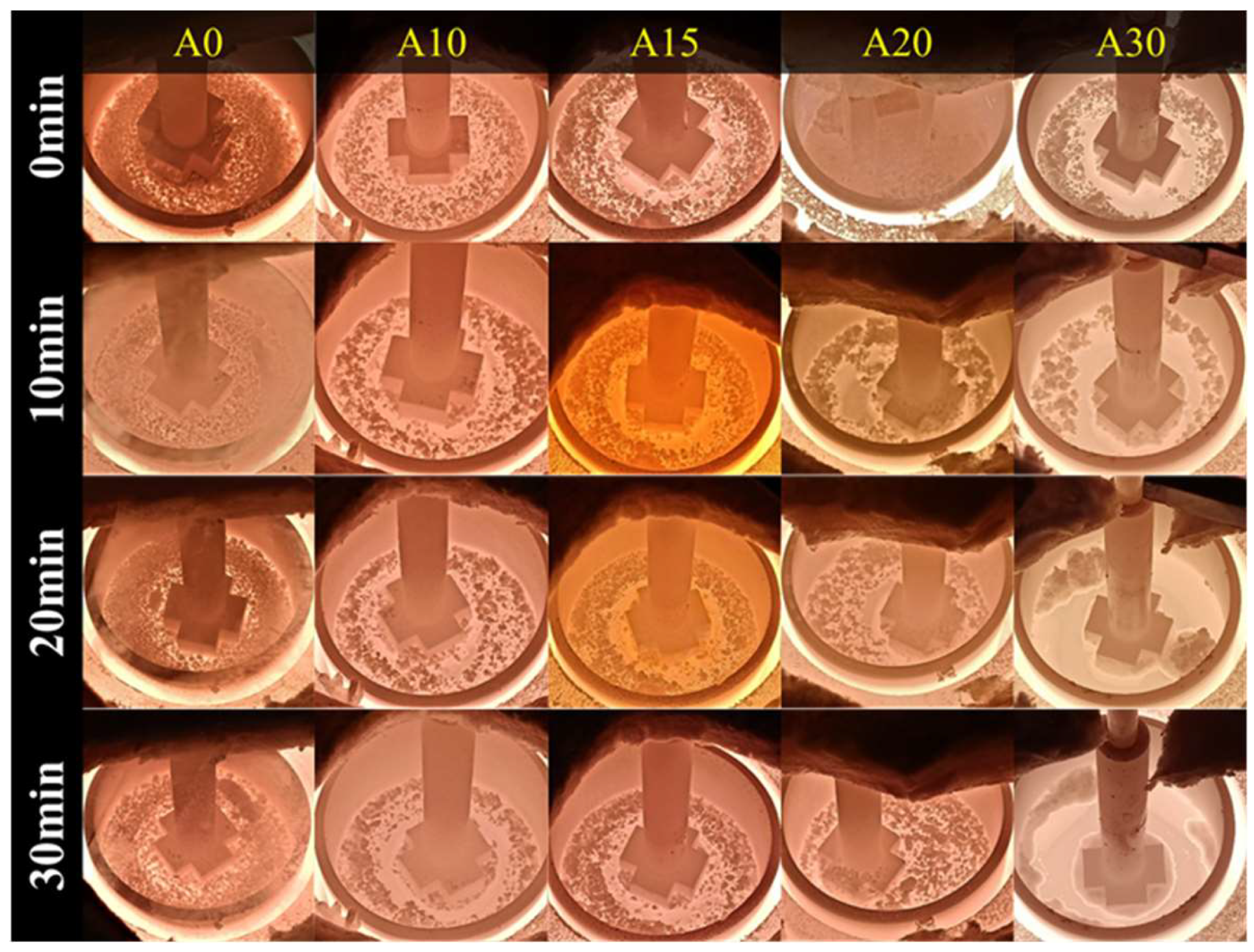
2.2. Desulfurization Test
3. Results
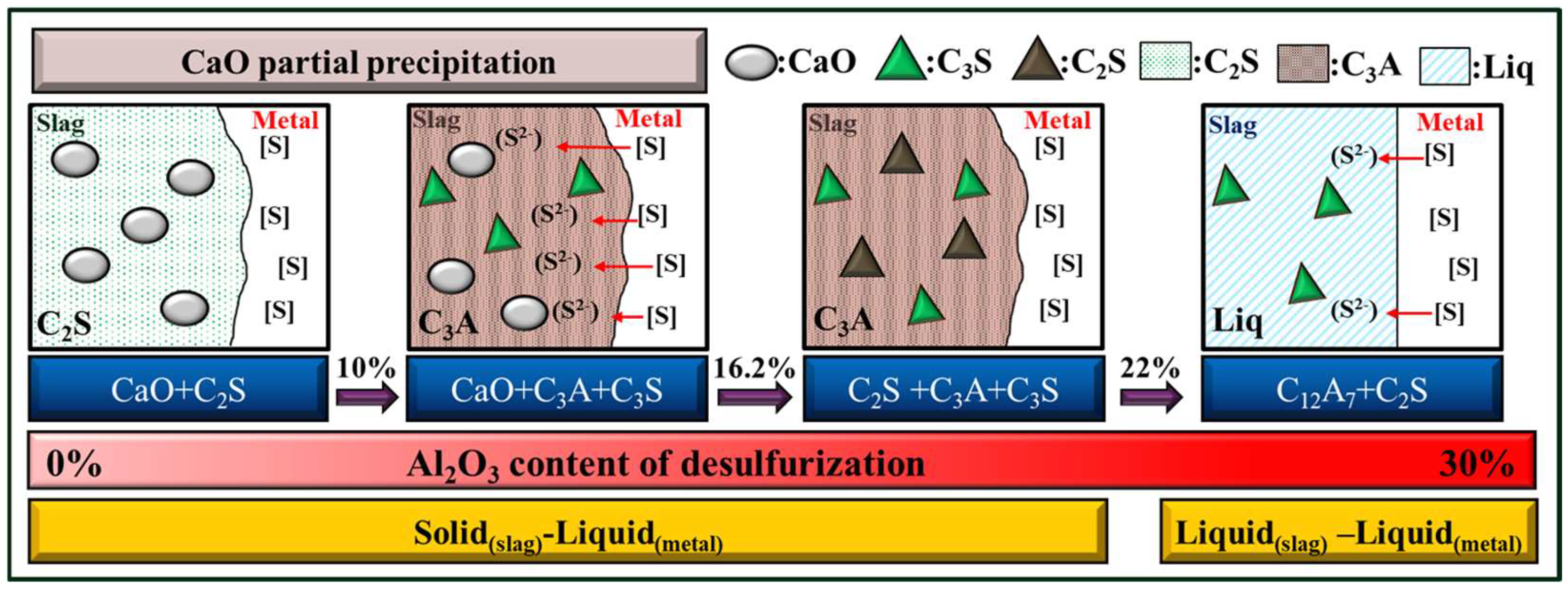
3.1. Effect of the CaO/Al2O3 Ratio on the Phase Composition
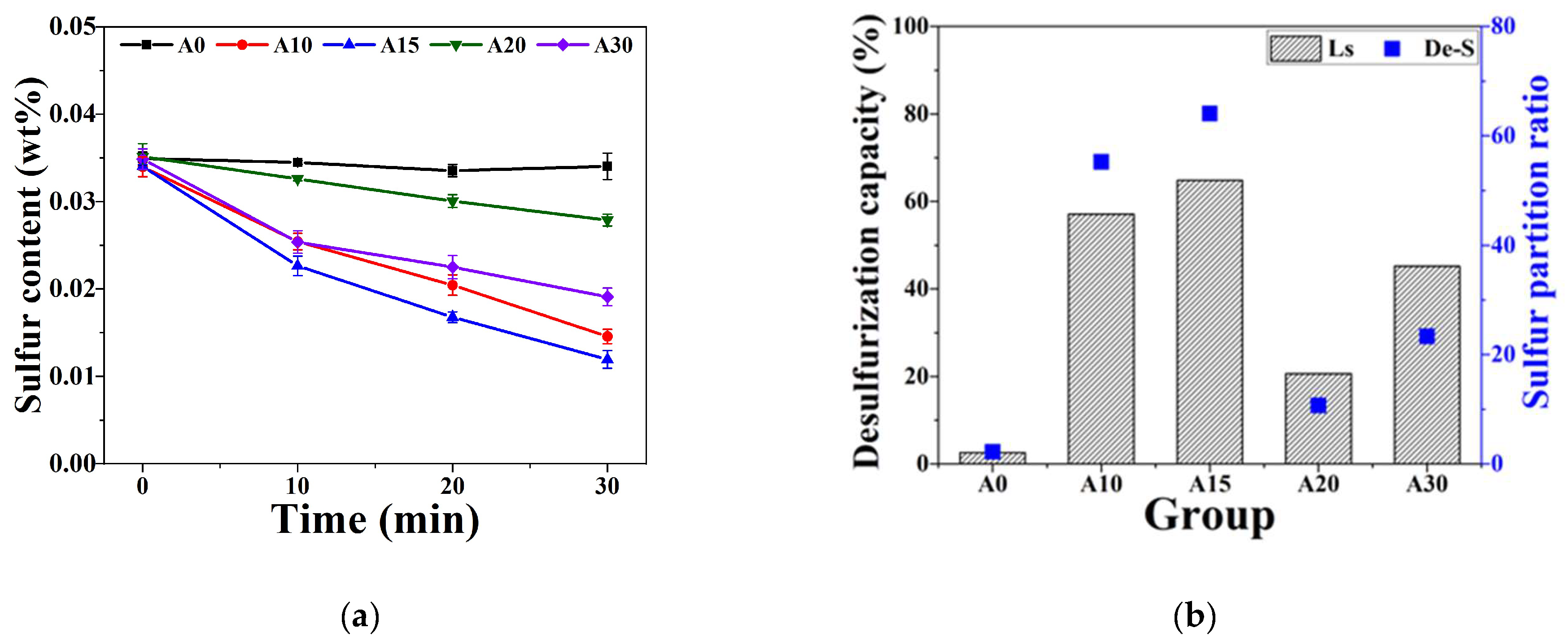
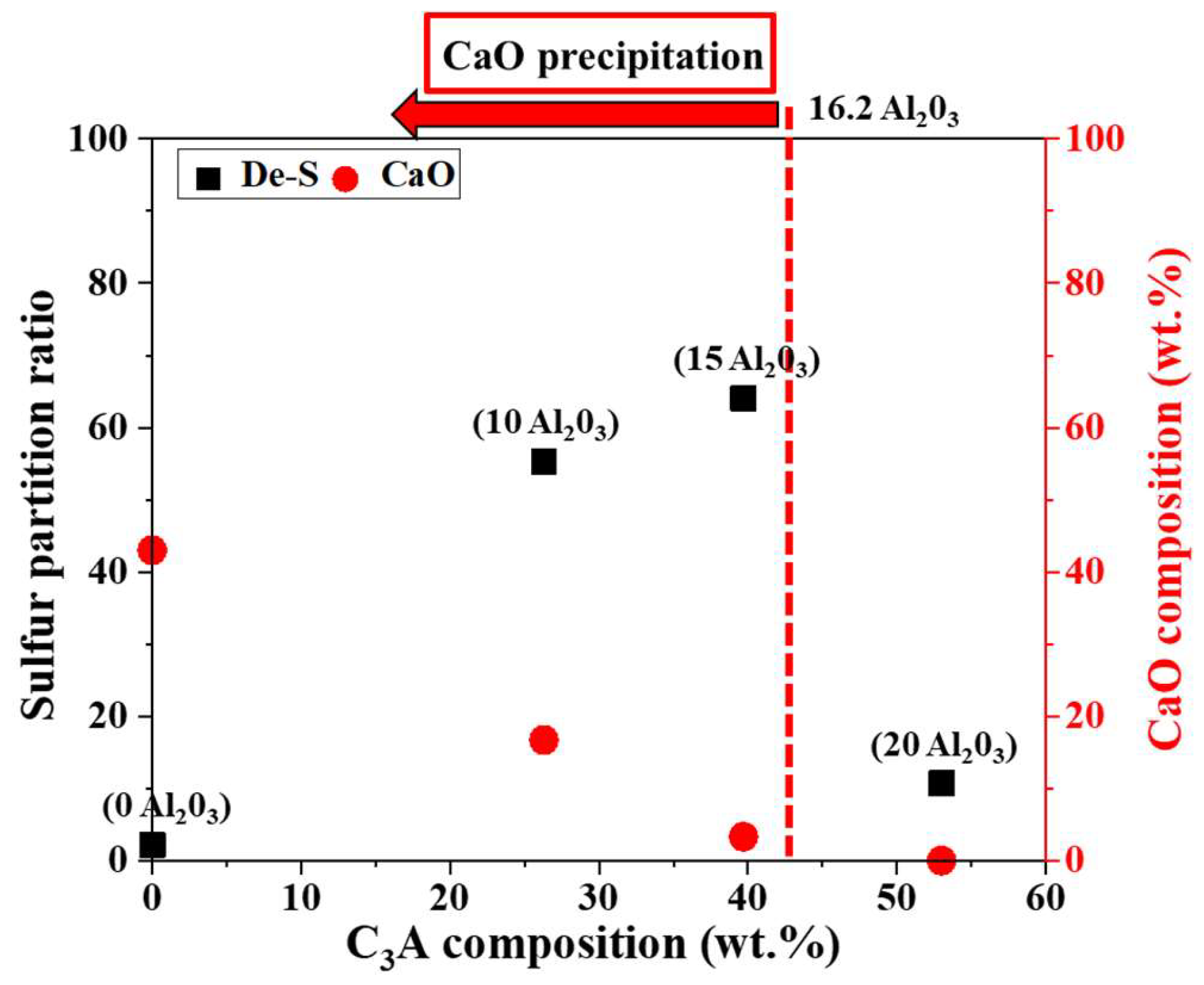
3.2. Comparison of Desulfurization Capacity
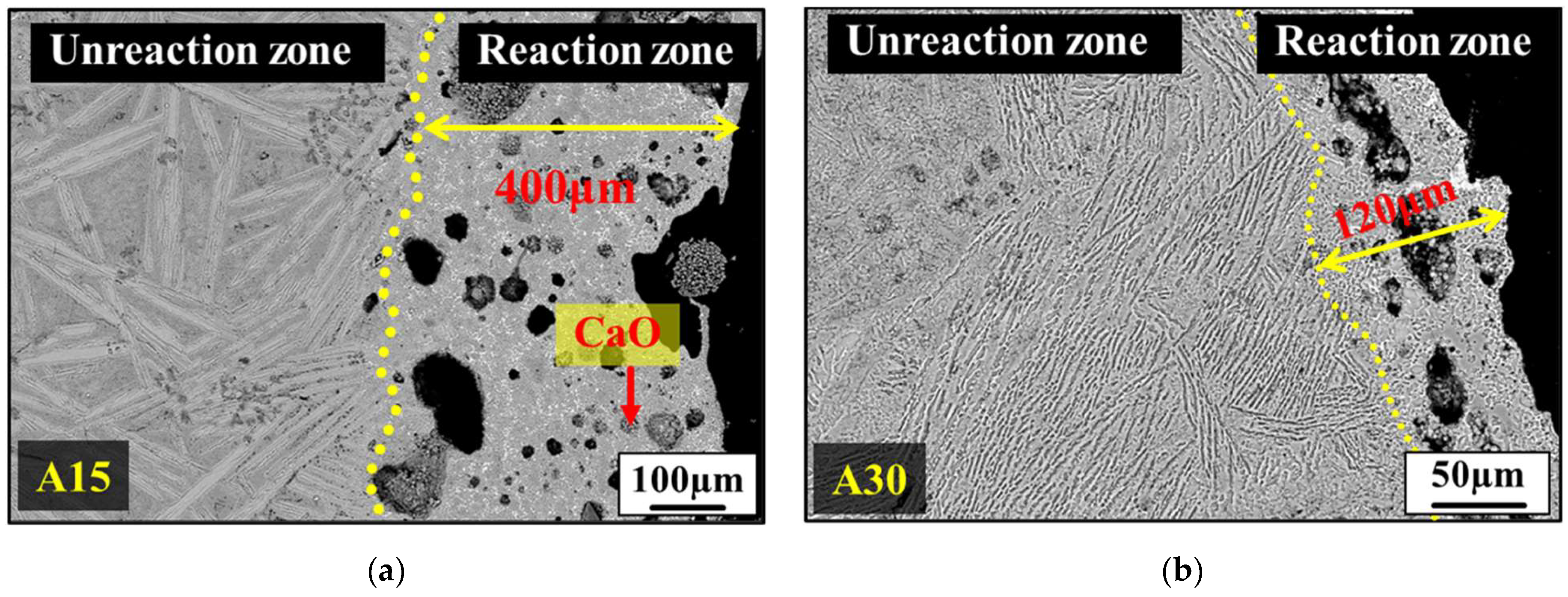
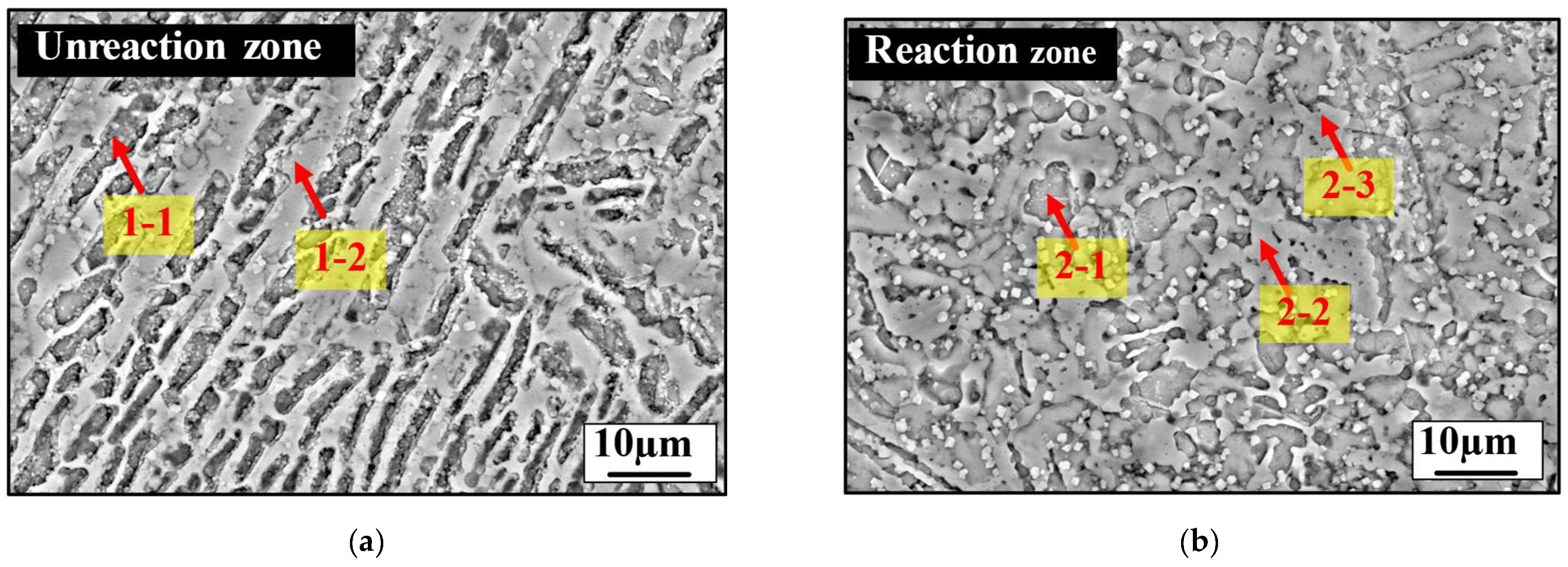
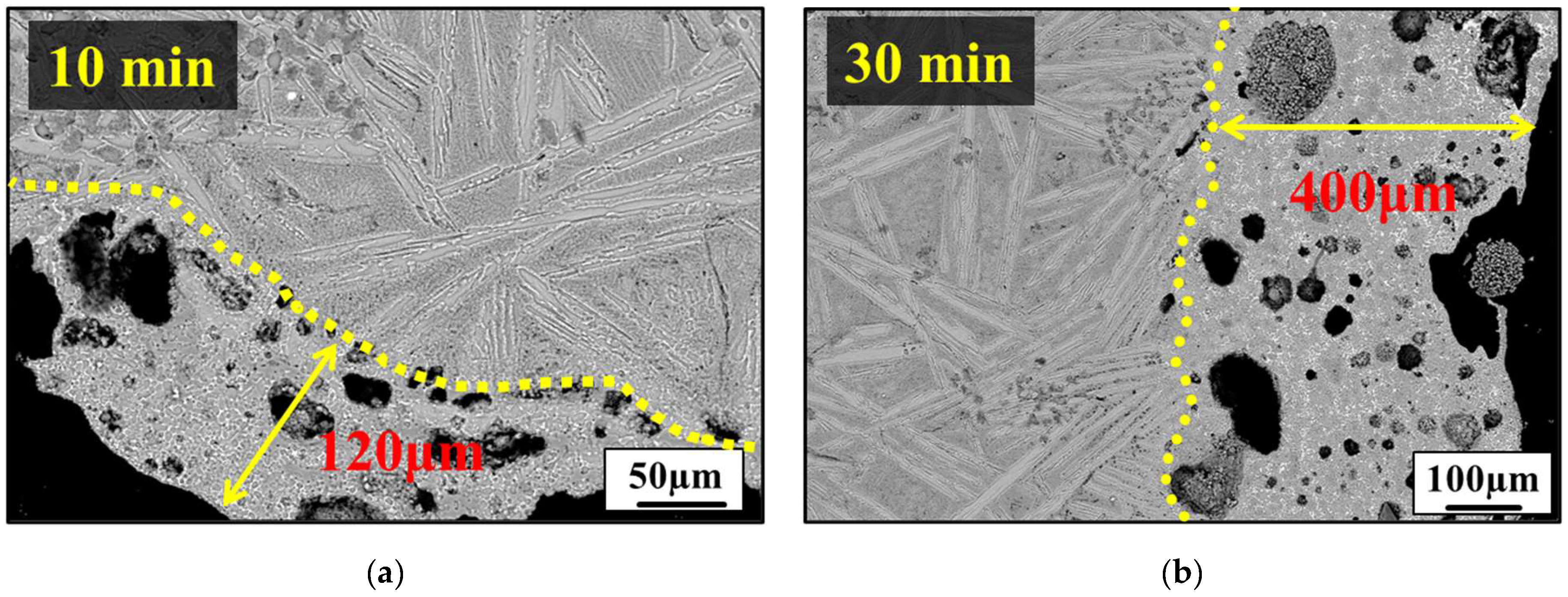
3.3. Analysis of the Sulfur-Tolerant Phase
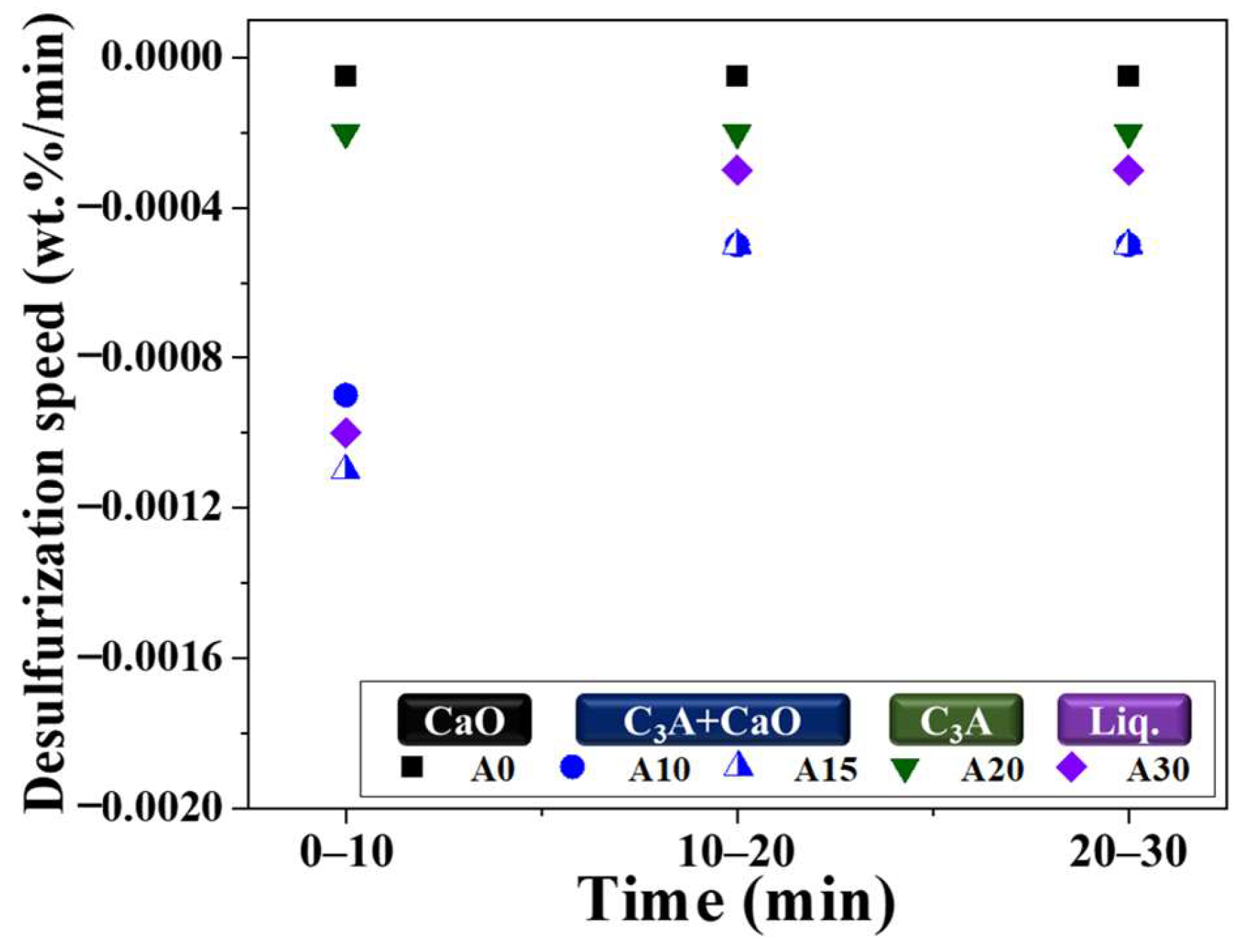
3.4. Effect of Composition on Desulfurization Speed
3.5. Calcium Aluminate Desulfurization
4. Conclusions
- The composition and phase characteristics of different CaO/Al2O3 KR desulfurizers identified via XRD closely align with the FactSage simulation results, validating the accuracy of the simulation. The phase characteristic shifts from the C3A phase when the Al2O3 content is between 10 and 20 wt.% to the C12A7 phase as the Al2O3 content reaches 30 wt.%.
- The desulfurization process utilizing solid(slag)–liquid(metal) diffusion indicates that, with an Al2O3 content of 20 wt.%, the Ls is 10.7. However, when the Al2O3 content is reduced to 15 wt.%, CaO particle precipitation occurs alongside it, enhancing the ability to attract sulfur ions from the molten iron to the sulfur-accommodating phase.
- With an Al2O3 content of 30 wt.%, desulfurization operates through liquid(slag)–liquid(metal) diffusion. The sulfur capacity of C12A7 is found to be lower than that of C3A, averaging about 0.1 at% with an Ls of 23.4.
- To replace fluorspar with Al2O3 as the KR desulfurizer and achieve effective desulfurization performance, it is essential that the total CaO content exceeds 65 wt.% and the Al2O3 content is maintained within 10~16.2 wt.%. This ensures that the desulfurizer possesses a high C3A phase, a high-capacity sulfur phase, and CaO particle precipitation.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Visuri, V.-V.; Vuolio, T.; Haas, T.; Fabritius, T. A review of modeling hot metal desulfurization. Steel Res. Int. 2020, 91, 1900454. [Google Scholar] [CrossRef]
- Li, Q.; Ma, S.; Feng, M.; Lei, H.; Zou, Z. Energy efficiency characterization and optimization of mechanical stirring multiphase dispersion processes: Applied to kanbara reactors for hot metal desulfurization. J. Mater. Res. Technol. 2023, 24, 5642–5659. [Google Scholar] [CrossRef]
- Wang, R.; Jia, S.; He, Z. Numerical investigation on the effects of impeller structures in hot metal desulfurization processes by mechanical stirring. Metals 2022, 12, 229. [Google Scholar] [CrossRef]
- Lee, S.; Min, D.J. A study on the formation mechanism of the interfacial layer between solid CaO and molten iron alloys. Met. Mater. Int. 2019, 25, 248–256. [Google Scholar] [CrossRef]
- Mitsuo, T.; Shoji, T.; Hatta, Y.; Ono, H.; Mori, H.; Kai, T. Improvement of desulfurization by addition of aluminum to hot metal in the lime injection process. Trans. Jpn. Inst. Met. 1982, 23, 768–779. [Google Scholar] [CrossRef]
- Kawai, Y.; Mori, K.; San-Nomiya, Y. Fundamental study on the rate of desulphurization of pig iron by solid lime. Tetsu-to-Hagane 1975, 61, 29–35. [Google Scholar] [CrossRef]
- Niekerk, W.H.V.; Dippenaar, R.J. Thermodynamic aspects of Na2O and CaF2 containing lime-based slags used for the desulphurization of hot-metal. ISIJ Int. 1993, 33, 59–65. [Google Scholar] [CrossRef]
- Amini, S.; Brungs, M.; Ostrovski, O. Dissolution of dense lime in molten slags under static conditions. ISIJ Int. 2007, 47, 32–37. [Google Scholar] [CrossRef]
- Andersson, E.; Sichen, D. The effect of caf2 in the slag in ladle refining. Steel Res. Int. 2009, 80, 544–551. [Google Scholar] [CrossRef]
- Jeong, T.S.; Oh, M.K.; Chung, Y.; Park, J.H. Effect of white mud addition on desulfurization rate of molten steel. Metall. Mater. Trans. B 2021, 52, 3596–3605. [Google Scholar] [CrossRef]
- Pak, J.J.; Fruehan, R.J. The effect of na2o on dephosphorization by cao-based steelmaking slags. Metall. Trans. B 1991, 22, 39–46. [Google Scholar] [CrossRef]
- Pezzin, R.d.O.; Berger, A.P.L.; Grillo, F.F.; Junca, E.; Furtado, H.S.; Oliveira, J.R.d. Analysis of the influence of the solid and liquid phases on steel desulfurization with slags from the CaO–Al2O3 systems using computational thermodynamics. J. Mater. Res. Technol. 2020, 9, 838–846. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, P.; Men, G.; McLean, A. Hot metal desulphurization using waste residues from the aluminum industry. High Temp. Mater. Process. 2012, 31, 519–528. [Google Scholar] [CrossRef]
- Yajima, K.; Matsuura, H.; Tsukihashi, F. Effect of simultaneous addition of Al2O3 and MgO on the liquidus of the CaO-SiO2-FeOX system with various oxygen partial pressures at 1573 k. ISIJ Int. 2010, 50, 191–194. [Google Scholar] [CrossRef]
- Takahashi, K.; Utagawa, K.; Shibata, H.; Kitamura, S.-y.; Kikuchi, N.; Kishimoto, Y. Influence of solid CaO and liquid slag on hot metal desulfurization. ISIJ Int. 2012, 52, 10–17. [Google Scholar] [CrossRef]
- Tanaka, T.; Ogiso, Y.; Ueda, M.; Lee, J. Trial on the application of capillary phenomenon of solid CaO to desulfurization of liquid fe. ISIJ Int. 2010, 50, 1071–1077. [Google Scholar] [CrossRef][Green Version]
- Oktay, E.; Fruehan, R.J. On the hot metal desulfurization. Steel Res. 1995, 66, 93–95. [Google Scholar] [CrossRef]
- Hong, J.-p.; Wang, J.; Chen, H.-y.; Sun, B.-d.; Li, J.-j.; Chen, C. Process of aluminum dross recycling and life cycle assessment for Al-Si alloys and brown fused alumina. Trans. Nonferrous Met. Soc. China 2010, 20, 2155–2161. [Google Scholar] [CrossRef]
- Srivastava, A.; Meshram, A. A hydrometallurgical perspective of aluminium dross recycling. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Hu, K.; Reed, D.; Robshaw, T.J.; Smith, R.M.; Ogden, M.D. Characterisation of aluminium black dross before and after stepwise salt-phase dissolution in non-aqueous solvents. J. Hazard. Mater. 2021, 401, 123351. [Google Scholar] [CrossRef]
- Lv, H.; Zhao, H.; Zuo, Z.; Li, R.; Liu, F. A thermodynamic and kinetic study of catalyzed hydrolysis of aluminum nitride in secondary aluminum dross. J. Mater. Res. Technol. 2020, 9, 9735–9745. [Google Scholar] [CrossRef]
- Verma, S.K.; Dwivedi, V.K.; Dwivedi, S.P. Utilization of aluminium dross for the development of valuable product—A review. Mater. Today Proc. 2021, 43, 547–550. [Google Scholar] [CrossRef]
- Xie, M.; Lv, H.; An, H.; Liu, F.; Zhao, H. Effect of alkali doping on the preparation of calcium aluminate by aluminum dross calcification process. Environ. Technol. Innov. 2023, 32, 103312. [Google Scholar] [CrossRef]
- Li, R.; Zhong, J.; Xie, M.; Huang, Z.; Zhao, H.; Liu, F. Quantitative evaluation on aln transformation and volatilization of chlorides and fluorides in pyrometallurgical treatment for aluminum dross. J. Mater. Res. Technol. 2024, 29, 2879–2888. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Lu, F.-H. Oxidation behavior of aln films at high temperature under controlled atmosphere. J. Eur. Ceram. Soc. 2008, 28, 691–698. [Google Scholar] [CrossRef]
- Su, Z.; Liu, K.; Lin, K.; Liu, S.; Zhang, Y.; Jiang, T. A novel route to denitrify, recover chlorines and prepare premelted refine slag of 12cao·7Al2O3 (C12A7) from secondary aluminum dross (SAD). J. Mater. Res. Technol. 2022, 19, 1203–1216. [Google Scholar] [CrossRef]
- Hu, S.; Wang, D.; Hou, D.; Zhao, W.; Li, X.; Qu, T.; Zhu, Q. Research on the preparation parameters and basic properties of premelted calcium aluminate slag prepared from secondary aluminum dross. Materials 2021, 14, 5855. [Google Scholar] [CrossRef]
- López, F.A.; Martín, M.I.; Alguacil, F.J.; Sergio Ramírez, M.; González, J.R. Synthesis of calcium aluminates from non-saline aluminum dross. Materials 2019, 12, 1837. [Google Scholar] [CrossRef]
- Jerebtsov, D.A.; Mikhailov, G.G. Phase diagram of CaO-Al2O3 system. Ceram. Int. 2001, 27, 25–28. [Google Scholar] [CrossRef]
- Darban, S.; Reynaert, C.; Ludwig, M.; Prorok, R.; Jastrzębska, I.; Szczerba, J. Corrosion of alumina-spinel refractory by secondary metallurgical slag using coating corrosion test. Materials 2022, 15, 3425. [Google Scholar] [CrossRef]
- Kim, S.-j.; Kageyama, M.; Gao, X.; Ueda, S.; Kitamura, S.-y. Solubility of sulfur in the solid oxide of the calcium-aluminate system. ISIJ Int. 2019, 59, 1752–1755. [Google Scholar] [CrossRef]
- Lazou, A.; Kolbeinsen, L.; Safarian, J. Evaluation of calcium aluminate slags and pig irons produced from the smelting-reduction of diasporic bauxite. Materials 2021, 14, 7740. [Google Scholar] [CrossRef] [PubMed]
- Shu, Q.; Wang, Y.; Li, J.; Liu, Y.; Li, P.; Chou, K. Effect of Na2O on dissolution rate of alumina in CaO–Al2O3–MgO–SiO2 slag. ISIJ Int. 2015, 55, 2297–2303. [Google Scholar] [CrossRef]
- Jeong, B.-K.; Bang, G.-H.; Kang, Y.-B. Role of liquid and solid particles in solid-liquid mixed fluxes on sulfur removal from molten iron under centrifugal stirring by an impeller. Powder Technol. 2022, 396, 1–12. [Google Scholar] [CrossRef]
- Do Espírito Santo, E.V.; Soares, S.G.; de Oliveira, H.C.C.; Junca, E.; Grillo, F.F.; de Oliveira, J.R. Influence of industrial wastes and different fluxes on hot metal desulfurization efficiency. JOM 2021, 73, 1909–1918. [Google Scholar] [CrossRef]
- Baba, Y.; Gao, X.; Ueda, S.; Kitamura, S. Sulfide capacities of solid oxides in calcium-aluminate systems. ISIJ Int. 2020, 60, 1617–1623. [Google Scholar] [CrossRef]






| No. | Desulfurizer Composition, wt% | ||
|---|---|---|---|
| CaO | SiO2 | Al2O3 | |
| A0 | 85 | 15 | -- |
| A10 | 75 | 15 | 10 |
| A15 | 70 | 15 | 15 |
| A20 | 65 | 15 | 20 |
| A30 | 55 | 15 | 30 |
| Fe | C | Si | Mn | P | S |
|---|---|---|---|---|---|
| 94.97 | 4.5 | 0.4 | 0.25 | 0.1 | 0.035 |
| No. | FactSage(1400) | XRD |
|---|---|---|
| A0 | 43CaO + 57C3S | C2S, CaO |
| A10 | 16.7CaO + 26.3C3A + 57C3S | C3S, CaO, C3A |
| A15 | 3.3CaO + 39.7C3A + 57C3S | C3S, CaO, C3A |
| A20 | 53C3A + 30.7C2S + 16.3C3S | C2S, C3S, C3A |
| A30 | 34C2S + 1CA + 65Liq | C2S, C12A7, C2AS |
| No. | Chemical Composition, at.% | Phase | |||||
|---|---|---|---|---|---|---|---|
| Ca | Si | Al | O | S | Mg | ||
| 1–1 | 29.48 | 9.94 | 0.83 | 58.88 | - | 0.87 | C3S |
| 1–2 | 29.07 | 2.17 | 15.4 | 59.00 | - | 0.35 | Rich (Ca, Al) |
| 2–1 | 27.52 | 13.93 | 0.30 | 58.21 | - | 0.05 | C2S |
| 2–2 | 18.19 | 3.87 | 16.31 | 60.79 | 0.62 | 0.21 | Rich (Ca, Al) |
| 2–3 | 17.40 | 4.70 | 13.45 | 63.77 | 0.47 | 0.21 | Rich (Ca, Al) |
| 2–4 | 43.95 | 8.20 | 3.20 | 43.42 | - | 1.23 | CaO |
| No. | Chemical Composition, at.% | Phase | |||||
|---|---|---|---|---|---|---|---|
| Ca | Si | Al | O | S | Mg | ||
| 1–1 | 37.75 | 10.94 | 3.12 | 47.09 | - | 1.09 | C3S |
| 1–2 | 24.21 | 2.36 | 15.53 | 56.67 | - | 1.23 | Rich (Ca, Al) |
| 2–1 | 26.90 | 12.35 | 1.64 | 58.48 | - | 0.62 | C2S |
| 2–2 | 19.41 | 2.53 | 19.64 | 56.42 | 0.12 | 1.88 | Rich (Ca, Al) |
| 2–3 | 19.43 | 5.77 | 12.91 | 60.99 | 0.10 | 0.81 | Rich (Ca, Al) |
| No. | Chemical Composition, at.% | Phase | |||||
|---|---|---|---|---|---|---|---|
| Ca | Si | Al | O | S | Mg | ||
| 1–1 | 23.86 | 3.27 | 13.82 | 58.87 | - | 0.19 | Rich (Ca, Al) |
| 1–2 | 20.85 | 1.88 | 14.79 | 62.23 | - | 0.25 | Rich (Ca, Al) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, J.-M.; Lin, C.-M.; Chang, Y.-E.; Lin, H.-J.; Wu, W. Effects of Different CaO/Al2O3 Ratios on the Phase Composition and Desulfurization Ability of CaO-Based Desulfurizers in Hot Metal. Metals 2024, 14, 363. https://doi.org/10.3390/met14030363
Shen J-M, Lin C-M, Chang Y-E, Lin H-J, Wu W. Effects of Different CaO/Al2O3 Ratios on the Phase Composition and Desulfurization Ability of CaO-Based Desulfurizers in Hot Metal. Metals. 2024; 14(3):363. https://doi.org/10.3390/met14030363
Chicago/Turabian StyleShen, Jyun-Ming, Chi-Ming Lin, Yu-En Chang, Hui-Jan Lin, and Weite Wu. 2024. "Effects of Different CaO/Al2O3 Ratios on the Phase Composition and Desulfurization Ability of CaO-Based Desulfurizers in Hot Metal" Metals 14, no. 3: 363. https://doi.org/10.3390/met14030363
APA StyleShen, J.-M., Lin, C.-M., Chang, Y.-E., Lin, H.-J., & Wu, W. (2024). Effects of Different CaO/Al2O3 Ratios on the Phase Composition and Desulfurization Ability of CaO-Based Desulfurizers in Hot Metal. Metals, 14(3), 363. https://doi.org/10.3390/met14030363






