Machine Learning-Assisted Prediction of Corrosion Behavior of 7XXX Aluminum Alloys
Abstract
:1. Introduction
2. Experimental Method and Dataset
2.1. Corrosion Dataset
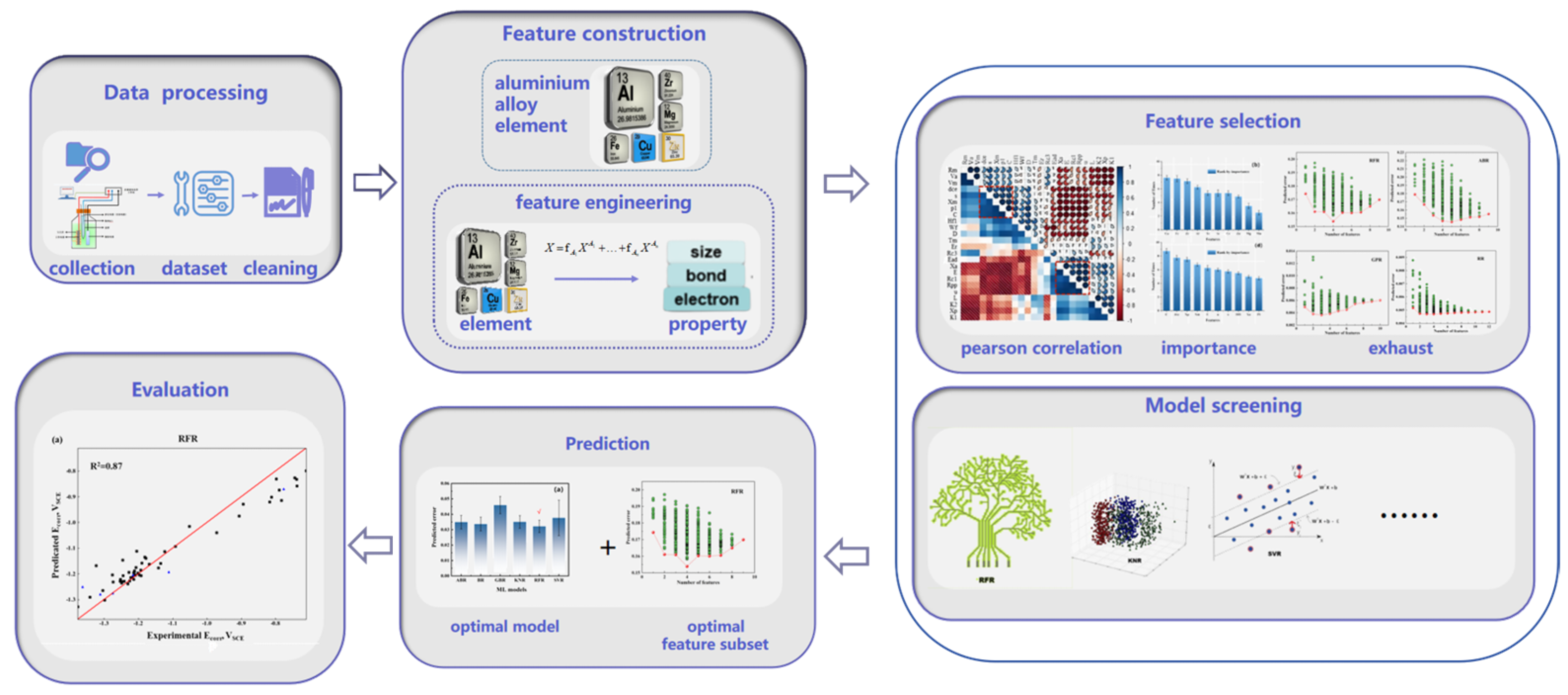
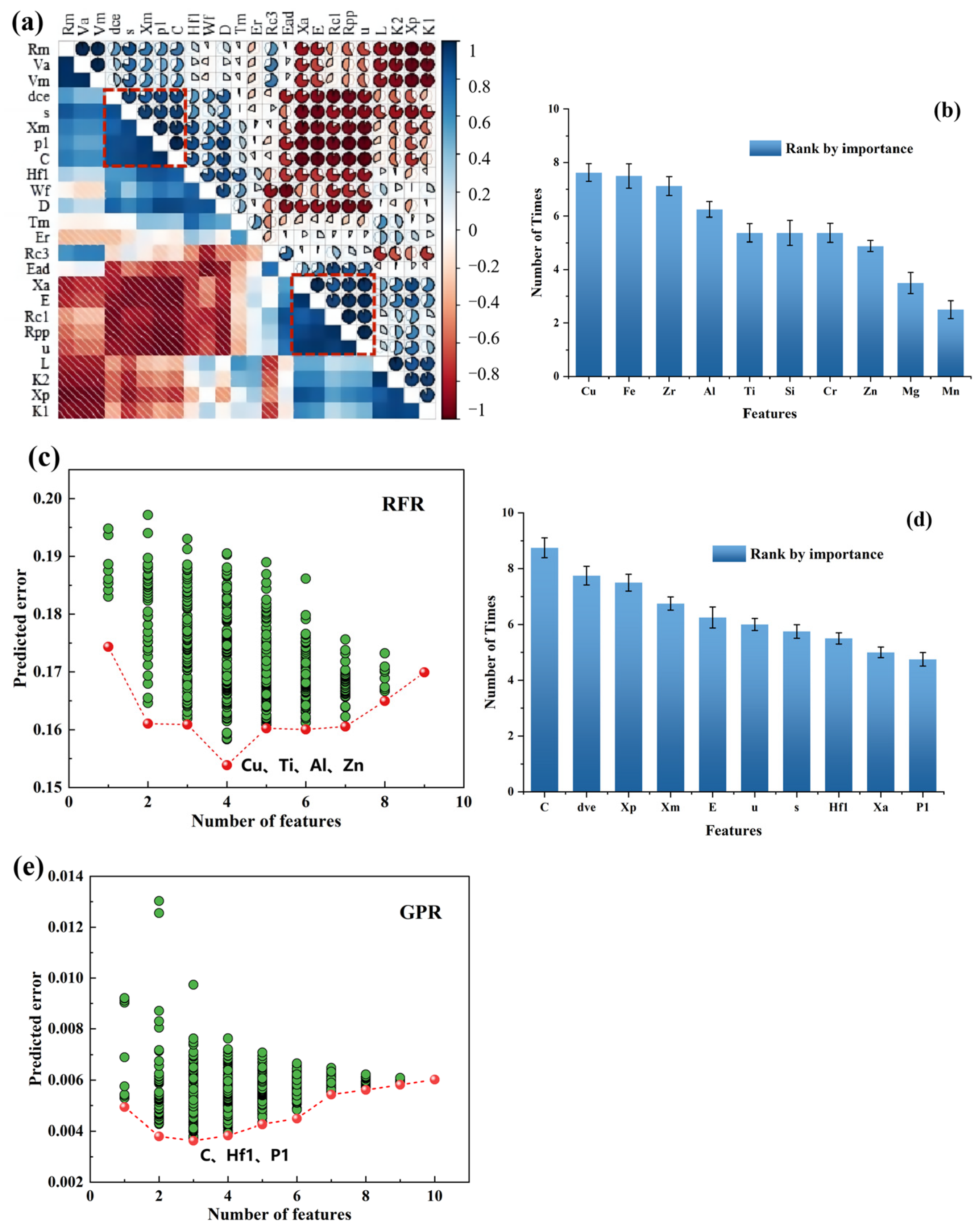
2.2. Feature Creation and Selection
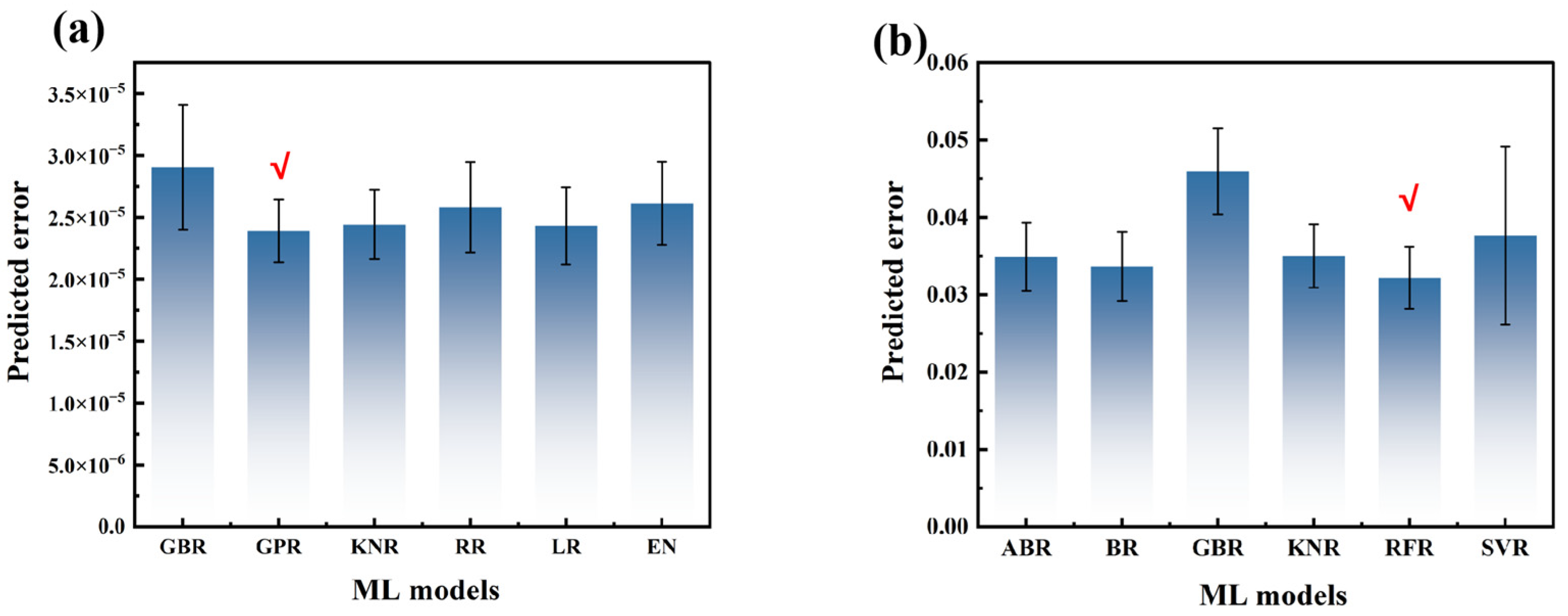
2.3. Evaluation of Model Performance
- GridSearchCV was utilized for parameter tuning, and the optimal model and parameter combination were obtained based on the grid search results.
- The process was repeated 1000 times for each model with bootstrapping. After each iteration, the fine-tuning of mesh search parameters was carried out on the training set to determine the best model and parameter combination. Subsequently, the predictions were made on the test set to calculate the mean and standard deviation of the evaluation metrics, including R2 and MSE. Origin, a professional drawing software recognized in the industry, and authoritative tools in the field of statistical testing, such as SciPy library in R and Python, were mainly adopted for statistical analysis and chart drawing in this study. In addition, the Matplotlib library was also used to assist in the drawing of charts.
3. Results and Discussion
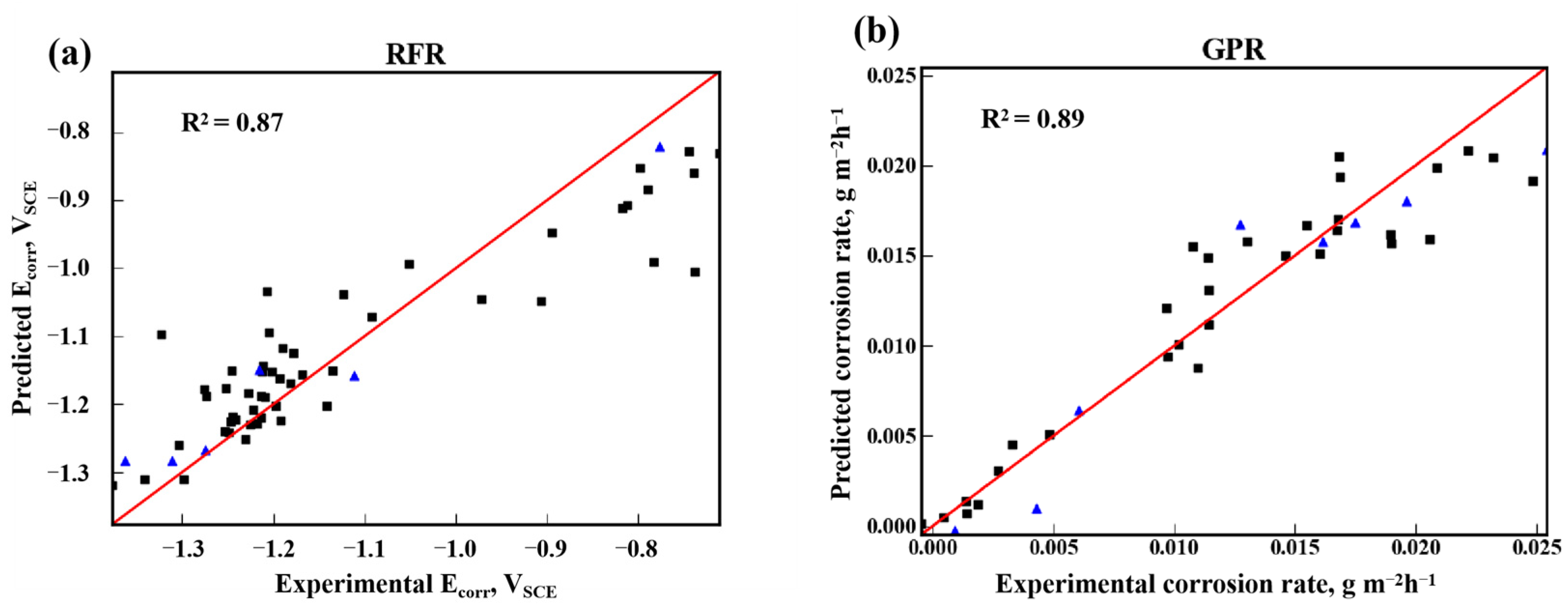
3.1. Prediction of Ecorr and Corrosion Rate
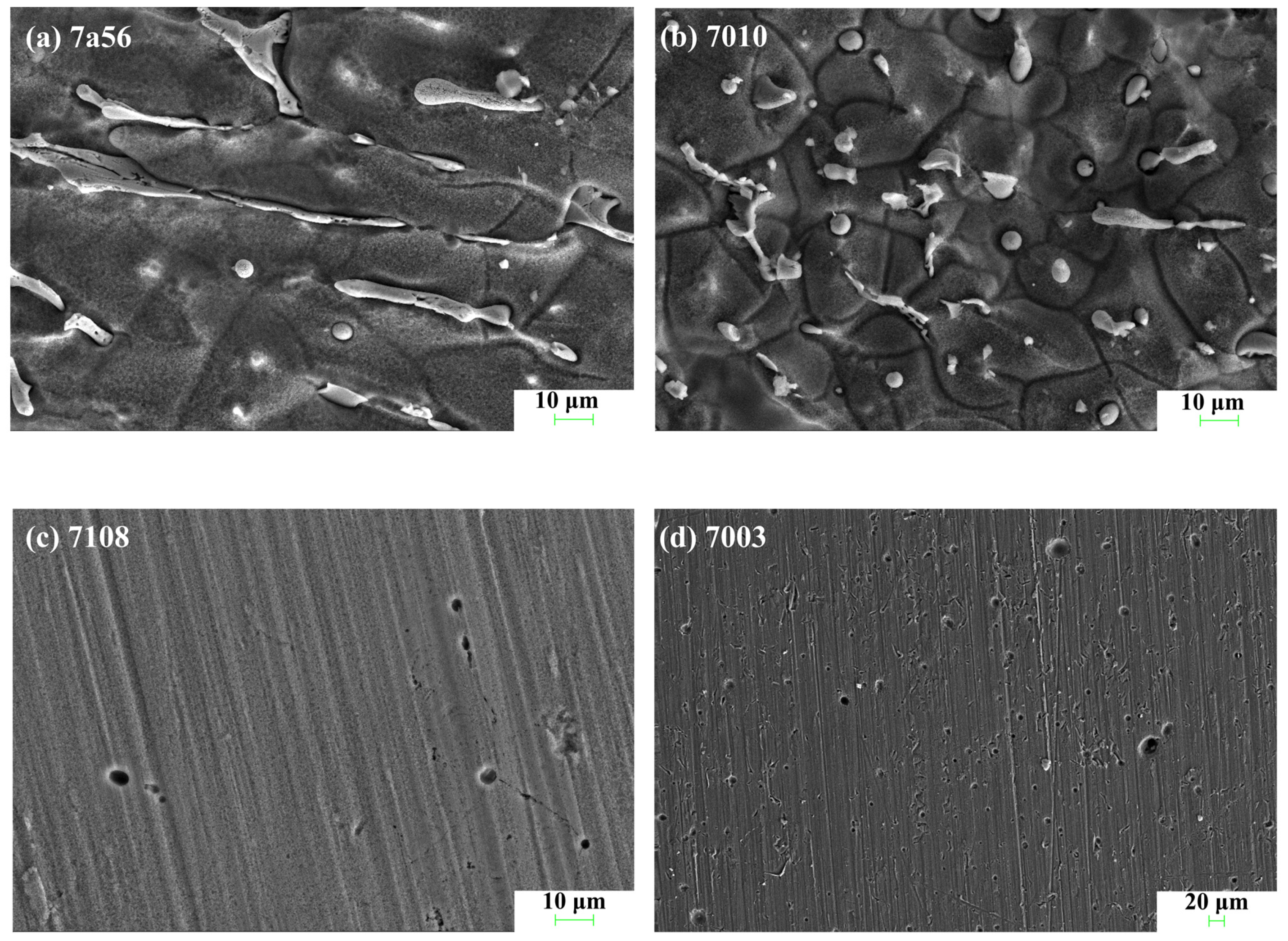
3.2. Characterization of Corrosion Morphology
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gest, R.J.; Troiano, A.R. Stress Corrosion and Hydrogen Embrittlement in an Aluminum Alloy. Corrosion 2013, 30, 274–279. [Google Scholar] [CrossRef]
- Rao, A.C.U.; Vasu, V.; Govindaraju, M.; Srinadh, K.V.S. Stress corrosion cracking behaviour of 7xxx aluminum alloys: A literature review. Trans. Nonferrous Met. Soc. China 2016, 26, 1447–1471. [Google Scholar] [CrossRef]
- Meng, S.; Yu, Y.; Zhang, X.; Zhou, L.; Liang, X.; Liu, P. Investigations on electrochemical corrosion behavior of 7075 aluminum alloy with femtosecond laser modification. Vacuum 2024, 221, 112911. [Google Scholar] [CrossRef]
- Ji, Y.; Dong, C.; Chen, L.; Xiao, K.; Li, X. High-throughput computing for screening the potential alloying elements of a 7xxx aluminum alloy for increasing the alloy resistance to stress corrosion cracking. Corros. Sci. 2021, 183, 109304. [Google Scholar] [CrossRef]
- Rout, P.K.; Ghosh, M.M.; Ghosh, K.S. Effect of solution pH on electrochemical and stress corrosion cracking behaviour of a 7150 Al–Zn–Mg–Cu alloy. Mater. Sci. Eng. A 2014, 604, 156–165. [Google Scholar] [CrossRef]
- Staley, J.T. Aging kinetics of aluminum alloy 7050. Metall. Trans. 1974, 5, 929–932. [Google Scholar] [CrossRef]
- Xue, D.; Balachandran, P.V.; Hogden, J.; Theiler, J.; Xue, D.; Lookman, T. Accelerated search for materials with targeted properties by adaptive design. Nat. Commun. 2016, 7, 11241. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, Y.; Wang, C.; Xue, D.; Bai, Y.; Antonov, S.; Dai, L.; Lookman, T.; Su, Y. Machine learning assisted design of high entropy alloys with desired property. Acta Mater. 2019, 170, 109–117. [Google Scholar] [CrossRef]
- Stanev, V.; Oses, C.; Kusne, A.G.; Rodriguez, E.; Paglione, J.; Curtarolo, S.; Takeuchi, I. Machine learning modeling of superconducting critical temperature. Npj Comput. Mater. 2018, 4, 29. [Google Scholar] [CrossRef]
- Wen, C.; Wang, C.; Zhang, Y.; Antonov, S.; Xue, D.; Lookman, T.; Su, Y. Modeling solid solution strengthening in high entropy alloys using machine learning. Acta Mater. 2021, 212, 116917. [Google Scholar] [CrossRef]
- Xue, D.; Xue, D.; Yuan, R.; Zhou, Y.; Balachandran, P.V.; Ding, X.; Sun, J.; Lookman, T. An informatics approach to transformation temperatures of NiTi-based shape memory alloys. Acta Mater. 2017, 125, 532–541. [Google Scholar] [CrossRef]
- Wei, X.; Fu, D.; Chen, M.; Wu, W.; Wu, D.; Liu, C. Data mining to effect of key alloying elements on corrosion resistance of low alloy steels in Sanya seawater environmentAlloying Elements. J. Mater. Sci. Technol. 2021, 64, 222–232. [Google Scholar] [CrossRef]
- Lv, Y.-J.; Wang, J.-W.; Wang, J.; Xiong, C.; Zou, L.; Li, L.; Li, D.-W. Steel corrosion prediction based on support vector machines. Chaos Solitons Fractals 2020, 136, 109807. [Google Scholar] [CrossRef]
- Liu, M.; Li, W. Prediction and analysis of corrosion rate of 3C steel using interpretable machine learning methods. Mater. Today Commun. 2023, 35, 106408. [Google Scholar] [CrossRef]
- Feng, X.; Wang, Z.; Jiang, L.; Zhao, F.; Zhang, Z. Simultaneous enhancement in mechanical and corrosion properties of Al-Mg-Si alloys using machine learning. J. Mater. Sci. Technol. 2023, 167, 1–13. [Google Scholar] [CrossRef]
- Ao, M.; Ji, Y.; Sun, X.; Guo, F.; Xiao, K.; Dong, C. Image Deep Learning Assisted Prediction of Mechanical and Corrosion Behavior for Al-Zn-Mg Alloys. IEEE Access 2022, 10, 35620–35631. [Google Scholar] [CrossRef]
- Messina, J.; Luo, R.; Xu, K.; Lu, G.; Deng, H.; Tschopp, M.A.; Gao, F. Machine learning to predict aluminum segregation to magnesium grain boundaries. Scr. Mater. 2021, 204, 114150. [Google Scholar] [CrossRef]
- Ji, Y.; Li, N.; Cheng, Z.; Fu, X.; Ao, M.; Li, M.; Sun, X.; Chowwanonthapunya, T.; Zhang, D.; Xiao, K.; et al. Random forest incorporating ab-initio calculations for corrosion rate prediction with small sample Al alloys data. Npj Mater. Degrad. 2022, 6, 83. [Google Scholar] [CrossRef]
- Takara, Y.; Ozawa, T.; Yamaguchi, M. Analysis of the elemental effects on the surface potential of aluminum alloy using machine learning. Jpn. J. Appl. Phys. 2022, 61, SL1008. [Google Scholar] [CrossRef]
- Blanc, C.; Mankowski, G. Susceptibility to pitting corrosion of 6056 aluminium alloy. Corros. Sci. 1997, 39, 949–959. [Google Scholar] [CrossRef]
- Zaid, B.; Saidi, D.; Benzaid, A.; Hadji, S. Effects of pH and chloride concentration on pitting corrosion of AA6061 aluminum alloy. Corros. Sci. 2008, 50, 1841–1847. [Google Scholar] [CrossRef]
- Blanc, C.; Lavelle, B.; Mankowski, G. The role of precipitates enriched with copper on the susceptibility to pitting corrosion of the 2024 aluminium alloy. Corros. Sci. 1997, 39, 495–510. [Google Scholar] [CrossRef]
- ASTM G1-03; Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens. American Society of Testing Materials: West Conshohocken, PA, USA, 2017.
- Pattanayak, S.; Dey, S.; Chatterjee, S.; Chowdhury, S.G.; Datta, S. Computational intelligence based designing of microalloyed pipeline steel. Comput. Mater. Sci. 2015, 104, 60–68. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, C.; Fu, H.; Shen, J.; Zhang, Z.; Xie, J. Discovery of aluminum alloys with ultra-strength and high-toughness via a property-oriented design strategy. J. Mater. Sci. Technol. 2022, 98, 33–43. [Google Scholar] [CrossRef]
- Zhang, H.; Fu, H.; He, X.; Wang, C.; Jiang, L.; Chen, L.-Q.; Xie, J. Dramatically Enhanced Combination of Ultimate Tensile Strength and Electric Conductivity of Alloys via Machine Learning Screening. Acta Mater. 2020, 200, 803–810. [Google Scholar] [CrossRef]
- Qu, Z.; Tang, D.; Wang, Z.; Li, X.; Chen, H.; Lv, Y. Pitting Judgment Model Based on Machine Learning and Feature Optimization Methods. Front. Mater. 2021, 8, 733813. [Google Scholar] [CrossRef]
- Diao, Y.; Yan, L.; Gao, K. Improvement of the machine learning-based corrosion rate prediction model through the optimization of input features. Mater. Des. 2021, 198, 109326. [Google Scholar] [CrossRef]
- Xie, J.; Su, Y.; Xue, D.; Jiang, X.; Fu, H.; Hung, H. Machine Learning for Materials Research and Development. Acta Metall. Sinca 2021, 57, 1343–1361. [Google Scholar]
- Kang, Q.; Mi, X.; Wang, H.; Wu, L.; Sun, K.; Tang, A. Research Progress of Artificial Neural Networks in Material Science. Mater. Rep. 2020, 34, 11. [Google Scholar]
- Saber, D.; Taha, I.B.M.; El-Aziz, K.A. Prediction of the Corrosion Rate of Al-Si Alloys Using Optimal Regression Methods. Intell. Autom. Soft Comput. 2021, 29, 757–769. [Google Scholar] [CrossRef]
- Honysz, R. Modeling the Chemical Composition of Ferritic Stainless Steels with the Use of Artificial Neural Networks. Metals 2021, 11, 724. [Google Scholar] [CrossRef]
- Churyumov, A.Y.; Kazakova, A.A. Prediction of True Stress at Hot Deformation of High Manganese Steel by Artificial Neural Network Modeling. Materials 2023, 16, 1083. [Google Scholar] [CrossRef]

| Al Alloy | Composition | Ecorr | Corrosion Rate | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | Fe | Si | Mn | Cu | Ti | Zn | Mg | Cr | Zr | VSCE | g m−2h−1 | |
| 7001 | 86.59 | 0.04 | 0.02 | 0 | 2.2 | 0 | 7.58 | 3.4 | 0.18 | 0 | −0.91 | 0.016764943 |
| 7003 | 93.41 | 0.01 | 0.1 | 0 | 0 | 0 | 5.53 | 0.8 | 0 | 0.15 | −1.3 | −0.000462364 |
| 7004 | 93.34 | 0.02 | 0.01 | 0.43 | 0 | 0 | 4.2 | 1.86 | 0 | 0.13 | −1.36 | 0.001860988 |
| 7008 | 93.81 | 0.05 | 0.01 | 0 | 0.09 | 0 | 4.61 | 1.24 | 0.02 | 0.17 | −1.27 | −0.000466587 |
| 7010 | 88.94 | 0.2 | 0.11 | 0.01 | 1.81 | 0.01 | 6.2 | 2.57 | 0.01 | 0.14 | −1.19 | 0.017504447 |
| 7020 | 91.91 | 0.52 | 0.44 | 0.48 | 0.22 | 0 | 4.67 | 1.42 | 0.34 | 0 | −1.14 | 0.011413508 |
| 7021 | 93.17 | 0.01 | 0.01 | 0 | 0 | 0 | 5.21 | 1.6 | 0 | 0 | −1.34 | 0.001402624 |
| 7034 | 84.76 | 0.18 | 0.09 | 0.01 | 1 | 0 | 11.12 | 2.67 | 0 | 0.17 | −1.21 | 0.020883803 |
| 7049 | 87.97 | 0.21 | 0.11 | 0.01 | 1.65 | 0.01 | 7.21 | 2.71 | 0.12 | 0 | −1.22 | 0.016025025 |
| 7049A | 87.97 | 0.02 | 0.01 | 0 | 1.59 | 0 | 7.64 | 2.64 | 0.13 | 0 | −1.21 | 0.009662373 |
| 7055 | 85.97 | 0.32 | 0.18 | 0.07 | 2.88 | 0.04 | 8.32 | 1.98 | 0.05 | 0.2 | −0.97 | 0.012719807 |
| 7065 | 88.39 | 0.21 | 0.1 | 0.01 | 2.2 | 0.01 | 7.1 | 1.79 | 0 | 0.18 | −1.2 | 0.019599506 |
| 7075 | 90.31 | 0.09 | 0.03 | 0 | 1.59 | 0 | 5.13 | 2.64 | 0.2 | 0 | −0.79 | 0.018946766 |
| 7076 | 89.08 | 0.06 | 0.03 | 0.5 | 0.82 | 0 | 7.68 | 1.83 | 0 | 0 | −1.14 | 0.010975013 |
| 7085 | 88.42 | 0.08 | 0.04 | 0 | 1.87 | 0 | 7.76 | 1.71 | 0 | 0.12 | −1.22 | 0.013017443 |
| 7108 | 93.14 | 0.07 | 0.02 | 0 | 0.01 | 0 | 5.29 | 1.25 | 0 | 0.21 | −1.23 | 0.004287823 |
| 7150 | 88.89 | 0.05 | 0.03 | 0 | 2.44 | 0 | 6.08 | 2.42 | 0 | 0.09 | −1.12 | 0.020571823 |
| 7175 | 89.94 | 0.1 | 0.06 | 0.01 | 1.73 | 0 | 5.39 | 2.63 | 0.14 | 0 | −0.8 | 0.015501338 |
| 7255 | 86.8 | 0.07 | 0.04 | 0 | 2.5 | 0 | 8.2 | 2.21 | 0 | 0.18 | −1.21 | 0.022177866 |
| 7A01 | 98.75 | 0.18 | 0.05 | 0 | 0.04 | 0 | 0.98 | 0 | 0 | 0 | −0.78 | 0.000929177 |
| 7A02 | 97.36 | 0.03 | 0.01 | 0 | 0.2 | 0.08 | 1.6 | 0.63 | 0.08 | 0 | −1.05 | 0.000460826 |
| 7A04 | 89.68 | 0.01 | 0.01 | 0.36 | 1.76 | 0 | 5.73 | 2.33 | 0.13 | 0 | −0.74 | 0.016159255 |
| 7A05 | 91.22 | 0.48 | 0.3 | 0.39 | 0.26 | 0.05 | 5.29 | 1.81 | 0.04 | 0.16 | −1.11 | 0.010170653 |
| 7A09 | 90.38 | 0.03 | 0.01 | 0.04 | 1.75 | 0 | 4.95 | 2.65 | 0 | 0.19 | −0.78 | 0.018981165 |
| 7A10 | 91.58 | 0.02 | 0.01 | 0.28 | 0.77 | 0 | 3.59 | 3.63 | 0.12 | 0 | −0.81 | 0.006033941 |
| 7A11 | 97.03 | 0.07 | 0.02 | 1.19 | 0.12 | 0 | 1.56 | 0 | 0 | 0 | −0.82 | 0.00138087 |
| 7A33 | 92.25 | 0.07 | 0.02 | 0.01 | 0.39 | 0 | 4.76 | 2.43 | 0.07 | 0 | −1.25 | 0.003284987 |
| 7A36 | 86.72 | 0.06 | 0.03 | 0.01 | 2.24 | 0 | 8.57 | 2.23 | 0 | 0.15 | −1.23 | 0.028420891 |
| 7A46 | 91.64 | 0 | 0.01 | 0 | 0.27 | 0 | 6.56 | 1.51 | 0 | 0 | −1.28 | 0.00272138 |
| 7A55 | 87.31 | 0.04 | 0.02 | 0.01 | 2.24 | 0.03 | 7.83 | 2.41 | 0 | 0.12 | −1.21 | 0.024831672 |
| 7A56 | 87.03 | 0.04 | 0.03 | 0.01 | 1.78 | 0 | 8.83 | 2.12 | 0 | 0.16 | −1.23 | 0.023217957 |
| 7A85 | 88.63 | 0.02 | 0.01 | 0 | 1.78 | 0 | 7.52 | 1.91 | 0.01 | 0.13 | −1.24 | 0.011437292 |
| 7A88 | 90.9 | 0.06 | 0.03 | 0.41 | 1.45 | 0 | 4.84 | 2.19 | 0.11 | 0 | −0.9 | 0.011410403 |
| 7A93 | 85.97 | 0.04 | 0.02 | 0 | 1.97 | 0 | 9.46 | 2.32 | 0 | 0.22 | −1.25 | 0.016879487 |
| 7A99 | 87.96 | 0.03 | 0.01 | 0 | 1.84 | 0 | 7.66 | 2.35 | 0 | 0.15 | −1.25 | 0.014618998 |
| 7B04 | 88.98 | 0.12 | 0.01 | 0.44 | 1.94 | 0 | 5.73 | 2.64 | 0.14 | 0 | −0.74 | 0.010751195 |
| 7B68 | 86.98 | 0.02 | 0.01 | 0 | 2.44 | 0.03 | 7.91 | 2.4 | 0 | 0.21 | −1.21 | 0.016831389 |
| 7B85 | 85.77 | 0.24 | 0.12 | 0.01 | 1.53 | 0.01 | 8.38 | 2.24 | 0 | 1.7 | −1.17 | 0.016737208 |
| 7C04 | 91.92 | 0.01 | 0.01 | 0.4 | 1.79 | 0 | 3.54 | 2.2 | 0.15 | 0 | −0.71 | 0.009723648 |
| 7E49 | 88.45 | 0.04 | 0.02 | 0.3 | 0.67 | 0 | 7.42 | 2.81 | 0 | 0.14 | −1.25 | 0.004823047 |
| R2 of Training Set | R2 of Test Set | MSE of Training Set | MSE of Test Set | |
|---|---|---|---|---|
| Ecorr | 0.91 | 0.87 | 3.21 × 10−3 | 4.8 × 10−3 |
| Corrosion rate | 0.91 | 0.89 | 5.12 × 10−6 | 6.64 × 10−6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, X.; Zhang, N.; Yang, J.; Chen, T.; Niu, T. Machine Learning-Assisted Prediction of Corrosion Behavior of 7XXX Aluminum Alloys. Metals 2024, 14, 401. https://doi.org/10.3390/met14040401
Xiong X, Zhang N, Yang J, Chen T, Niu T. Machine Learning-Assisted Prediction of Corrosion Behavior of 7XXX Aluminum Alloys. Metals. 2024; 14(4):401. https://doi.org/10.3390/met14040401
Chicago/Turabian StyleXiong, Xilin, Na Zhang, Jingjing Yang, Tongqian Chen, and Tong Niu. 2024. "Machine Learning-Assisted Prediction of Corrosion Behavior of 7XXX Aluminum Alloys" Metals 14, no. 4: 401. https://doi.org/10.3390/met14040401
APA StyleXiong, X., Zhang, N., Yang, J., Chen, T., & Niu, T. (2024). Machine Learning-Assisted Prediction of Corrosion Behavior of 7XXX Aluminum Alloys. Metals, 14(4), 401. https://doi.org/10.3390/met14040401






