Microstructure and Wear Resistance of Grx-Ti-BN Composite Coating on TC4 by Argon Arc Cladding
Abstract
:1. Introduction
2. Experimental Section
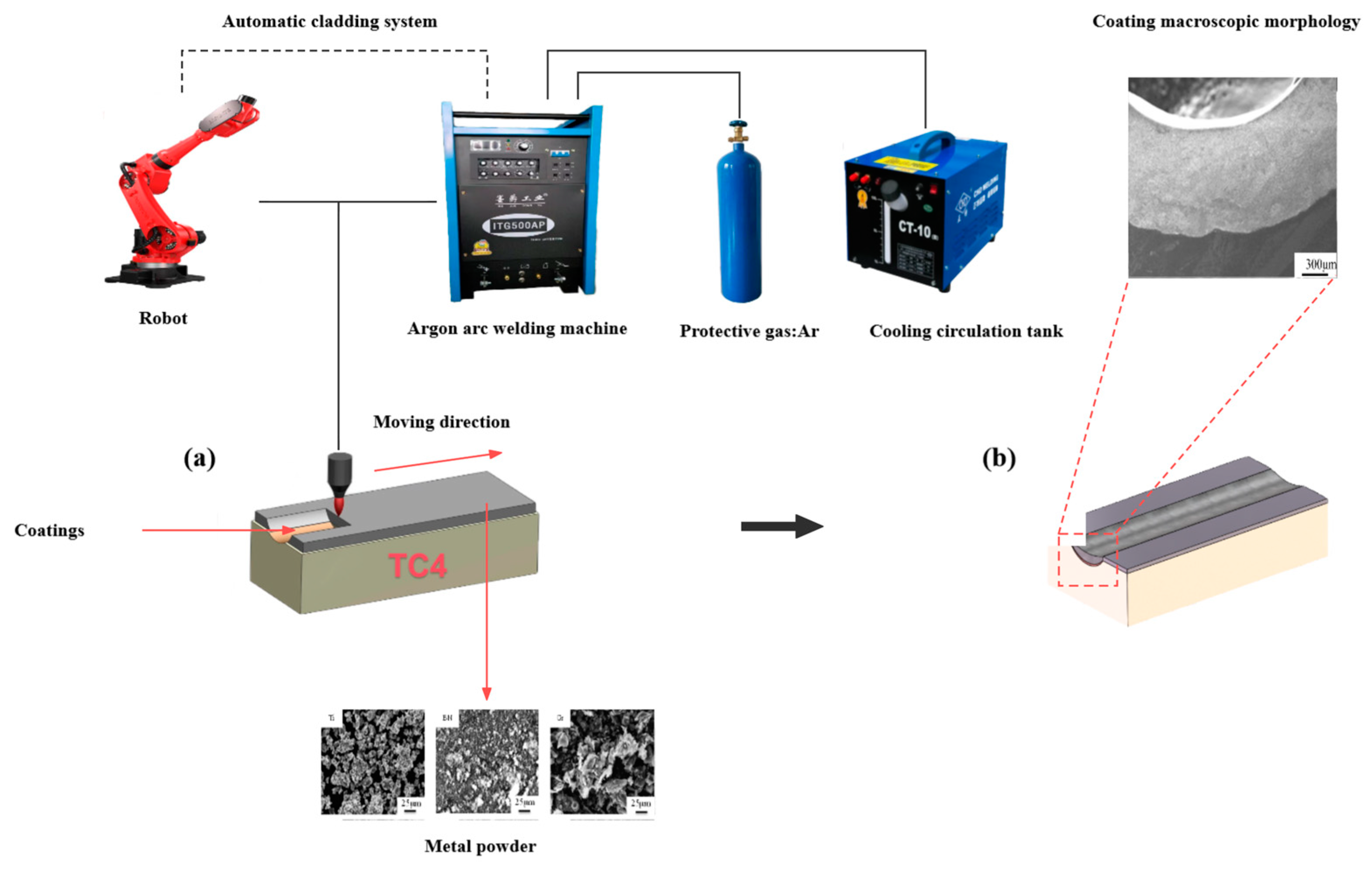
2.1. Material and Preparation
2.2. Microstructure Observation and Performance Testing
3. Results and Discussion
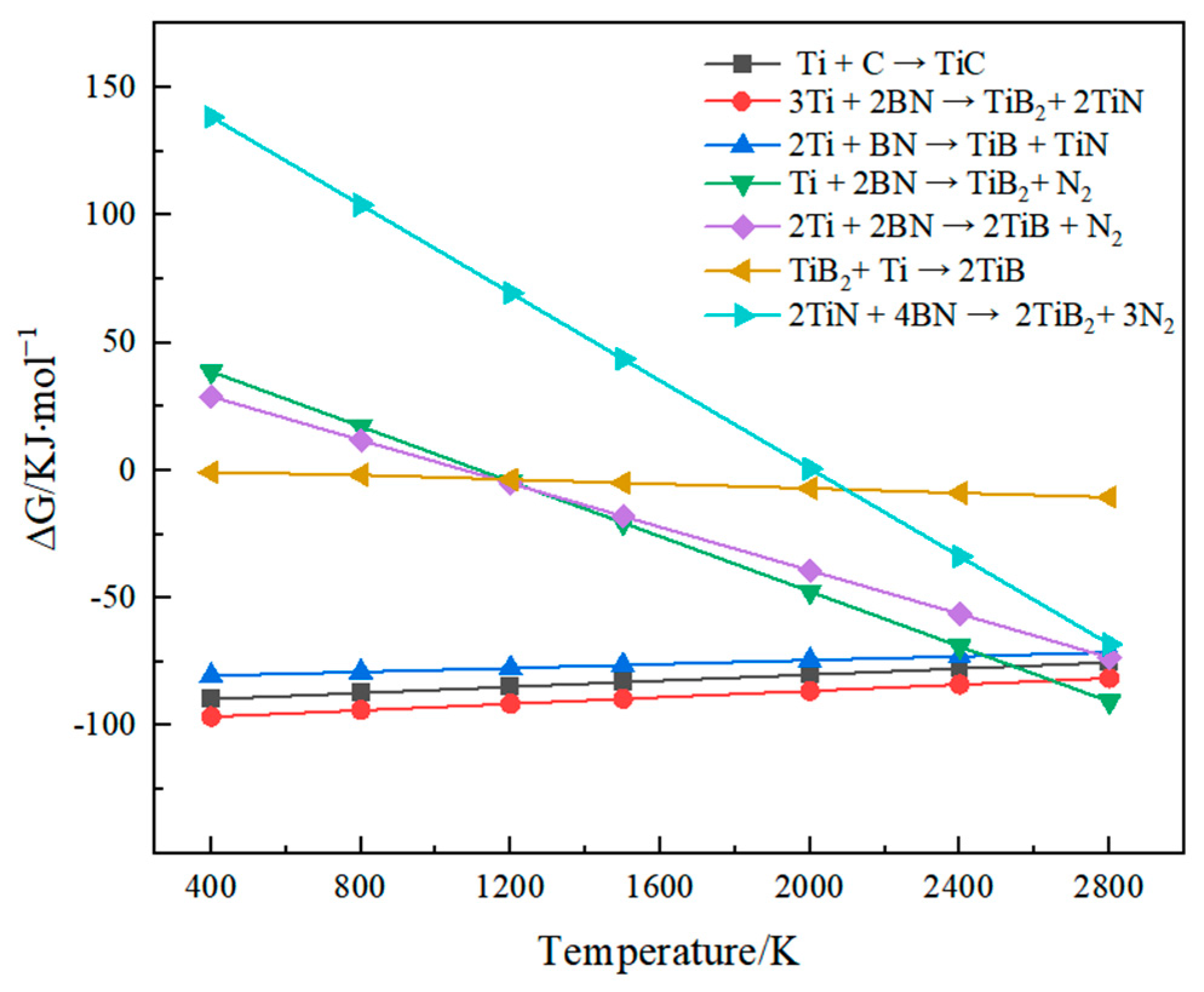
3.1. Coating Thermodynamic Analysis and Phase Composition
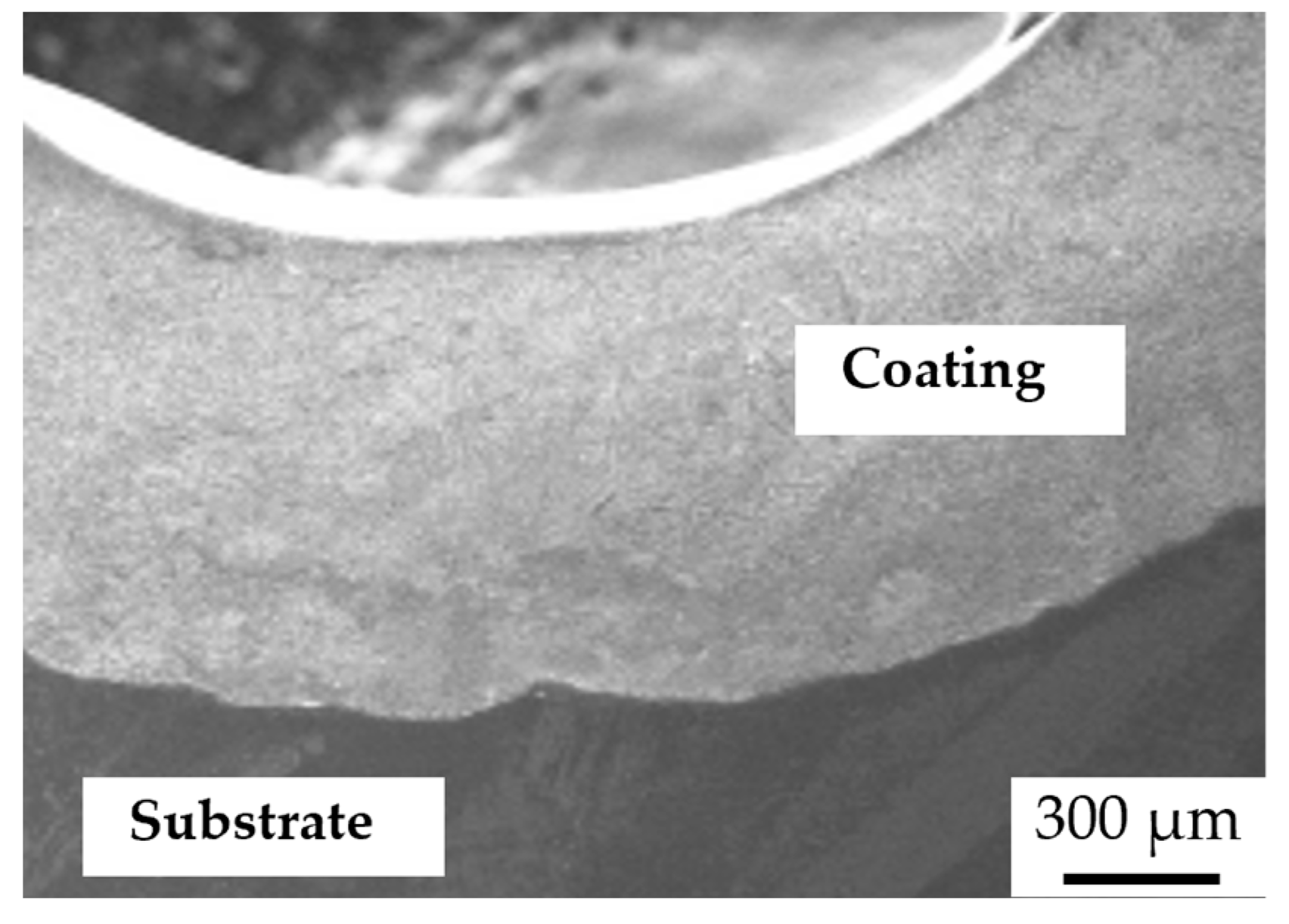
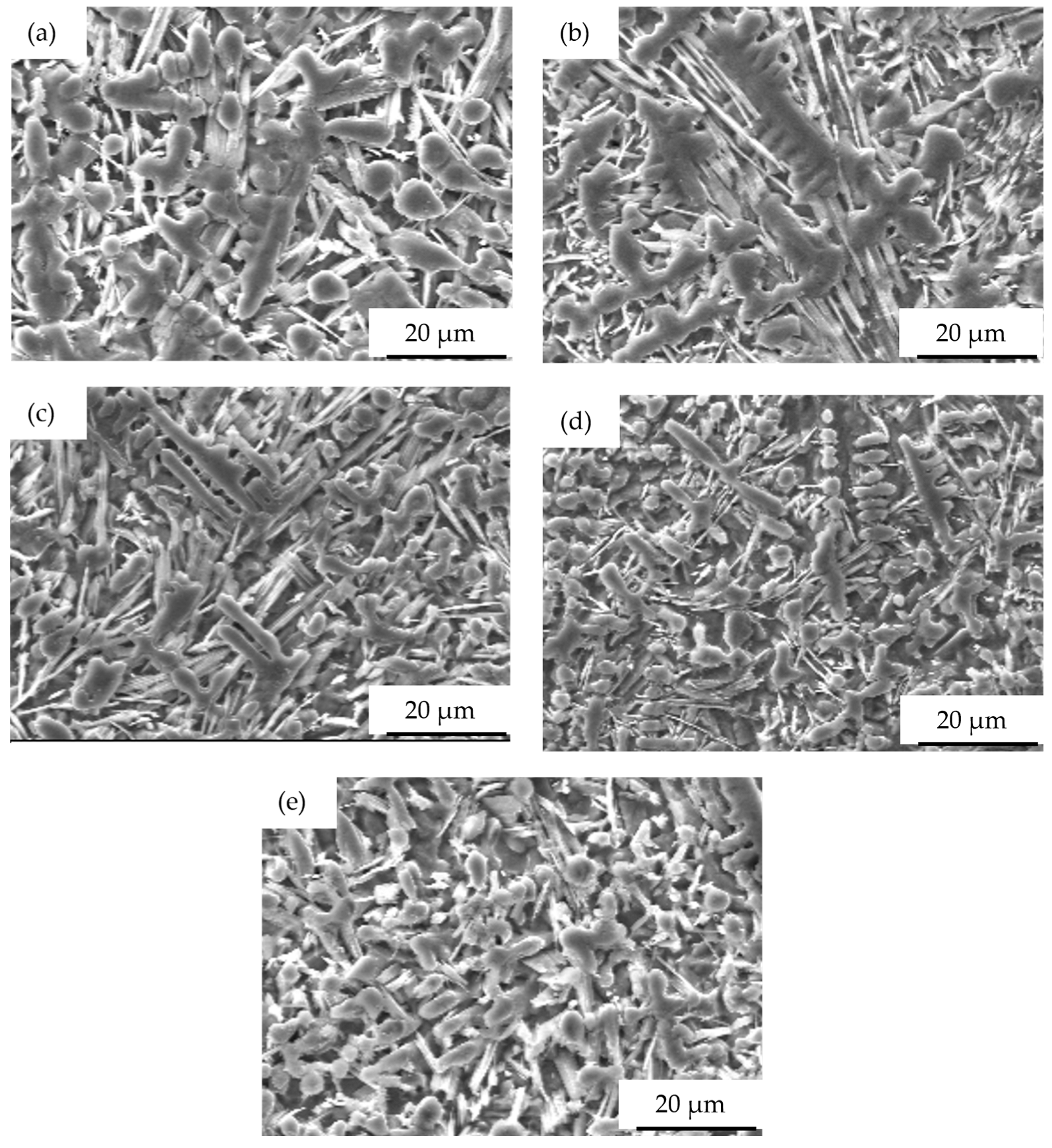
3.2. Microstructure of the Coatings
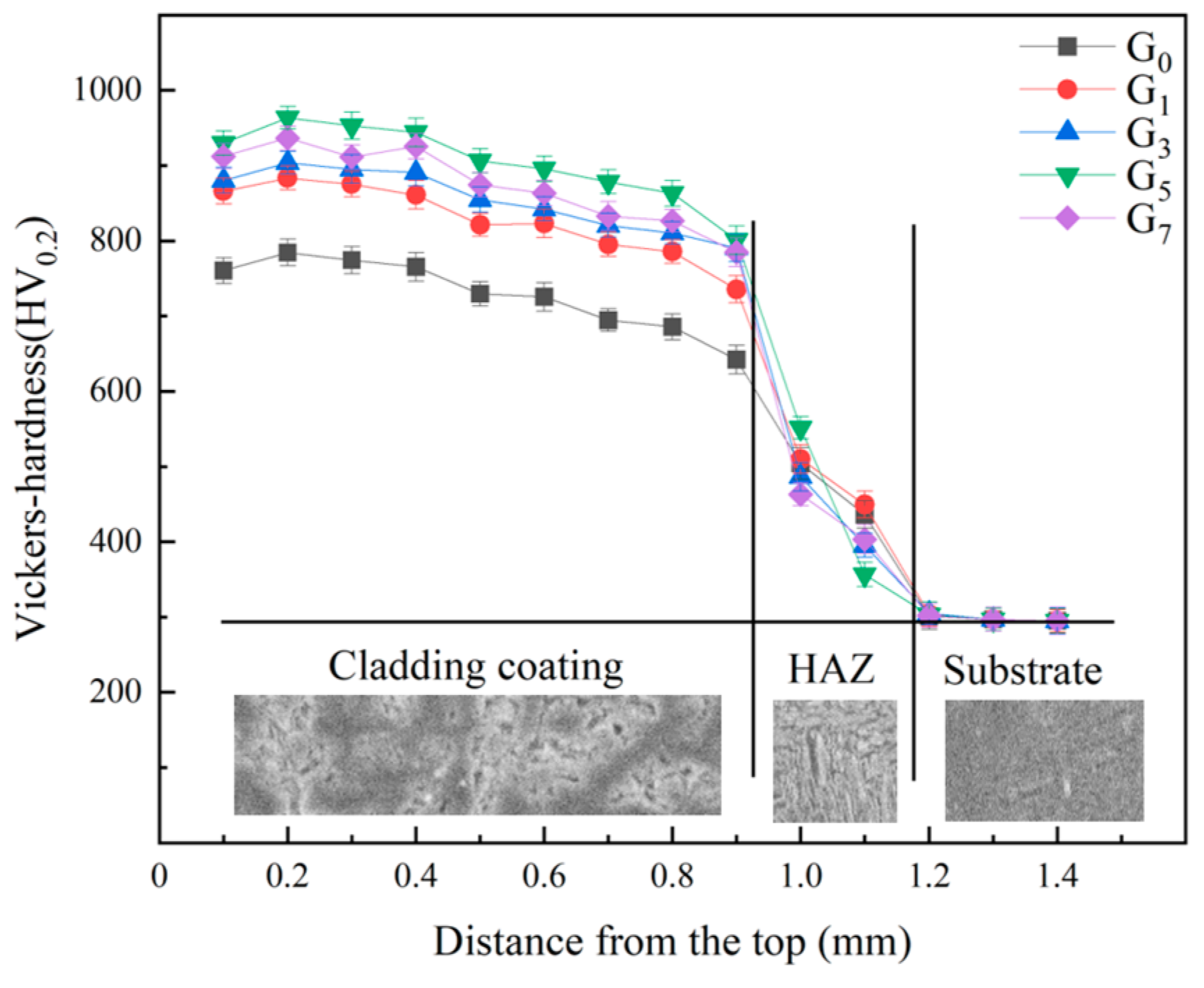
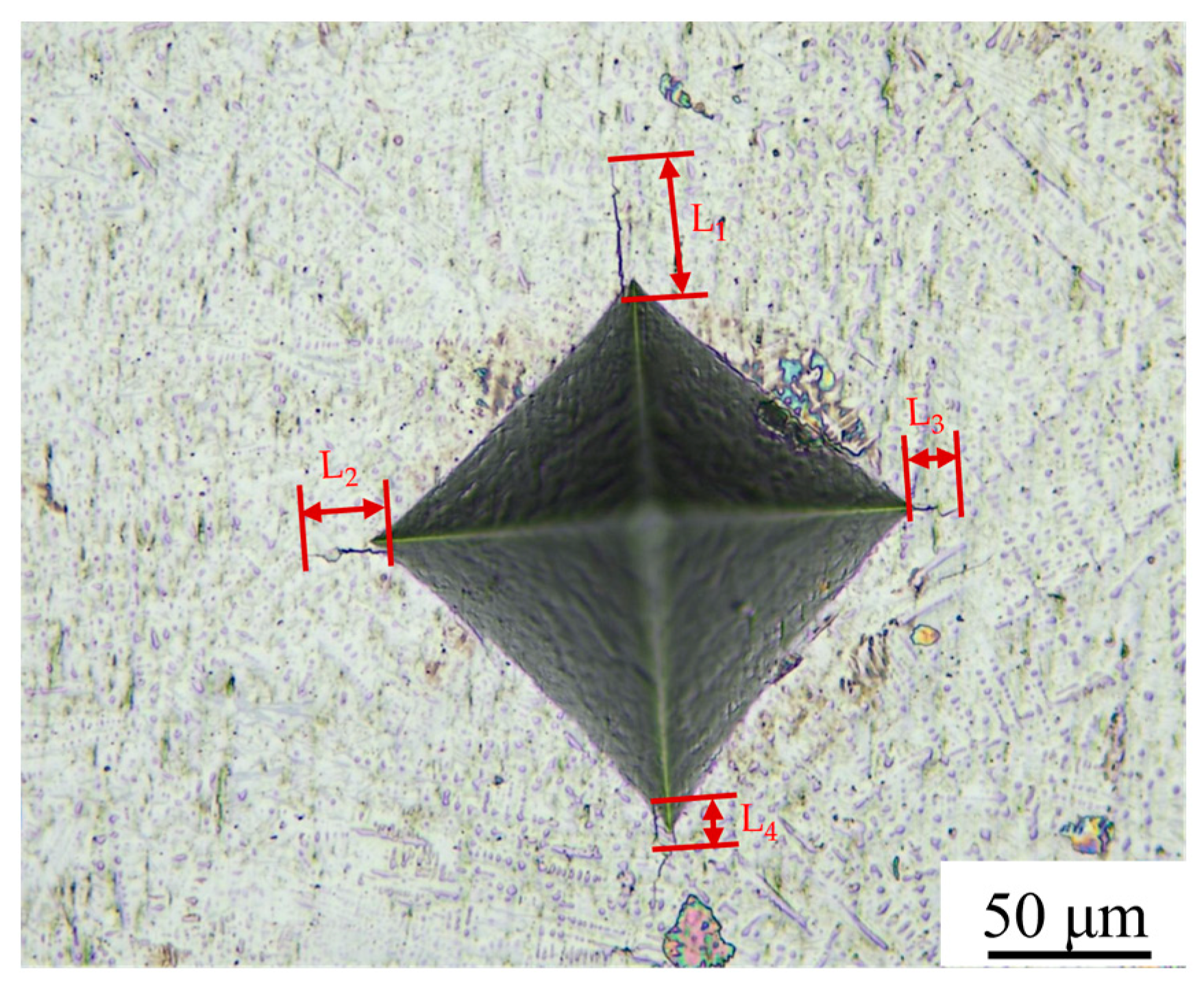
3.3. Microhardness of the Cladding Layer
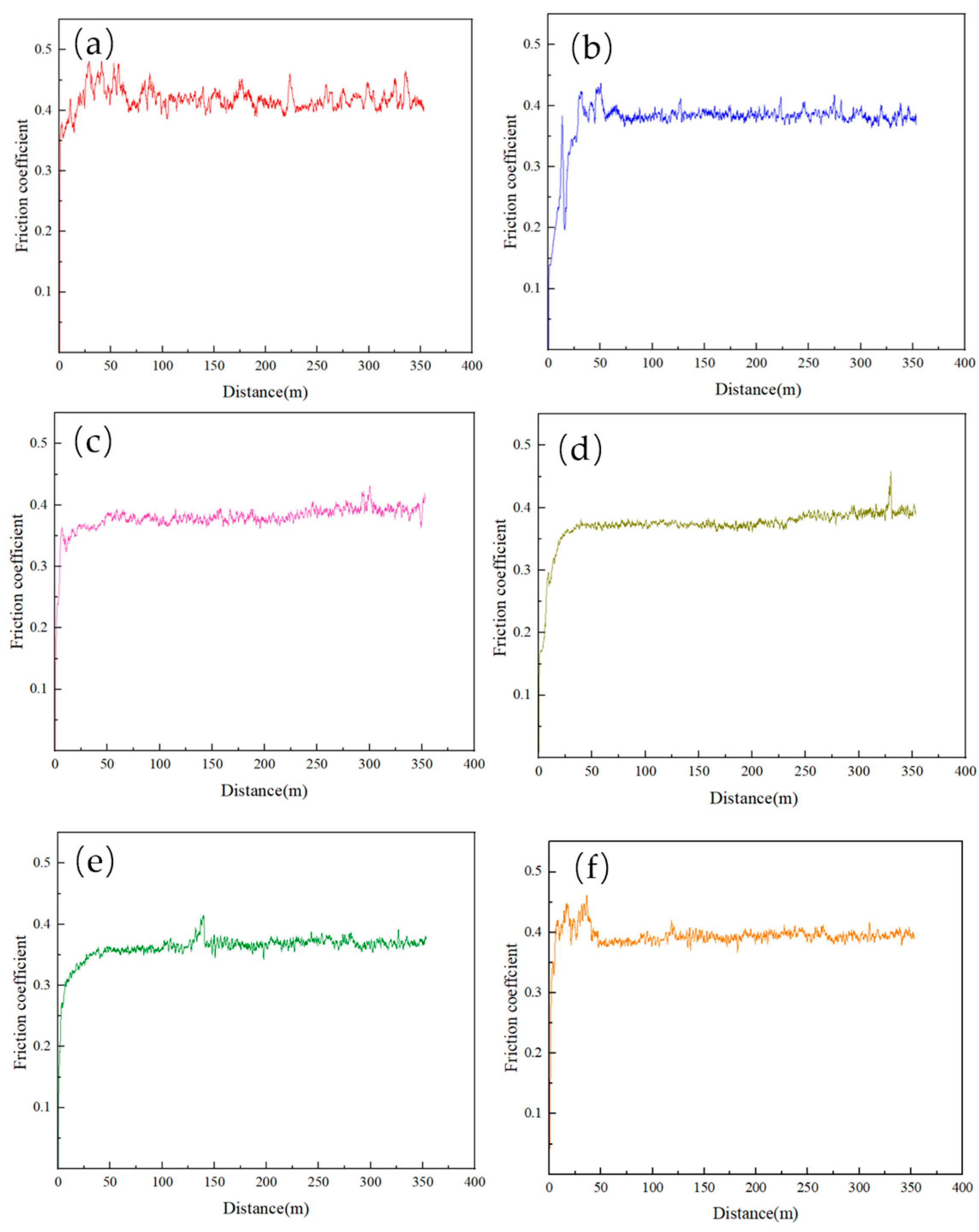
3.4. Wear Behavior
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Li, J.; Dang, C.; Wang, Y.; Zhu, Y. Influence of carbon contents on the structure and tribocorrosion properties of TiSiCN coatings on Ti6Al4V. Tribol. Int. 2017, 109, 285296. [Google Scholar] [CrossRef]
- Li, M.C.; Zhang, P.L.; Yu, Z.S.; Hua, Y.; Xin, Y.; Shaowei, L. Microstructure and Micromechanical Properties of Ni-Ti-Si Coating Deposited by Laser Cladding. Rare Met. Mater. Eng. 2019, 48, 1225. [Google Scholar]
- Mao, X.; Dong, L.; Zhang, Y.; Qin, S.; Sun, G.; Wang, L. Superior strength-ductility combination in TiC/TC4 composites via in situ construction Ti2Cu nanoparticles. Carbon 2024, 219, 118805. [Google Scholar] [CrossRef]
- Wang, J.H.; Peng, F.D.; Xu, Q.; Zhao, X.; Hu, Z.; Zhang, J.; Liu, Y. Enhanced dry machinability of TC4 titanium alloy by longitudinal-bending hybrid ultrasonic vibration-assisted milling. J. Clean. Prod. 2022, 379, 134866. [Google Scholar]
- Zhao, X.; Liu, H.; Li, S.; Wang, X.; Sheng, Y.; Zhang, P.; Li, W. Corrigendum to “Combined effect of TiN coating and surface texture on corrosion-wear behavior of selective laser melted CP-titanium in simulated body fluid”. J. Alloys Compd. 2020, 842, 155750. [Google Scholar] [CrossRef]
- Sun, J.; Qi, Y.J.; Liu, H.; Zeng, R.C. Research Progress on Tribo-Corrosion of Titanium and Titani-um Alloys in Seawater Environment. Mater. Prot. 2020, 53, 151–156. [Google Scholar]
- Zhang, L.T.; Liu, D.X.; Zhang, W.Q.; Wang, X.S.; Dai, X.Y.; Xu, J.F. Research Progress of Laser Cladding Coating on Titanium Alloy Surface. Surf. Technol. 2020, 49, 97–104. [Google Scholar]
- Zhang, Z.Q.; Yang, F.; Zhang, H.W. Microstructure and wear resistance of TiCx reinforced Ti-based laser cladding coating with race earth. Aeronaut. Astronaut. Sin. 2021, 42, 43–56. [Google Scholar]
- He, Y.N.; Yu, S.R.; Li, S.X.; Chen, Q. Evolution of Tribological Characteristics of TC4 Alloy in Fretting Wear Partial Slip Regime. Mater. Rep. 2021, 35, 14096–14100. [Google Scholar]
- Liu, J.D.; Zhang, S.Q.; Wang, H.M. Microstructure and wear resistance of laser cladding WC particles reinforced composite coatings. Chin. J. Nonferrous Met. 2012, 22, 2600–2607. [Google Scholar]
- Zhao, X.; Lv, P.; Fang, S.; Tu, X.; Ren, P.; Liu, D.; Chen, L.; Xiao, L.; Liu, S. Microstructure and Wear Behavior of Ti-xFe-SiC In Situ Composite Ceramic Coatings on TC4 Substrate from Laser Cladding. Materials 2023, 17, 100. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Shen, K.; Wang, X. Microstructural evolution of low-pressure plasma-sprayed Ti–6Al–4V coatings after heat treatment. Surf. Coat. Technol. 2020, 393, 125792. [Google Scholar] [CrossRef]
- Koseki, S.; Inoue, K.; Sekiya, K.; Morito, S.; Ohba, T.; Usuki, H. Wear mechanisms of PVD-coated cutting tools during continuous turning of Ti-6Al-4V alloy. Precis. Eng. 2017, 47, 434. [Google Scholar] [CrossRef]
- Liu, L.; Liu, H.; Zhang, X.; Wang, Y.; Hao, X. Corrosion Behavior of TiMoNbX (X = Ta, Cr, Zr) Refractory High Entropy Alloy Coating Prepared by Laser Cladding Based on TC4 Titanium Alloy. Materials 2023, 16, 3860. [Google Scholar] [CrossRef] [PubMed]
- Vaz, R.F.; Garfias, A.; Albaladejo, V.; Sanchez, J.; Cano, I.G. A Review of Advances in Cold Spray Additive Manufacturing. Coatings 2023, 13, 267. [Google Scholar] [CrossRef]
- Yang, C.Y.; Cheng, X.; Tang, H.B.; Tian, X.; Liu, D. Influence of microstructures and wear behaviors of the microstructures coatings on TC11 alloy surface using laser cladding technique. Surf. Coat. Technol. 2018, 337, 97. [Google Scholar] [CrossRef]
- Wen, R.H.; Ming, P. Microstructure and wear resistance of laser cladding NiCrAlY/TC4 composite coatings with in-situ synthesized transition layer by modulating thermal boundary in nitrogen atmosphere. J. Mater. Sci. 2023, 58, 16119–16143. [Google Scholar]
- Meng, J.; Ji, Z. Microstructure and Technology Research of In-situ Synthesis TiN-TiB2/Ni Composite Coating by Argon Arc Cladding. Phys. Procedia 2013, 50, 253–260. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Ma, G.Z.; Xiao, Y.F.; Wang, X.H.; Yu, T.Y.; Wang, H.P.; Wang, H.D. Research Status of Graphene Friction Reduction and Anti-wear Coating Technology. Surf. Technol. 2023, 52, 78–87+121. [Google Scholar]
- Berman, D.; Erdemir, A.; Sumant, A.V. Graphene: A new emerging lubricant. Mater. Today 2014, 17, 31–42. [Google Scholar] [CrossRef]
- Xiao, P.; Yang, L.; Liu, J.; Zhang, X.; Chen, D. A non-fluorinated superhydrophobic composite coating with excellent anticorrosion and wear-resistant performance. Front. Chem. 2022, 10, 952919. [Google Scholar] [CrossRef] [PubMed]
- Baig, Z.; Mamat, O.; Mustapha, M. Recent Progress on the Dispersion and the Strengthening Effect of Carbon Nanotubes and Graphene-Reinforced Metal Nanocomposites Critical. Rev. Solid State Mater. Sci. 2016, 43, 1–46. [Google Scholar]
- Rashad, M.; Pan, F.; Tang, A.; Asif, M.; She, J.; Gou, J.; Mao, J.; Hu, H. Development of magnesium-graphene nanoplatelets composite. J. Compos. Mater. 2014, 49, 285–293. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Xiao, B.L.; Wang, W.G.; Ma, Z.Y. Analysis of carbon nanotube shortening and composite strengthening in carbon nanotube/aluminum composites fabricated by multi-pass friction stir processing. Carbon 2012, 50, 1843. [Google Scholar] [CrossRef]
- Liu, X.H.; Li, J.J.; Liu, E.Z.; He, C.; Shi, C.; Zhao, N. Towards strength-ductility synergy with favorable strengthening effect through the formation of a quasi-continuous graphene nanosheets coated Ni structure in aluminum matrix composite. Mater. Sci. Eng. A 2019, 748, 52. [Google Scholar] [CrossRef]
- Liang, B.M.; Luo, M.Y.; Zhang, Z.J.; Zhang, X.H. Research Progress on Preparation and Strengthening Mechanism of Graphene Raw Materials for Graphene Metal Matrix Composites. Light Ind. Sci. Technol. 2017, 8, 5. [Google Scholar]
- Meng, J.S.; Li, C.S.; Mi, D.Z.; Liu, B.B.; Xue, F.; Cui, W.C. Structure and Properties of Graphene Reinforced Ti-bas-ed Composite Coatings on TC4 Alloy. Surf. Technol. 2021, 50, 79–85. [Google Scholar]
- Zhang, M.C.; Guo, R.P.; Zhang, Y. Preparation and Characterization of High-entropy Cemented Carbide WC-AlCo0.4CrFeNi2.7. Mater. Rep. 2024, 38, 168–173. [Google Scholar]
- Ye, D.L.; Hu, J.H. Practical Thermodynamic Numbers for Inorganic Substances According to the Manual; Metallurgical Industry Press: Dongcheng, China, 2002. [Google Scholar]
- Ehira, M.; Egami, A. Mechanical properties and microstructures of submicroncemets. Int. J. Refract. Met. Hard Mater. 1995, 13, 313–319. [Google Scholar] [CrossRef]
- Song, B.X.; Yu, T.B.; Jiang, X.Y.; Xi, W.C. Analysis of Molten Pool Temperatures and Convection Caused by Laser Cladding. J. North-East. Univ. 2020, 41, 1427–1431. [Google Scholar]
- Zhang, C.W. Study on the Structure and Performance of Ti(B, C, N) Powders on Steel and Titanium Alloy Substrate by Laser Cladding; Jilin University: Changchun, China, 2015. [Google Scholar]
- Xiong, L.S. Fundamentals of Welding Engineering; China Machine Press: Beijing, China, 2007; pp. 113–154. [Google Scholar]
- Meng, J.S.; Shi, X.P.; Zhang, S.J.; Wang, M.; Xue, F.; Liu, B.; Bian, L. Friction and wear properties of TiN-TiB2-Ni based composite coatings by argon arc cladding technology. Surf. Coat. Technol. 2019, 374, 437–447. [Google Scholar] [CrossRef]
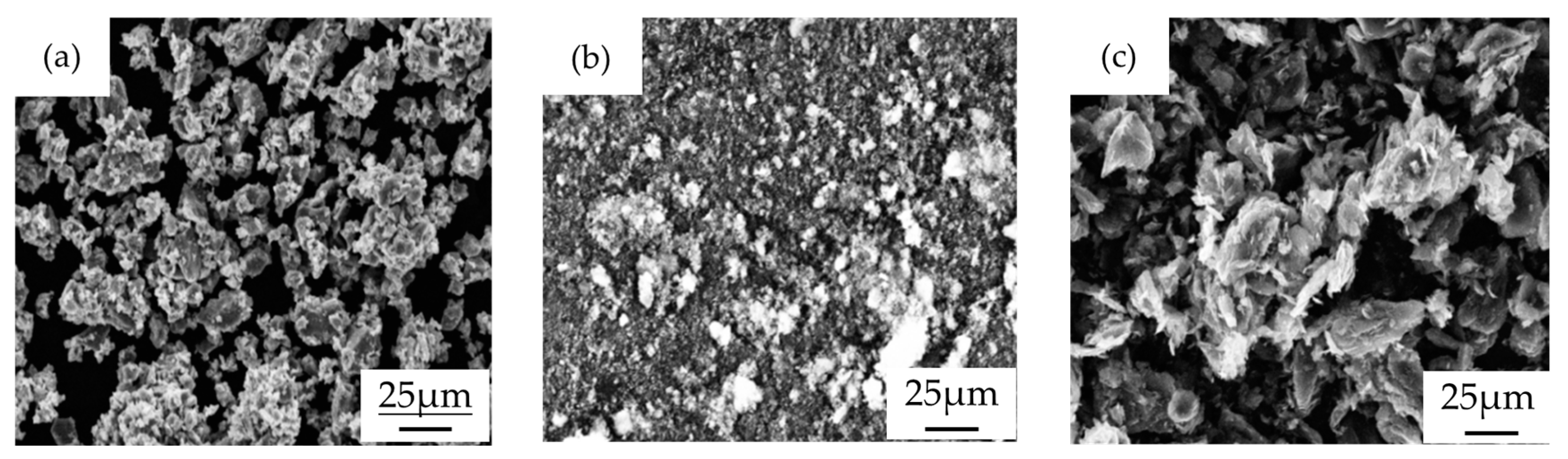





| Constituencies | Powder Mass (g) | ||
|---|---|---|---|
| Ti | BN | Gr | |
| Ti-BN-G0 | 3.71 | 1.29 | 0 |
| Ti-BN-G1 | 3.67 | 1.28 | 0.05 |
| Ti-BN-G3 | 3.60 | 1.25 | 0.15 |
| Ti-BN-G5 | 3.52 | 1.23 | 0.25 |
| Ti-BN-G7 | 3.45 | 1.20 | 0.35 |
| Current/A | Voltage/V | Cladding Speed/mm·s−1 | Argon Flow/L·min−1 | Argon Purity/% |
|---|---|---|---|---|
| 100 | 14.5 | 3.0 | 10 | 99.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Meng, J.; Ding, H.; Hou, Y.; Li, S.; Hao, C.; Shi, X. Microstructure and Wear Resistance of Grx-Ti-BN Composite Coating on TC4 by Argon Arc Cladding. Metals 2024, 14, 403. https://doi.org/10.3390/met14040403
Li Q, Meng J, Ding H, Hou Y, Li S, Hao C, Shi X. Microstructure and Wear Resistance of Grx-Ti-BN Composite Coating on TC4 by Argon Arc Cladding. Metals. 2024; 14(4):403. https://doi.org/10.3390/met14040403
Chicago/Turabian StyleLi, Qindong, Junsheng Meng, Hao Ding, Yilin Hou, Sijie Li, Chenfan Hao, and Xiaoping Shi. 2024. "Microstructure and Wear Resistance of Grx-Ti-BN Composite Coating on TC4 by Argon Arc Cladding" Metals 14, no. 4: 403. https://doi.org/10.3390/met14040403




