Zr as an Alternative Grain Refiner in the Novel AlSi5Cu2Mg Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results
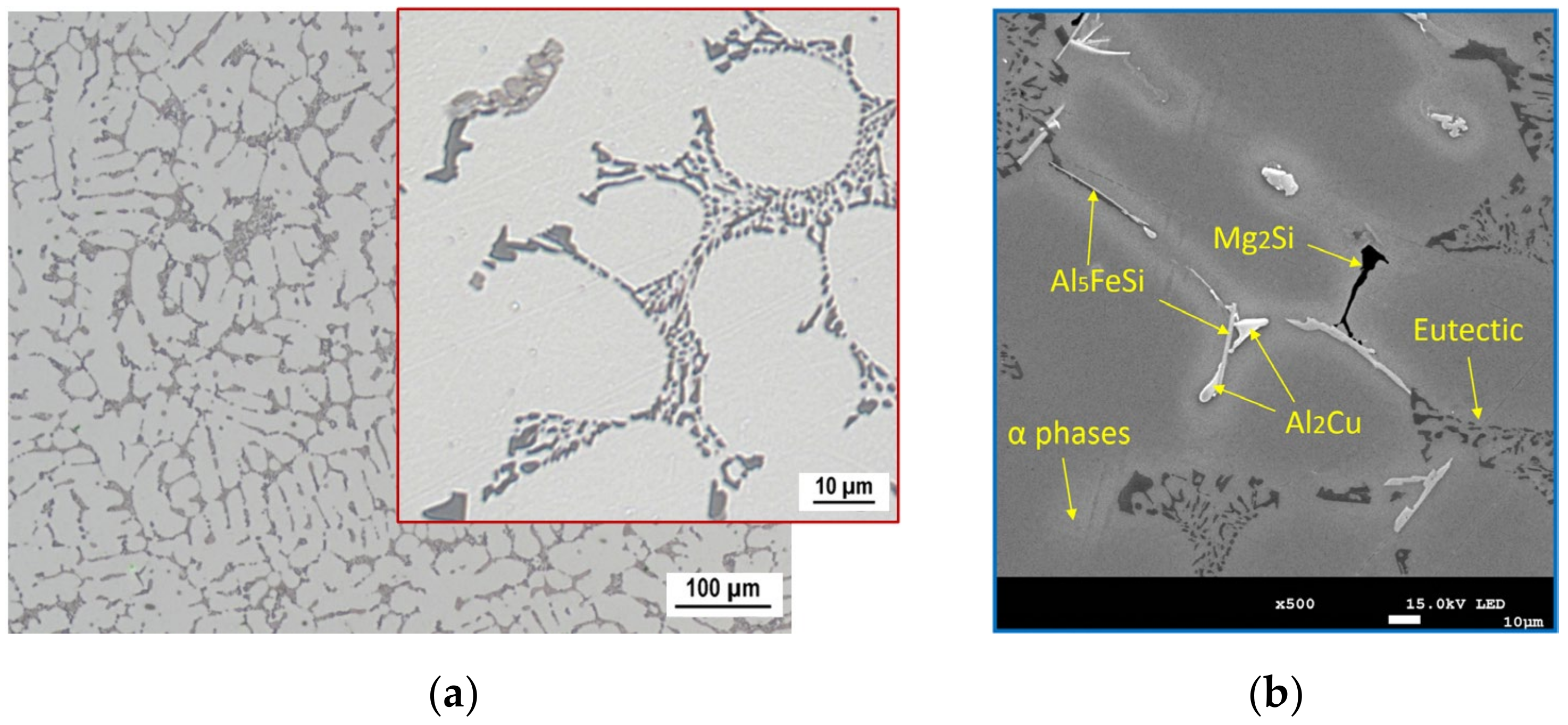
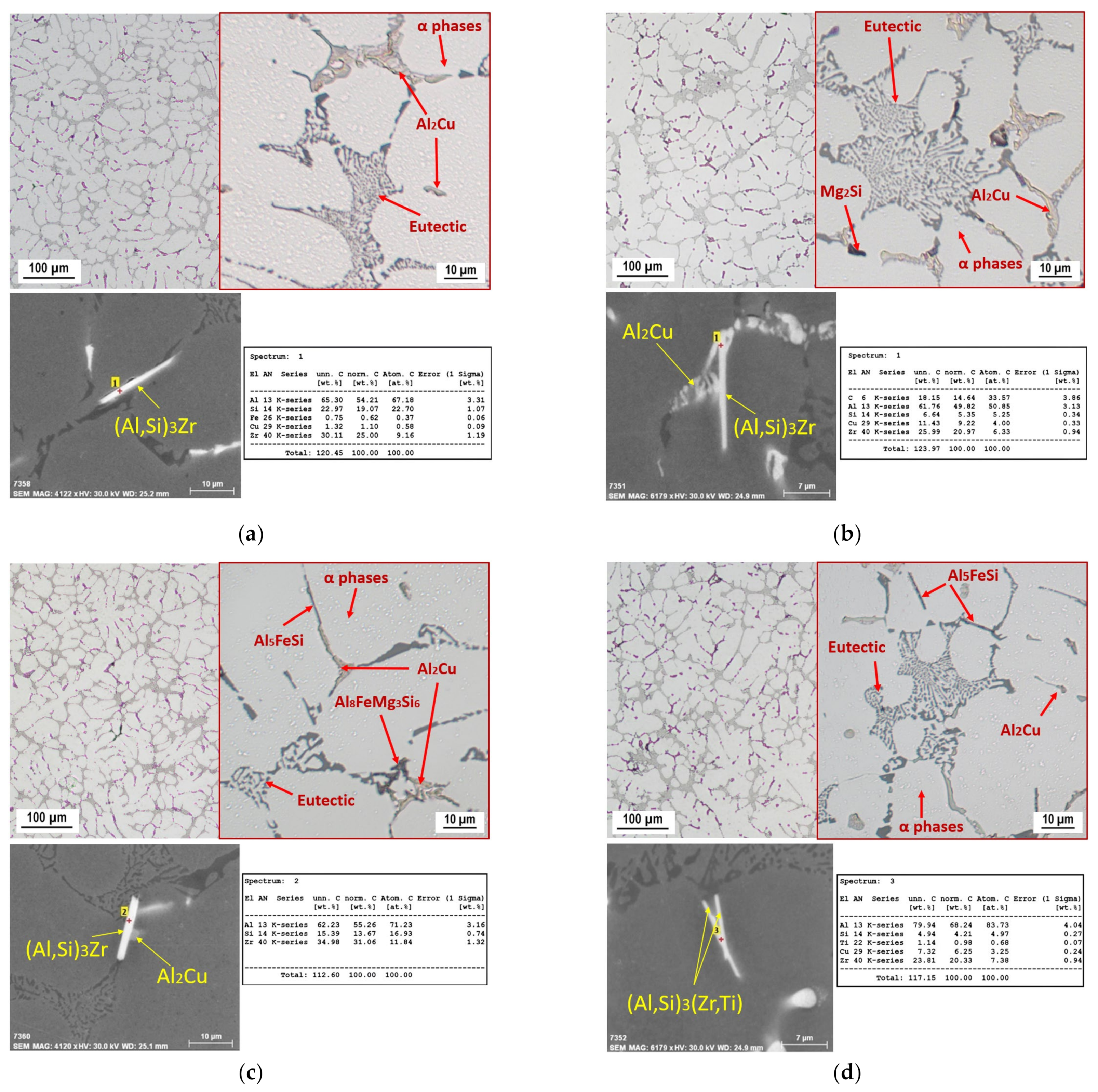
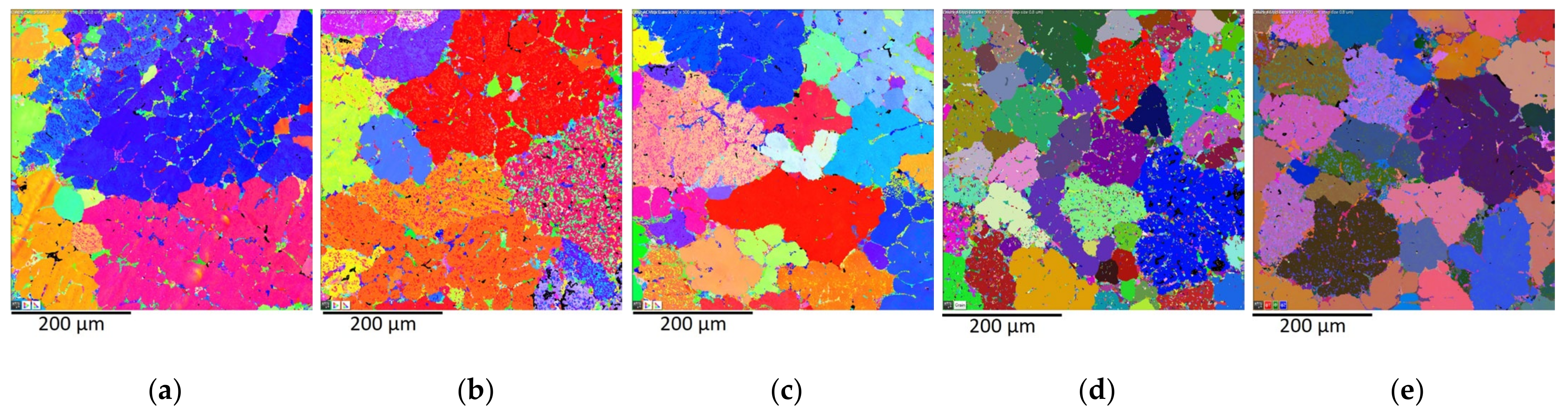
3.1. Microstructure
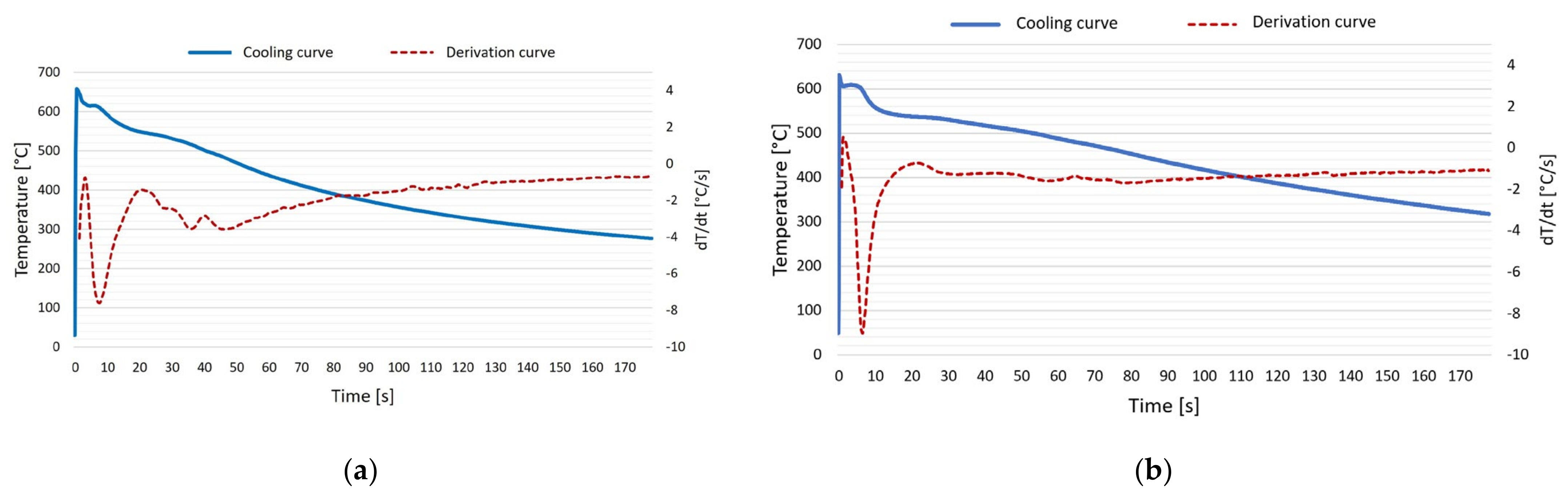
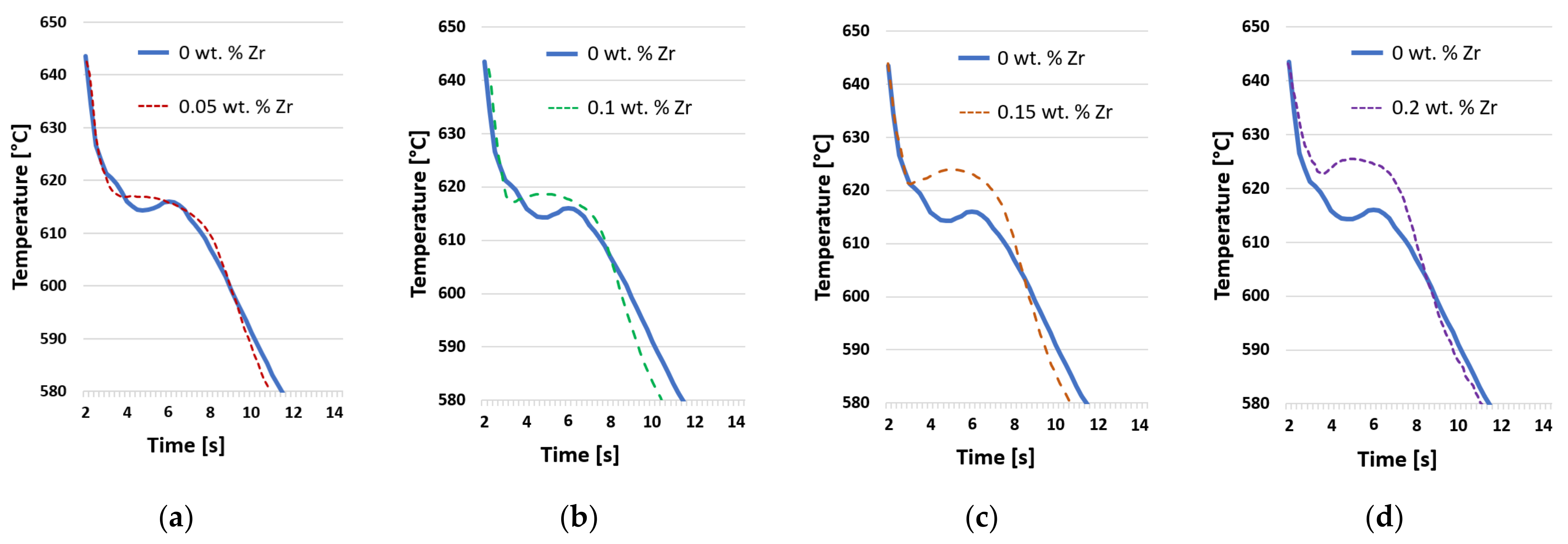
3.2. Crystallization Process
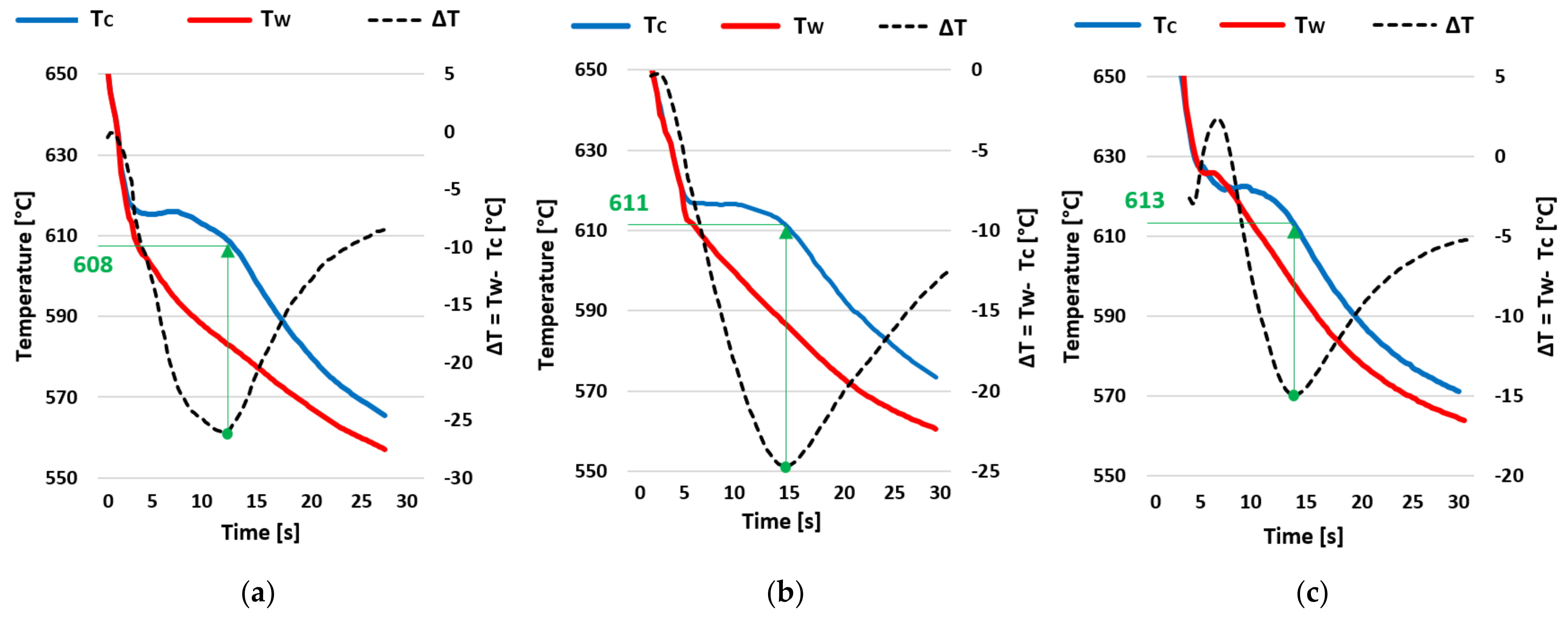
3.3. Dendrite Coherency Temperature (DCT)
3.4. Microhardness
4. Conclusions
- Due to the addition of Zr in amounts ranging from 0.05 to 0.20 wt.% into the AlSi5Cu2Mg alloy, the crystallization of Zr-based phases occurred in the form of (Al,Si)3Zr or (Al,Si)3(Zr,Ti), with a crystal structure of the DO22 type. These phases were observed to have a needle-faced morphology with an average length of 16.5 μm. With the addition of increasing amounts of Zr, the number of needles present in the microstructure increased, but their morphology and length did not change.
- Newly formed intermetallic phases based on Zr acted as strong nucleation particles for α-Al grain growth in the AlSi5Cu2Mg alloy. As the amount of Zr added to the alloy increased, the inoculation effect also increased. With the addition of 0.20 wt.% Zr, grain reduction occurred by up to 53% compared to the alloy without inoculation. Grain refinement in the AlSi5Cu2Mg alloy led to an increase in the number of grain boundaries that limit the movement of dislocations, as demonstrated by an increase in the microhardness of the α-Al grains.
- The main grain-refining effect can be attributed to the particle size of Zr-based nucleants. With the increase in the number of sufficiently large Zr-based nucleates, less undercooling was required for primary grain growth on the nucleating particles, which is consistent with the theory of free growth.
- The evaluation of the crystallization process pointed to a gradual increase in the temperature of the liquidus and the associated expansion of the solidification interval of the AlSi5Cu2Mg alloy due to the increase in the amount of wt.% Zr in the alloy. For an alloy with 0.20 wt.% Zr, the temperature of the liquidus was 7 °C higher than that of the AlSi5Cu2Mg alloy without the addition of Zr. By gradually increasing the addition of Zr to the AlSi5Cu2Mg alloy, the dendrite coherence temperature (DCT) also gradually increased. A temperature rise of 5 °C occurred between the AlSi5Cu2Mg alloy without added Zr and the alloy with the addition of 0.20 wt.% Zr.
- The addition of Zr to the AlSi5Cu2Mg alloy provided benefits in the form of the grain refinement of the α-Al phase and the positive outcomes associated with this phenomenon. The addition of 0.20 wt.% Zr proved to be the ideal amount.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Medlen, D.; Bolibruchová, D. The Influence of Remelting on the Properties of AlSi6Cu4 Alloy Modified by Antimony. Arch. Foundry Eng. 2021, 12, 81–86. [Google Scholar] [CrossRef]
- Czerwinski, F.; Emadi, D.; Sediako, D.G.; Shaha, S.K. High-Temperature Aluminum Alloys for Automotive Powertrain. Adv. Mater. Process. 2016, 174, 16–20. [Google Scholar] [CrossRef]
- Bruna, M.; Remisová, A.; Sládek, A. Effect of Filter Thickness on Reoxidation and Mechanical Properties of Aluminium Alloy AlSi7Mg0.3. Arch. Metall. Mater. 2019, 64, 1100–1106. [Google Scholar] [CrossRef]
- Sigworth, G.; Campbell, J.; Jorstad, J. The Modification of Al-Si Casting Alloys: Important Practical and Theoretical Aspects. Int. J. Met. 2009, 3, 65–78. [Google Scholar] [CrossRef]
- Lei, Z.; Wen, S.; Huang, H.; Wei, W.; Nie, Z. Grain Refinement of Aluminum and Aluminum Alloys by Sc and Zr. Metals 2023, 13, 751. [Google Scholar] [CrossRef]
- Rathi, S.K.; Sharma, A.; Sabatino, M.D. Grain Refinement of Al-Si Alloys: Scientific and Industrial Aspects. J. Mod. Thermodyn. Mech. Syst. 2019, 1, 7–15. [Google Scholar]
- Dong, X.; Amirkhanlou, S.; Ji, S. Formation of strength platform in cast Al–Si–Mg–Cu alloys. Sci. Rep. 2019, 9, 9582. [Google Scholar] [CrossRef] [PubMed]
- Farkoosh, A.R.; Chen, X.G.; Pekguleryuz, M. Dispersoid strengthening of a high temperature Al–Si–Cu–Mg alloy via Mo addition. Mater. Sci. Eng. 2015, 620, 181–189. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, L.; Wang, Z.; Gao, A. Achievement of High Strength and Ductility in Al–Si–Cu–Mg Alloys by Intermediate Phase Optimization in As-Cast and Heat Treatment Conditions. Materials 2020, 13, 647. [Google Scholar] [CrossRef] [PubMed]
- Samuel, E.; Tahiri, H.; Samuel, A.M.; Songmene, V.; Samuel, F.H. A Review on Fundamentals of Grain Refining of Al-Si Cast Alloys; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Samuel, A.M.; Samuel, E.; Songmene, V.; Samuel, F.H. A Comparative Study of Grain Refining of Al-(7–17%) Si Cast Alloys Using Al-10% Ti and Al-4% B Master Alloys. Materials 2023, 16, 2867. [Google Scholar] [CrossRef]
- Ait El Haj, B.; Hamadellah, A.; Bouayad, A.; El Akili, C. Review of Grain Refinement Performance of Aluminium Cast Alloys. Metall. Mater. Eng. 2023, 29, 1–15. [Google Scholar] [CrossRef]
- Xie, H.; Lv, J. Precipation of TiAl3 in remelting Al-5Ti-1B and the grain refinement of 7050 alloy. Mater. Res. Express 2024, 8, 066513. [Google Scholar] [CrossRef]
- Xu, C.; Xiao, W.L.; Wen, S.Z.; Zhen, Y.J.; Ma, C.L. Microstructures and grain refinement performance of Al–Ti–B–C grain refiner via powder metallurgy. Rare Met. 2022, 41, 2357–2362. [Google Scholar] [CrossRef]
- Choudhary, C.; Bar, H.N.; Pramanick, A.K.; Sahoo, K.L.; Mandal, D. Effect of Strain Induced Melt Activation Process on the Microstructure and Mechanical Properties of Al-5Ti-1B Treated Al-7Si Alloy. Met. Mater. Int. 2022, 28, 2529–2542. [Google Scholar] [CrossRef]
- Chen, Z.; Yan, K. Grain refinement of commercially pure aluminum with addition of Ti and Zr elements based on crystallography orientation. Sci. Rep. 2020, 10, 16591. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.M.; Zhang, M.X. Edge-to-Edge Matching—A New Approach to the Morphology and Crystallography of Precipitates; The University of Queensland: Brisbane, QLD, Australia, 1999; pp. 41–62. [Google Scholar]
- Samuel, E.; Samuel, A.M.; Songmene, V.; Samuel, F.H. A Review on the Analysis of Thermal and Thermodynamic Aspects of Grain Refinement of Aluminum-Silicon-Based Alloys. Materials 2023, 16, 5639. [Google Scholar] [CrossRef]
- Sujith, S.V.; Kim, H.; Mulik, R.S.; Park, H.; Lee, J. Synergistic Effect of In-Situ Al-7075/Al3Ti Metal Matrix Composites Prepared via Stir-Assisted Ultrasonic Melt Processing Under Dynamic Nucleation. Met. Mater. Int. 2022, 28, 2288–2303. [Google Scholar] [CrossRef]
- Mao, G.; Tong, G.; Gao, W.; Liu, S.; Zhong, L. The poisoning effect of Sc or Zr in grain refinement of Al-Si-Mg alloy with Al-Ti-B. Mater. Lett. 2021, 301, 130428. [Google Scholar] [CrossRef]
- Wang, F.; Qiu, D.; Liu, Z.L.; Taylor, J.A.; Easton, M.A.; Zhang, M.X. The grain refinement mechanism of cast aluminium by zirconium. Acta Mater. 2013, 61, 5636–5645. [Google Scholar] [CrossRef]
- Wang, F.; Qiu, D.; Liu, Z.L.; Taylor, J.; Easton, M.; Zhang, M.X. Crystallographic study of Al3Zr and Al3Nb as grain refiners for Al alloys. Trans. Nonferrous Met. Soc. China 2014, 24, 2034–2040. [Google Scholar] [CrossRef]
- Jia, Z.H.; Couzinié, J.-P.; Cherdoudi, N.; Guillot, I.; Arnberg, L.; Asholt, P.; Brusethaug, S.; Barlas, B.; Massinin, D. Precipitation behaviour of Al3Zr precipitate in Al–Cu–Zr and Al–Cu–Zr–Ti–V alloys. Trans. Nonferrous Met. Soc. China 2012, 22, 1860–1865. [Google Scholar] [CrossRef]
- Tang, H.; Geng, Y.; Luo, J.; Xu, J.; Ju, H.; Yu, L. Mechanical Properties of High Mg-Content Al-Mg-Sc-Zr Alloy Fabricated by Selective Laser Melting. Met. Mater. Int. 2021, 27, 2592–2599. [Google Scholar] [CrossRef]
- Vončina, M.; Kores, S.; Ernecl, M.; Medved, J. The role of Zr and T6 heat treatment on microstructure evolution and hardness of AlSi9Cu3(Fe) diecasting alloy. J. Min. Metall. Sect. B-Metal. 2017, 53, 423–428. [Google Scholar] [CrossRef]
- Rahimian, M.; Amirkhanlou, S.; Blake, P.; Ji, S. Nanoscale Zr-containing precipitates; a solution for significant improvement of high-temperature strength in Al-Si-Cu-Mg alloys. Mater. Sci. Eng. A 2018, 721, 328–338. [Google Scholar] [CrossRef]
- Samuel, E.; Nabawy, A.M.; Samuel, A.M.; Doty, H.W.; Songmene, V.; Samuel, F.H. Effect of Zr and Ti Addition and Aging Treatment on the Microstructure and Tensile Properties of Al-2%Cu-Based Alloys. Materials 2022, 15, 4511. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zeng, H.; Wang, C.; Tang, Z. Effect of Al3Zr Dispersoid on Microstructure and Mechanical Properties of Al-Cu-Li Alloy During Composite Spinning-Extrusion Forming. Front. Mater. 2022, 9, 822589. [Google Scholar] [CrossRef]
- Pan, S.; Qian, F.; Li, C.; Wang, Z.; Li, Y. Synergistic strengthening by nano-sized α-Al(Mn,Fe)Si and Al3Zr dispersoids in a heat-resistant Al–Mn–Fe–Si–Zr alloy. Mater. Sci. Eng. A 2021, 819, 141460. [Google Scholar] [CrossRef]
- Kong, Y.; Pu, Q.; Jia, Z.; Liu, M.; Roven, H.J.; Jia, J.; Liu, Q. Microstructure and property evolution of Al-0.4Fe-0.15Zr-0.25Er alloy processed by high pressure torsion. J. Alloys Compd. 2020, 824, 153949. [Google Scholar] [CrossRef]
- Backerud, S.L.; Sigworth, G.K. Recent Developments in the Thermal. Analysis of Aluminum Casting Alloys. AFS Trans. 1989, 97, 459–464. [Google Scholar]
- Kaiser, M.; Rashed, M. Precipitation behavior prior to hot roll in the cold-rolled Al-Mg and Al-Mg-Zr alloys. Met. Mater. 2022, 60, 247–256. [Google Scholar] [CrossRef]
- Bolibruchová, D.; Sýkorová, M.; Brůna, M.; Matejka, M.; Širanec, L. Effect of Zr Addition on Selected Properties and Microstructure of Aluminum Alloy AlSi5Cu2Mg. Inter. J. Metalcast. 2023, 17, 2598–2611. [Google Scholar] [CrossRef]
- Campbell, J. Complete Casting Handbook. In Metal Casting Processes, Metallurgy, Techniques and Design, 2nd ed.; Butterworth-Heinemann: Oxford, UK, 2015; p. 1141. [Google Scholar]
- Hermandez-Sandoval, J.; Garza-Elizondo, G.H.; Samuel, A.M.; Valtiierra, S.; Samuel, F.H. The ambient and high temperature deformation behavior of Al–Si–Cu–Mg alloy with minor Ti, Zr, Ni additions. Mater. Des. 2014, 58, 89–101. [Google Scholar] [CrossRef]
- Michna, Š. Encyclopedia of Aluminum; Adin s.r.o.: Prešov, Slovakia, 2014; p. 700. (In Czech) [Google Scholar]
- Chen, Z.; He, Z.; Jie, W. Growth restriction effects during solidification of aluminium alloys. Trans. Nonferrous Met. Soc. China 2008, 19, 410–413. [Google Scholar] [CrossRef]
- Djurdjevic, M.B.; Byczynski, G. The impact of chemistry on the dendrite coherency point of the 3XX series of Al alloys. In Proceedings of the ICAA_11, Aachen, Germany, 22–26 September 2008; Volume 1, pp. 1–12. [Google Scholar]
- Chai, G.; Backerud, L.; Rolland, T.; Arnberg, L. Dendrite Coherency during Equiaxed Solidification in Binary Aluminum Alloys. Met. Trans. A 1995, 26, 965–970. [Google Scholar] [CrossRef]
- Huber, G.; Djurdjevic, M.B.; Manasijević, S. Quantification of Feeding Regions of Hypoeutectic Al-(5, 7, 9)Si-(0-4)Cu (wt.%) Alloys Using Cooling Curve Analysis; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Gómez, I.V.; Viteri, E.V.; Montero, J.; Djurdjevic, M.; Huber, G. The Determination of Dendrite Coherency Point Characteristics Using Three New Methods for Aluminum Alloys. Appl. Sci. 2018, 8, 1236. [Google Scholar] [CrossRef]
- Pan, H.; He, Y.; Zhang, X. Interactions between Dislocations and Boundaries during Deformation. Materials 2021, 14, 1012. [Google Scholar] [CrossRef] [PubMed]
- Lanjewar, H.; Kestens, L.A.I.; Verleysen, P. Damage and strengthening mechanisms in severely deformed commercially pure Al: Experiments and modeling. Mater. Sci. Eng. A 2021, 800, 140224. [Google Scholar] [CrossRef]
- Shaokun, T.; Jingyuan, L.; Junlong, Z.; Zhumabieke, W.; Dan, L. Effect of Zr and Sc on microstructure and properties of 7136 aluminum alloy. J. Mater. Res. Technol. 2019, 8, 130–4140. [Google Scholar] [CrossRef]
- Bolibruchová, D.; Matejka, M.; Brůna, M.; Kuriš, M.; Michalcová, A. Investigation of Microstructure and Mechanical Properties of AlSi7Mg0.3Cu0.5 Alloy with Addition of Zr, Ti and Sr. Inter. J. Metalcast. 2023, 17, 2584–2597. [Google Scholar] [CrossRef]

| Zr Addition (wt.%) | Si | Cu | Mg | Fe | Mn | Ti | Sr | Zr | Al |
|---|---|---|---|---|---|---|---|---|---|
| 0 | 5.47 | 1.91 | 0.29 | 0.18 | 0.02 | 0.013 | 0.01 | 0.0009 | Bal. |
| 0.05 | 5.67 | 1.91 | 0.29 | 0.19 | 0.02 | 0.013 | 0.01 | 0.05 | Bal. |
| 0.10 | 5.65 | 1.92 | 0.29 | 0.19 | 0.02 | 0.014 | 0.01 | 0.10 | Bal. |
| 0.15 | 5.55 | 1.91 | 0.29 | 0.19 | 0.02 | 0.014 | 0.01 | 0.12 | Bal. |
| 0.20 | 5.43 | 1.90 | 0.29 | 0.18 | 0.02 | 0.014 | 0.01 | 0.19 | Bal. |
| Zr Addition (wt.%) | Tliq (°C) | Tsol (°C) |
|---|---|---|
| 0 | 615 | 517 |
| 0.05 | 617 | 518 |
| 0.10 | 618 | 518 |
| 0.15 | 620 | 517 |
| 0.20 | 621 | 518 |
| Zr Addition (wt.%) | 0 | 0.05 | 0.10 | 0.15 | 0.20 |
|---|---|---|---|---|---|
| DCT | 608 | 610 | 612 | 613 | 613 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolibruchová, D.; Matejka, M.; Širanec, L.; Švec, M. Zr as an Alternative Grain Refiner in the Novel AlSi5Cu2Mg Alloy. Metals 2024, 14, 581. https://doi.org/10.3390/met14050581
Bolibruchová D, Matejka M, Širanec L, Švec M. Zr as an Alternative Grain Refiner in the Novel AlSi5Cu2Mg Alloy. Metals. 2024; 14(5):581. https://doi.org/10.3390/met14050581
Chicago/Turabian StyleBolibruchová, Dana, Marek Matejka, Lukáš Širanec, and Martin Švec. 2024. "Zr as an Alternative Grain Refiner in the Novel AlSi5Cu2Mg Alloy" Metals 14, no. 5: 581. https://doi.org/10.3390/met14050581
APA StyleBolibruchová, D., Matejka, M., Širanec, L., & Švec, M. (2024). Zr as an Alternative Grain Refiner in the Novel AlSi5Cu2Mg Alloy. Metals, 14(5), 581. https://doi.org/10.3390/met14050581








