Hybrid Zinc Coatings with Improved Corrosion Resistance Based on Chitosan Oligosaccharides
Abstract
1. Introduction
2. Materials and Methods
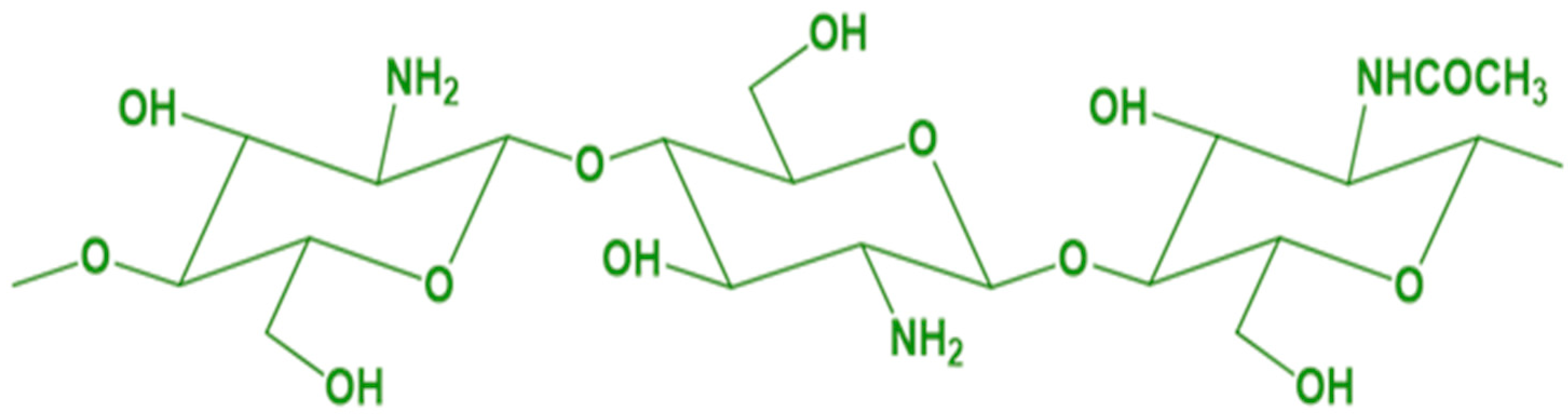
2.1. Materials
2.2. Preparation of Dispersions to Obtain Hybrid Coatings
2.3. TEM Studies
2.4. Electrodeposition of the Hybrid Coatings
- -
- COS1—hybrid coatings electrodeposited from a starting electrolyte with a concentration 0.02 wt.% ZnO;
- -
- COS2—hybrid coatings electrodeposited from a starting electrolyte with a concen tration of 0.04 wt.% ZnO.
2.5. Surface Morphology
2.6. Corrosion Characterization and CVA Studies
2.7. Atomic Force Microscopy (AFM) and Water Contact Angle Measurements
2.8. Chemical and Phase Composition (XPS Analysis)
2.9. XRD Analyses
2.10. Corrosive Medium and Reproducibility of the Investigation Results
3. Results
3.1. TEM Studies
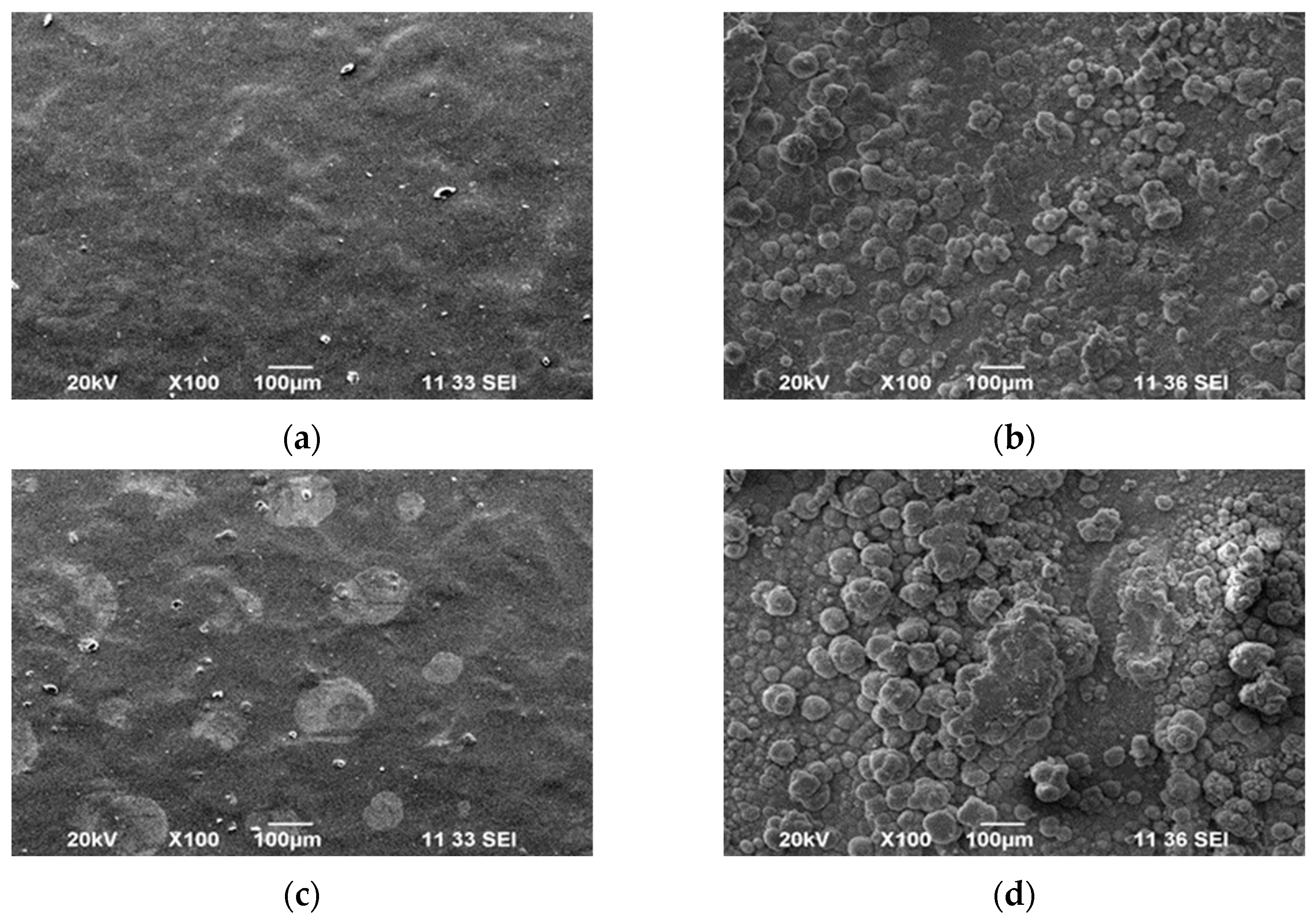
3.2. Surface Morphology
3.3. Cyclic Voltammetry (CVA) Studies
3.4. Potentiodynamic Polarization (PDP) Curves
3.5. Polarization Resistance (Rp) Measurements
3.6. Surface Topography and Wettability
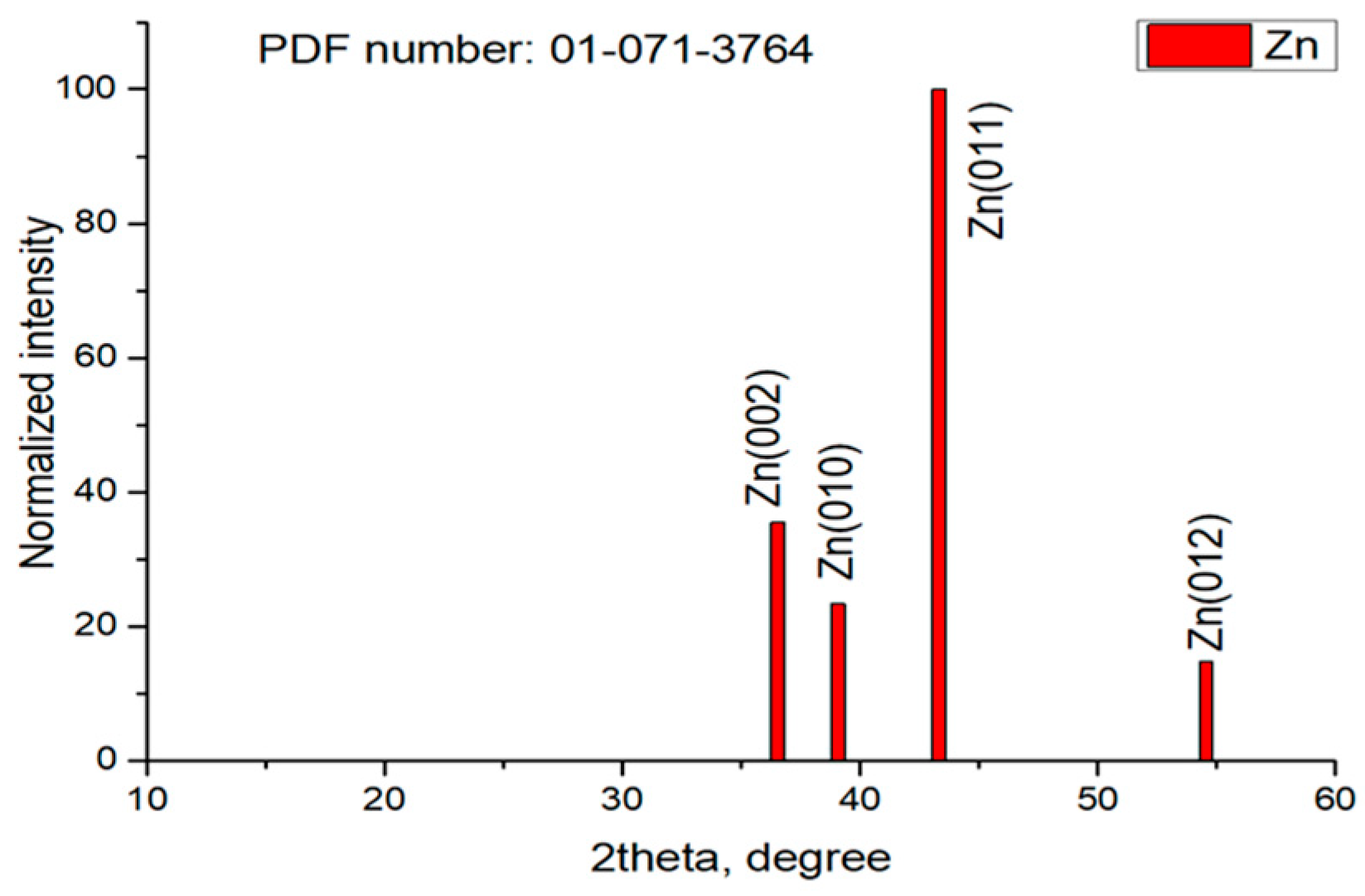
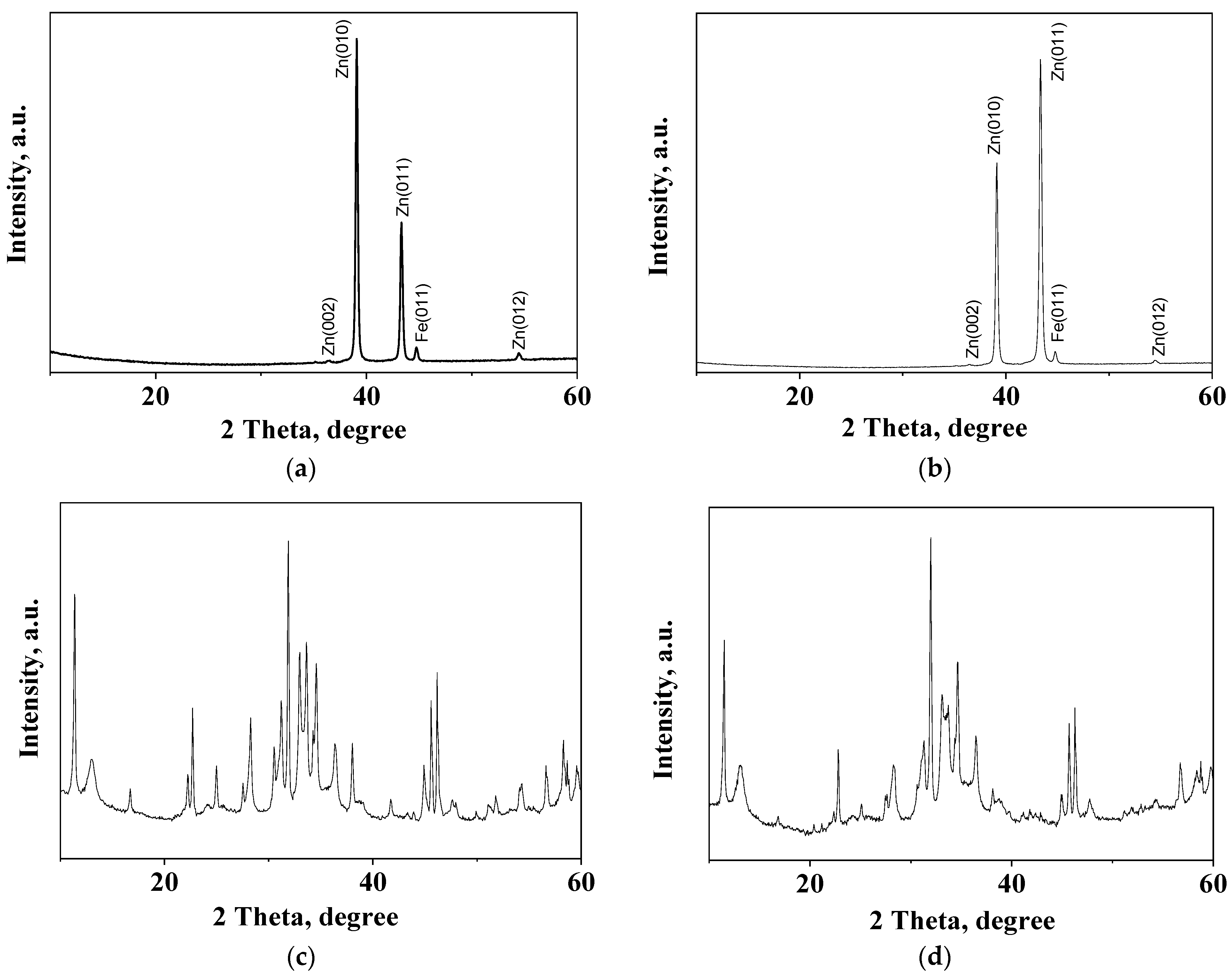
3.7. XRD Studies
3.8. XPS Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koch, G.; Brongers, M.; Thomson, N.; Virmani, Y.; Payer, J. Corrosion Cost and Preventive Strategies in the United States; NACE International: Houston, TX, USA, 2016. [Google Scholar]
- Zhang, X.G. Corrosion and Electrochemistry of Zinc; Plenum Press: New York, NY, USA, 1996; ISBN 978-1-4757-9877-7. [Google Scholar]
- Popoola, P.A.I.; Malatji, N.; Fayomi, O.S. Fabrication and properties of zinc composite coatings for mitigation of corrosion in coastal and marine zone. In Applied Studies of Coastal and Marine Environments; InTech: London, UK, 2016; Volume 32, pp. 137–144. [Google Scholar]
- De La Fuente, D.; Castano, J.G.; Morcillo, M. Long-term atmospheric corrosion of zinc. Corros. Sci. 2007, 9, 1420–1436. [Google Scholar] [CrossRef]
- Maniam, K.K.; Paul, S. Corrosion performance of electrodeposited zinc and zinc-alloy coatings in marine environment. Corros. Mater. Degrad. 2021, 2, 163–189. [Google Scholar] [CrossRef]
- Sorensen, P.A.; Kiil, S.; Dam-Johansen, K.; Weinell, C.E. Anticorrosive coatings: A Review. J. Coat. Technol. Res. 2009, 6, 135–176. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, B.; Banfield, J.F. Particle size and pH effects on nanoparticle dissolution. J. Phys. Chem. C 2010, 114, 14876–14884. [Google Scholar] [CrossRef]
- Srivastava, M.; Srivastava, S.K.; Ji, N.G.; Prakash, R. Chitosan based new nanocomposites for corrosion protection of mild steel in aggressive chloride media. Intern. J. Biolog. Macromol. 2019, 140, 177–187. [Google Scholar] [CrossRef]
- Azizi, M.; Schneider, W.; Plieth, W. Electrolytic co-deposition of silicate and mica particles with zinc. J. Solid State Electrochem. 2005, 9, 429–437. [Google Scholar] [CrossRef]
- Xia, X.; Zhitomirsky, I.; McDermid, J.R. Electrodeposition of zinc and composite zinc-yttria stabilized zirconia coating. J. Mater. Process. Technol. 2009, 209, 2632–2640. [Google Scholar] [CrossRef]
- Adriana, V.; Simona, V.; Aurel, P.; Caius, B.; Liana, M.M. Electrodeposited Zn–TiO2 nanocomposite coatings and their corrosion behavior. J. Appl. Electrochem. 2010, 40, 1519–1527. [Google Scholar]
- Behzadnasab, M.; Mirabedini, S.M.; Kabiri, K.; Jamali, S. Corrosion performance of epoxy coatings containing silane treated ZrO2 nanoparticles on mild steel in 3.5% NaCl solution. Corr. Sci. 2011, 53, 89–98. [Google Scholar] [CrossRef]
- Mohammad, B.; Mahamood, A.; Mehdi, A. Cu-Zn-Al2O3 nanocomposites: Study of microstructure, corrosion, and wear properties. Int. J. Met. Mater. 2017, 24, 462–472. [Google Scholar]
- Kamburova, K.; Boshkova, N.; Boshkov, N.; Radeva, T. Composite coatings with polymeric modified ZnO nanoparticles andnanocontainers with inhibitor for corrosion protection of low carbon steel. Colloids Surf. A Physicochem. Eng. Asp. 2021, 609, 125741. [Google Scholar] [CrossRef]
- Romero, R.; Martin, F.; Ramos-Barrado, J.R.; Leinen, D. Study of different inorganic oxide thin films as barrier coatings against the corrosion of galvanized steel. Surf. Coat. Technol. 2010, 204, 2060–2063. [Google Scholar] [CrossRef]
- Lopez Ibanez, R.; Romero, R.; Martın, F.; Ramos-Barrado, J.R.; Leinen, D. ZnO thin films on aluminized steel by spray pyrolysis. Surf. Interface Anal. 2006, 38, 789–792. [Google Scholar] [CrossRef]
- Tang, F.; Uchikoshi, T.; Sakka, Y. Electrophoretic deposition behavior of aqueous nanosized zinc oxide suspensions. J. Am. Ceram. Soc. 2002, 85, 2161–2165. [Google Scholar] [CrossRef]
- Boshkova, N.; Kamburova, K.; Radeva, T.; Simeonova, S.; Grozev, N.; Shipochka, M.; Boshkov, N. Comparative corrosion characterization of hybrid zinc coatings in Cl−-contaning medium and artificial sea water. Coatings 2022, 12, 1798. [Google Scholar] [CrossRef]
- Anwar, S.; Khan, F.; Zhang, Y.; Caines, S. Zn composite corrosion resistance coatings: What works and what does not work? J. Loss Prev. Process Ind. 2021, 69, 104376. [Google Scholar] [CrossRef]
- Zhang, X.; Liang, J.; Liu, B.; Peng, Z. Preparation of superhydrophobic zinc coating for corrosion protection. Colloids Surf. A Physicochem. Eng. Asp. 2014, 454, 113–118. [Google Scholar] [CrossRef]
- Patel, A.K.; Michaud, P.; de Baynast, H.; Grédiac, M.; Mathias, J.D. Preparation of chitosan-based adhesives and assessment of their mechanical properties. J. Appl. Polym. Sci. 2013, 127, 3869–3876. [Google Scholar] [CrossRef]
- Mati-Baouche, N.; Elchinger, P.H.; de Baynast, H.; Pierre, G.; Delattre, C.; Michaud, P. Chitosan as an adhesive. Eur. Polym. J. 2014, 60, 198–212. [Google Scholar] [CrossRef]
- Varma, A.J.; Deshpande, S.V.; Kennedy, J.F. Metal complexation by chitosan and its derivatives: A review. Carbohydr. Polym. 2004, 55, 77–93. [Google Scholar] [CrossRef]
- Kamburova, K.; Boshkova, N.; Boshkov, N.; Radeva, T.; Atanasova, G. Corrosion protection of electrogalvanised steel by application of non-conducting polyaniline-silica particles. Trans. IMF 2021, 99, 181–187. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. Vesta 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Takayama, Y.; Sato, M.; Watanabe, H. Crystallographic Orientation Dependence of Corrosion Behavior of 5N Purity Aluminum in Different Concentrations of HCl Aqueous Solutions; Weiland, H., Rollett, A.D., Cassada, W.A., Eds.; ICAA13 Pittsburgh; Springer: Berlin/Heidelberg, Germany, 2012; pp. 391–396. [Google Scholar]
- Takayama, Y.; Nohara, K.; Kato, H. Influence of Crystallographic Orientation on Corrosion Behavior of 5N Purity Aluminum. In Proceedings of the 12th International Conference on Aluminium Alloys, Yokohama, Japan, 5–9 September 2010; pp. 1469–1474. [Google Scholar]
- Liu, M.; Qiu, D.; Zhao, M.-C.; Song, G.-L.; Atrens, A. The effect of crystallographic orientation on the active corrosion of pure magnesium. Scr. Mater. 2008, 58, 421–424. [Google Scholar] [CrossRef]
- Shin, K.S.; Bian, M.Z.; Nam, N.D. Effects of Crystallographic Orientation on Corrosion Behavior of Magnesium Single Crystals. JOM 2012, 64, 664–670. [Google Scholar] [CrossRef]
- Yamasaki, M.; Shi, Z.; Atrens, A.; Furukawa, A.; Kawamura, Y. Influence of crystallographic orientation and Al alloying on the corrosion behavior of extruded α-Mg/LPSO two-phase MgZn-Y alloys with multimodal microstructure. Corros. Sci. 2022, 200, 110237. [Google Scholar] [CrossRef]
- Al-Gaashani, R.; Radiman, S.; Daud, A.R.; Tabet, N.; Al-Douri, Y. XPS and optical studies of different morphologies of ZnO nanostructures prepared by microwave methods. Ceram. Int. 2013, 39, 2283–2292. [Google Scholar] [CrossRef]

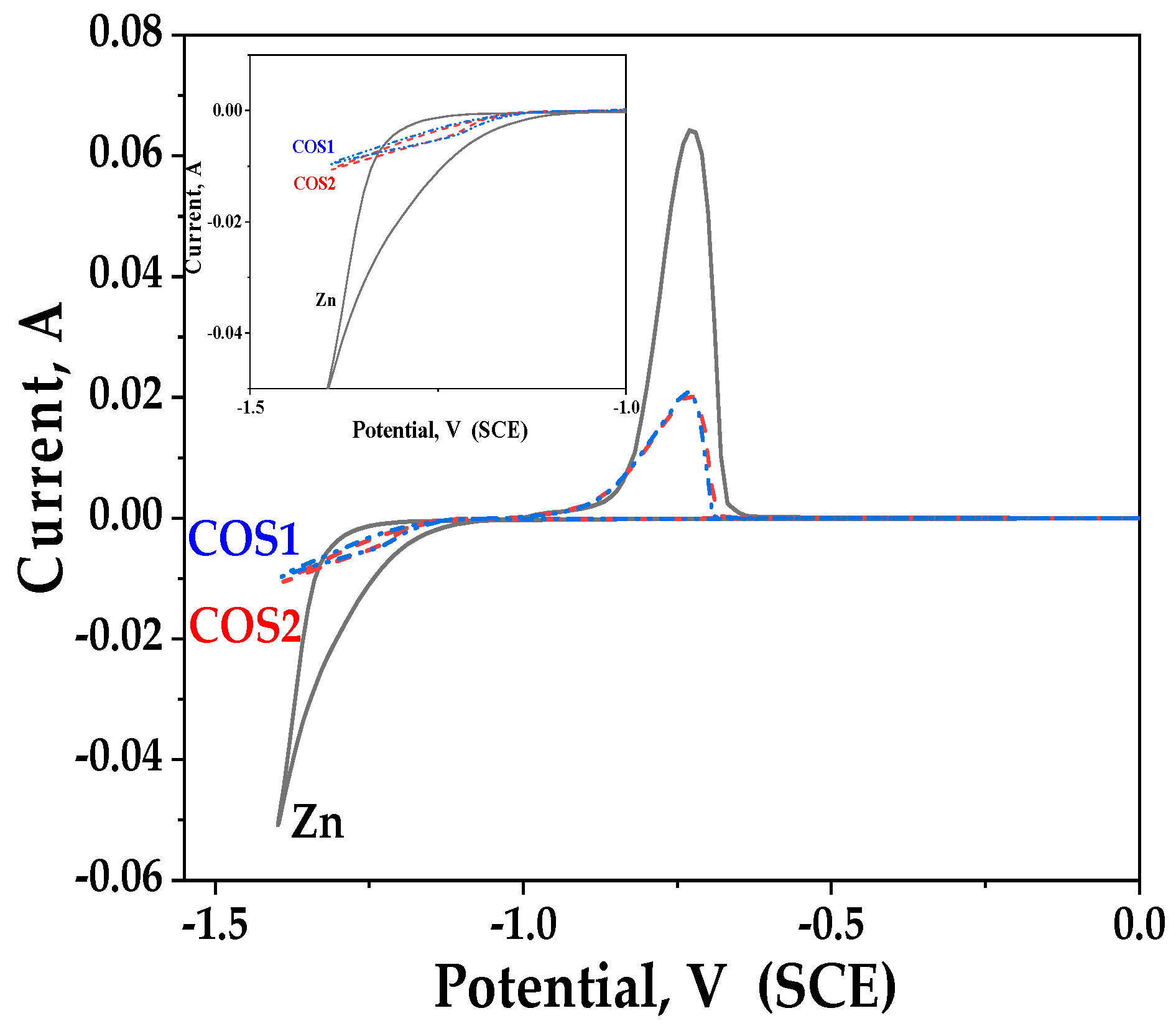



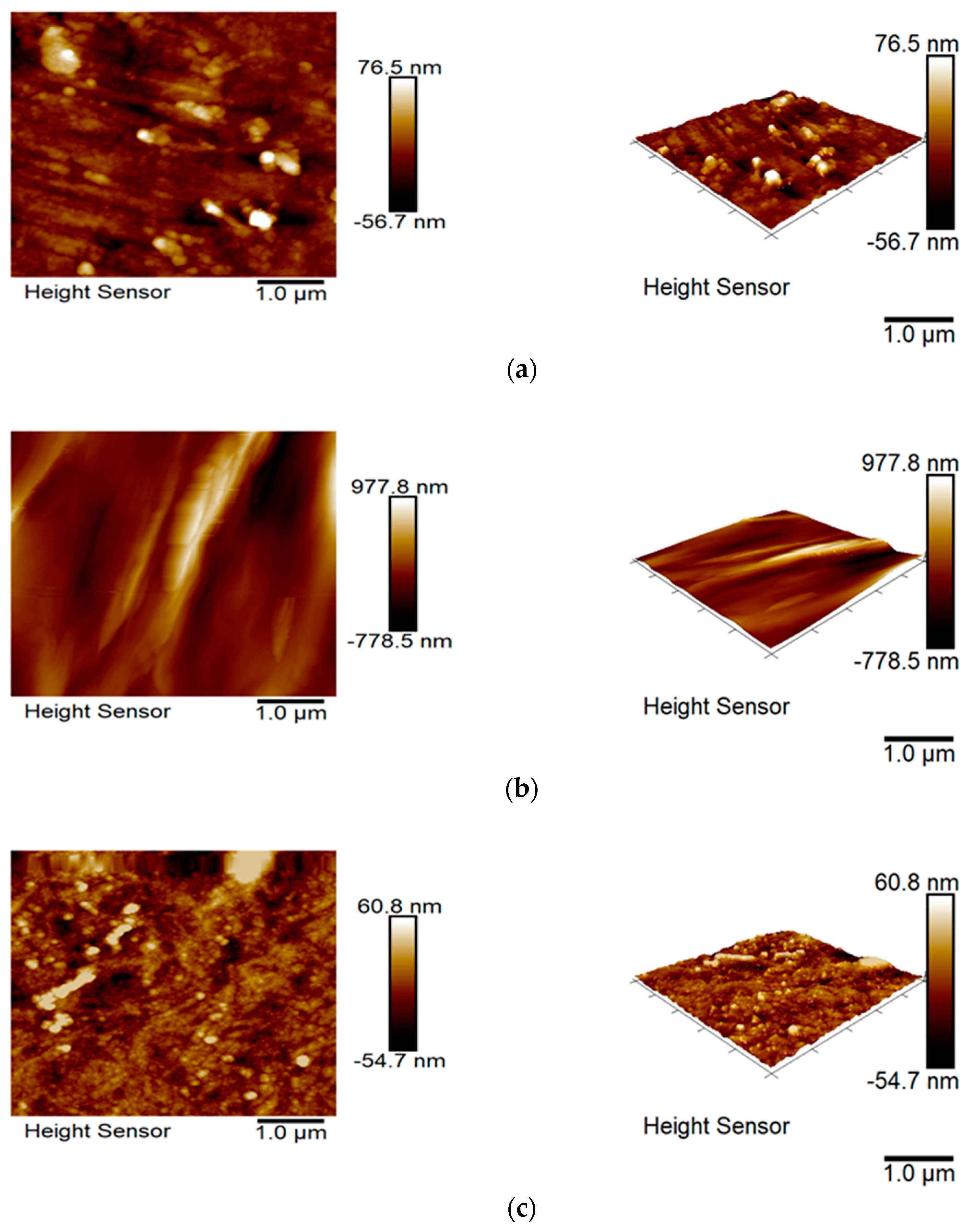



| Sample | Icorr, A·cm−2 | Ecorr, V |
|---|---|---|
| Zn | 1.7 × 10−5 | −1.07 |
| COS1 | 8.2 × 10−6 | −1.07 |
| COS2 | 1.3 × 10−5 | −1.05 |
| Sample | COS1 | COS1T * | COS2 | COS2T * |
|---|---|---|---|---|
| Image (µm × µm) | 5 × 5 | 5 × 5 | 5 × 5 | 5 × 5 |
| Rq (nm) | 15.9 | 213.0 | 14.7 | 128.0 |
| Ra (nm) | 10.8 | 153.0 | 11.0 | 104.0 |
| Sample | Peaks, 2 Theta Degree |
|---|---|
| Zn | 39.08; 39.11; 43.33; 43.35; 54.46 |
| ZHC | 11.36; 16.69; 22.23; 22.69; 24.98; 30.52; 31.22; 32.96; 33.63; 34.57; 36.39; 38.02; 43.92; 45.61; 46.20; 48.00; 51.80; 54.30; 58.28; 58.70; 59.58 |
| HZ | 13.00; 28.27; 30.52; 31.22; 32.96; 41.72; 45.61; 51.10; 56.64; 58.70; 59.57 |
| Z | 34.57; 36.39; 47.60; 56.64 |
| Samples | C, at. % | O, at. % | Zn, at. % | Cl, at. % | Na, at. % |
|---|---|---|---|---|---|
| COS1 | 71.7 | 20.2 | 8.1 | - | - |
| COS2 | 51.3 | 33.7 | 15.0 | - | - |
| COS1T | 27.0 | 29.0 | 15.6 | 16.5 | 11.9 |
| COS2T | 28.2 | 36.6 | 20.6 | 8.6 | 6.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boshkova, N.; Grancharov, G.; Shipochka, M.; Avdeev, G.; Atanasova-Vladimirova, S.; Stoilova, O.; Boshkov, N. Hybrid Zinc Coatings with Improved Corrosion Resistance Based on Chitosan Oligosaccharides. Metals 2024, 14, 636. https://doi.org/10.3390/met14060636
Boshkova N, Grancharov G, Shipochka M, Avdeev G, Atanasova-Vladimirova S, Stoilova O, Boshkov N. Hybrid Zinc Coatings with Improved Corrosion Resistance Based on Chitosan Oligosaccharides. Metals. 2024; 14(6):636. https://doi.org/10.3390/met14060636
Chicago/Turabian StyleBoshkova, Nelly, Georgy Grancharov, Maria Shipochka, Georgy Avdeev, Stela Atanasova-Vladimirova, Olya Stoilova, and Nikolai Boshkov. 2024. "Hybrid Zinc Coatings with Improved Corrosion Resistance Based on Chitosan Oligosaccharides" Metals 14, no. 6: 636. https://doi.org/10.3390/met14060636
APA StyleBoshkova, N., Grancharov, G., Shipochka, M., Avdeev, G., Atanasova-Vladimirova, S., Stoilova, O., & Boshkov, N. (2024). Hybrid Zinc Coatings with Improved Corrosion Resistance Based on Chitosan Oligosaccharides. Metals, 14(6), 636. https://doi.org/10.3390/met14060636








