Electrochemical Behaviour of an Au-Ge Alloy in an Artificial Saliva and Sweat Solution
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Artificial Sweat
3.1.1. Open-Circuit Potential Measurement—Artificial Sweat
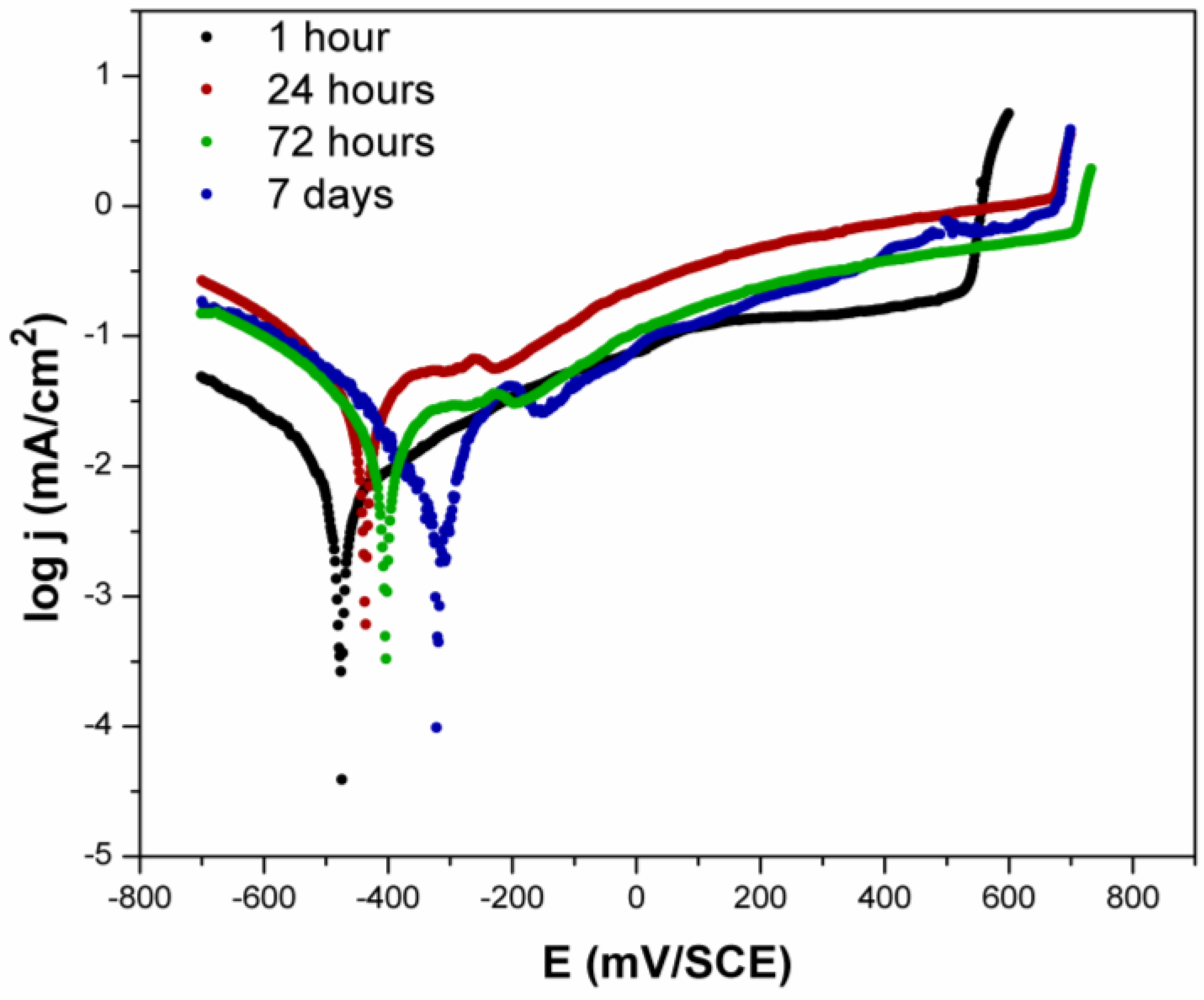
3.1.2. Potentiodynamic Measurements—Artificial Sweat
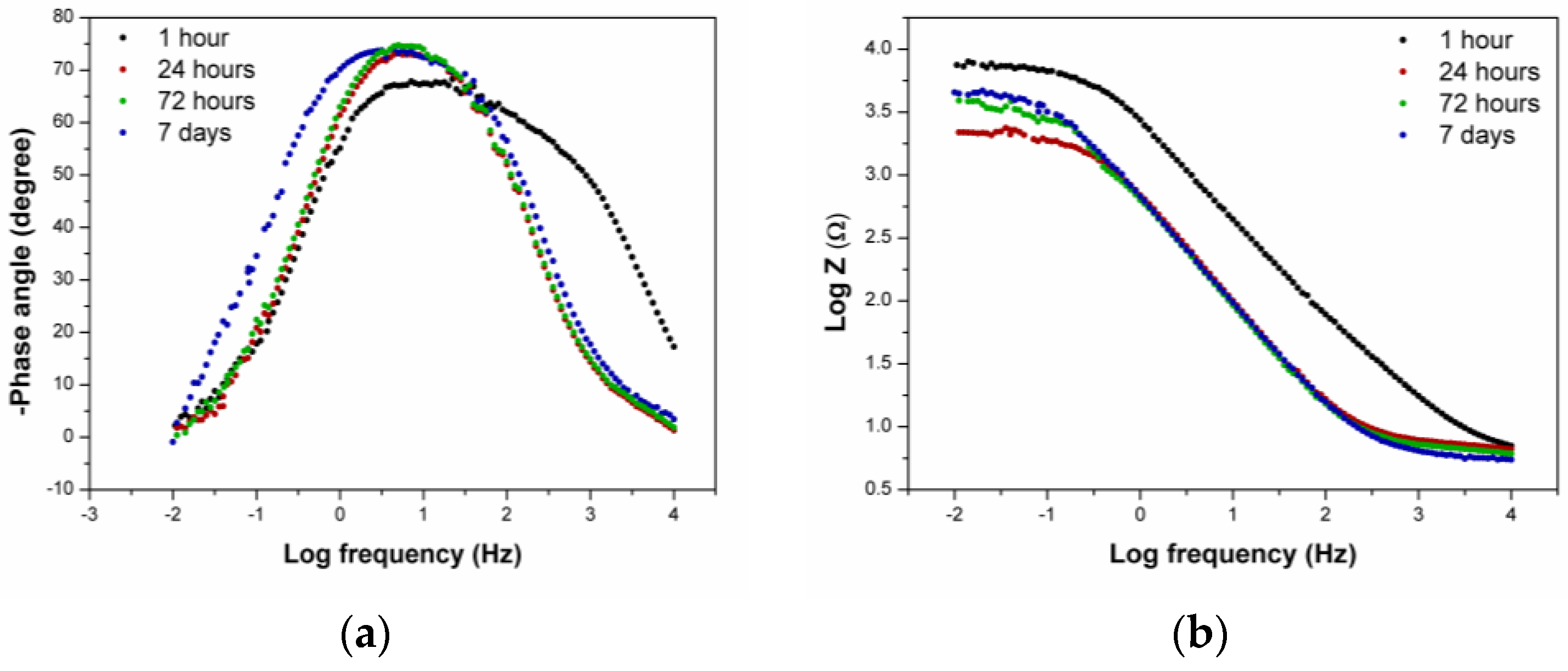
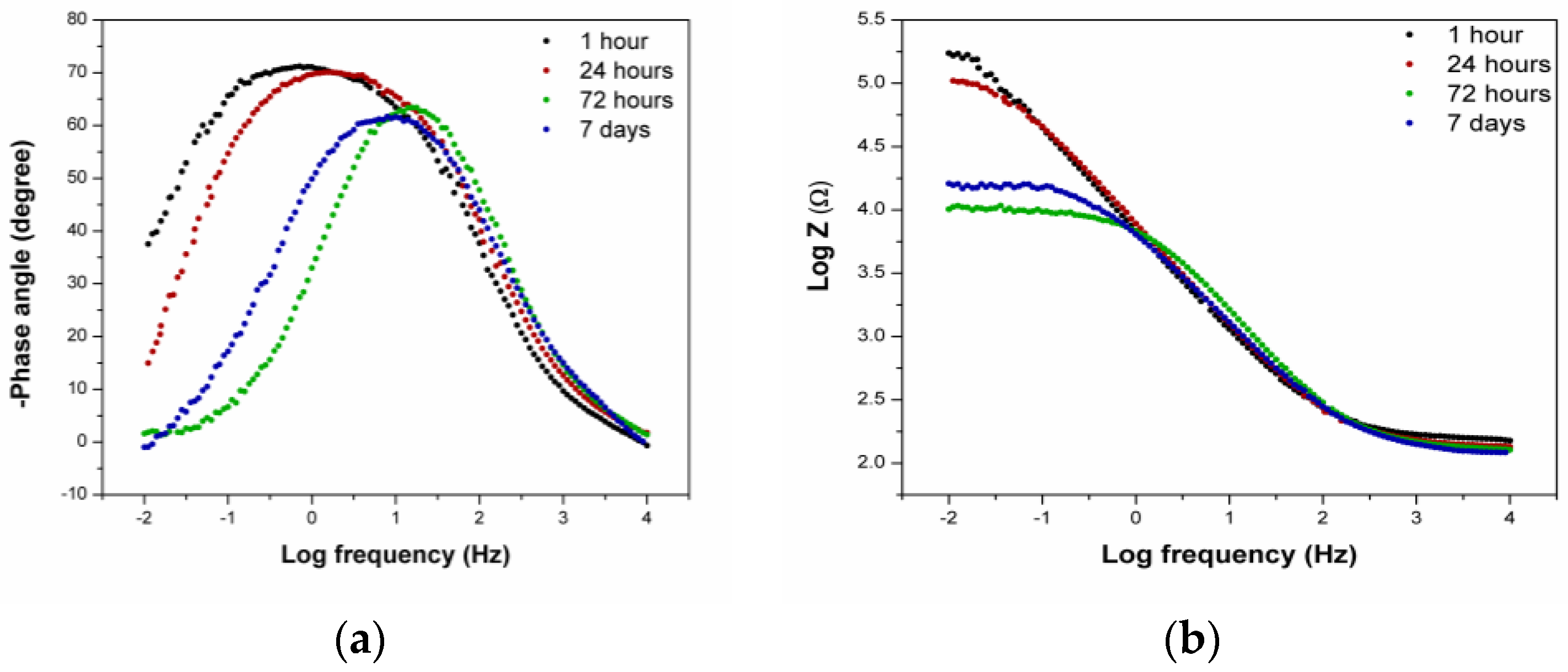
3.1.3. EIS Measurements—Artificial Sweat
3.2. Artificial Saliva
3.2.1. Open-Circuit Potential Measurement—Artificial Saliva
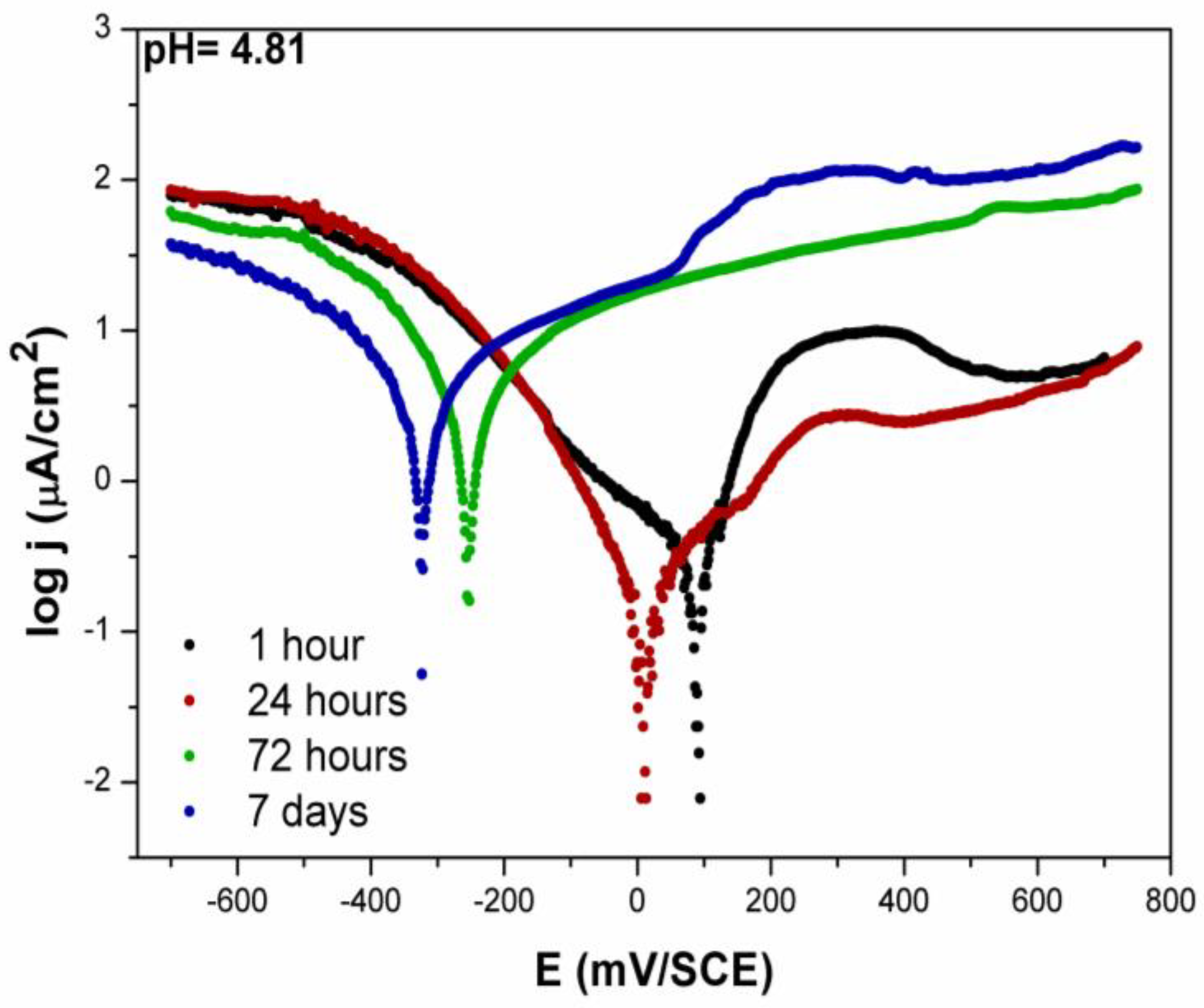
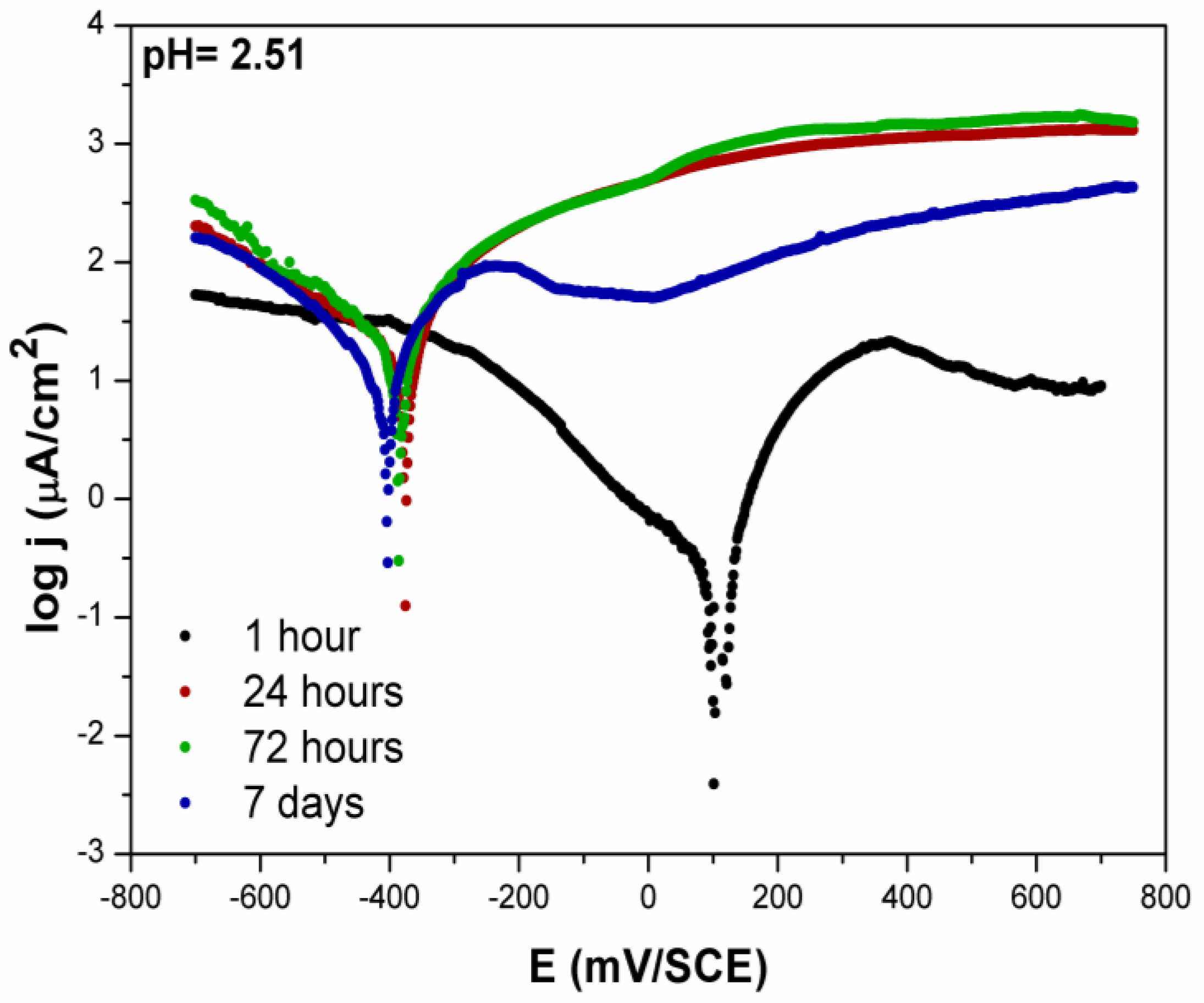
3.2.2. Potentiodynamic Measurements—Artificial Saliva
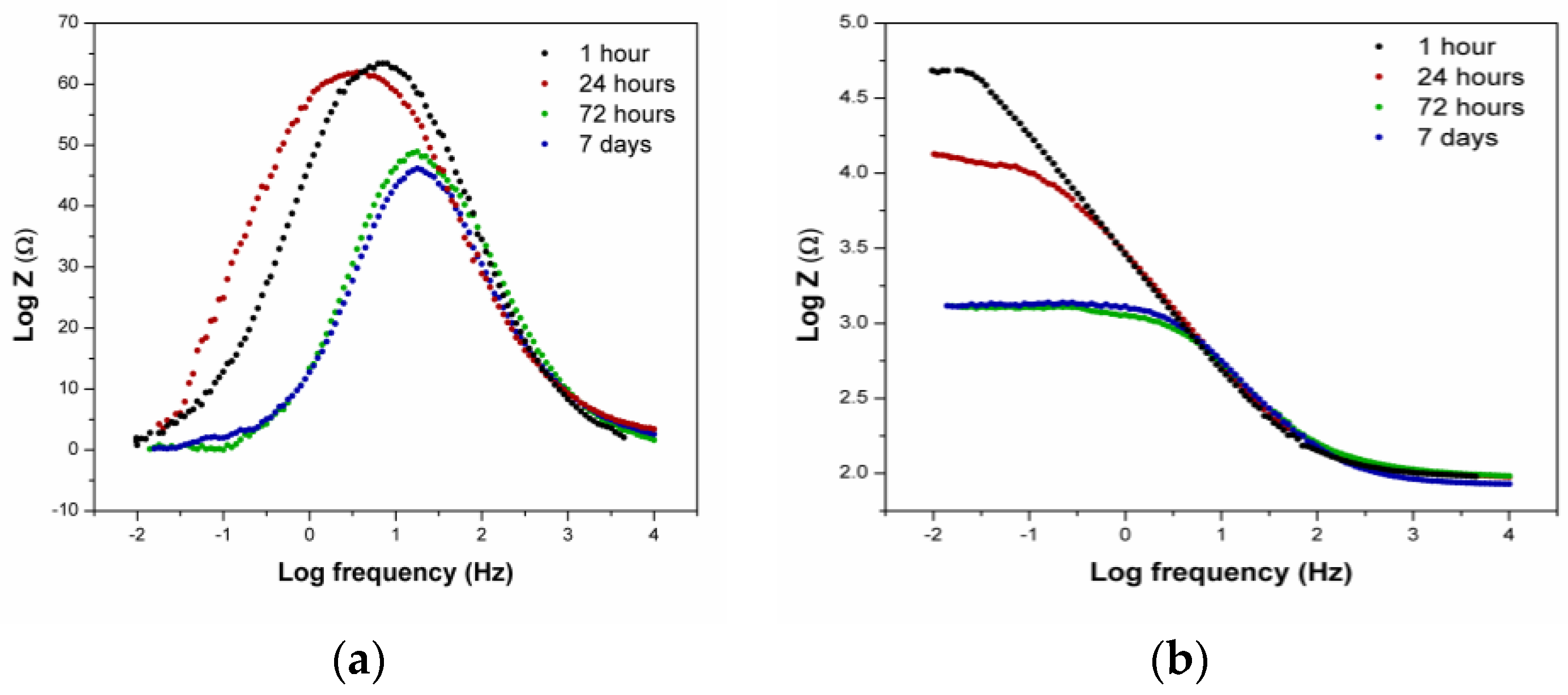
3.2.3. EIS Measurements—Artificial Saliva
3.3. SEM/EDX Analysis
4. Conclusions
- -
- The obtained results showed that the polarisation curves of the tested alloy were similar in all measurements, suggesting that the main corrosion mechanism was the same, regardless of the immersion times. The difference in behaviour occurred only in the length of the Tafel’s, mixed, and passive region, as well as in the value of the passive current density.
- -
- As a function of immersion time, a shift in EOCP and Ecorr towards the anodic direction was also recorded, as well as an increase in the corrosion current density. This kind of behaviour suggests that the layer formed on the alloy’s surface in the artificial sweat solution formed a kind of insulating barrier, but it was, nevertheless, permeable to ions’ exchange.
- -
- The EIS measurements confirmed that, after one hour in the artificial sweat solution, the Au-Ge alloy surface did not stabilise. Because of that, the immersion time of 1 h differed in the behaviour when compared to the later period, and was described by a model with two time constants in contrast to the later measurements, where only one time constant occurred.
- -
- The slight increase in the polarisation resistance and the double layer capacitance over time also suggests that the layer formed on the alloy’s surface in the artificial sweat solution behaved as a kind of insulating barrier, but it was still permeable to ions from the solution.
- -
- The potentiodynamic polarisation measurement showed that, in both saliva solutions during the immersion time, a shift in the EOCP and Ecorr towards the cathodic direction was recorded, as well as an increase in the corrosion current density.
- -
- After 7 days, the corrosion potential in both solutions was not much different, but, in the acidic saliva solution, the corrosion current density was significantly higher (higher corrosion rate) than in the original artificial saliva solution.
- -
- Only one time constant appeared in the EIS measurements of both tested saliva solutions, independent of the immersion time.
- -
- The results obtained at different immersion times showed that the corrosion resistance of the tested alloy decreased as a function of time.
- -
- The results also showed that, in the acidic saliva solution (pH = 2.51), the corrosion rate of the studied alloy was higher, which was accompanied by a decrease in the impedance (Z), phase angle, and polarisation resistance, and an increase in the double layer capacitance over time when compared to the original saliva solution (pH = 4.81).
- -
- The SEM/EDX analysis results are in good agreement with the results obtained through the electrochemical measurements in the artificial sweat and the artificial saliva solutions.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nikpour, S.; Henderson, J.D.; Matin, S.; Nie, H.-Y.; Hedberg, J.; Dehnavi, V.; Hosein, Y.K.; Holdsworth, D.W.; Biesinger, M.; Hedberg, Y.S. Effect of Passivation and Surface Treatment of a Laser Powder Bed Fusion Biomedical Titanium Alloy on Corrosion Resistance and Protein Adsorption. Electrochim. Acta 2024, 475, 143650. [Google Scholar] [CrossRef]
- Atapour, M.; Sanaei, S.; Wei, Z.; Sheikholeslam, M.; Henderson, J.D.; Eduok, U.; Hosein, Y.K.; Holdsworth, D.W.; Hedberg, Y.S.; Ghorbani, H.R. In Vitro Corrosion and Biocompatibility Behavior of CoCrMo Alloy Manufactured by Laser Powder Bed Fusion Parallel and Perpendicular to the Build Direction. Electrochim. Acta 2023, 445, 142059. [Google Scholar] [CrossRef]
- Caporali, S.; Bardi, U. Corrosion Mechanism in Artificial Sweat Solution of In-Bearing White Bronze Alloy. Corrosion 2012, 68, 025001-1–025001-8. [Google Scholar] [CrossRef]
- Markel, K.; Silverberg, N.; Pelletier, J.L.; Watsky, K.L.; Jacob, S.E. Art of Prevention: A Piercing Article about Nickel. Int. J. Women’s Dermatol. 2020, 6, 203–205. [Google Scholar] [CrossRef] [PubMed]
- Rudolf, R.; Majerič, P.; Lazić, V.; Grgur, B. Development of a New AuCuZnGe Alloy and Determination of Its Corrosion Properties. Metals 2022, 12, 1284. [Google Scholar] [CrossRef]
- Cho, J.M.; Chae, J.; Jeong, S.R.; Moon, M.J.; Shin, D.Y.; Lee, J.H. Immune Activation of Bio-Germanium in a Randomized, Double-Blind, Placebo-Controlled Clinical Trial with 130 Human Subjects: Therapeutic Opportunities from New Insights. PLoS ONE 2020, 15, e0240358. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Yang, S.; Sun, J.; Ning, C. Realizing Both Antibacterial Activity and Cytocompatibility in Silicocarnotite Bioceramic via Germanium Incorporation. J. Funct. Biomater. 2023, 14, 154. [Google Scholar] [CrossRef] [PubMed]
- Zemek, J.; Jiricek, P.; Houdkova, J.; Ledinsky, M.; Jelinek, M.; Kocourek, T. On the Origin of Reduced Cytotoxicity of Germanium-Doped Diamond-Like Carbon: Role of Top Surface Composition and Bonding. Nanomaterials 2021, 11, 567. [Google Scholar] [CrossRef]
- Wang, Y.; Teng, G.; Zhou, H.; Dong, C. Germanium Reduces Inflammatory Damage in Mammary Glands During Lipopolysaccharide-Induced Mastitis in Mice. Biol. Trace Elem. Res. 2020, 198, 617–626. [Google Scholar] [CrossRef]
- Elango, J.; Bushin, R.; Lijnev, A.; De Aza, P.N.; Martínez, C.P.-A.; Marín, J.M.G.; Hernandez, A.B.; Olmo, L.R.M.; Val, J.E.M.S. De The Effect of Germanium-Loaded Hydroxyapatite Biomaterials on Bone Marrow Mesenchymal Stem Cells Growth. Cells 2022, 11, 2993. [Google Scholar] [CrossRef]
- Kurt, M.Ş.; Arslan, M.E.; Yazici, A.; Mudu, İ.; Arslan, E. Tribological, Biocompatibility, and Antibiofilm Properties of Tungsten–Germanium Coating Using Magnetron Sputtering. J. Mater. Sci. Mater. Med. 2021, 32, 6. [Google Scholar] [CrossRef] [PubMed]
- Porcayo-Calderon, J.; Rodríguez-Díaz, R.A.; Porcayo-Palafox, E.; Martinez-Gomez, L. Corrosion Performance of Cu-Based Coins in Artificial Sweat. J. Chem. 2016, 2016, 9542942. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Kuan, W.-H.; Liu, C.-L. Comparative Study of the Composition of Sweat from Eccrine and Apocrine Sweat Glands during Exercise and in Heat. Int. J. Environ. Res. Public Health 2020, 17, 3377. [Google Scholar] [CrossRef] [PubMed]
- Mareci, D.; Chelariu, R.; Dan, I.; Gordin, D.-M.; Gloriant, T. Corrosion Behaviour of β-Ti20Mo Alloy in Artificial Saliva. J. Mater. Sci. Mater. Med. 2010, 21, 2907–2913. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.R.; Pinto, A.M.P.; Toptan, F.; Alves, A.C. Impact of Simulated Inflammation and Food Breakdown on the Synergistic Interaction between Corrosion and Wear on Titanium. Corros. Sci. 2024, 228, 111839. [Google Scholar] [CrossRef]
- Bodunrin, M.O.; Chown, L.H.; Van Der Merwe, J.W.; Alaneme, K.K.; Oganbule, C.; Klenam, D.E.P.; Mphasha, N.P. Corrosion Behavior of Titanium Alloys in Acidic and Saline Media: Role of Alloy Design, Passivation Integrity, and Electrolyte Modification. Corros. Rev. 2020, 38, 25–47. [Google Scholar] [CrossRef]
- Begum, A.A.S.; Vahith, R.M.A.; Mohamed, M.K.V.; Kotra, V.; Shaik, B.; Al-Kahtani, A. Corrosion Mitigation on Orthodontic Wire Made of SS 18/8 Alloy Using Esomeprazole Tablet (Esiloc-40 Mg) in Artificial Saliva. J. Saudi Chem. Soc. 2023, 27, 101681. [Google Scholar] [CrossRef]
- ISO 3160-2:2015; Watch-Cases and Accessories—Gold Alloy Coverings. International Organization for Standardization: Geneva, Switzerland, 2015.
- Rathish, R.J.; Rajendran, S.; Christy, J.L.; Devi, B.S.; Johnmary, S.; Manivannan, M.; Rajam, K.; Rengan, P. Corrosion Behaviour of Metals in Artificial Sweat. Open Corros. J. 2010, 3, 38–44. [Google Scholar] [CrossRef]
- Jimenez-Marcos, C.; Mirza-Rosca, J.C.; Baltatu, M.S.; Vizureanu, P. Effect of Si Contents on the Properties of Ti15Mo7ZrxSi Alloys. Materials 2023, 16, 4906. [Google Scholar] [CrossRef]
- Porcayo-Calderon, J.; Rodriguez-Diaz, R.A.; Porcayo-Palafox, E.; Colin, J.; Molina-Ocampo, A.; Martinez-Gomez, L. Effect of Cu Addition on the Electrochemical Corrosion Performance of Ni3Al in 1.0 M H2SO4. Adv. Mater. Sci. Eng. 2015, 2015, 209286. [Google Scholar] [CrossRef]
- Canto, J.; Rodríguez-Díaz, R.A.; Martinez-de-la-Escalera, L.M.; Neri, A.; Porcayo-Calderon, J. Corrosion Inhibition in CO2-Saturated Brine by Nd3+ Ions. Molecules 2023, 28, 6593. [Google Scholar] [CrossRef] [PubMed]
- Dutta Chowdhury, N.; Ghosh, K.S. Electrochemical Behaviour of Dental Amalgam in Natural, Artificial Saliva and in 0.90 Wt.% NaCl Solution. Corros. Sci. 2018, 133, 217–230. [Google Scholar] [CrossRef]
- Falodun, O.E.; Oke, S.R.; Solomon, M.M.; Bayode, A. Microstructural Evolution and Electrochemical Corrosion Characteristics of Ti–Ni Matrix Composite in NaCl and HCl Solutions. Ceram. Int. 2024, 50, 15124–15133. [Google Scholar] [CrossRef]
- Torbati-Sarraf, H.; Ding, L.; Khakpour, I.; Daviran, G.; Poursaee, A. Unraveling the Corrosion of the Ti–6Al–4V Orthopedic Alloy in Phosphate-Buffered Saline (PBS) Solution: Influence of Frequency and Potential. Corros. Mater. Degrad. 2024, 5, 276–288. [Google Scholar] [CrossRef]
- Fekry, A.M.; El-Sherif, R.M. Electrochemical Corrosion Behavior of Magnesium and Titanium Alloys in Simulated Body Fluid. Electrochim. Acta 2009, 54, 7280–7285. [Google Scholar] [CrossRef]
- Arrieta-González, C.D.; Perez-Arizmendi, F.J.; Dorta-Leon, M.A.; Porcayo-Calderón, J. Effect of Temperature on the Corrosion Resistance of Ni5Al Coating Deposited by Electric Arc in 3.5% NaCl Solution. Coatings 2023, 13, 1349. [Google Scholar] [CrossRef]
- Alves, V.A.; Reis, R.Q.; Santos, I.C.B.; Souza, D.G.; de Gonçalves, F.T.; Pereira-da-Silva, M.A.; Rossi, A.; da Silva, L.A. In Situ Impedance Spectroscopy Study of the Electrochemical Corrosion of Ti and Ti–6Al–4V in Simulated Body Fluid at 25 °C and 37 °C. Corros. Sci. 2009, 51, 2473–2482. [Google Scholar] [CrossRef]
- Matemadombo, F.; Nyokong, T. Characterization of Self-Assembled Monolayers of Iron and Cobalt Octaalkylthiosubstituted Phthalocyanines and Their Use in Nitrite Electrocatalytic Oxidation. Electrochim. Acta 2007, 52, 6856–6864. [Google Scholar] [CrossRef]
- Shao, P.; Xiao, H.; Liu, K.; Chen, X.; Hou, M.; Zhang, Q.; Qian, C.; Huang, S. Influence of Annealing Temperature on the Mechanical Properties and Corrosion Behavior of Ti-5.5Al-2.0Zr-1.5Sn-0.5Mo-1.5Nb Alloy. Arab. J. Chem. 2024, 17, 105790. [Google Scholar] [CrossRef]


| Time | EOCP (mV) | Ecorr (mV) | jcorr (µA/cm2) |
|---|---|---|---|
| 1 h | −333 ± 10 | −423 ± 5 | 9.42 ± 1.20 |
| 24 h | −307 ± 8 | −404 ± 7 | 35.2 ± 2.10 |
| 72 h | −289 ± 4 | −383 ± 4 | 17.4 ± 1.50 |
| 7 days | −230 ± 5 | −325 ± 5 | 16.6 ± 1.80 |
| Time | Tafel reg. (mV) from Ecorr to | Mixed reg. (mV) from Tafel reg. to | Passive reg. (mV) from Mix. reg. to | jpass (µA/cm2) |
|---|---|---|---|---|
| 1 h | −400 ± 25 | +100 ± 25 | 530 ± 10 | 0.14 ± 0.07 |
| 24 h | −360 ± 15 | +200 ± 10 | 670 ± 5 | 0.80 ± 0.18 |
| 72 h | −320 ± 20 | +200 ± 20 | 700 ± 6 | 0.40 ± 0.20 |
| 7 days | −200 ± 15 | +450 ± 15 | 670 ± 8 | 0.70 ± 0.16 |
| Time | Rs (Ohm/cm2) | Rp (kOhm/cm2) | Cdl (mF/cm2) | n | Rf (kOhm/cm2) | Cf (µF/cm2) | χ2·10−3 |
|---|---|---|---|---|---|---|---|
| 1 h | 15 ± 1.0 | 5.80 ± 0.02 | 1.05 ± 0.10 | 0.85 ± 0.005 | 2.0 ± 0.2 | 2.0 ± 0.02 | 2.20 |
| 24 h | 5 ± 0.5 | 2.15 ± 0.03 | 3.67 ± 0.11 | 0.87 ± 0.001 | - | - | 1.15 |
| 72 h | 5 ± 0.5 | 4.53 ± 0.10 | 4.55 ± 0.21 | 0.84 ± 0.002 | - | - | 2.82 |
| 7 days | 5 ± 0.5 | 4.85 ± 0.08 | 4.45 ± 0.18 | 0.85 ± 0.002 | - | - | 1.87 |
| pH = 4.81 | pH = 2.51 | |||||
|---|---|---|---|---|---|---|
| Time | EOCP (mV) | Ecorr (mV) | jcorr (µA/cm2) | EOCP (mV) | Ecorr (mV) | jcorr (µA/cm2) |
| 1 h | −24 ± 5 | 96 ± 10 | 0.30 ± 0.15 | 20 ± 8 | 112 ± 10 | 0.26 ± 0.10 |
| 24 h | −50 ± 3 | 8 ± 5 | 0.15 ± 0.09 | −362 ± 10 | −361 ± 10 | 12.94 ± 1.85 |
| 72 h | −268 ± 7 | −247 ± 9 | 1.56 ± 0.50 | −367 ± 7 | −372 ± 7 | 14.09 ± 1.26 |
| 7 days | −339 ± 4 | −320 ± 4 | 1.95 ± 0.80 | −376 ± 9 | −388 ± 9 | 9.37 ± 0.98 |
| pH = 4.81 | pH = 2.51 | |||
|---|---|---|---|---|
| Time | Tafel reg. (mV) from Ecorr to | jpass (µA/cm2) | Tafel reg. (mV) from Ecorr to | jpass (µA/cm2) |
| 1 h | 230 ± 12 | 5.0 ± 2.0 | 370 ± 20 | 9.0 ± 1.8 |
| 24 h | 280 ± 25 | 3.2 ± 1.8 | −270 ± 18 | 1100 ± 50 |
| 72 h | −100 ± 14 | 39 ± 7.0 | −270 ± 16 | 1400 ± 75 |
| 7 days | −220 ± 8 | 110 ± 2.0 | −240 ± 10 | 250 ± 10 |
| pH = 4.81 | pH = 2.51 | |||
|---|---|---|---|---|
| Time | Log Z | −phase | Log Z | −phase |
| 1 h | 5.2 | 72 | 4.7 | 63 |
| 24 h | 5.2 | 70 | 4.0 | 62 |
| 72 h | 4.2 | 64 | 3.1 | 48 |
| 7 days | 4.0 | 62 | 3.0 | 46 |
| pH = 4.81 | pH = 2.51 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time | Rs (Ohm/cm2) | Rp (kOhm/cm2) | Cdl (mF/cm2) | n | χ2·10−3 | Rs (Ohm/cm2) | Rp (kOhm/cm2) | Cdl (mF/cm2) | n | χ2·103 |
| 1 h | 5 ± 1 | 280 ± 0.2 | 0.43 ± 0.01 | 0.84 ± 0.002 | 5.2 | 5 ± 2 | 270 ± 0.4 | 1.10 ± 0.02 | 0.83 ± 0.001 | 1.1 |
| 24 h | 5 ± 1 | 120 ± 1.0 | 0.37 ± 0.02 | 0.84 ± 0.001 | 2.5 | 80 ± 5 | 2.50 ± 0.1 | 1.24 ± 0.017 | 0.81 ± 0.001 | 4.3 |
| 72 h | 15 ± 0.2 | 10.25 ± 0.5 | 0.24 ± 0.01 | 0.80 ± 0.003 | 1.8 | 80 ± 4 | 1.30 ± 0.08 | 2.37 ± 0.032 | 0.86 ± 0.001 | 5.9 |
| 7 days | 15 ± 0.2 | 16.00 ± 0.4 | 0.11 ± 0.008 | 0.80 ± 0.001 | 2.2 | 80 ± 5 | 1.20 ± 0.15 | 2.51 ± 0.022 | 0.81 ± 0.002 | 2.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vastag, G.; Majerič, P.; Lazić, V.; Rudolf, R. Electrochemical Behaviour of an Au-Ge Alloy in an Artificial Saliva and Sweat Solution. Metals 2024, 14, 668. https://doi.org/10.3390/met14060668
Vastag G, Majerič P, Lazić V, Rudolf R. Electrochemical Behaviour of an Au-Ge Alloy in an Artificial Saliva and Sweat Solution. Metals. 2024; 14(6):668. https://doi.org/10.3390/met14060668
Chicago/Turabian StyleVastag, Gyöngyi, Peter Majerič, Vojkan Lazić, and Rebeka Rudolf. 2024. "Electrochemical Behaviour of an Au-Ge Alloy in an Artificial Saliva and Sweat Solution" Metals 14, no. 6: 668. https://doi.org/10.3390/met14060668





