The Initial Assessment of the Possibility of Simulation Fire Standard Curve in the Electric Furnace with the Assessment of Chemical Composition Changes in Steel after Cooling Phase
Abstract
:1. Introduction
- Assessment of the possibility of using an electric furnace for more advanced research (mechanical properties of steel, microstructural changes, nonstandard fire curves).
- Assessment of chemical composition changes of steel after cooling phase.
- Assessment of the influence of the sample cooling method and rate on the experiment results.
2. Materials and Methods
2.1. Characterization and Preparation of the Furnace
2.2. The Methods of Samples Cooling
- In water;
- In the furnace;
- In water mixed with the particular unique polymer AQUA-QUENCH® 320, produced by Quaker Houghton Cracow, Poland (intensity: 11%).
2.3. The Investigation of the Chemical Component of Samples
- Multipoint analysis—This method allows multiple localizations to be examined simultaneously, which is especially convenient when examining both basic materials components and foreign particles.
- Multilayer analysis—Thanks to constant laser exposure and drilling, analyzing the layers beneath the surface is possible.
3. Results
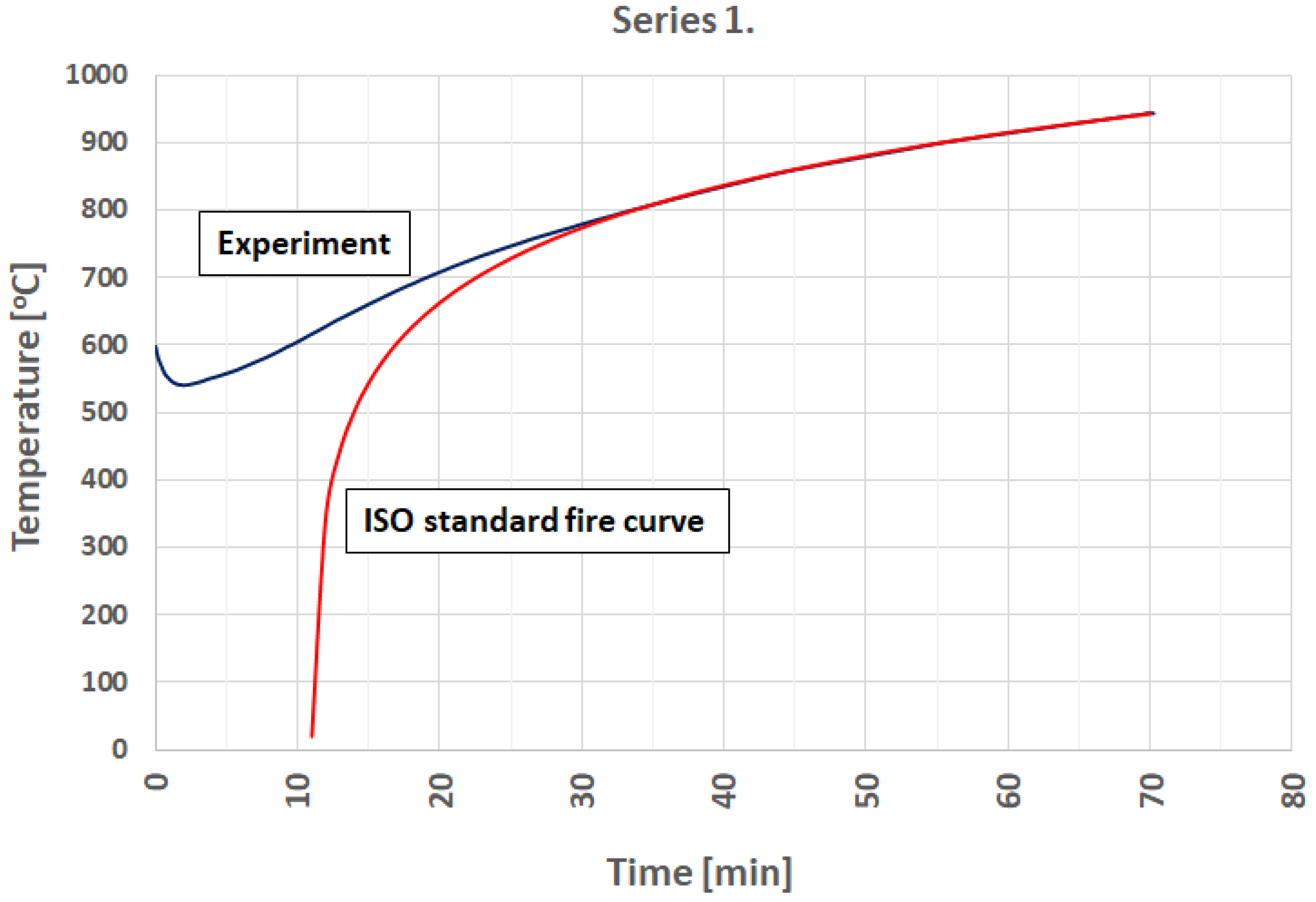
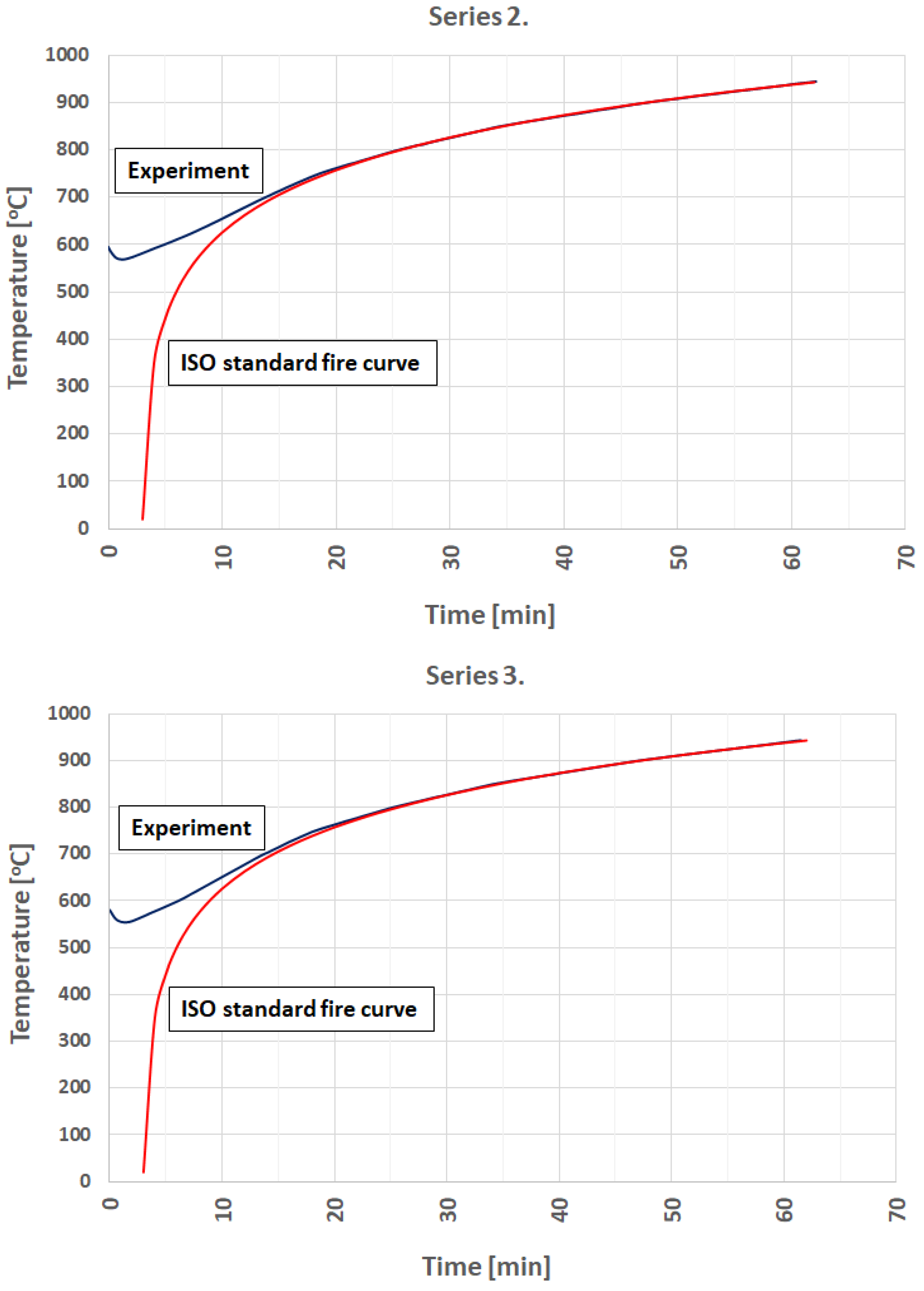
3.1. Fire Simulation
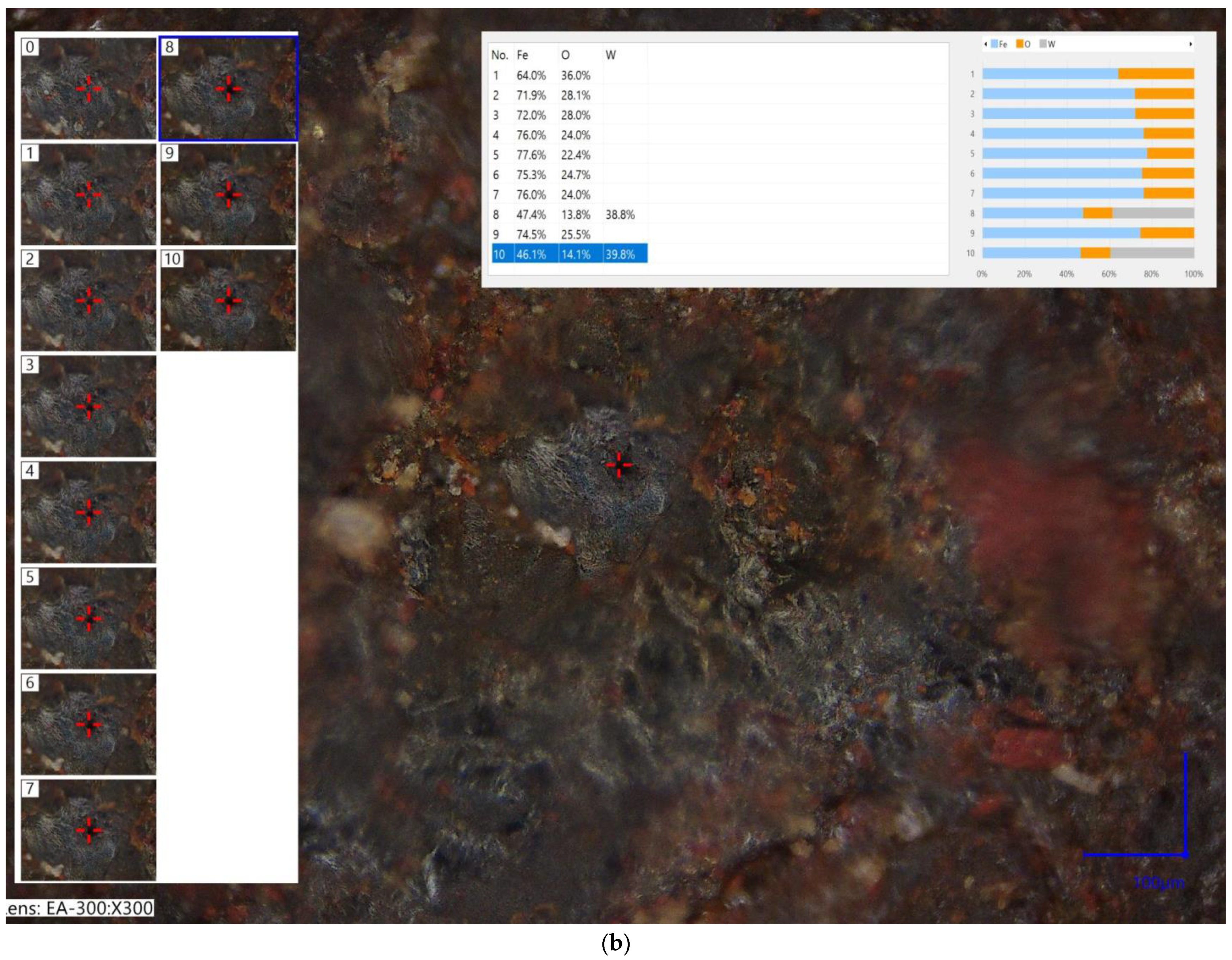
3.2. Chemical Analysis and Component Analysis
4. Discussion
5. Conclusions
- It is possible to model a developed phase of the standard fire curve using an electric furnace.
- Preceding research indicates that it is possible to simulate any fire curve provided that the rapid growth of the temperature phase will be omitted.
- There is no notable change in the chemical composition of specimens after a fire test, and a cooling regime does not influence it.
- A laser microscope is an effective device for the chemical composition analysis of specimens after a cooling phase.
- It is necessary to investigate the influence of omitting the initial phase of fire; alternatively, the authors intend to model a nonstandard fire curve if it turns out that the rate of change of temperature in the initial phase is crucial for the obtained results.
- The issue mentioned above should also be expressed quantitatively—the authors plan to compare the results obtained using the electric furnace with those presented by other authors using gas furnaces.
- An analysis of microstructural changes in specimens, which can also be performed using a laser microscope.
- An analysis of changes in the mechanical properties of steel—this task involves strength tests before and after fire.
- Investigations of more significantly prominent structural members with the use of a larger electric furnace.
- A finite element modeling of a fire situation and comparison of laboratory and numerical results; some effort, in this case, has already been presented by Szczecina and Nowak [29].
- Assessment of structural members after the fire, as presented by Kossakowski et al. [30].
- Laboratory tests with several types of fire curves, including those defined by Eurocodes; some theoretical research is presented in Kubicka et al. [31].
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EN 1993-1-2; Eurocode 3: Design of Steel Structures—Part 1–2: General Rules—Structural Fire Design. CEN: Brussels, Belgium, 2005.
- EN 1991-1-2; Eurocode 1: Actions on Structures—Part 1–2: General Actions—Actions on Structures Exposed to Fire. CEN: Brussels, Belgium, 2002.
- Yu, H.; Burgess, I.W.; Davison, J.B.; Plank, R.J. Experimental and Numerical Investigations of the Behavior of Flush End Plate Connections at Elevated Temperatures. J. Struct. Eng. 2011, 137, 80–87. [Google Scholar] [CrossRef]
- Wang, W.; Xia, Y.; Li, G. Fire Resistance Studies on High Strength Steel Structures. Int. J. High Rise Build. 2018, 7, 287–298. [Google Scholar] [CrossRef]
- Liu, D.; Liu, X.; Wang, W.; Fu, F. Nondestructive Post-fire Damage Assessment of Structural Steel Members Using Leeb Harness Method. Fire Technol. 2020, 56, 1777–1799. [Google Scholar] [CrossRef]
- Król, P.A.; Wachowski, M. Effect of Fire Temperature and Exposure Time on High-Strength Steel Bolts Microstructure and Residual Mechanical. Materials 2021, 14, 3116. [Google Scholar] [CrossRef] [PubMed]
- Almand, K.H. Structural Fire Resistance Experimental Research—Priority Needs of U.S. Industry; Fire Protection Research Foundation: Quincy, MA, USA, 2012. [Google Scholar]
- Hasburgh, L.E.; Bourne, K.J.; Stone, S.D.; Zelinka, S.L. Simulating real compartment fire conditions in a furnace. Fire Mater. 2020, 44, 333. [Google Scholar] [CrossRef]
- Molkens, P.; Cashell, K.A.; Rossi, B. Post-fire mechanical properties of carbon steel and safety factors for the reinstatement of steel structures. Eng. Struct. 2021, 234, 111975. [Google Scholar] [CrossRef]
- Molkens, P.; Cashell, K.A.; Rossi, B. Performance of structural stainless steel following a fire. Eng. Struct. 2021, 235, 112001. [Google Scholar] [CrossRef]
- Radzi, N.A.M.; Hamid, R.; Mutalib, A.A.; Kaish, A.B.M.A. A Review of the Structural Fire Performance Testing Methods for Beam-to-Column Connections. Adv. Civ. Eng. 2021, 2021, 5432746. [Google Scholar] [CrossRef]
- Bisby, L.; Gales, J.; Maluk, C. A contemporary review of large-scale non-standard structural fire testing. Fire Sci. Rev. 2013, 2, 1–27. [Google Scholar] [CrossRef]
- Winful, D.A.; Cashell, K.A.; Afshan, S.; Barnes, A.M.; Pargeter, R.J. Material properties of high strength steel under fire conditions. In Proceedings of the Eurosteel 2017 Conference, Copenhagen, Denmark, 13–15 September 2017. [Google Scholar] [CrossRef]
- Maraveas, C.; Fasoulakis, Z.C.; Tsavdaridis, K.D. Mechanical properties of High and Very High Steel at elevated temperatures and after cooling down. Fire Sci. Rev. 2017, 6, 1–13. [Google Scholar] [CrossRef]
- Maślak, M.; Żwirski, G. Zmiany strukturalne w stali konstrukcyjnej wywołane epizodami jej nagrzewania i stygnięcia podczas pożaru (Changes in Structural Steel Microstructures Following Heating and Cooling Episodes in Fires). Saf. Fire Technol. 2017, 48, 34–52. [Google Scholar] [CrossRef]
- Thanaraj, D.P.; Tattukolla, K.; Balamurali, K.; Anand, N.; Andrushia, D.; Beulah, G.A.P.; Krishanu, R. Influence of Heating–Cooling Regime on the Engineering Properties of Structural Concrete Subjected to Elevated Temperature. Buildings 2023, 13, 295. [Google Scholar] [CrossRef]
- Blancs, M.C.; Sanchez, A.O.; Navarro, I.F. Evaluation of steel structures integrity in a post-fire condition: Case study of the Serradells sports centre in Andorra. Fire Saf. J. 2022, 133, 103668. [Google Scholar] [CrossRef]
- Cábová, K.; Lišková, N.; Zeman, F.; Benýšek, M. Virtual test of fire resistance of a steel beam. In Proceedings of the Engineering Mechanics Conference, Svratka, Czech Republic, 14–17 May 2018. [Google Scholar] [CrossRef]
- Shaheen, M.A.; Presswood, R.; Afshan, S.H. Application of Machine Learning to predict the mechanical properties of high strength steel at elevated temperatures based on the chemical compostion. Structures 2023, 52, 17–29. [Google Scholar] [CrossRef]
- Jiang, B.; Qu, Y.; Wang, M.; Lou, G.; Li, G. Mechanical properties of Q355 hot-rolled steel during the entire fire process. J. Constr. Steel Res. 2024, 215, 108565. [Google Scholar] [CrossRef]
- Jiang, B.; Qu, Y.; Wang, M.; Lou, G.; Li, G. Mechanical properties and constitutive model of Q460 steel during the fire-cooling stage. Thin-Walled Struct. 2023, 189, 110904. [Google Scholar] [CrossRef]
- Maślak, M.; Skiba, R. Fire resistance increase of structural steel through the modification of its chemical composition. Procedia Eng. 2015, 108, 277–284. [Google Scholar] [CrossRef]
- Trzaska, J.; Sitek, W.A. Hybrid Method for Calculating the Chemical Composition of Steel with the Required Hardness after Cooling from the Austenitizing Temperature. Materials 2023, 17, 97. [Google Scholar] [CrossRef]
- Dani, A.A.; Roy, K.; Masood, R.; Fang, Z.; Lim, J.B.P. A Comparative Study on the Life Cycle Assessment of New Zealand Residential Buildings. Buildings 2022, 12, 50. [Google Scholar] [CrossRef]
- Burchart-Korol, D. Life cycle assessment of steel production in Poland: A case study. J. Clean. Prod. 2013, 54, 235–243. [Google Scholar] [CrossRef]
- Roy, K.; Dani, A.A.; Ichhpuni, H.; Fang, Z.; Lim, J.B.P. Improving Sustainability of Steel Roofs: Life Cycle Assessment of a Case Study Roof. Appl. Sci. 2022, 12, 5943. [Google Scholar] [CrossRef]
- Available online: www.keyence.eu (accessed on 10 March 2024).
- Available online: https://www.thermofisher.com/pl/en/home/industrial/spectroscopy-elemental-isotope-analysis/portable-analysis-material-id/industrial-elemental-radiation-solutions/how-does-libs-work.html (accessed on 10 March 2024).
- Szczecina, M.; Nowak, K. Numerical analysis of a steel frame in a fire situation. Inżynieria Bezpieczeństwa Obiektów Antropogenicznych 2022, 4, 42–53. [Google Scholar] [CrossRef]
- Kossakowski, P.; Piotrowski, J.Z.; Świt, G.; Trampczyński, W. The feasibility assessment of the exibition hall after the fire. Struct. Environ. 2010, 2, 20–28. [Google Scholar]
- Kubicka, K.; Radoń, U.; Szaniec, W. The influence of description of fire curves on the thermal and reliability response of steel structure. MATEC Web Conf. 2017, 117, 00095. [Google Scholar] [CrossRef]






| Reference No. | Findings/Scope of Research | Gaps/Limitations | |||
|---|---|---|---|---|---|
| Use of Electric [e]/Gas [g] Furnace | Chemical Composition Analysis | Numerical Analysis | Other/Additional Information | ||
| [3] | [e] | x | v | x | * |
| [4] | [e] | x | x | Creep strain, residual stress in welds, local buckling and behavior of steel columns after fire exposure. | * |
| [5] | [e] | x | x | Post-fire mechanical properties of steel; Leeb hardness method. | * |
| [6] | [e] | x | x | Microstructural analysis of samples, correlation between hardness and residual tensile strength. | * |
| [7] | [g] | x | x | Furnace built by authors with high efficiency (950 °C after 25 min.). | High costs |
| [8] | [g] | x | x | The furnace was programmed with 3 real compartment fire curves (obtained from a full-scale test). | |
| [9,10] | [g] [e] | x | x | The authors obtained steel’s retention factors and the stress–strain relationship in different temperatures. | |
| [11] | gas burner, electric furnace, ceramic pad | x | x | Beam-to-column connection tests, comparison of real fire–temperature curve with the standard curve. | x |
| [12] | [e] | x | x | Large-scale nonfire tests. | * |
| [13] | [e] | x | x | Investigation of mechanical properties. | * |
| [14] | [-] | x | x | Microstructure changes. | * |
| [15] | [-] | x | x | Influence of cooling on post-fire properties of steel/concrete. | * |
| [16] | [e] | x | x | Influence of cooling on post-fire properties of steel/concrete. | * |
| [17,18] | x | x | v | Case study and FEM modeling. | * |
| [19] | x | x | v | Use of machine learning to predict mechanical properties of steel. | * |
| [20,21] | [e] | x | x | Investigation of mechanical properties during the heating and cooling phase. | * |
| [22] | x | v | x | Modification of the chemical composition of steel to increase fire resistance. | ** |
| [23] | x | v | x | Use of artificial neural networks (ANNs). | ** |
| [24,25] | x | x | x | Reduction in costs, energy, and carbon footprint. | x |
| [26] | x | x | x | Sustainability of structures. | x |
| Value | ||
|---|---|---|
| The work temperature (°C) | Max. 1100 | |
| The power of the furnace (kW) | 32 | |
| The power of heating elements (kW) | 32 | |
| Unit power | (VAC) | 400 |
| (Hz) | 50 | |
| Deviation from the rated temperature (°C) | ±10 | |
| The dimensions of the furnace’s chamber l × d × h (mm) | 1000 × 600 × 500 | |
| The furnace dimensions l × d × h (mm) | 1800 × 1120 × 1850 | |
| The weight of the furnace (kg) | ~800 | |
| Heating elements—6 pcs | 5333 W/230 V | |
| Insulating elements | ||
| Ceramic fiber Alsiflex (°C) | 1260 | |
| CaSi plates (°C) | 1000 | |
| TC 26 (°C) | 1430 | |
| The maximum weight of batch (kg) | 250 | |
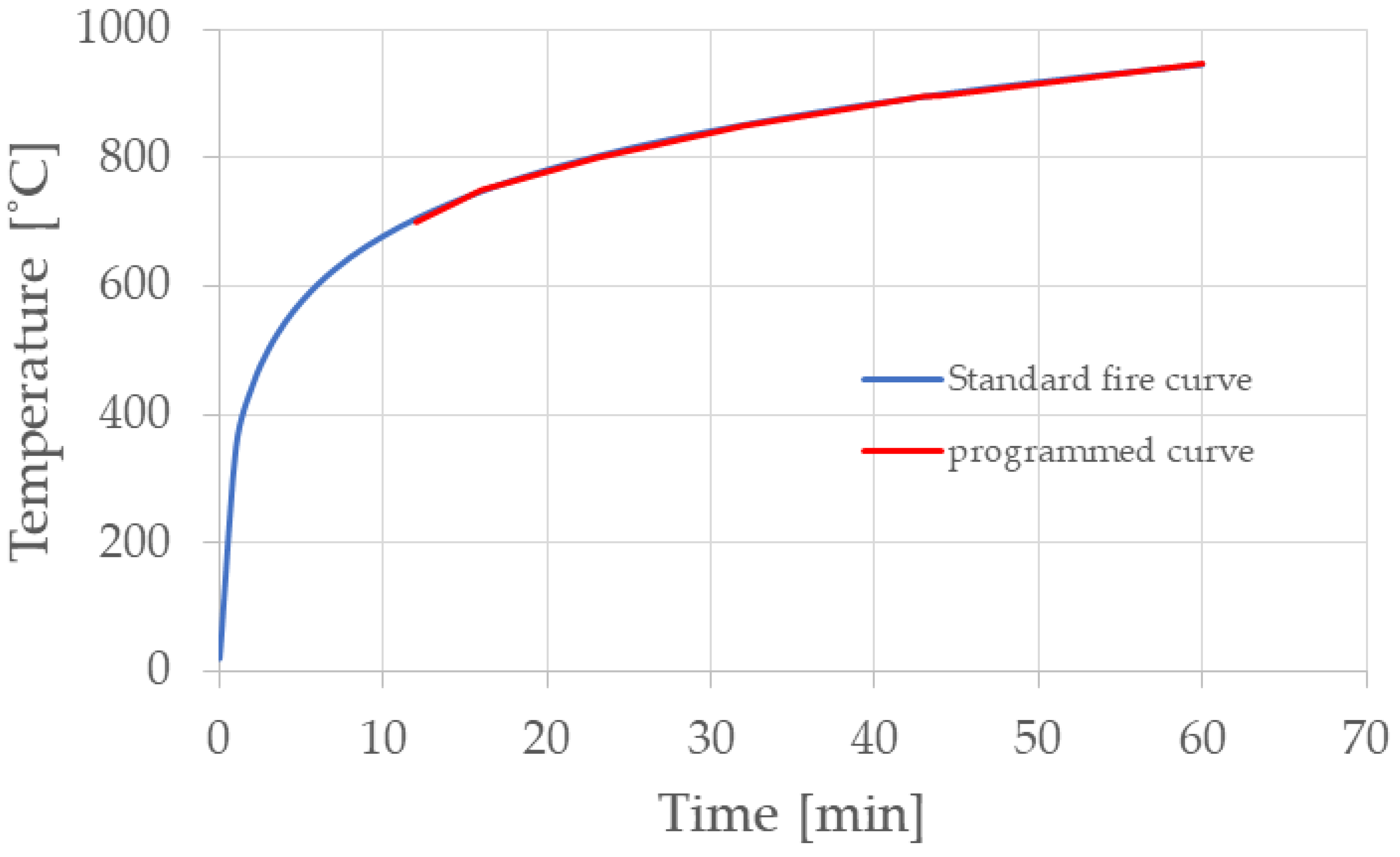
| Time (min) | 12 | 16 | 23 | 32 | 44 | 60 | |
| Temperature (°C) | EC Standard Fire Curve | 705.44 | 748.15 | 802.17 | 851.43 | 898.98 | 945.34 |
| Programmed Curve | 700 | 750 | 800 | 850 | 900 | 945 | |
| Properties | Typical Value |
|---|---|
| The appearance of concentrate | Straw-colored, semi-transparent |
| Specific gravity at 15.5 °C | 1.03 |
| Kinematic viscosity | 590 mm2/s |
| pH (concentration 20%) | 9.1 |
| Reframetric coefficient | 5.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubicka, K.; Szczecina, M.; Suckert, M.; Suckert, T. The Initial Assessment of the Possibility of Simulation Fire Standard Curve in the Electric Furnace with the Assessment of Chemical Composition Changes in Steel after Cooling Phase. Metals 2024, 14, 707. https://doi.org/10.3390/met14060707
Kubicka K, Szczecina M, Suckert M, Suckert T. The Initial Assessment of the Possibility of Simulation Fire Standard Curve in the Electric Furnace with the Assessment of Chemical Composition Changes in Steel after Cooling Phase. Metals. 2024; 14(6):707. https://doi.org/10.3390/met14060707
Chicago/Turabian StyleKubicka, Katarzyna, Michał Szczecina, Mariusz Suckert, and Tomasz Suckert. 2024. "The Initial Assessment of the Possibility of Simulation Fire Standard Curve in the Electric Furnace with the Assessment of Chemical Composition Changes in Steel after Cooling Phase" Metals 14, no. 6: 707. https://doi.org/10.3390/met14060707






