Influence of High-Temperature Deformation on the Dissolution of δ-Ferrite in Stainless Steels
Abstract
:1. Introduction
2. Materials and Methods
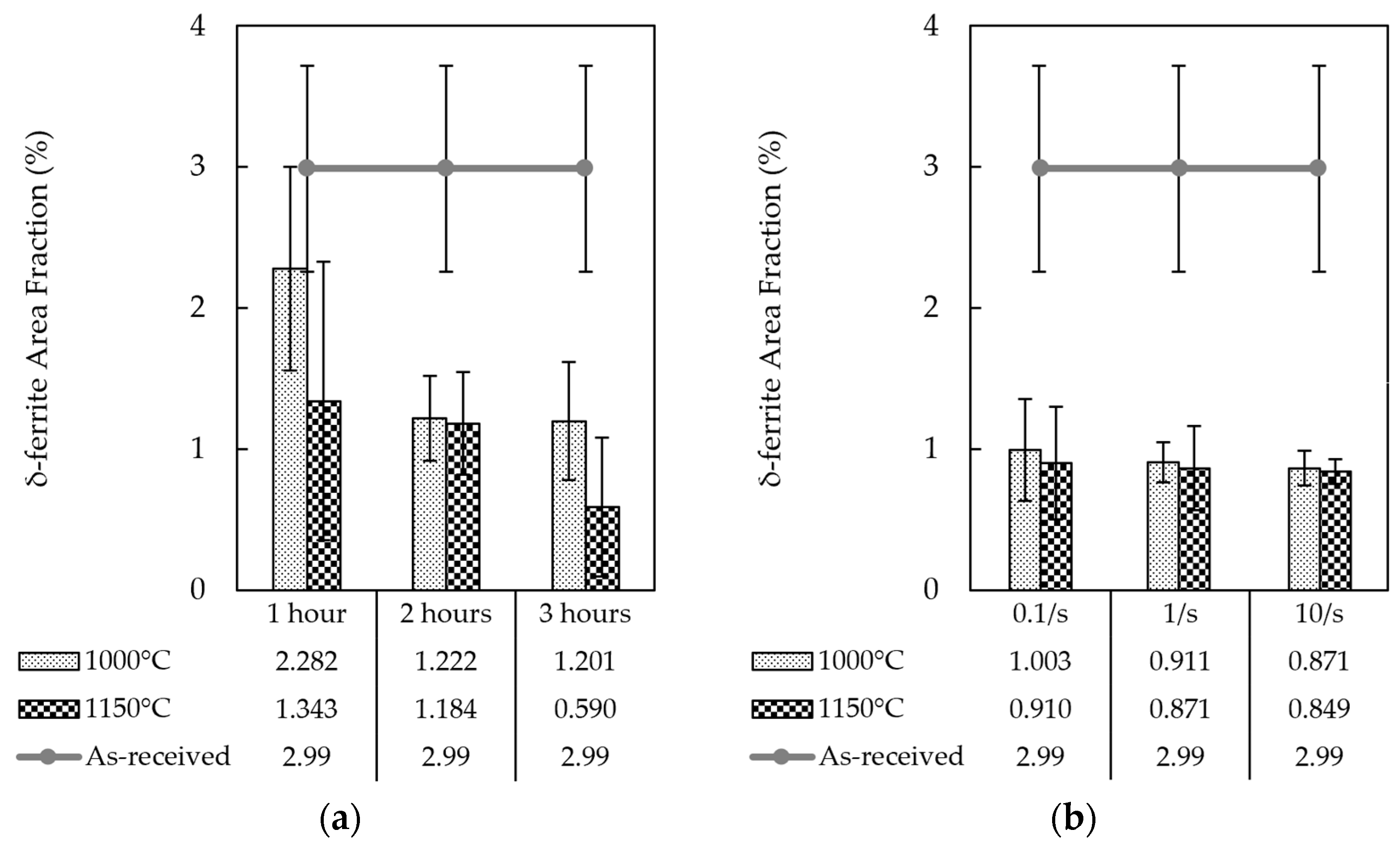
- Conventional Isothermal Annealing Treatments: The heat treatments were performed at two different temperatures of 1000 and 1150 °C, while the soaking times were chosen as 1, 2, and 3 h, respectively. After each treatment, the samples were air-cooled to room temperature. A computer-controlled 3-Zone Tube Furnace (LINDBERG CC58434C/LINDBERG, Watertown, WI, USA) was used for these heat treatments.
- Hot Compression Experiments: After heating the samples to the deformation temperature of 900 °C, a Universal Testing Machine (INSTRON 1000HDX/INSTRON, Norwood, MA, USA) equipped with tungsten carbide (WC) dies and an infrared furnace with four 1000 W lamps (ChambIR E4-D-01-A/ChambIR, Eden Prairie, MN, USA) was used to conduct compression tests under three distinct strain rates of 0.1, 1, and 10/s. The deformation strain was kept constant at 60% in all experiments. The post-process heat treatments were carried out at 1000 °C and 1150 °C for 10 min immediately after the deformation, followed by air cooling to room temperature. These annealing treatments were performed with the aid of a muffle furnace (Thermo Scientific Thermolyne F48028-80/Thermo Scientific, Asheville, NC, USA).
3. Results and Discussion
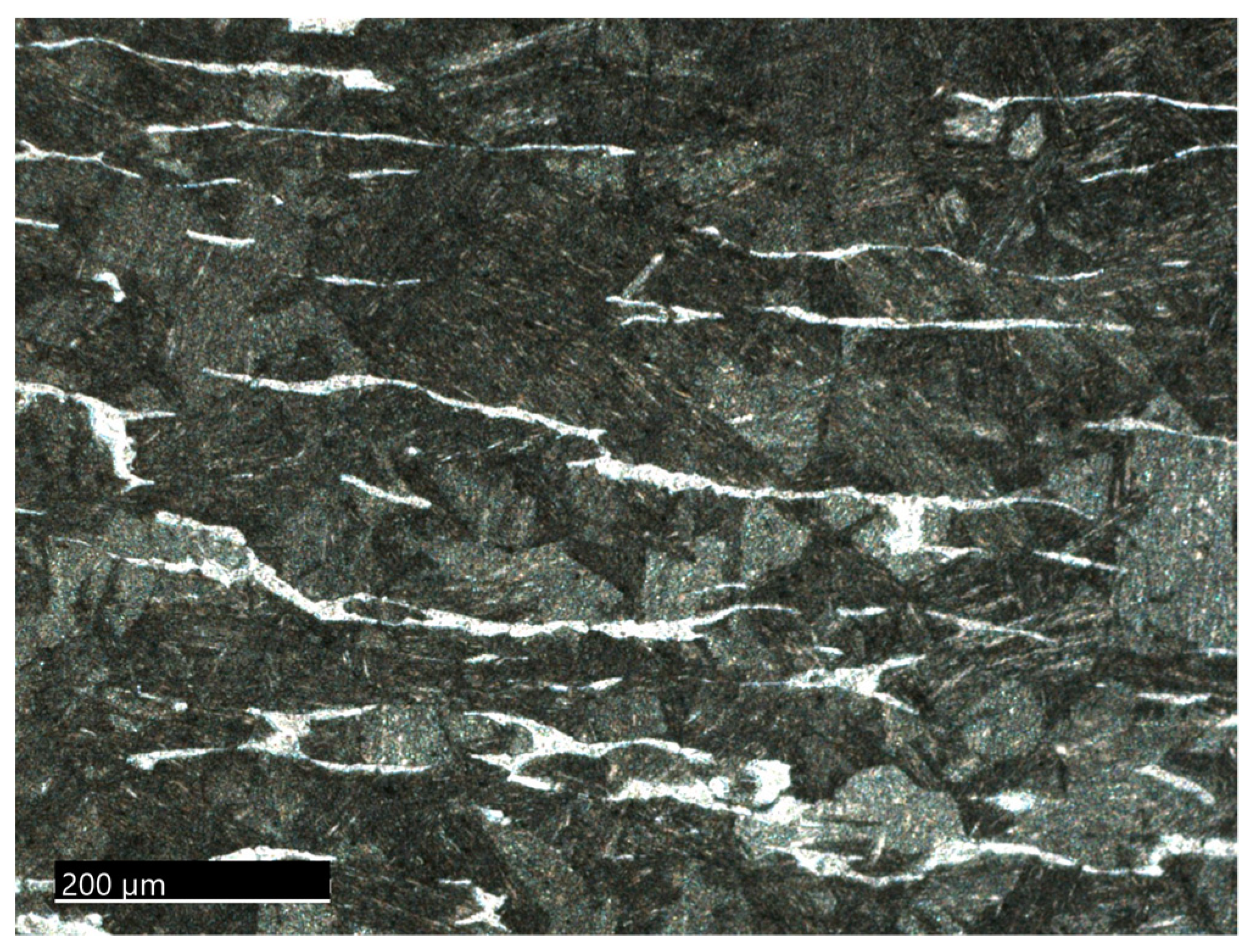
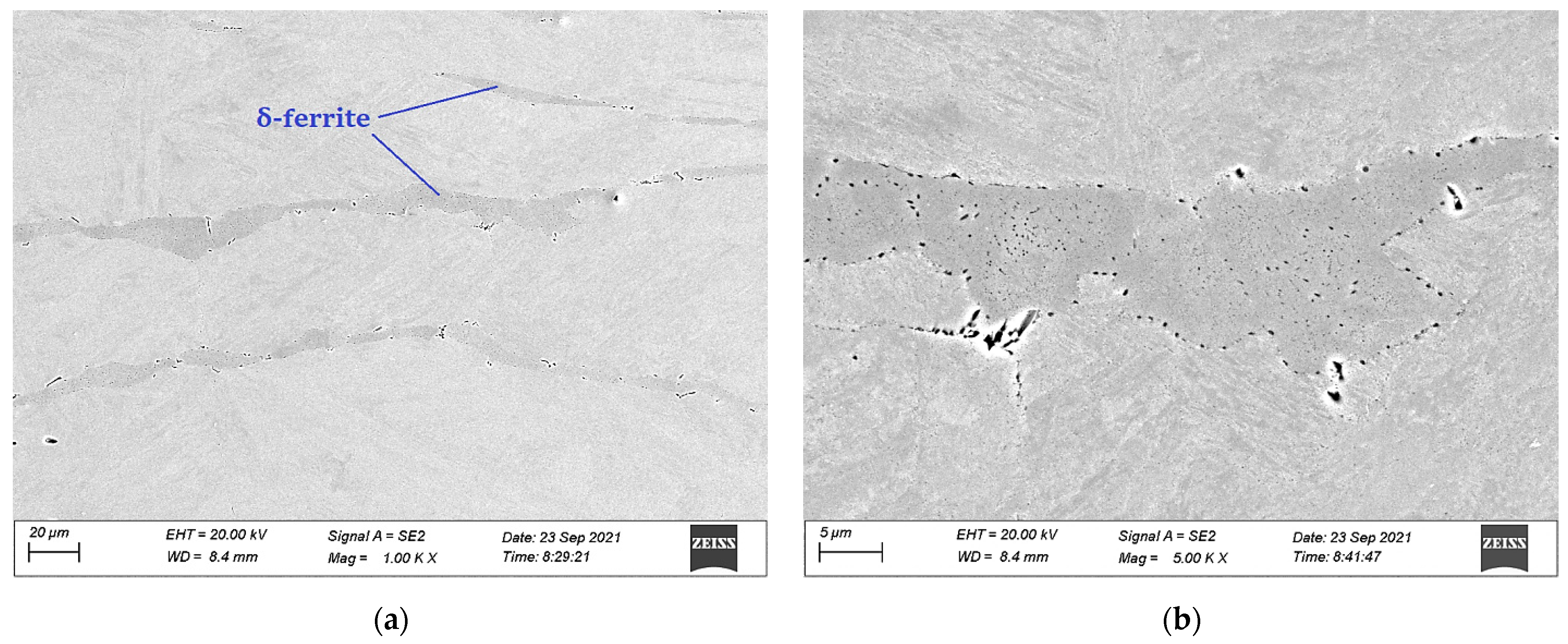
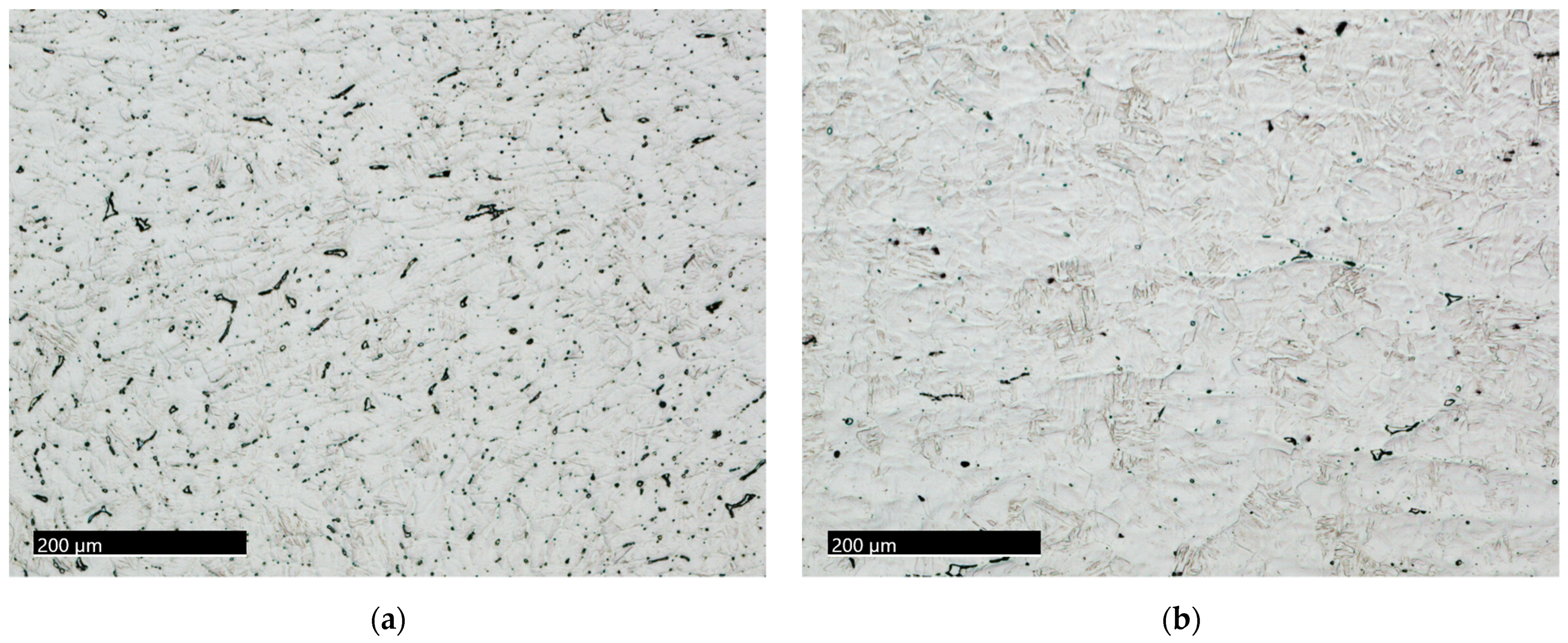
3.1. Microstructural Characterization
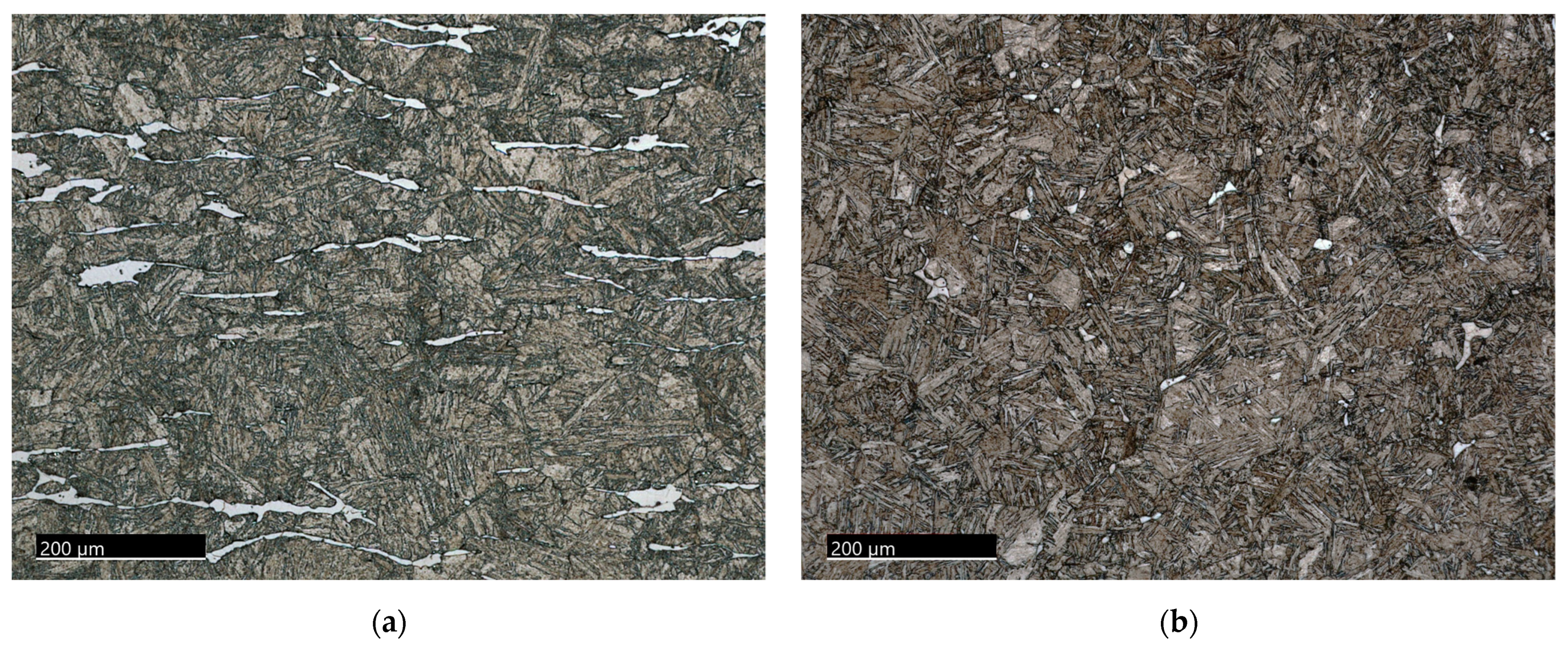
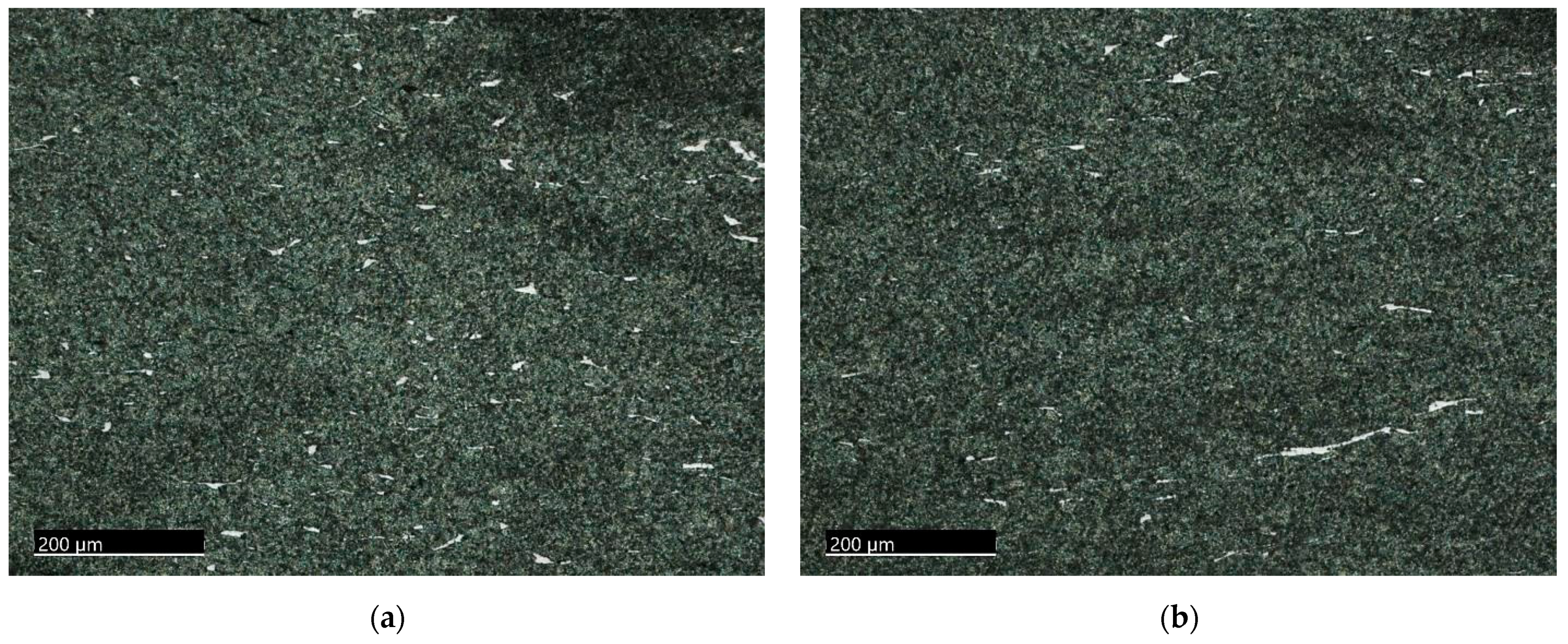
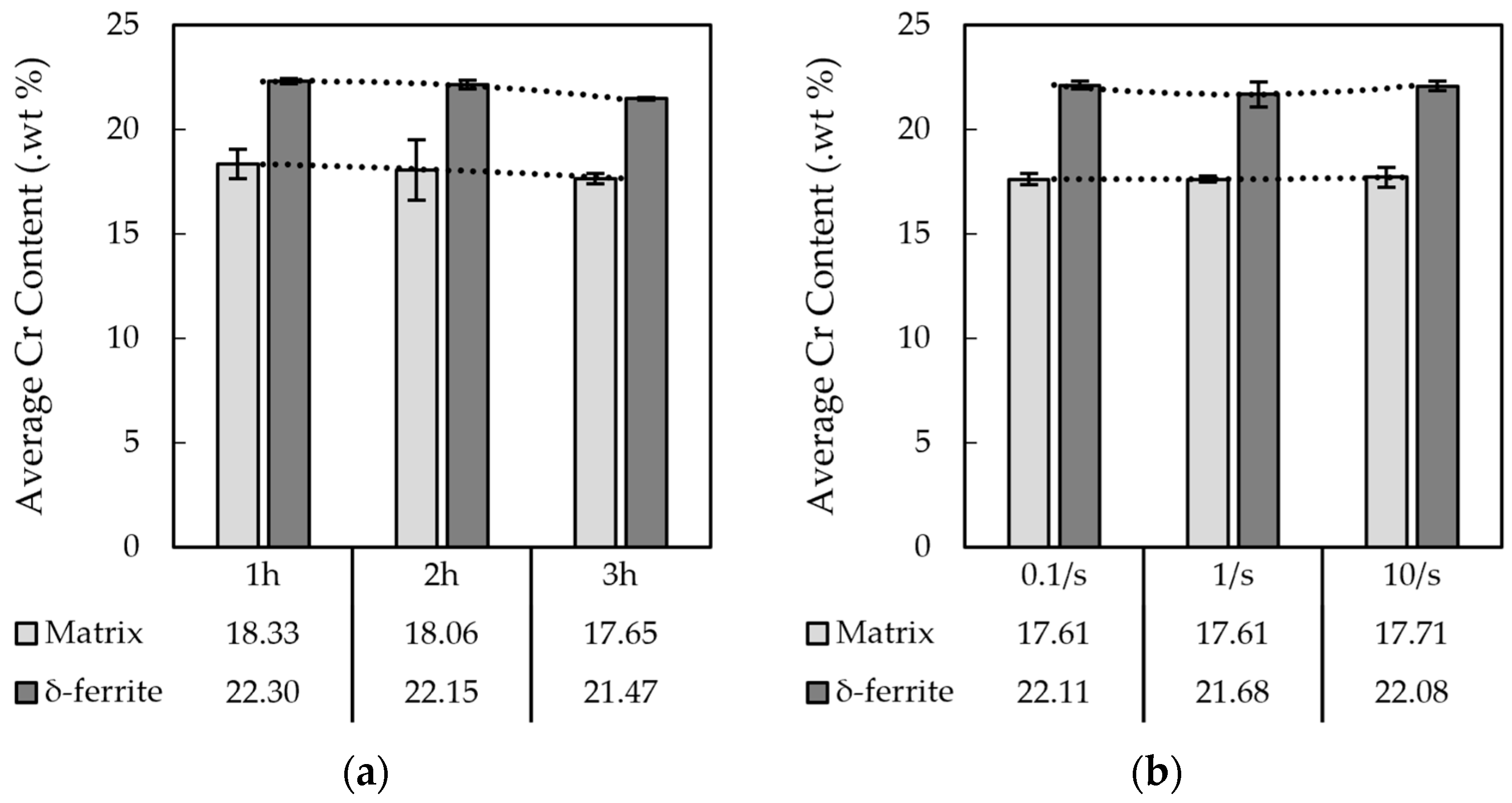
3.1.1. The 15-5PH Stainless Steel
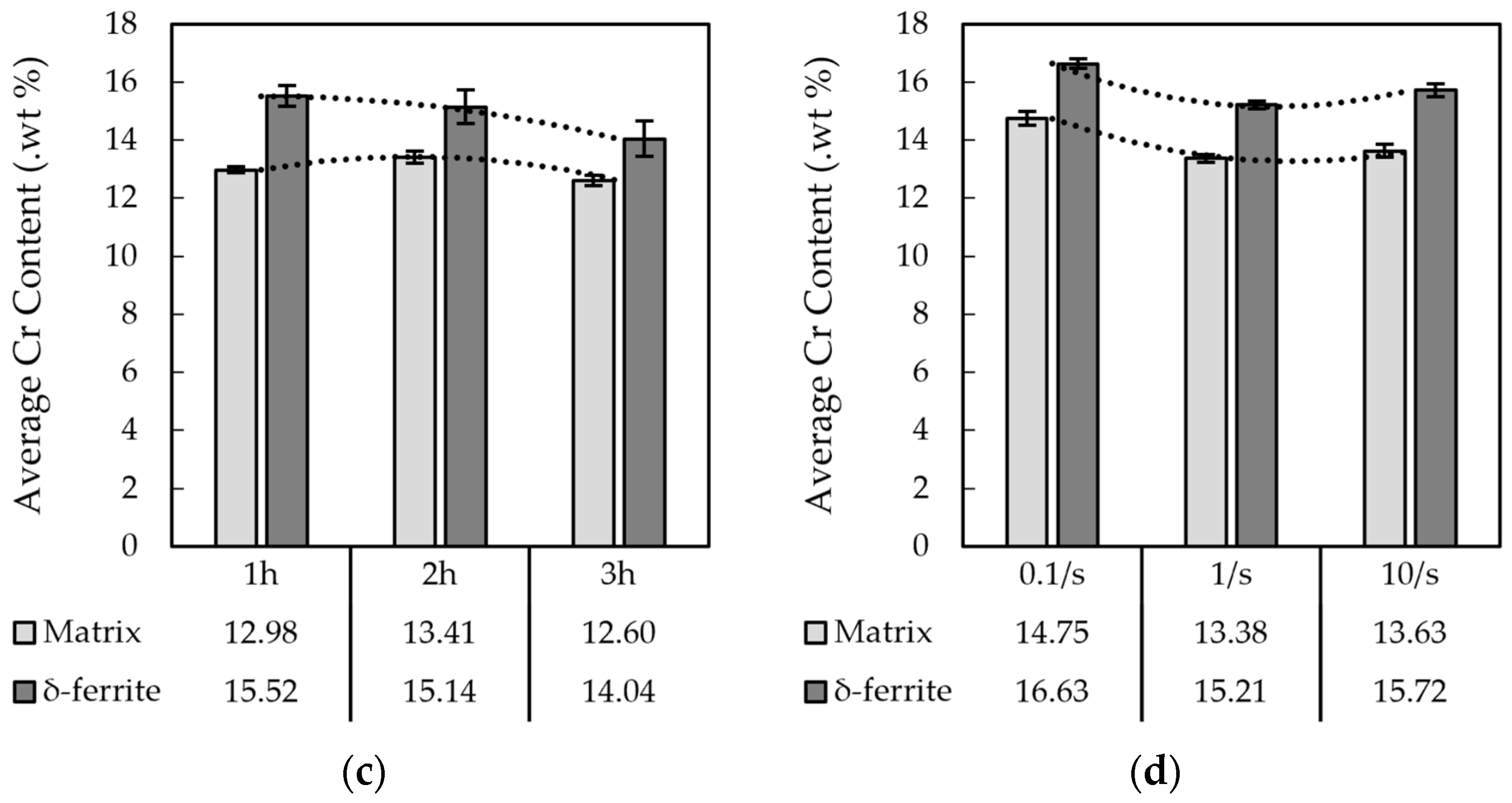
3.1.2. The M-154 Stainless Steel
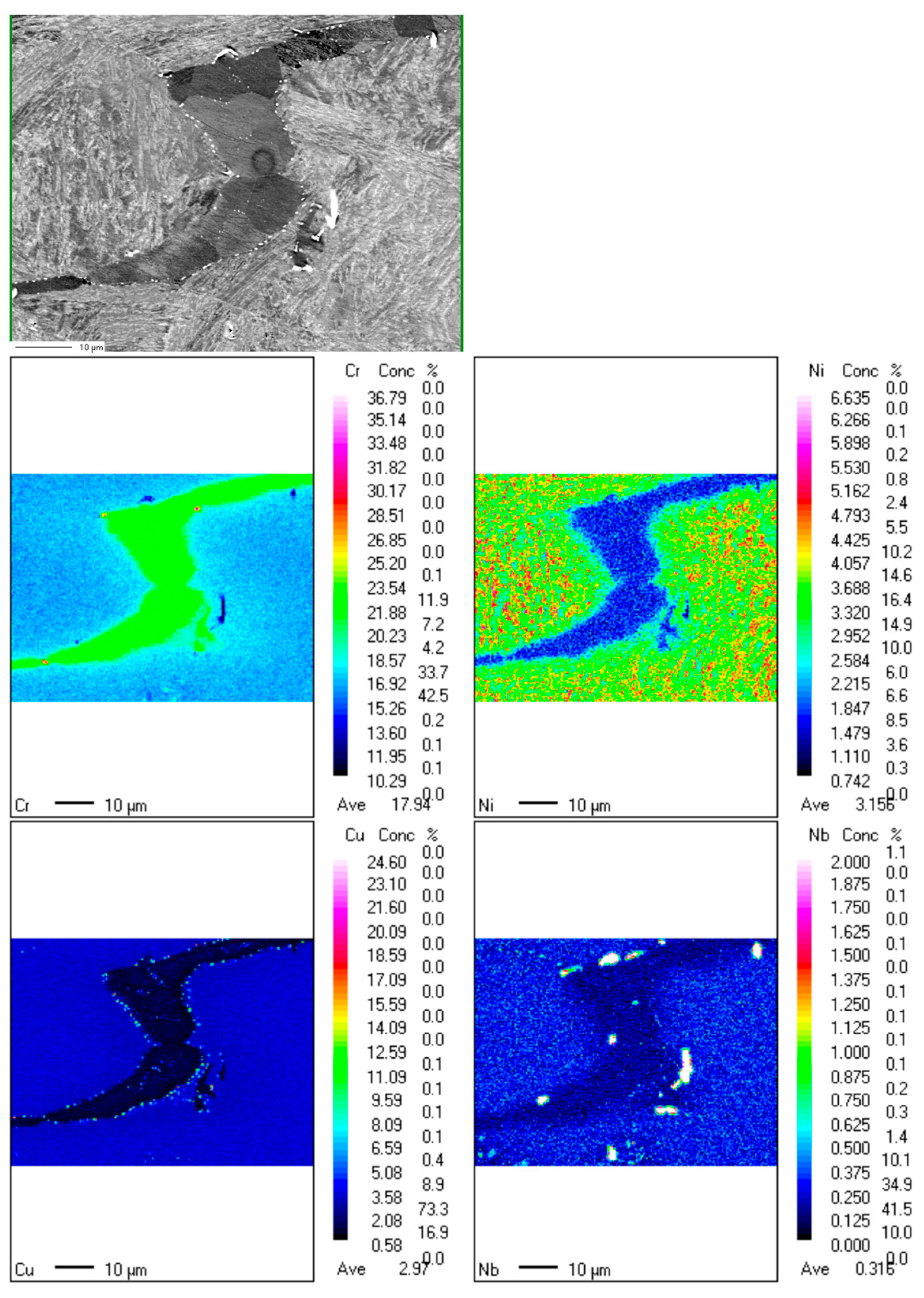
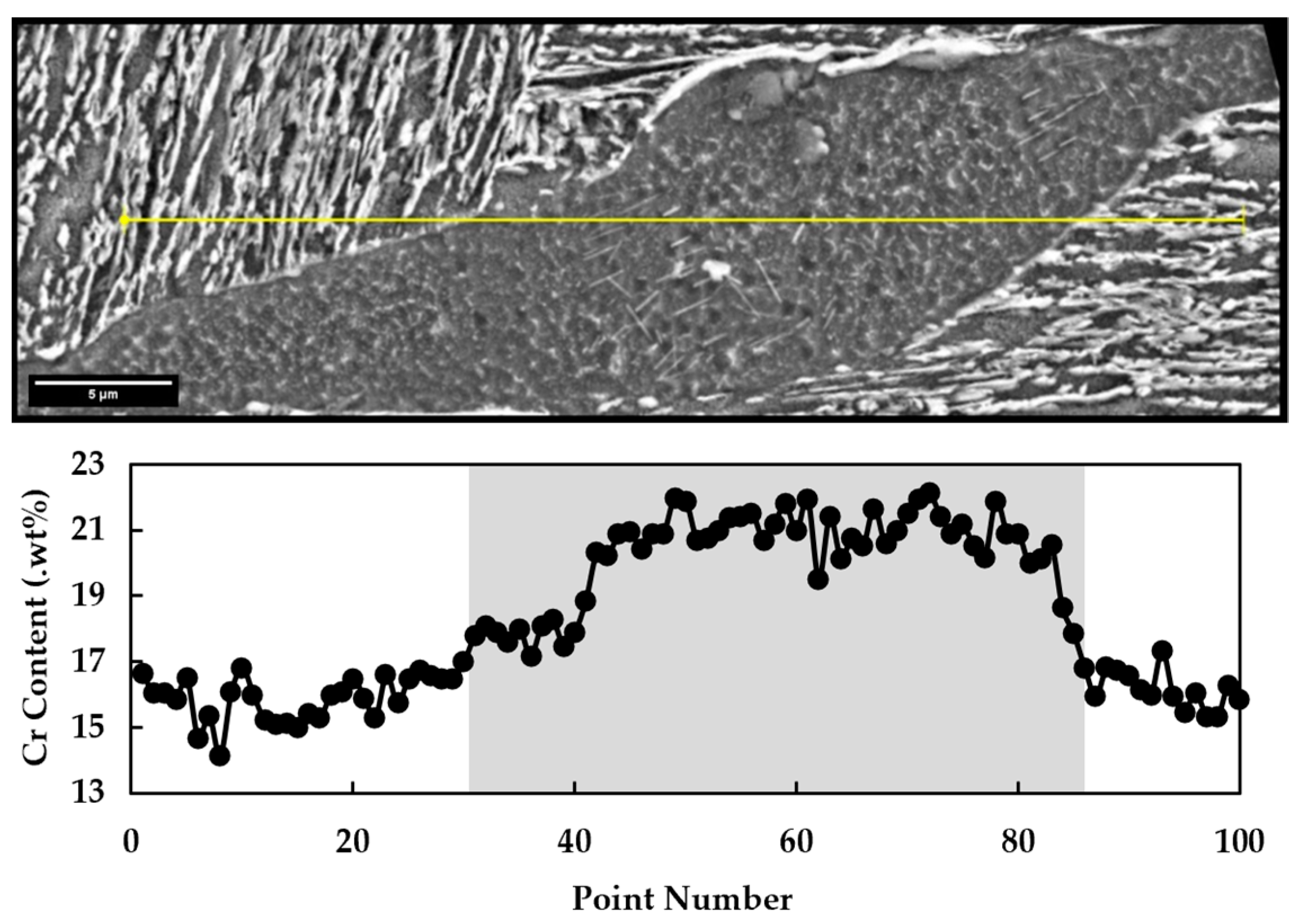
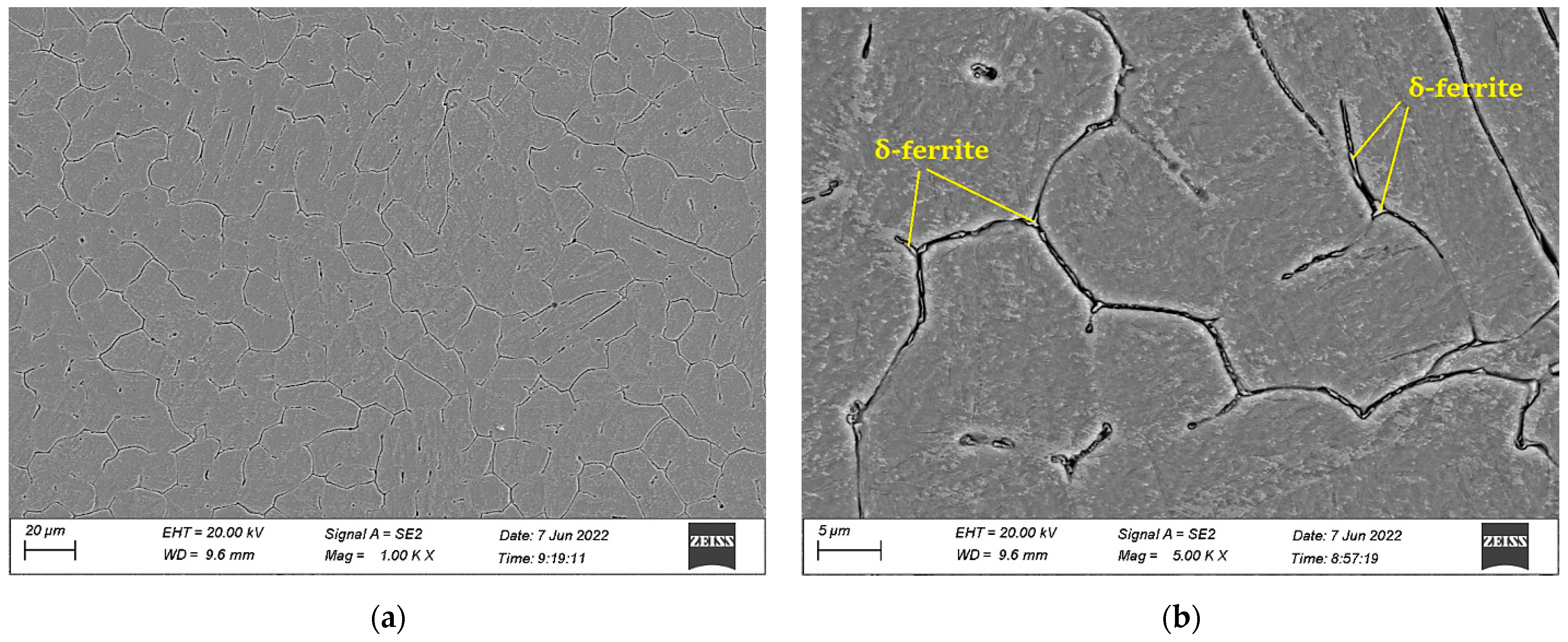
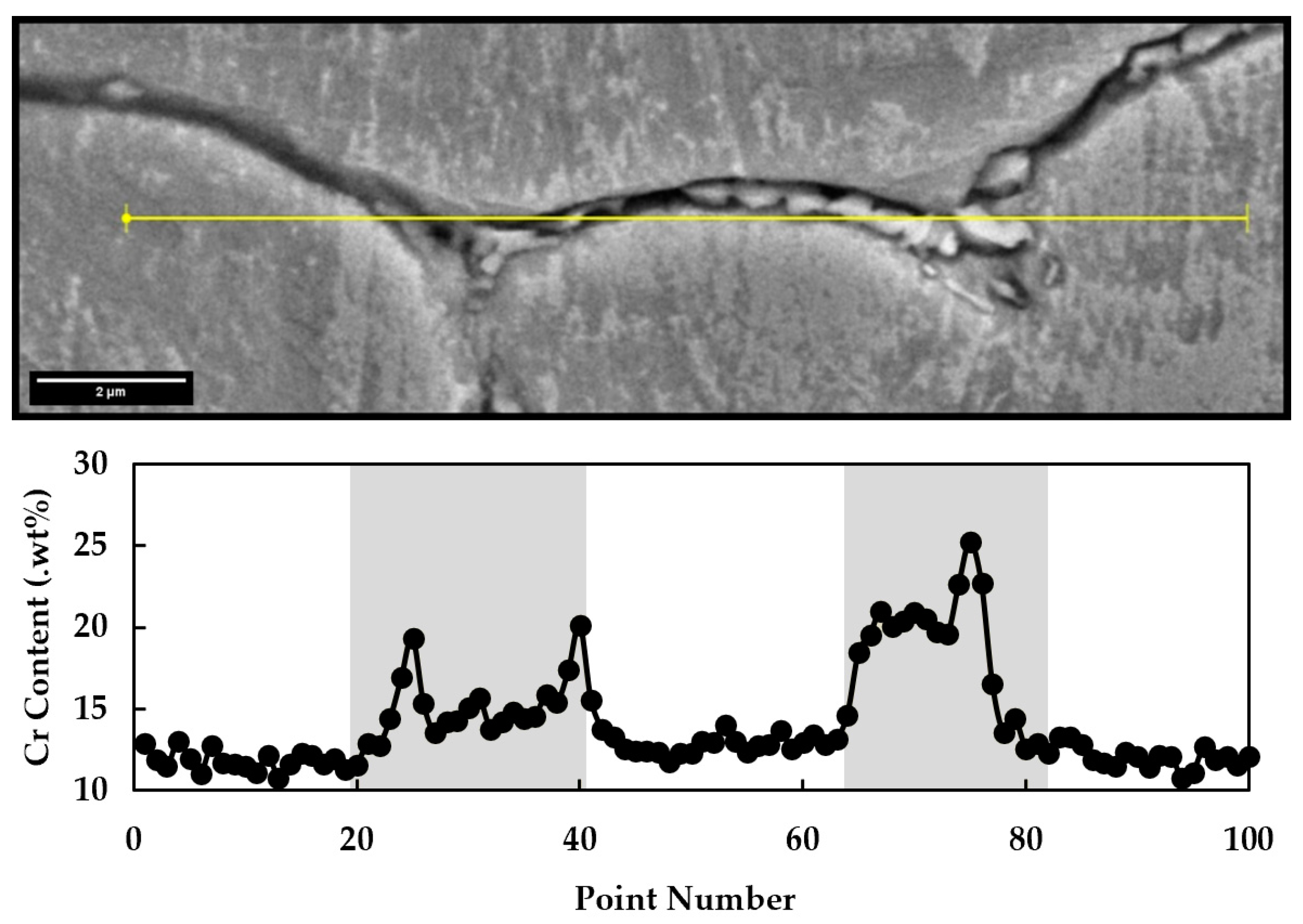
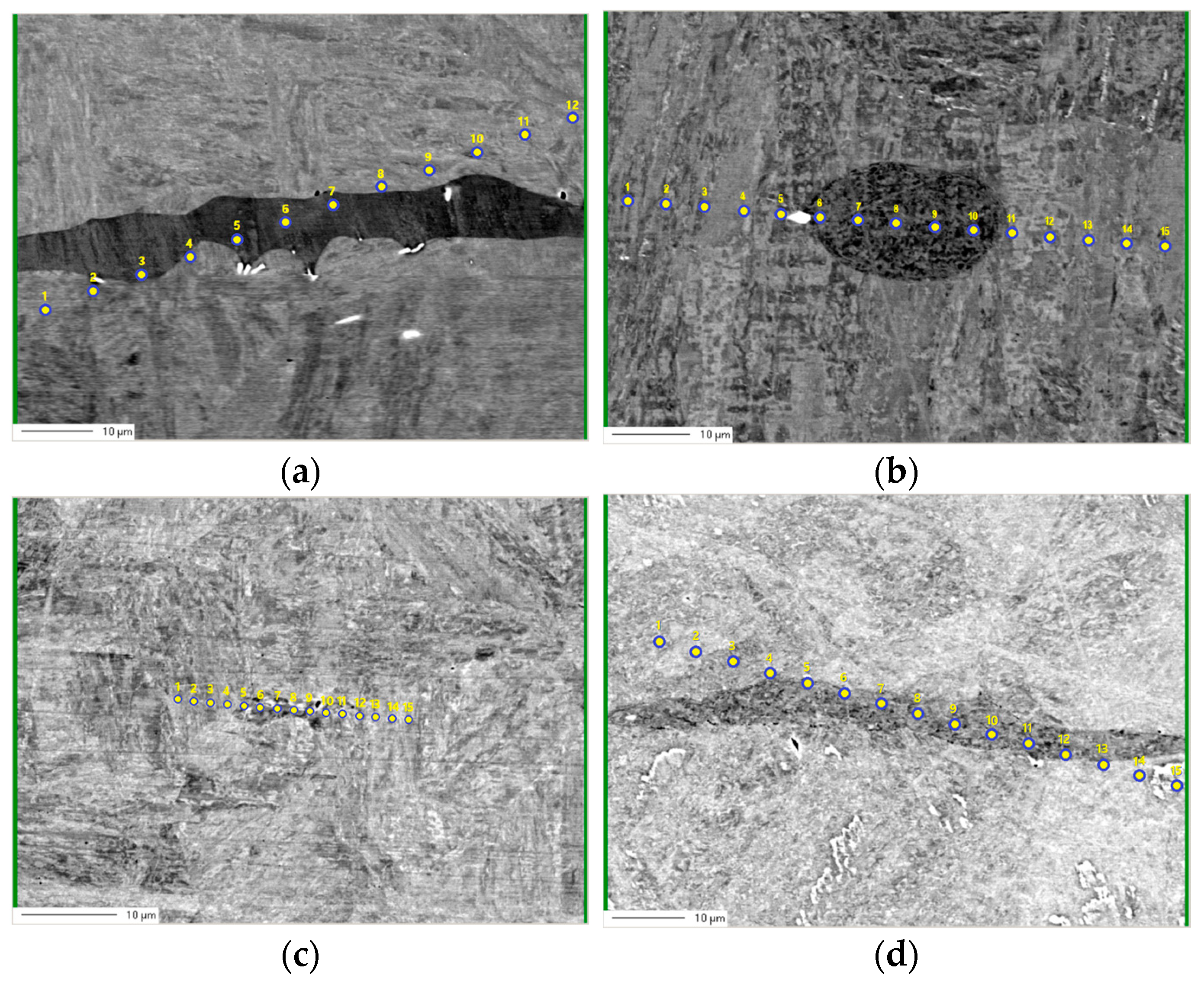
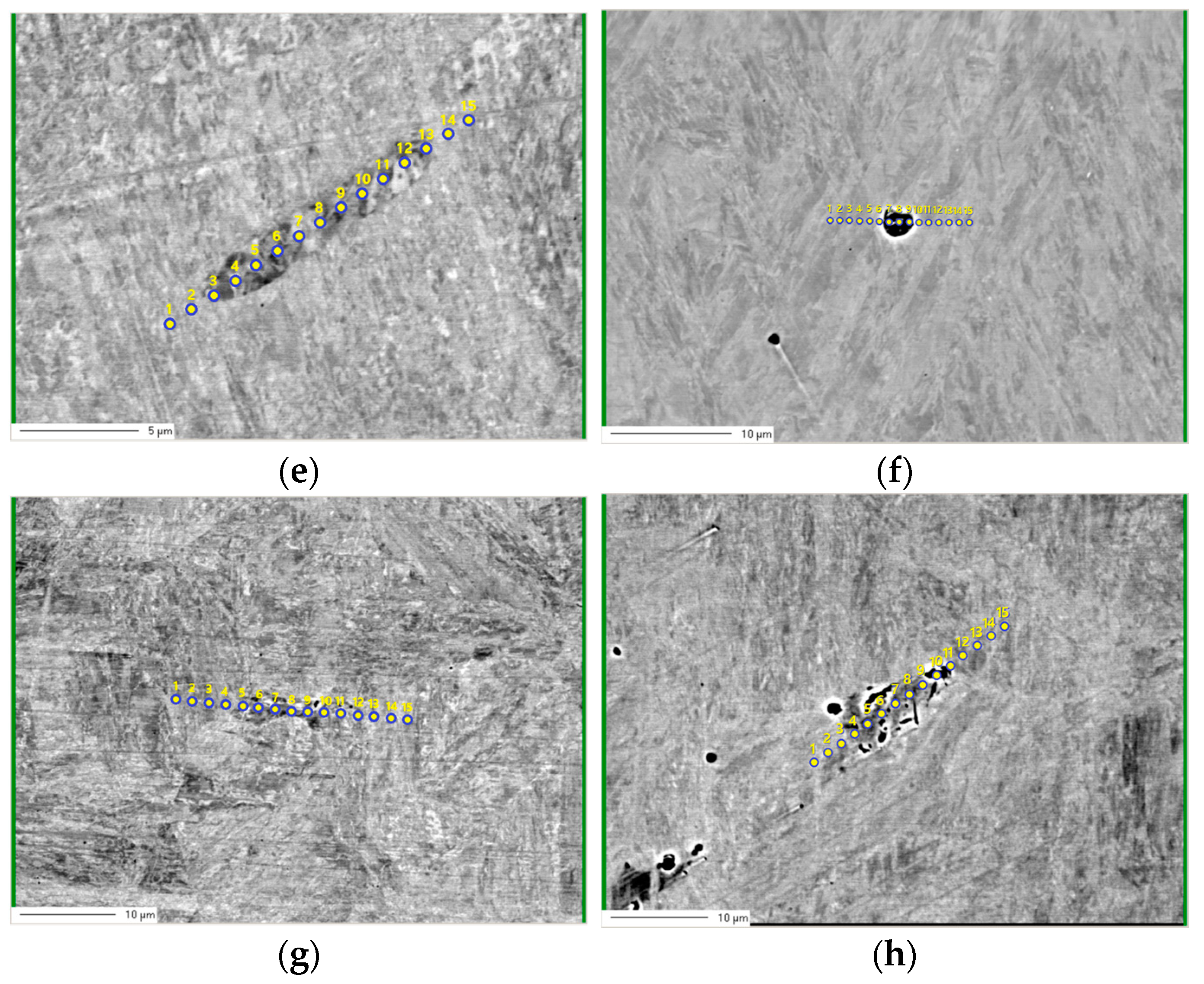
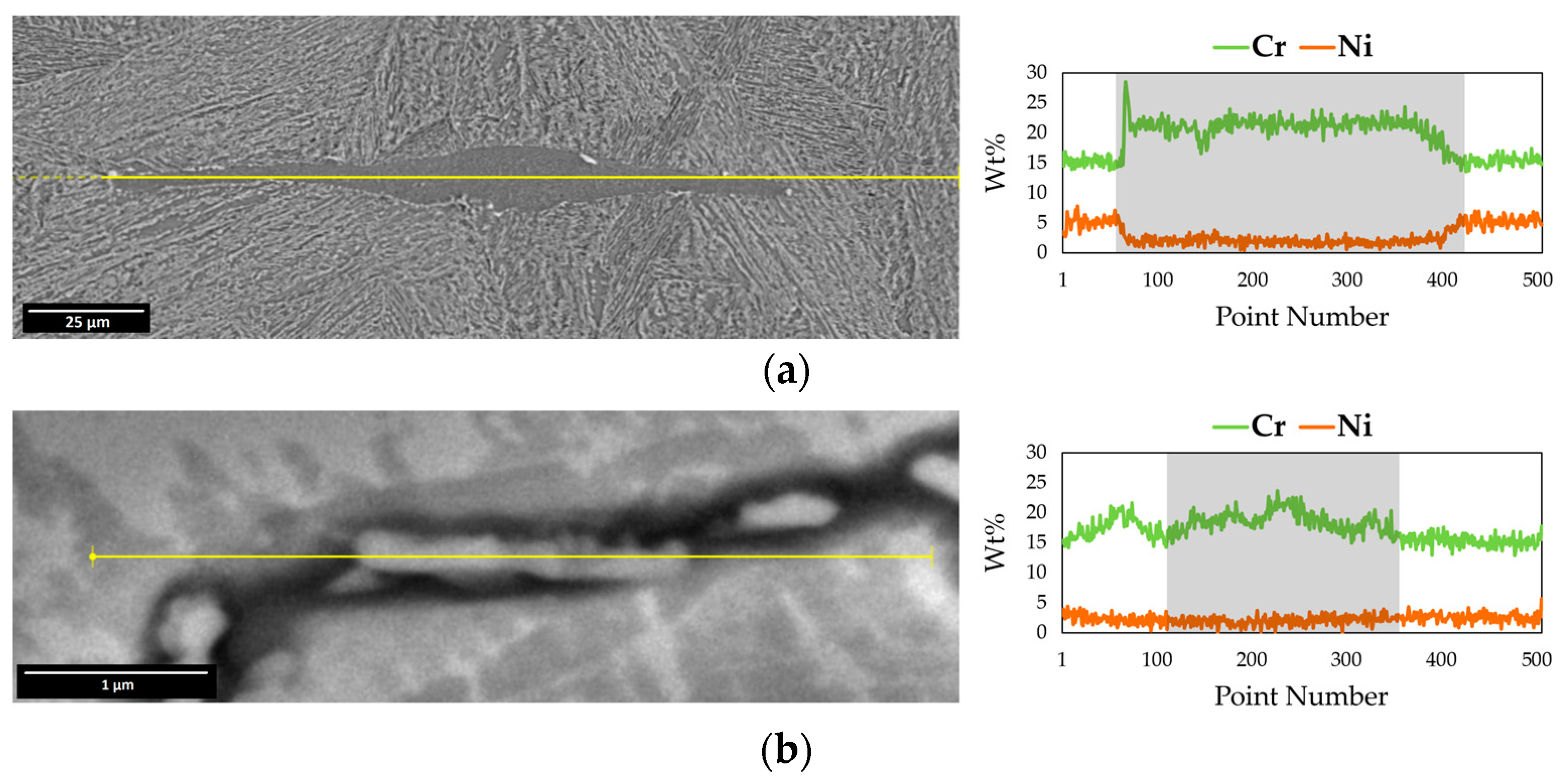
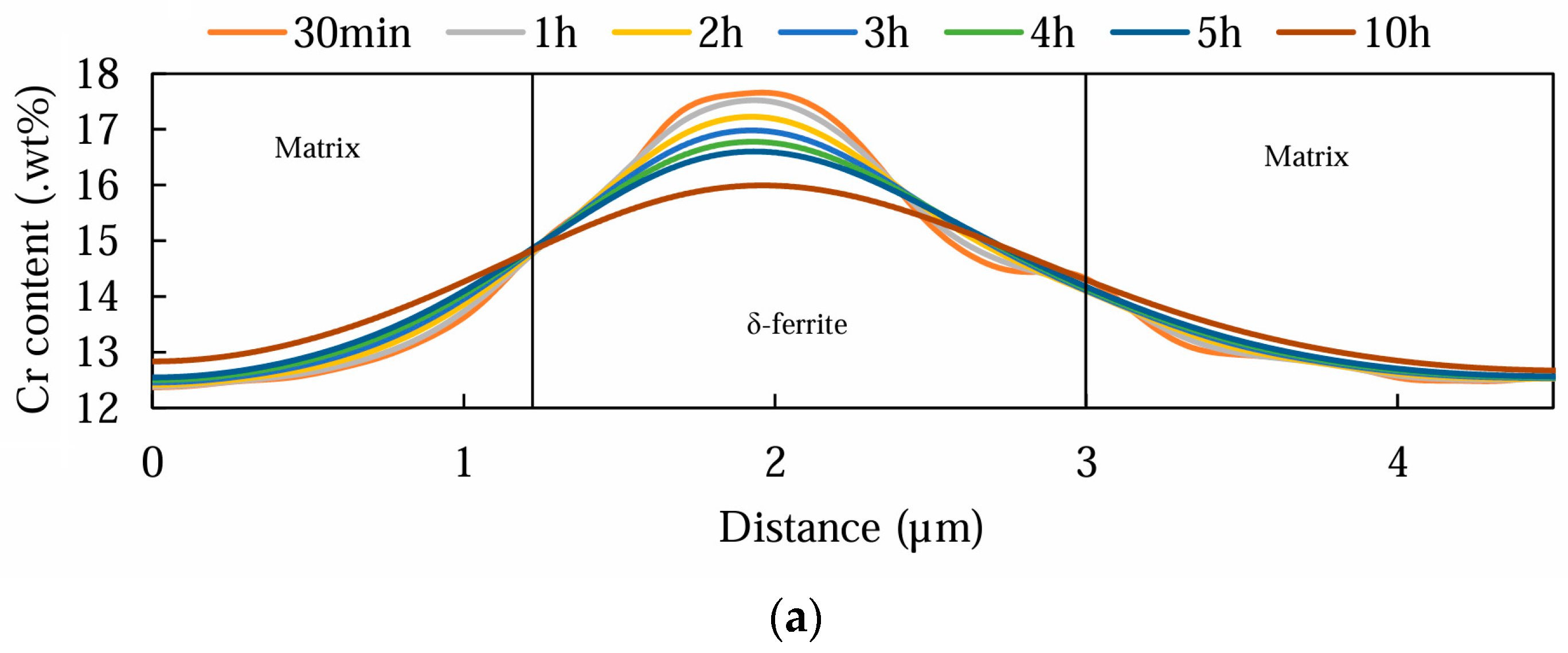
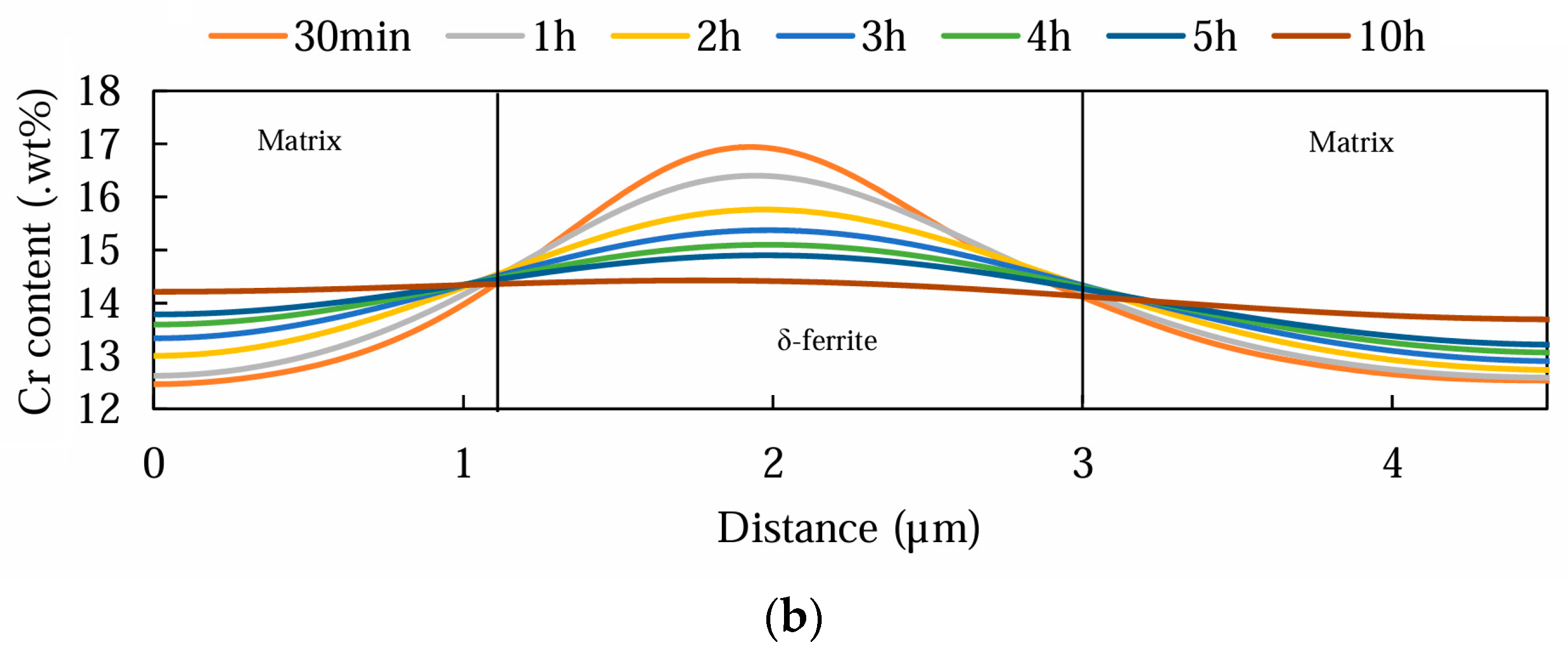
3.2. Electro Probe Micro Analysis (EPMA)
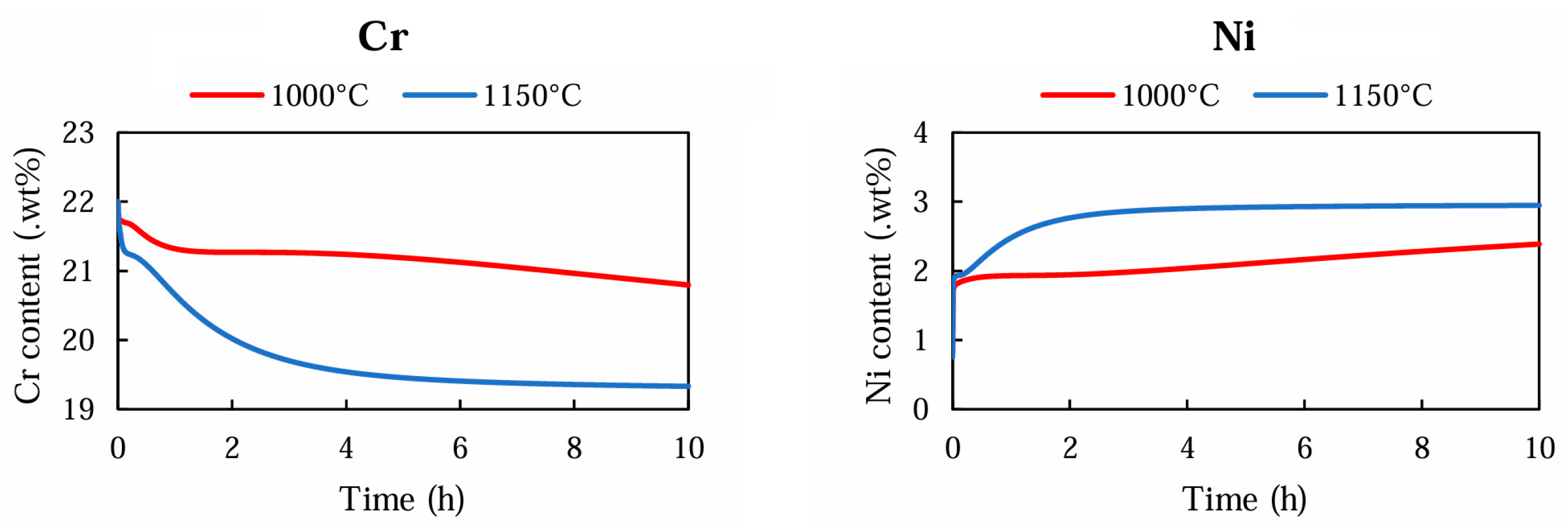
3.3. Thermodynamic Simulations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Winters, G.L.; Nutt, M.J. Stainless Steels for Medical and Surgical Applications; ASTM International: West Conshohocken, PA, USA, 2003; Volume 1438. [Google Scholar] [CrossRef]
- Baddoo, N. Stainless steel in construction: A review of research, applications, challenges and opportunities. J. Constr. Steel Res. 2008, 64, 1199–1206. [Google Scholar] [CrossRef]
- Emun, Y.; Zurob, H.; Kish, J. Corrosion Evaluation of Ferritic Stainless Steels for Automotive Exhaust Applications. Corrosion 2019, 75, 1276–1280. [Google Scholar] [CrossRef] [PubMed]
- Hamada, A.; Ghosh, S.; Ali, M.; Jaskari, M.; Järvenpää, A. Studying the strengthening mechanisms and mechanical properties of dissimilar laser-welded butt joints of medium-Mn stainless steel and automotive high-strength carbon steel. Mater. Sci. Eng. A 2022, 856, 143936. [Google Scholar] [CrossRef]
- Karabeyoglu, S.S.; Yaman, P. An Experimental Investigation of Martensitic Stainless Steel in Aircraft and Aerospace Industry for Thermal Wear Performance and Corrosion Potential. Pract. Metallogr. 2022, 59, 199–215. [Google Scholar] [CrossRef]
- Kumar, R.R.; Gupta, R.K.; Sarkar, A.; Prasad, M. Vacuum diffusion bonding of α-titanium alloy to stainless steel for aerospace applications: Interfacial microstructure and mechanical characteristics. Mater. Charact. 2022, 183, 111607. [Google Scholar] [CrossRef]
- Liu, T.; Zhu, X.; Chen, L.; Bi, H.; Lin, Y.; Long, J. Fatigue–creep behavior of two ferritic stainless steels in simulated automotive exhaust gas and argon. J. Mater. Sci. 2020, 55, 3684–3699. [Google Scholar] [CrossRef]
- Mishra, P.; Åkerfeldt, P.; Svahn, F.; Nilsson, E.; Forouzan, F.; Antti, M.-L. Microstructural characterization and mechanical properties of additively manufactured 21-6-9 stainless steel for aerospace applications. J. Mater. Res. Technol. 2023, 25, 1483–1494. [Google Scholar] [CrossRef]
- Bonagani, S.K.; Bathula, V.; Kain, V. Influence of tempering treatment on microstructure and pitting corrosion of 13 wt.% Cr martensitic stainless steel. Corros. Sci. 2018, 131, 340–354. [Google Scholar] [CrossRef]
- Ping, D.; Ohnuma, M.; Hirakawa, Y.; Kadoya, Y.; Hono, K. Microstructural evolution in 13Cr–8Ni–2.5 Mo–2Al martensitic precipitation-hardened stainless steel. Mater. Sci. Eng. A 2005, 394, 285–295. [Google Scholar] [CrossRef]
- Rho, B.S.; Hong, H.U.; Nam, S.W. The effect of δ-ferrite on fatigue cracks in 304L steels. Int. J. Fatigue 2000, 22, 683–690. [Google Scholar] [CrossRef]
- Du, D.; Wang, J.; Chen, K.; Zhang, L.; Andresen, P.L. Environmentally assisted cracking of forged 316LN stainless steel and its weld in high temperature water. Corros. Sci. 2019, 147, 69–80. [Google Scholar] [CrossRef]
- Cardoso, P.H.S.; Kwietniewski, C.; Porto, J.; Reguly, A.; Strohaecker, T.R. The influence of delta ferrite in the AISI 416 stainless steel hot workability. Mater. Sci. Eng. A 2003, 351, 1–8. [Google Scholar] [CrossRef]
- Bai, T.; Guan, K. Evaluation of stress corrosion cracking susceptibility of nanocrystallized stainless steel 304L welded joint by small punch test. Mater. Des. (1980–2015) 2013, 52, 849–860. [Google Scholar] [CrossRef]
- Mukherjee, M.; Pal, T.K. Role of microstructural constituents on surface crack formation during hot rolling of standard and low nickel austenitic stainless steels. Acta Metall. Sin. (Engl. Lett.) 2013, 26, 206–216. [Google Scholar] [CrossRef]
- Chandra, K.; Kumar, N.N.; Kain, V. Effect of retained δ-ferrite transforming to sigma phase on the hardness and corrosion resistance of stainless steel 321. Trans. Indian Inst. Met. 2022, 75, 959–966. [Google Scholar] [CrossRef]
- Lu, W.F.; Huang, J.Y.; Yung, T.Y.; Chen, T.C.; Tsai, K.C. Effects of thermal aging on the stress corrosion cracking behavior of cast stainless steel with different δ-ferrite levels in high temperature water environment. J. Nucl. Mater. 2022, 568, 153900. [Google Scholar] [CrossRef]
- Liu, B.B.; Zhang, H.; Xu, X.Y.; Tian, Q.R.; Fu, J.X. Analysis of Cracking Causes of AISI 416 Martensitic Stainless Steel During Hot Rolling. Trans. Indian Inst. Met. 2023, 76, 2643–2650. [Google Scholar] [CrossRef]
- Alcantar-Mondragón, N.; Reyes-Calderón, F.; García-García, V.; Garnica-González, P.; Estrada-Hernández, S. Thermal and diffusion conditions for delta ferrite formation in partially melted and heat-affected zones of multipass SAW joint performed in martensitic stainless steel 12Cr–1Mo. Results Mater. 2023, 20, 100489. [Google Scholar] [CrossRef]
- Cho, J.; Czerwinski, F.; Szpunar, J. The effect of reheating conditions and chemical composition on δ ferrite content in austenitic stainless steel slabs. J. Mater. Sci. 2000, 35, 1997–2003. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, D.; Guan, L.; Cao, L.; Wang, W. Effect of Si on δ-Ferrite/γ-Austenite Transformation and M23C6 Precipitation During Solidification of X10CrAlSi18 Ferritic Heat-Resistant Stainless Steel. Steel Res. Int. 2024, 95, 2300528. [Google Scholar] [CrossRef]
- Fukumoto, S.; Iwasaki, Y.; Motomura, H.; Fukuda, Y. Dissolution behavior of δ-ferrite in continuously cast slabs of SUS304 during heat treatment. ISIJ Int. 2012, 52, 74–79. [Google Scholar] [CrossRef]
- Arh, B.; Tehovnik, F.; Vode, F. Transformation of the δ-Ferrite in SS2343 Austenitic Stainless Steel upon Annealing at 1050 °C, 1150 °C and 1250 °C. Metals 2021, 11, 935. [Google Scholar] [CrossRef]
- de Souza Ciacco, P. Study of the Formation and Dissolution of δ-Ferrite during High Temperature Forging Processing of Austenitic, Martensitic and PH Stainless Steels. Master’s Thesis, University of Pittsburgh, Pittsburgh, PA, USA, 2023. Available online: http://d-scholarship.pitt.edu/id/eprint/44265 (accessed on 13 June 2023).
- Borgenstam, A.; Höglund, L.; Ågren, J.; Engström, A. DICTRA, a tool for simulation of diffusional transformations in alloys. J. Phase Equilibria 2000, 21, 269–280. [Google Scholar] [CrossRef]
- Schäfer, L. Influence of delta ferrite and dendritic carbides on the impact and tensile properties of a martensitic chromium steel. J. Nucl. Mater. 1998, 258, 1336–1339. [Google Scholar] [CrossRef]
- Kasana, S.S.; Pandey, O. Influence of Chemical Composition and Thermo-Mechanical Processing on the Formation of Delta Ferrite in 10.7 CrMoVNbN Steel. Trans. Indian Inst. Met. 2020, 73, 2491–2502. [Google Scholar] [CrossRef]
- Ravibharath, R.; Muthupandi, V.; Bala Srinivasan, P.; Devakumaran, K. Characterization of solidification cracking in 304HCu austenitic stainless steel welds. Trans. Indian Inst. Met. 2020, 73, 2345–2353. [Google Scholar] [CrossRef]
- Rezayat, M.; Mirzadeh, H.; Namdar, M.; Parsa, M.H. Unraveling the effect of thermomechanical treatment on the dissolution of delta ferrite in austenitic stainless steels. Metall. Mater. Trans. A 2016, 47, 641–648. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Mahata, D.; Roychaudhuri, R.; Mondal, R. Effect of rolling deformation and solution treatment on microstructure and mechanical properties of a cast duplex stainless steel. Bull. Mater. Sci. 2012, 35, 839–846. [Google Scholar] [CrossRef]
- Shaikh, H.; Khatak, H.S.; Seshadri, S.K.; Gnanamoorthy, J.B.; Rodriguez, P. Effect of ferrite transformation on the tensile and stress corrosion properties of type 316 L stainless steel weld metal thermally aged at 873 K. Metall. Mater. Trans. A 1995, 26, 1859–1868. [Google Scholar] [CrossRef]
- Cui, C.; Gao, X.; Su, G.; Gao, C.; Liu, Z.; Misra, R.D. Effect of thermal treatment on the evolution of delta ferrite in 11Cr–3Co–2.3 W steel. Mater. Sci. Technol. 2018, 34, 2087–2096. [Google Scholar] [CrossRef]





| Grade | C | Mn | Si | Cr | Ni | Mo | Cu | Nb | V |
|---|---|---|---|---|---|---|---|---|---|
| 15-5PH | 0.04 | 0.54 | 0.22 | 15.46 | 4.06 | 0.20 | 3.80 | 0.25 | 0.06 |
| M-154 | 0.13 | 0.72 | 0.20 | 11.50 | 2.80 | 1.72 | 0.17 | - | 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bajmalu Rostami, R.; de Souza Ciacco, P.; Viali Muñoz, M.C.; Simoes, L.F.; Garcia, C.I. Influence of High-Temperature Deformation on the Dissolution of δ-Ferrite in Stainless Steels. Metals 2024, 14, 783. https://doi.org/10.3390/met14070783
Bajmalu Rostami R, de Souza Ciacco P, Viali Muñoz MC, Simoes LF, Garcia CI. Influence of High-Temperature Deformation on the Dissolution of δ-Ferrite in Stainless Steels. Metals. 2024; 14(7):783. https://doi.org/10.3390/met14070783
Chicago/Turabian StyleBajmalu Rostami, Rahman, Pedro de Souza Ciacco, Mauricio Claudio Viali Muñoz, Luis Fellipe Simoes, and Calixto Isaac Garcia. 2024. "Influence of High-Temperature Deformation on the Dissolution of δ-Ferrite in Stainless Steels" Metals 14, no. 7: 783. https://doi.org/10.3390/met14070783






