Ultrafine Grain 316L Stainless Steel Manufactured by Ball Milling and Spark Plasma Sintering: Consequences on the Corrosion Resistance in Chloride Media
Abstract
1. Introduction
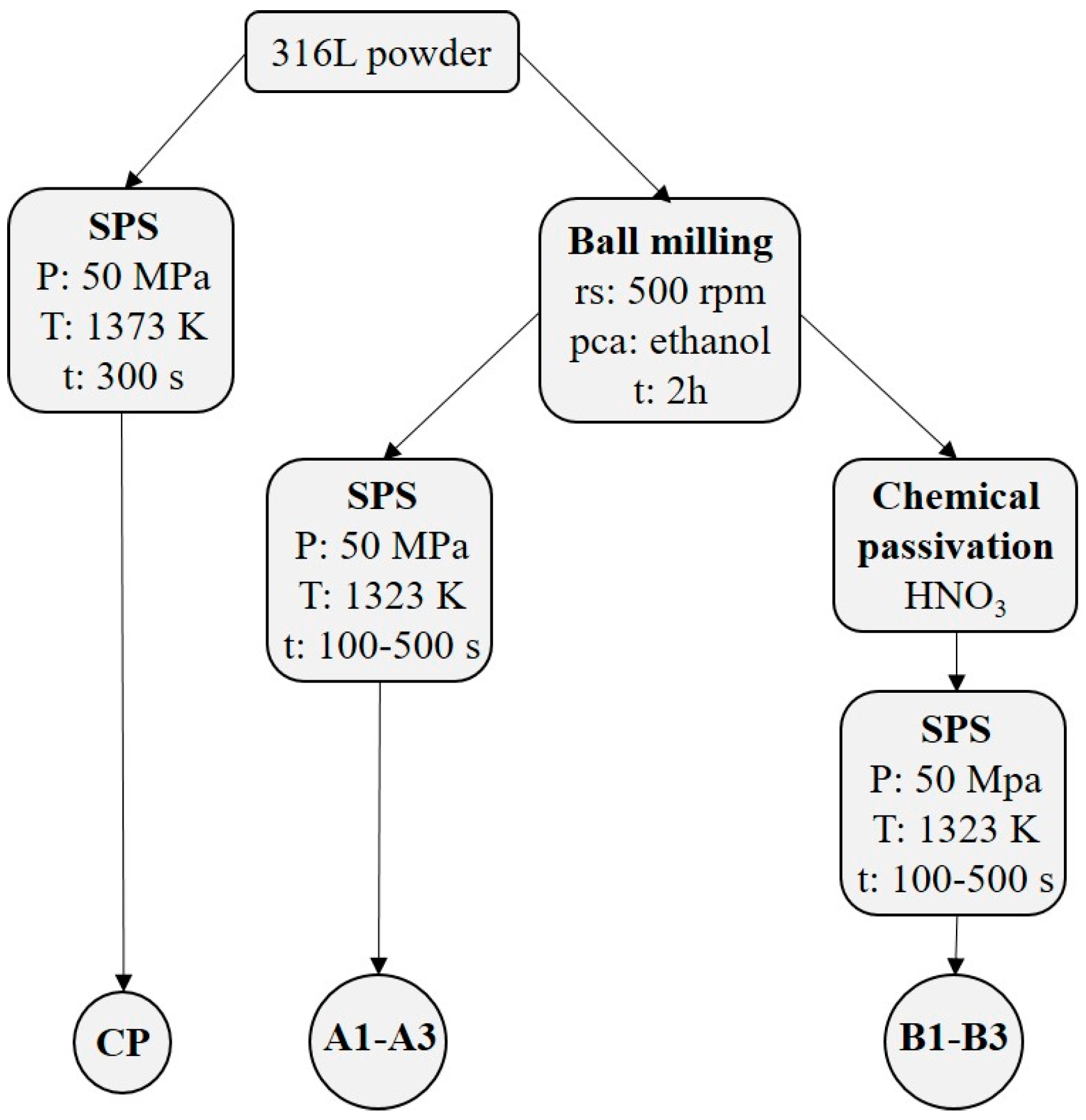
2. Experimental Details
2.1. Sintering Elaboration and Microstructure Observations
2.2. Electrochemical Experiments
3. Results and Discussion
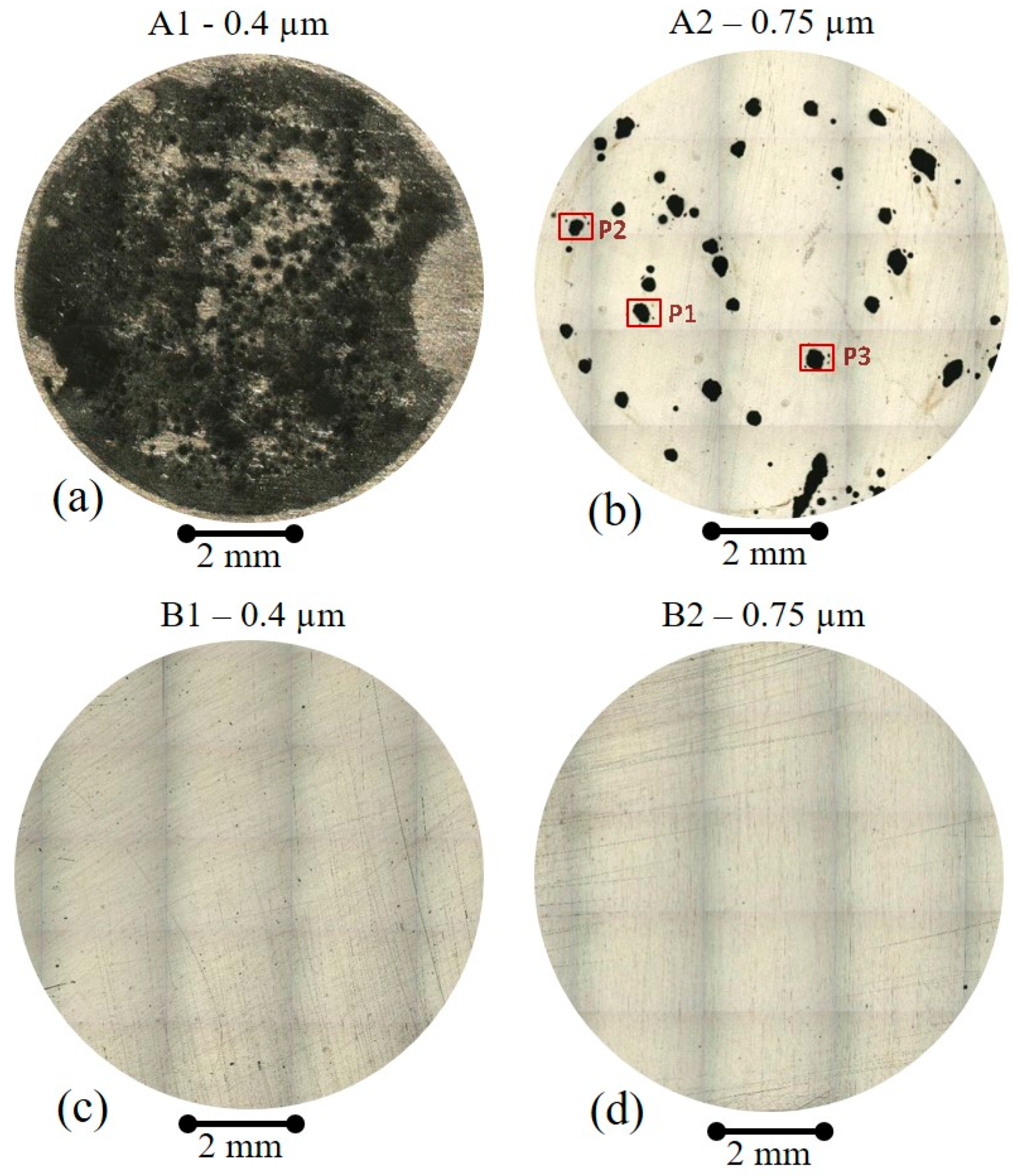
3.1. Brief Microstructural Overview of the Samples
3.2. Corrosion Behavior
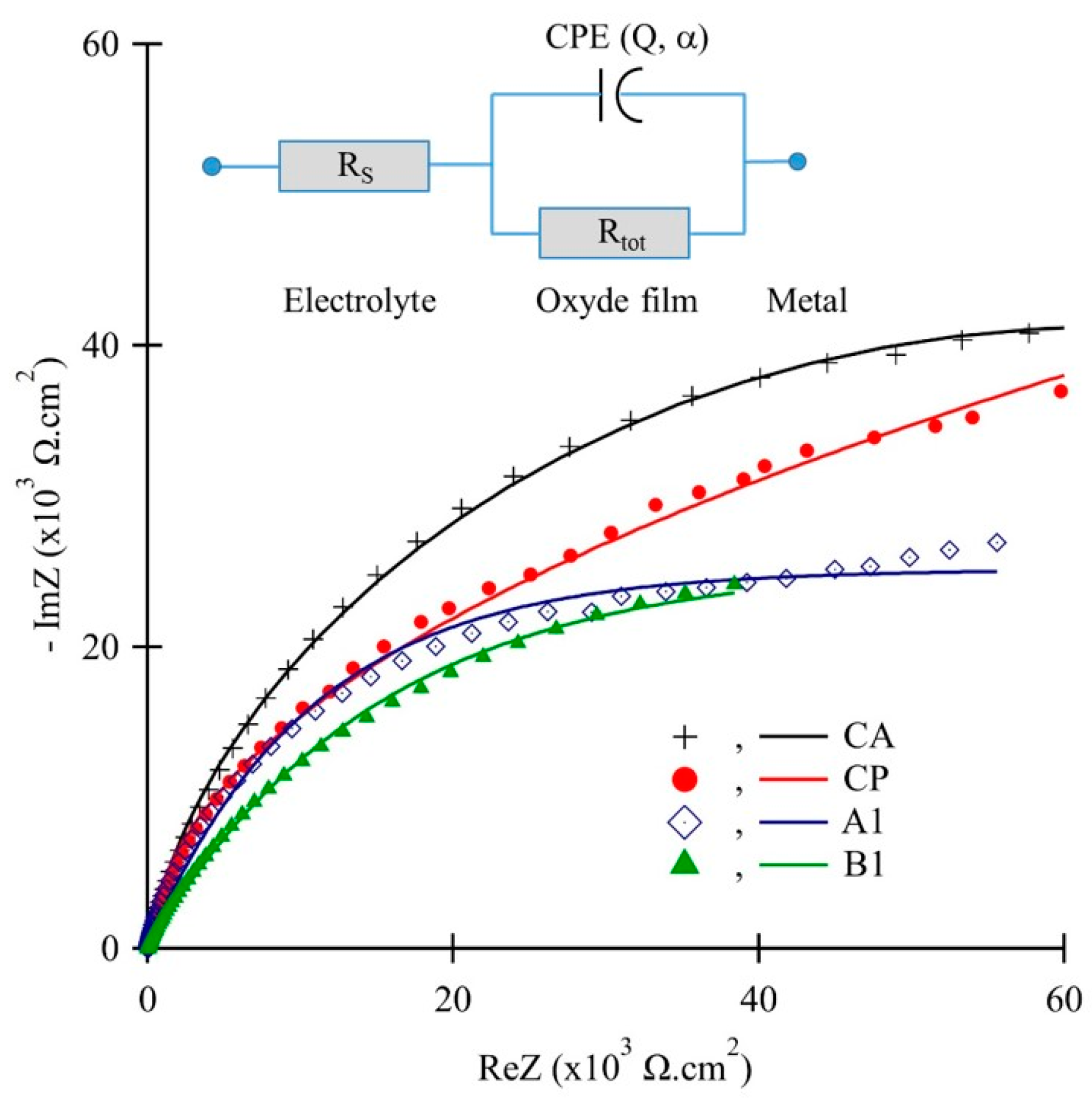
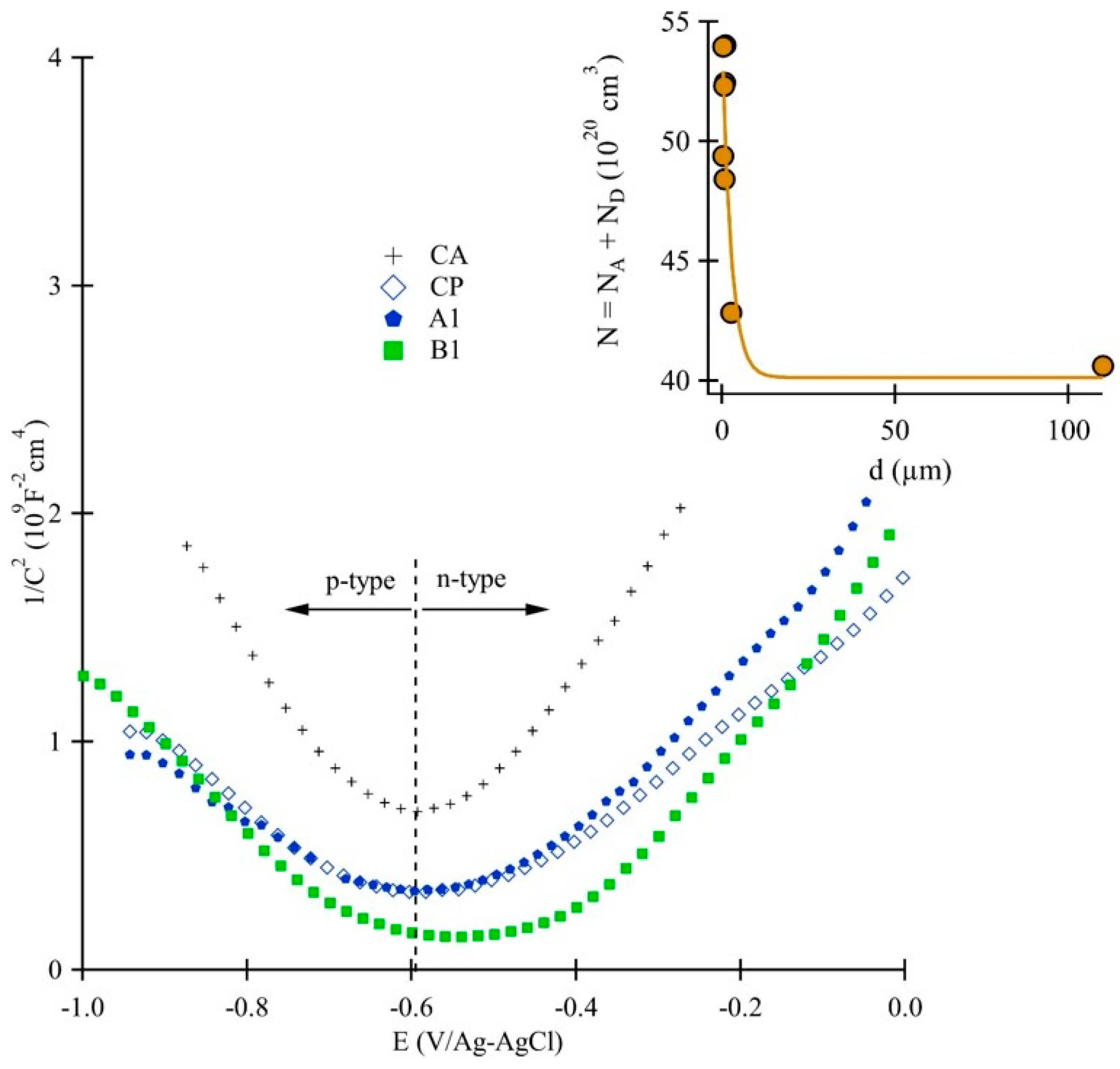
3.3. Electrical and Semiconducting Characteristics of the Surface Oxide
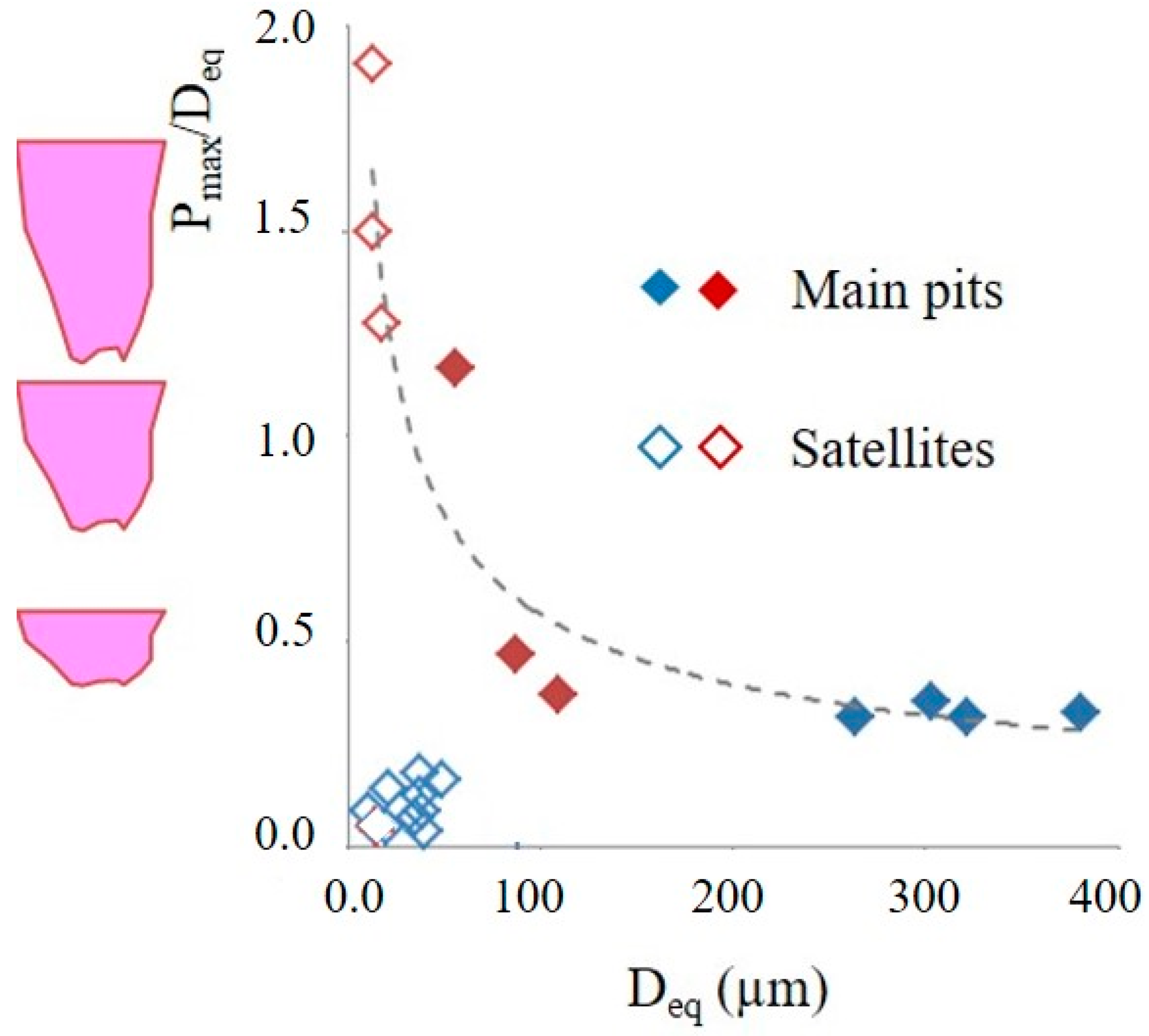
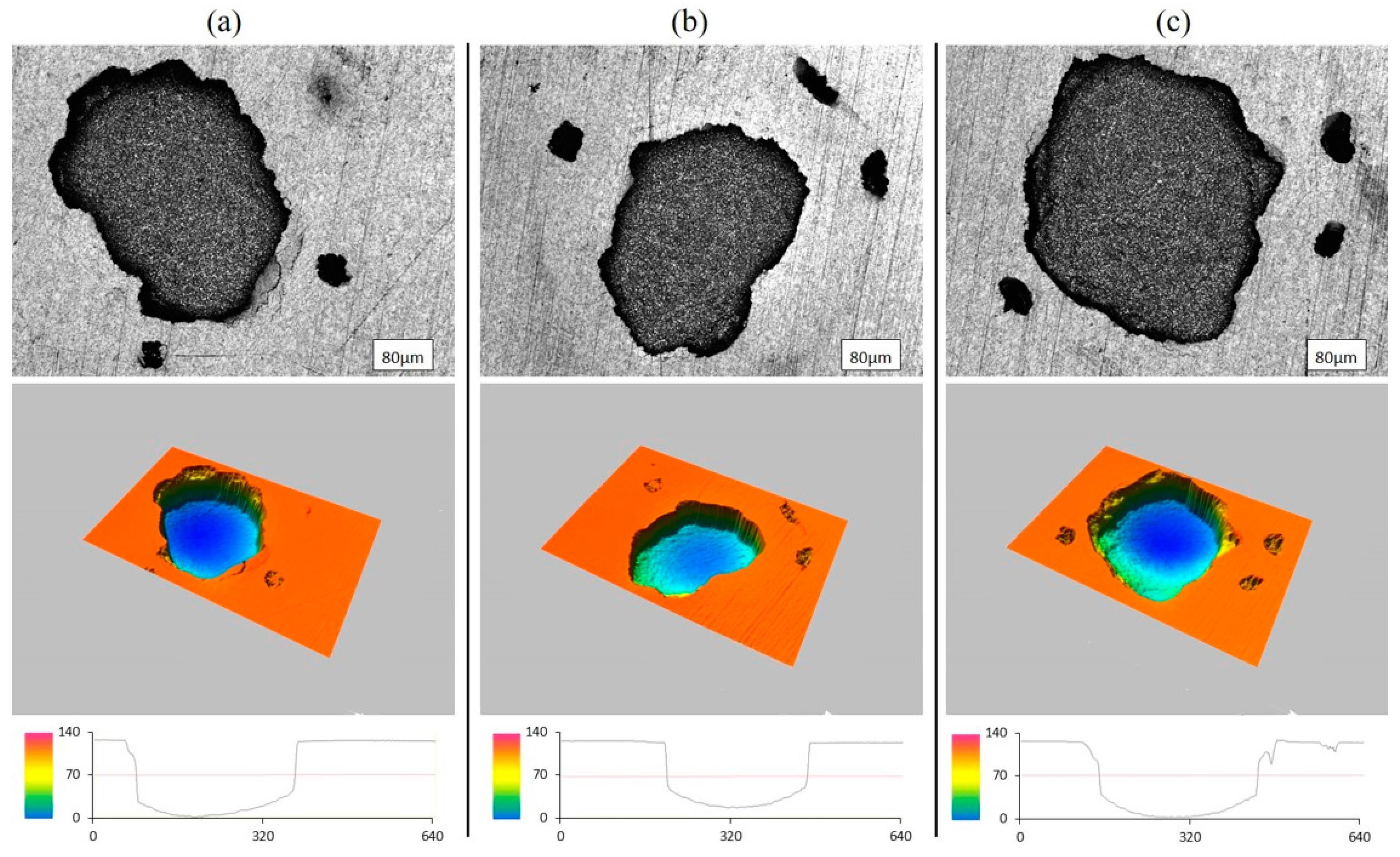
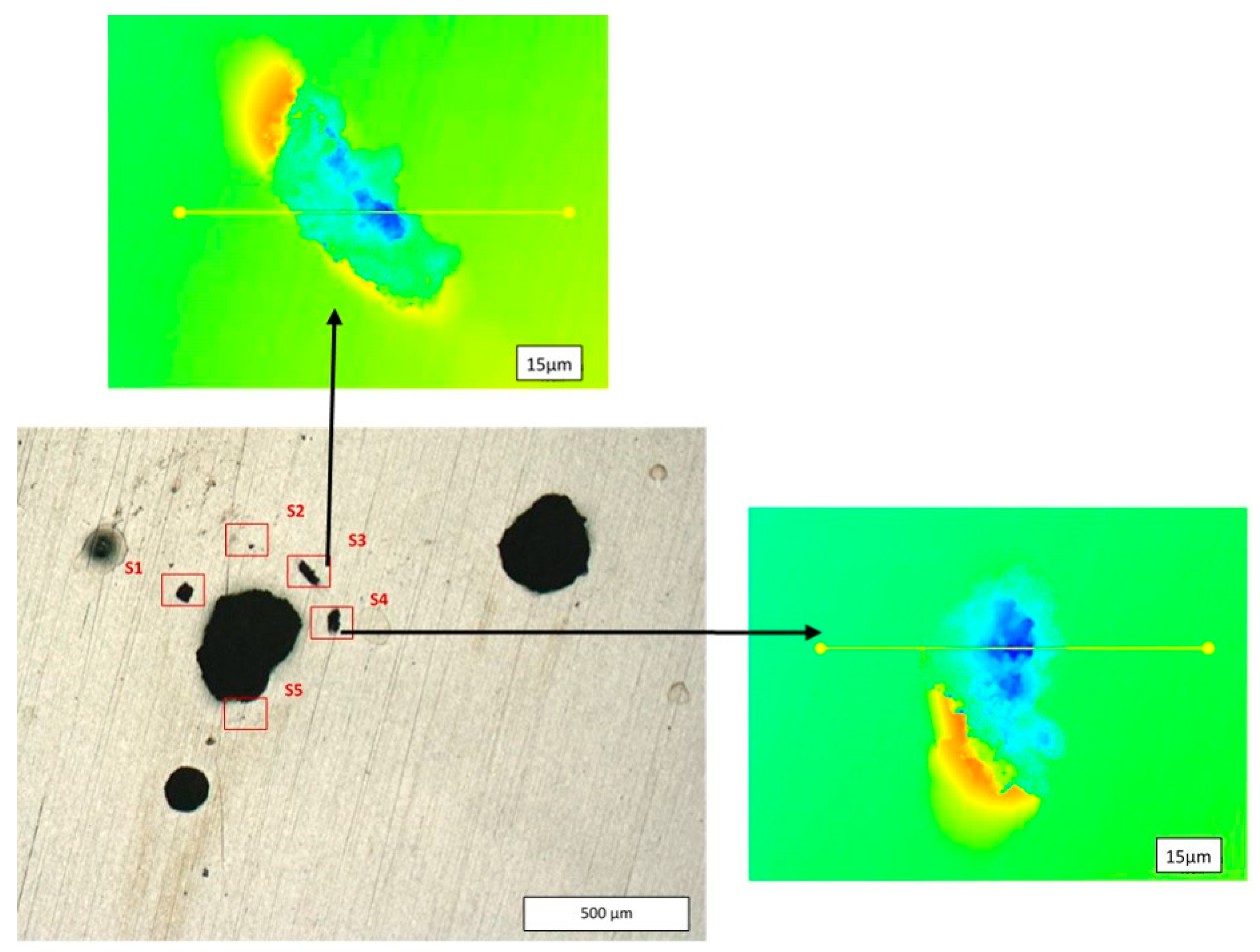
3.4. Role of the Prepassivation of the Ball-Milled Powder to Prevent Pitting Mechanisms
4. Conclusions
- -
- The passivity is improved when the powder is passivated in HNO3 prior to sintering, especially for the smallest grain sizes.
- -
- Reducing the grain diameter in the UFG range increases the electrical resistance of the oxide film.
- -
- The total charge carrier density of the oxide layer decreases as the grain size increases. Cl− anions are then less able to transfer through the thickness of the outer layer, and then the chemical breakdown of the passive layer is more difficult to achieve.
- -
- The mechanism of pitting consists of nucleation and subsurface growth of the main pits, followed by the formation of satellite pits distributed around the main pits.
- -
- The pre-passivation of the powder prevents this pitting nucleation and growth mechanism. As a result, the samples with the smallest grain diameters made with the pre-passivated powder develop a better resistance to pitting than those made with the commercial powder, as well as improved corrosion properties in chloride environments.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- He, Q.; Pan, F.; Wang, D.; Liu, H.; Guo, F.; Wang, Z.; Ma, Y. Microstructure and properties of 316L stainless steel foils for pressure sensor of pressurized water reactor. Nucl. Eng. Technol. 2021, 53, 172–177. [Google Scholar] [CrossRef]
- Zhang, E.; Zhao, X.; Hu, J.; Wang, R.; Fu, S.; Qin, G. Antibacterial metals and alloys for potential biomedical implants. Bioact. Mater. 2021, 6, 2569–2612. [Google Scholar] [CrossRef] [PubMed]
- Jena, G.; Anandkumar, B.; Sofia, S.; George, R.P.; Philip, J. Fabrication of silanized GO hybrid coating on 316L SS with enhanced corrosion resistance and antibacterial properties for marine applications. Surf. Coat. Technol. 2020, 402, 126295. [Google Scholar] [CrossRef]
- D’Andrea, D. Additive Manufacturing of AISI 316L Stainless Steel: A Review. Metals 2023, 13, 1370. [Google Scholar] [CrossRef]
- Liu, E.; Zhang, Y.; Zhu, L.; Zeng, Z.; Gao, R. Effect of strain-induced martensite on the tribocorrosion of AISI 316L austenitic stainless steel in seawater. RSC Adv. 2017, 7, 44923–44932. [Google Scholar] [CrossRef]
- Song, R.-B.; Xiang, J.-Y.; Hou, D.-P. Characteristics of Mechanical Properties and Microstructure for 316L Austenitic Stainless Steel. J. Iron Steel Res. Int. 2011, 18, 53–59. [Google Scholar] [CrossRef]
- Krasilnikov, N.; Lojkowski, W.; Pakiela, Z.; Valiev, R. Tensile strength and ductility of ultra-fine-grained nickel processed by severe plastic deformation. Mater. Sci. Eng. A 2005, 397, 330–337. [Google Scholar] [CrossRef]
- Sun, L.G.; Wu, G.; Wang, Q.; Lu, J. Nanostructural metallic materials: Structures and mechanical properties. Mater. Today 2020, 38, 114–135. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Estrin, Y.; Horita, Z.; Langdon, T.G.; Zehetbauer, M.J.; Zhu, Y.T. Fundamentals of Superior Properties in Bulk NanoSPD Materials. Mater. Res. Lett. 2016, 4, 1–21. [Google Scholar] [CrossRef]
- Choi, J.-P.; Lee, G.-Y.; Song, J.-I.; Lee, W.-S.; Lee, J.-S. Sintering behavior of 316L stainless steel micro–nanopowder compact fabricated by powder injection molding. Powder Technol. 2015, 279, 196–202. [Google Scholar] [CrossRef]
- Pandya, S.; Ramakrishna, K.S.; Annamalai, A.R.; Upadhyaya, A. Effect of sintering temperature on the mechanical and electrochemical properties of austenitic stainless steel. Mater. Sci. Eng. A 2012, 556, 271–277. [Google Scholar] [CrossRef]
- Kurgan, N. Effects of sintering atmosphere on microstructure and mechanical property of sintered powder metallurgy 316L stainless steel. Mater. Des. 2013, 52, 995–998. [Google Scholar] [CrossRef]
- Preston, A.D.; Ma, K. Insight into the effects of pore size and distribution on mechanical properties of austenite stainless steels. J. Mater. Sci. 2021, 56, 17278–17295. [Google Scholar] [CrossRef]
- Oleszak, D.; Grabias, A.; Pekala, M.; Swiderska-Sroda, A.; Kulik, T. Evolution of structure in austenitic steel powders during ball milling and subsequent sintering. J. Alloy Compd. 2007, 434–435, 340–343. [Google Scholar] [CrossRef]
- Trzaska, Z.; Cours, R.; Monchoux, J.-P. Densification of Ni and TiAl by SPS: Kinetics and Microscopic Mechanisms. Metall. Mater. Trans. A 2018, 49, 4849–4859. [Google Scholar] [CrossRef]
- Hu, Z.-Y.; Zhang, Z.-H.; Cheng, X.-W.; Wang, F.-C.; Zhang, Y.-F.; Li, S.-L. A review of multi-physical fields induced phenomena and effects in spark plasma sintering: Fundamentals and applications. Mater. Des. 2020, 191, 108662. [Google Scholar] [CrossRef]
- Orrù, R.; Licheri, R.; Locci, A.M.; Cincotti, A.; Cao, G. Consolidation/synthesis of materials by electric current activated/assisted sintering. Mater. Sci. Eng. R Rep. 2009, 63, 127–287. [Google Scholar] [CrossRef]
- Cipolloni, G.; Pellizzari, M.; Molinari, A.; Hebda, M.; Zadra, M. Contamination during the high-energy milling of atomized copper powder and its effects on spark plasma sintering. Powder Technol. 2015, 275, 51–59. [Google Scholar] [CrossRef]
- Keller, C.; Tabalaiev, K.; Marnier, G.; Noudem, J.; Sauvage, X.; Hug, E. Influence of spark plasma sintering conditions on the sintering and functional properties of an ultra-fine grained 316L stainless steel obtained from ball-milled powder. Mater. Sci. Eng. A 2016, 665, 125–134. [Google Scholar] [CrossRef]
- Marnier, G.; Keller, C.; Noudem, J.; Hug, E. Functional properties of a spark plasma sintered ultrafine-grained 316L steel. Mater. Des. 2014, 63, 633–640. [Google Scholar] [CrossRef]
- Flipon, B.; de la Cruz, L.G.; Hug, E.; Keller, C.; Barbe, F. Elaboration of austenitic stainless steel samples with bimodal grain size distributions and investigation of their mechanical behavior. AIP Conf. Proc. 2017, 1896, 200007. [Google Scholar]
- Flipon, B.; Keller, C.; Cruz, L.G.D.L.; Hug, E.; Barbe, F. Tensile properties of spark plasma sintered AISI 316L stainless steel with unimodal and bimodal grain size distributions. Mater. Sci. Eng. A 2018, 729, 249–256. [Google Scholar] [CrossRef]
- García, C.; Martín, F.; de Tiedra, P.; Cambronero, L.G. Pitting corrosion behaviour of PM austenitic stainless steels sintered in nitrogen–hydrogen atmosphere. Corros. Sci. 2007, 49, 1718–1736. [Google Scholar] [CrossRef]
- Hug, E.; Keller, C. Passivation properties of ultrafine grain 316L stainless steels manufactured by ball milling and spark plasma sintering. Mater. Chem. Phys. 2023, 309, 128398. [Google Scholar] [CrossRef]
- Kargar, B.S.; Moayed, M.H.; Babakhani, A.; Davoodi, A. Improving the corrosion behaviour of powder metallurgical 316L alloy by prepassivation in 20% nitric acid. Corros. Sci. 2011, 53, 135–146. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Wang, F. Pitting mechanism on an austenite stainless steel nanocrystalline coating investigated by electrochemical noise and in-situ AFM analysis. Electroch. Acta 2008, 54, 768–780. [Google Scholar] [CrossRef]
- Racot, A.; Aubert, I.; Touzet, M.; Thiebaut, S.; Demesy, M. Statistical analysis of the pitting corrosion induced by potentiostatic pulse tests of wrought and SLM 316L stainless steels. Corros. Sci. 2022, 197, 110036. [Google Scholar] [CrossRef]
- Orazem, M.E.; Tribollet, B. Electrochemical Impedance Spectroscopy; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Gomes, W.P.; Vanmaekelbergh, D. Impedance spectroscopy at semiconductor electrodes: Review and recent developments. Electroch. Acta 1996, 41, 967–973. [Google Scholar] [CrossRef]
- Trisnanto, S.R.; Wang, X.; Brochu, M.; Omanovic, S. Effects of crystallographic orientation on the corrosion behavior of stainless steel 316L manufactured by laser powder bed fusion. Corros. Sci. 2022, 196, 110009. [Google Scholar] [CrossRef]
- Wang, Z.; Paschalidou, E.-M.; Seyeux, A.; Zanna, S.; Maurice, V.; Marcus, P. Mechanisms of Cr and Mo Enrichments in the Passive Oxide Film on 316L Austenitic Stainless Steel. Front. Mater. 2019, 6, 232. [Google Scholar] [CrossRef]
- Yue, X.; Yang, Z.; Huang, L.; Zhang, L.; Li, J.; Xue, Z.; Pan, J. Passivation characteristics of ultra-thin 316L foil in NaCl solutions. J. Mater. Sci. Technol. 2022, 127, 192–205. [Google Scholar] [CrossRef]
- Lv, J.; Luo, H.; Liang, T.; Guo, W. The effects of grain refinement and deformation on corrosion resistance of passive film formed on the surface of 304 stainless steels. Mater. Res. Bull. 2015, 70, 896–907. [Google Scholar]
- Ebrahimzadeh, P.; Peral, L.B.; González-Martínez, R.; Mardaras, E.; Fernández-Pariente, I. Influence of Severe Surface Plastic Deformation Induced by Shot Peening on Microstructure and Corrosion Resistance of fine grained 316 L stainless steel. Corros. Sci. 2024, 231, 111988. [Google Scholar] [CrossRef]
- LTaveira, V.; Montemor, M.F.; Da Cunha Belo, M.; Ferreira, M.G.; Dick, L.F.P. Influence of incorporated Mo and Nb on the Mott–Schottky behaviour of anodic films formed on AISI 304L. Corros. Sci. 2010, 52, 2813–2818. [Google Scholar] [CrossRef]
- Dean, M.H.; Stimming, U. The electronic properties of disordered passive films. Corros. Sci. 1989, 29, 199–211. [Google Scholar] [CrossRef]
- Zhao, M.; Wu, H.; Lu, J.; Sun, G.; Du, L. Effect of grain size on mechanical property and corrosion behavior of a metastable austenitic stainless steel. Mater. Charact. 2022, 194, 112360. [Google Scholar] [CrossRef]
- Kale, A.B.; Kim, B.-K.; Kim, D.-I.; Castle, E.G.; Reece, M.; Choi, S.-H. An investigation of the corrosion behavior of 316L stainless steel fabricated by SLM and SPS techniques. Mater. Charact. 2020, 163, 110204. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Chen, Z.; Zhao, J.; Bobaru, F. Pitting, lacy covers, and pit merger in stainless steel: 3D peridynamic models. Corros. Sci. 2019, 150, 17–31. [Google Scholar] [CrossRef]
- Sun, D.; Jiang, Y.; Tang, Y.; Xiang, Q.; Zhong, C.; Liao, J.; Li, J. Pitting corrosion behavior of stainless steel in ultrasonic cell. Electroch. Acta 2009, 54, 1558–1563. [Google Scholar] [CrossRef]
- Ernst, P.; Newman, R.C. Pit growth studies in stainless steel foils. I. Introduction and pit growth kinetics. Corros. Sci. 2002, 44, 927–941. [Google Scholar] [CrossRef]
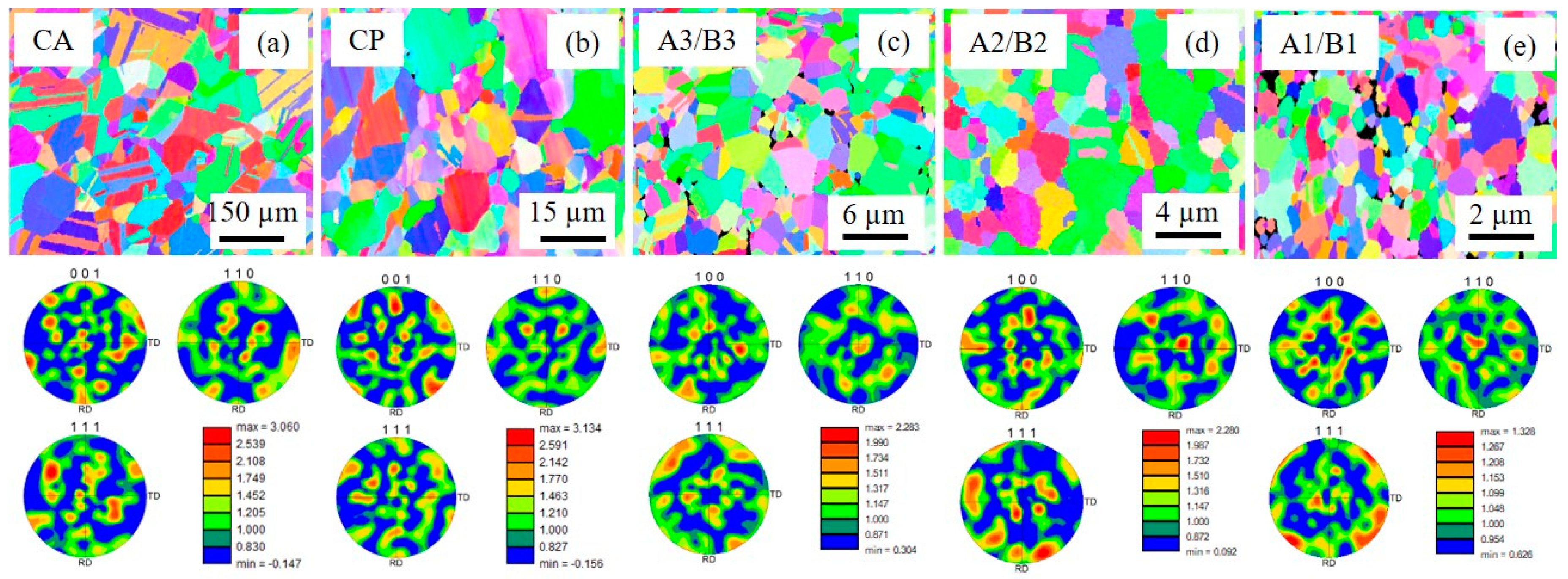
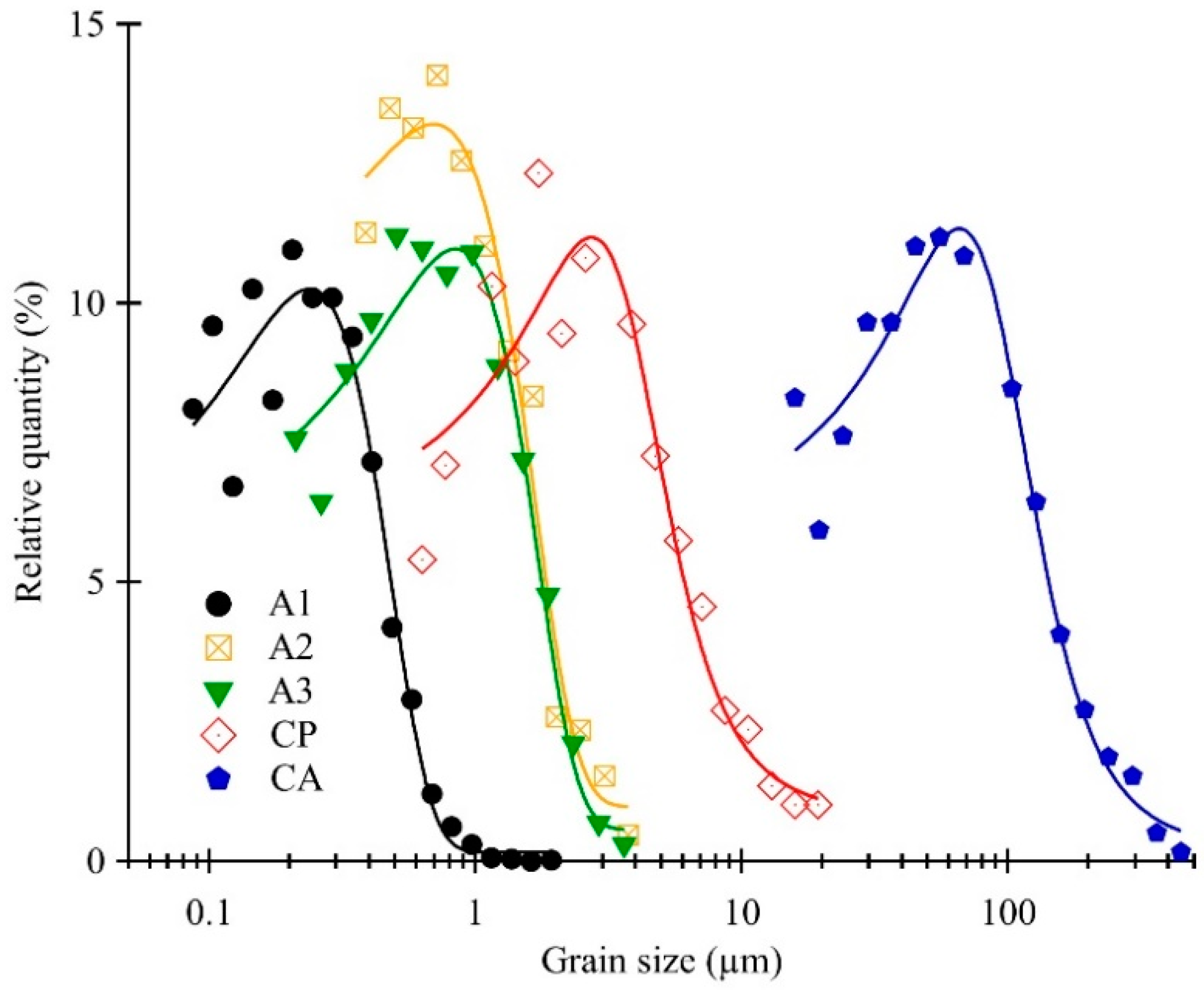



| Sample Code | ρ (%) | d (µm) | Σ3 (%) | LAGB (%) | HAGB (%) | MPD (MRD) |
|---|---|---|---|---|---|---|
| CA | 100 | 110 ± 20 | 34.6 | 28.9 | 71.1 | 3.35 |
| CP | 99.6 | 2.7 ± 1.8 | 28.7 | 23.2 | 76.8 | 3.13 |
| A1, B1 | 95.3 | 0.33 ± 0.09 | 19.1 | 13.2 | 86.8 | 1.33 |
| A2, B2 | 95.1 | 0.67 ± 0.07 | 28.4 | 18.8 | 81.2 | 2.28 |
| A3, B3 | 94.7 | 0.88 ± 0.08 | 29.7 | 10.2 | 89.8 | 2.28 |
| Sample Code | Ecor (mV) | Jcor * (µA/cm2) | Jpas (µA/cm2) | Epit (mV) | L (mV) |
|---|---|---|---|---|---|
| CA | −122 | 0.93 | 628 | 750 ± 175 | |
| CP | −136 | 1.71 | 267 | 416 ± 154 | |
| A1 | −52 | 0.85 | --------- | ----- | ----- |
| A2 | −25 | 0.95 | --------- | ----- | ----- |
| A3 | −59 | 0.77 | --------- | ----- | ----- |
| B1 | −82 | 0.65 | 296 | 438 ± 137 | |
| B2 | −97 | 0.57 | 137 | 234 ± 51 | |
| B3 | −91 | 0.61 | 105 | 196 ± 38 |
| Sample Code | Rs (Ω·cm2) | Rtot (kΩ·cm2) | Q (µF·sα−1/cm2) | α | Ceff (µF/cm2) | δ (nm) |
|---|---|---|---|---|---|---|
| CA | 22.9 | 201.28 | 16.1 | 0.90 | 31.0 | 2.6 |
| CP | 15.1 | 134.26 | 28.4 | 0.89 | 60.1 | 1.3 |
| A1 | 17.3 | 225.69 | 14.6 | 0.89 | 28.9 | 2.8 |
| A2 | 21.7 | 199.23 | 18.7 | 0.91 | 33.9 | 2.3 |
| A3 | 18.6 | 175.68 | 26.0 | 0.87 | 65.5 | 1.2 |
| B1 | 16.5 | 256.26 | 18.3 | 0.90 | 34.5 | 2.3 |
| B2 | 22.4 | 218.98 | 14.7 | 0.87 | 49.1 | 2.4 |
| B3 | 19.2 | 204.01 | 21.2 | 0.88 | 48.1 | 1.7 |
| Sample Code | NA (1020 cm3) | ND (1020 cm3) | EFB (mV) |
|---|---|---|---|
| CA | 15.22 | 25.39 | −590 |
| CP | 20.14 | 22.70 | −556 |
| A1 | 31.45 | 22.50 | −557 |
| A2 | 29.70 | 22.62 | −556 |
| A3 | 32.77 | 21.25 | −568 |
| B1 | 27.46 | 21.92 | −543 |
| B2 | 28.50 | 19.91 | −571 |
| B3 | 29.57 | 22.88 | −566 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hug, E.; Keller, C.; Folton, C.; Papin, J.; Tabalaiev, K.; Marnier, G. Ultrafine Grain 316L Stainless Steel Manufactured by Ball Milling and Spark Plasma Sintering: Consequences on the Corrosion Resistance in Chloride Media. Metals 2024, 14, 864. https://doi.org/10.3390/met14080864
Hug E, Keller C, Folton C, Papin J, Tabalaiev K, Marnier G. Ultrafine Grain 316L Stainless Steel Manufactured by Ball Milling and Spark Plasma Sintering: Consequences on the Corrosion Resistance in Chloride Media. Metals. 2024; 14(8):864. https://doi.org/10.3390/met14080864
Chicago/Turabian StyleHug, Eric, Clément Keller, Cendrine Folton, Jade Papin, Kostiantyn Tabalaiev, and Gaël Marnier. 2024. "Ultrafine Grain 316L Stainless Steel Manufactured by Ball Milling and Spark Plasma Sintering: Consequences on the Corrosion Resistance in Chloride Media" Metals 14, no. 8: 864. https://doi.org/10.3390/met14080864
APA StyleHug, E., Keller, C., Folton, C., Papin, J., Tabalaiev, K., & Marnier, G. (2024). Ultrafine Grain 316L Stainless Steel Manufactured by Ball Milling and Spark Plasma Sintering: Consequences on the Corrosion Resistance in Chloride Media. Metals, 14(8), 864. https://doi.org/10.3390/met14080864






