Effects of Nb on Creep Properties and Hot Corrosion Resistance of New Alumina-Forming Austenitic Steels at 700 °C
Abstract
:1. Introduction
2. Experimental
3. Results
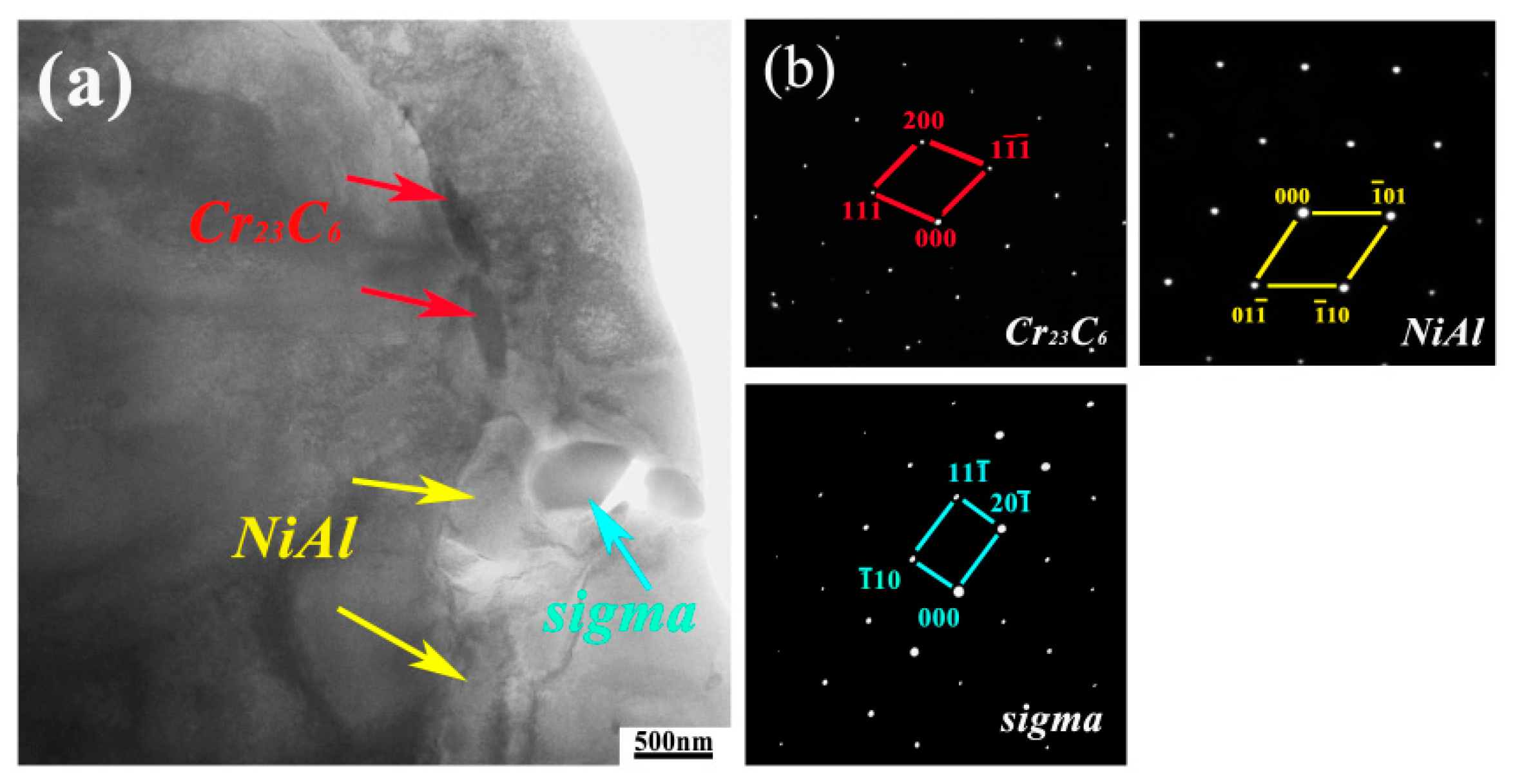
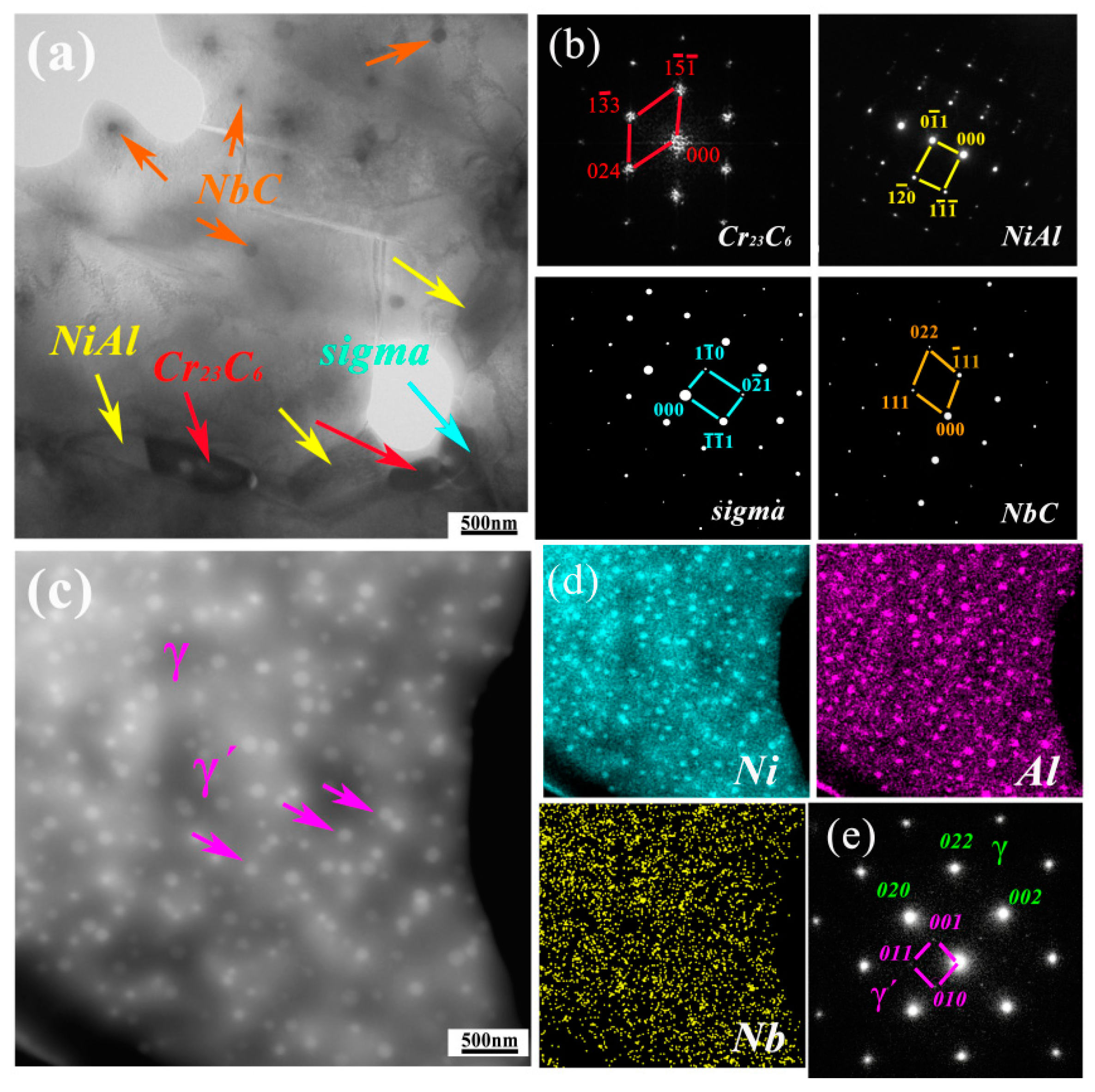
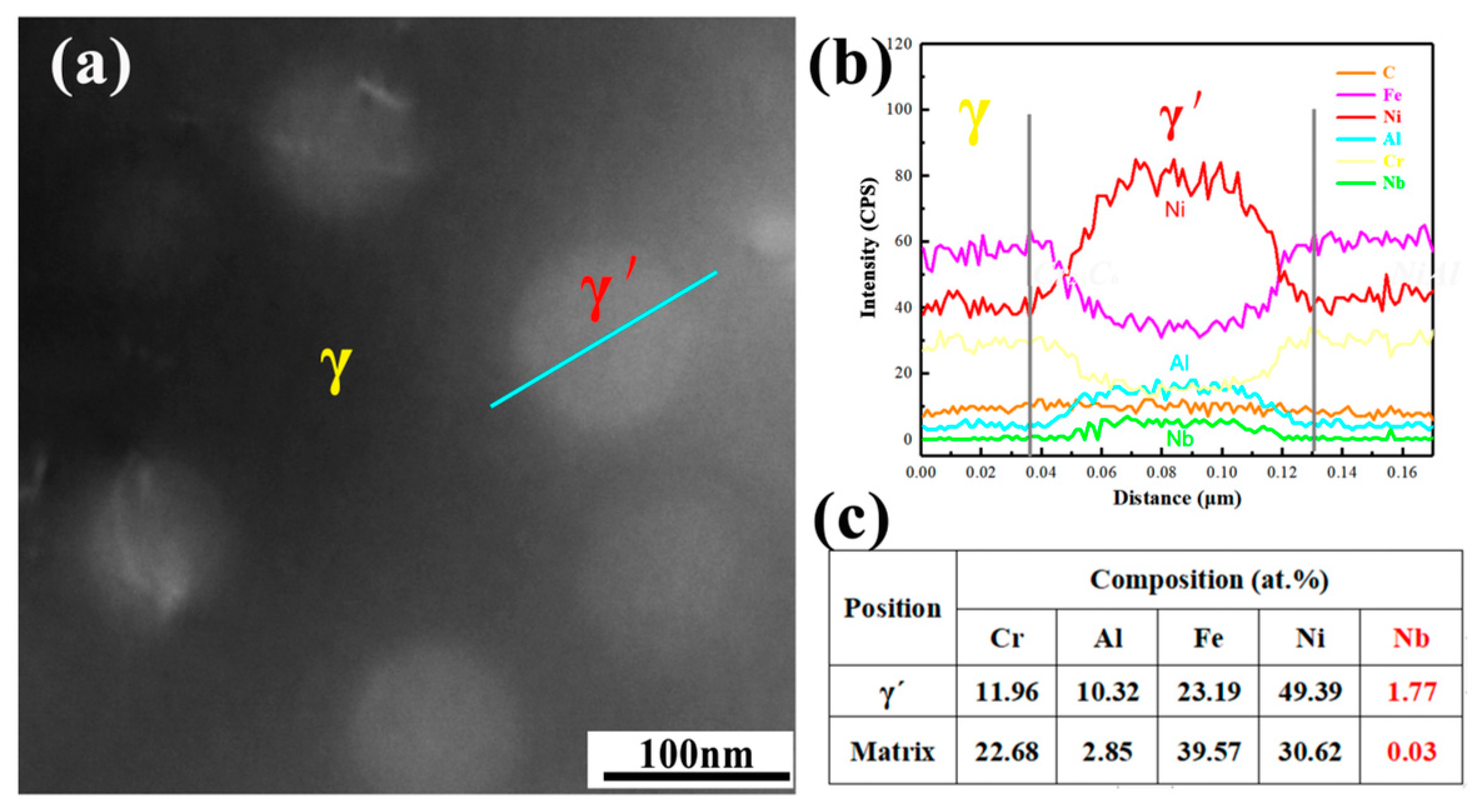
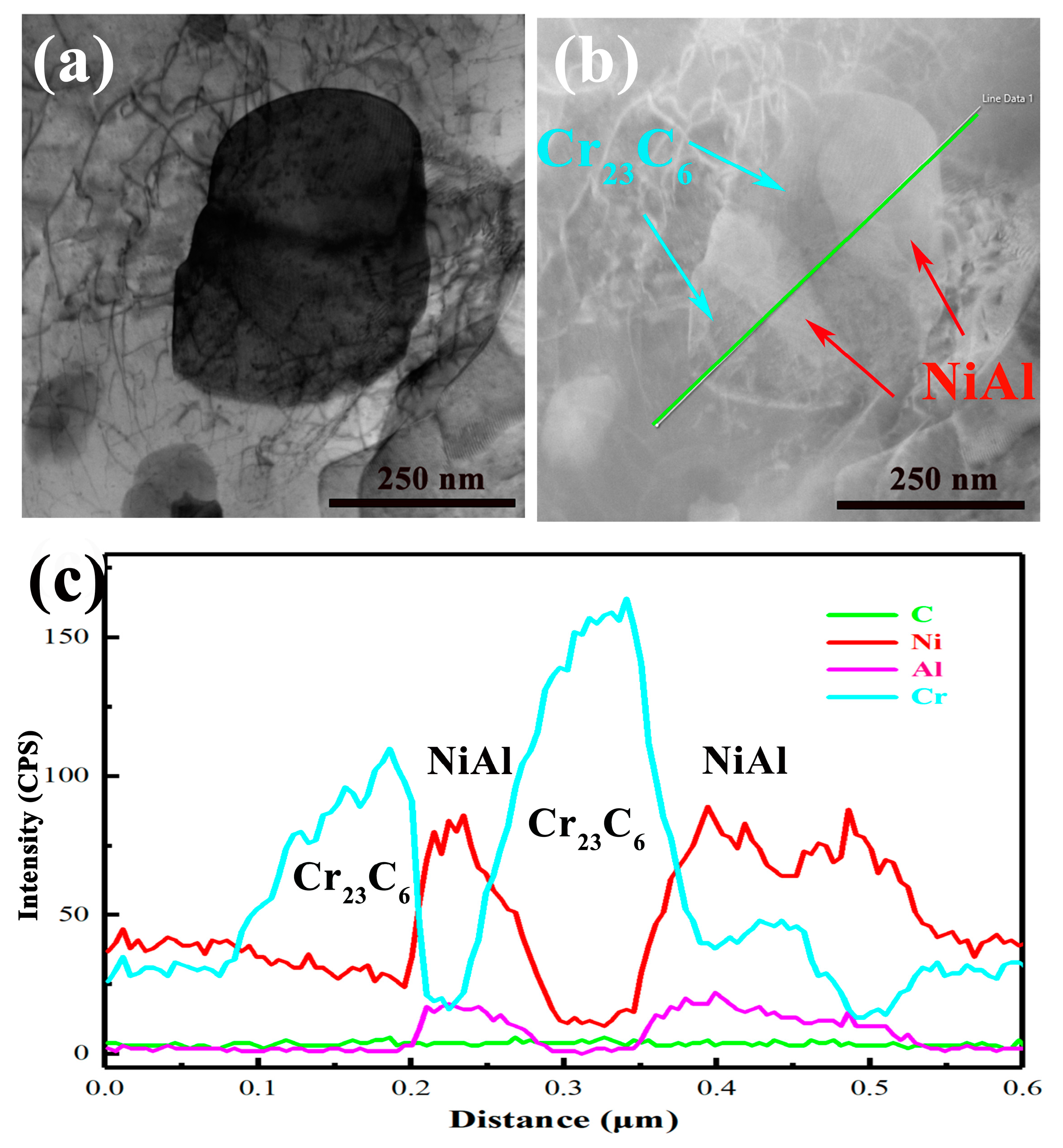
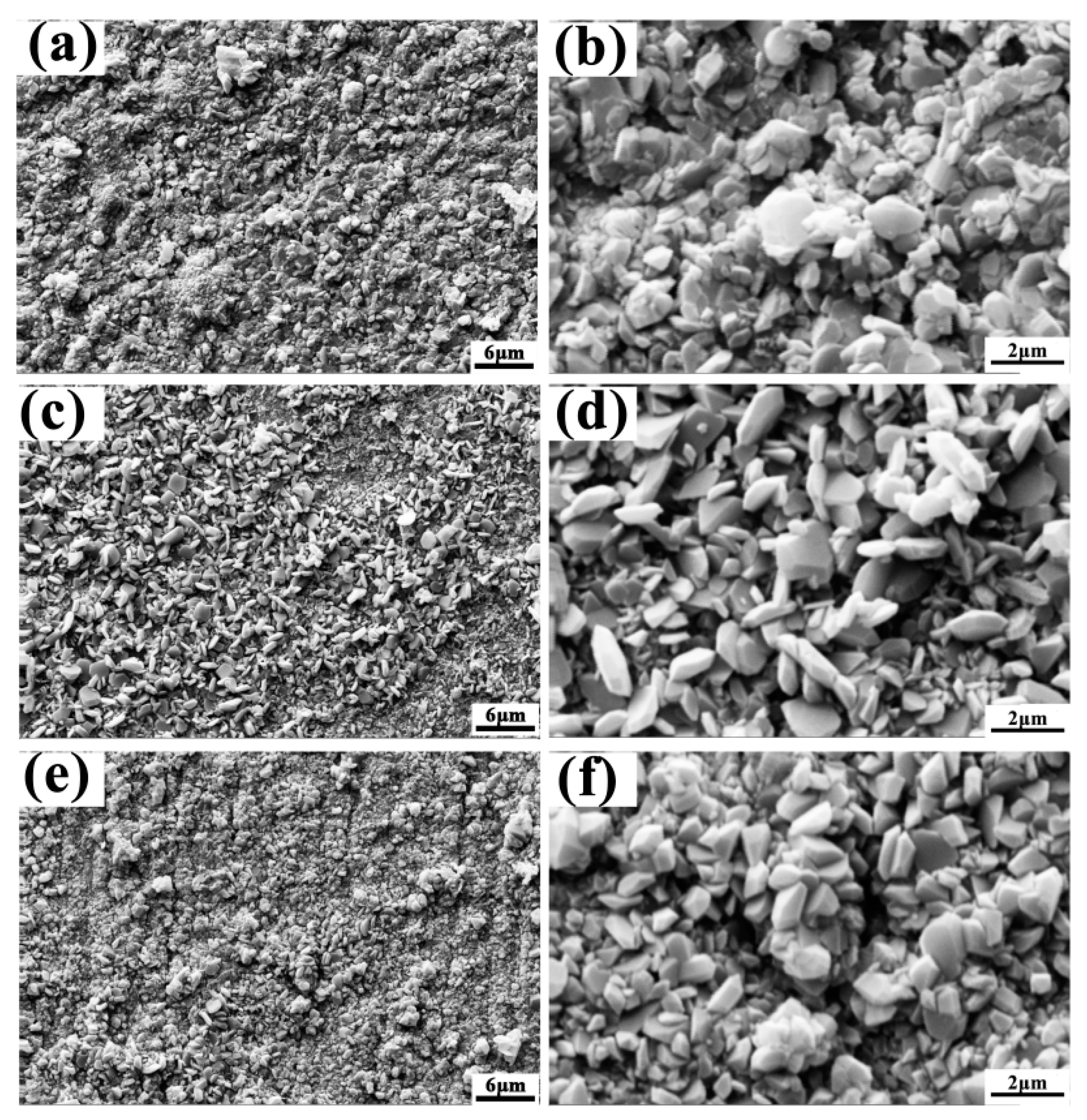
3.1. Microstructure
3.2. Creep Resistance
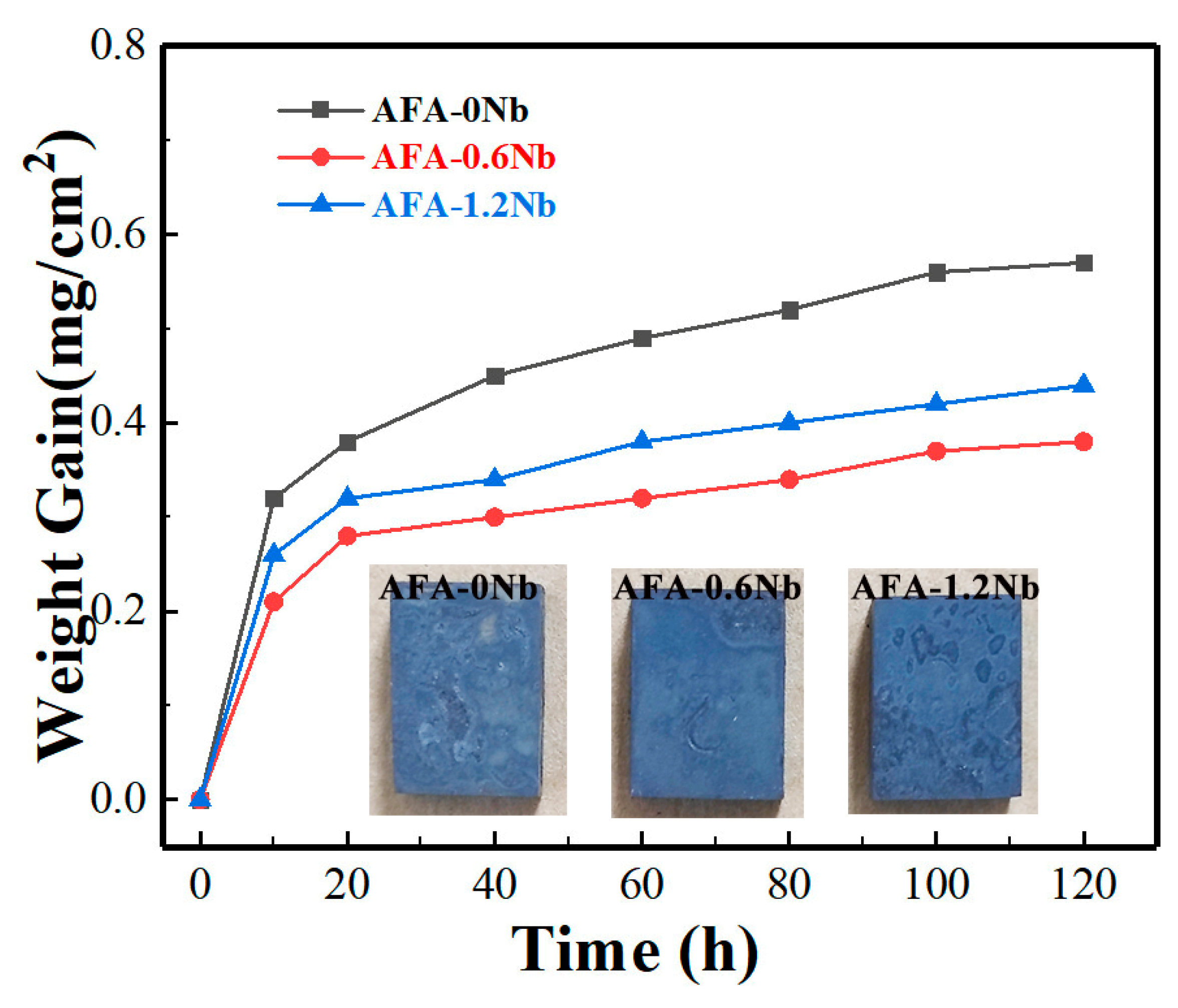
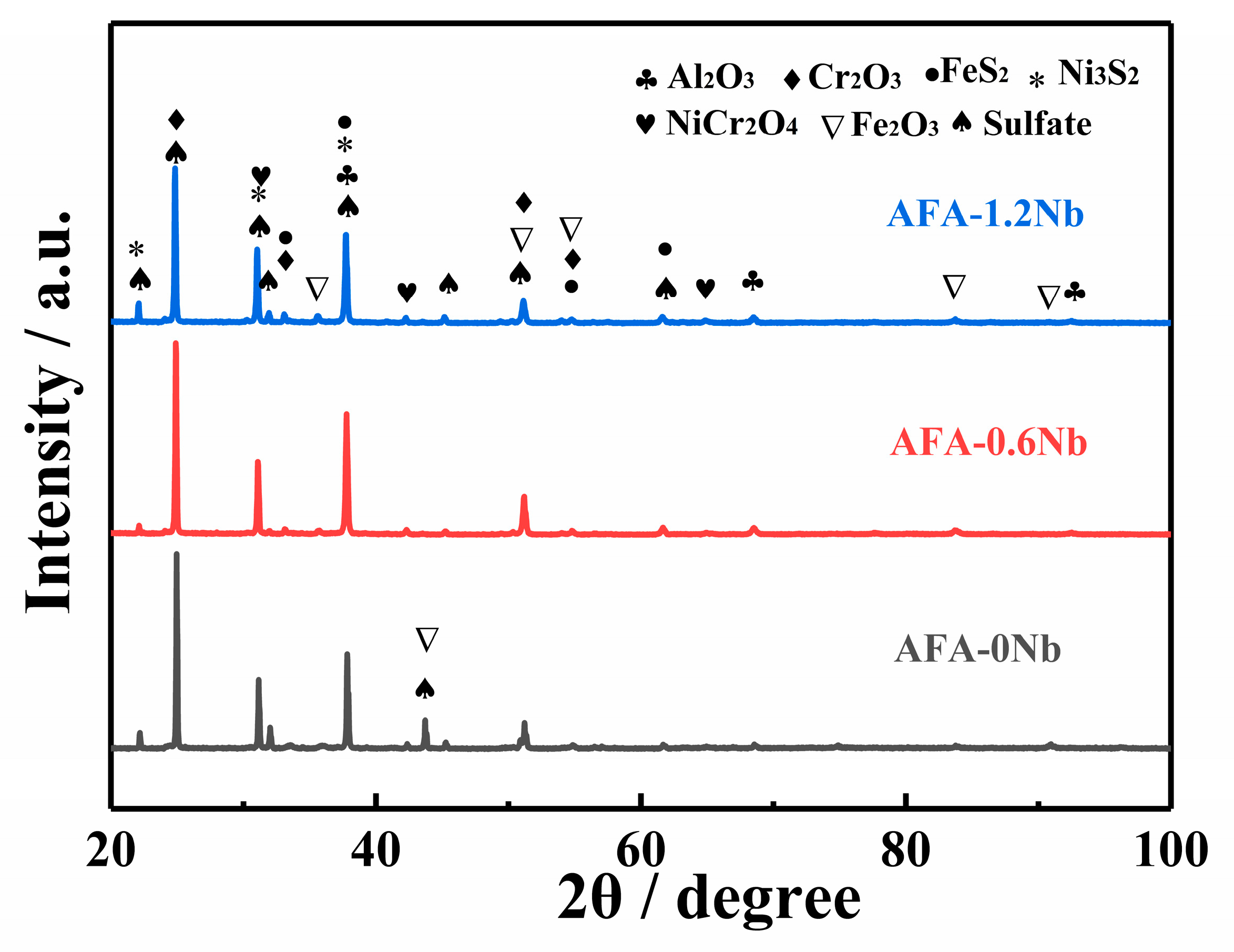
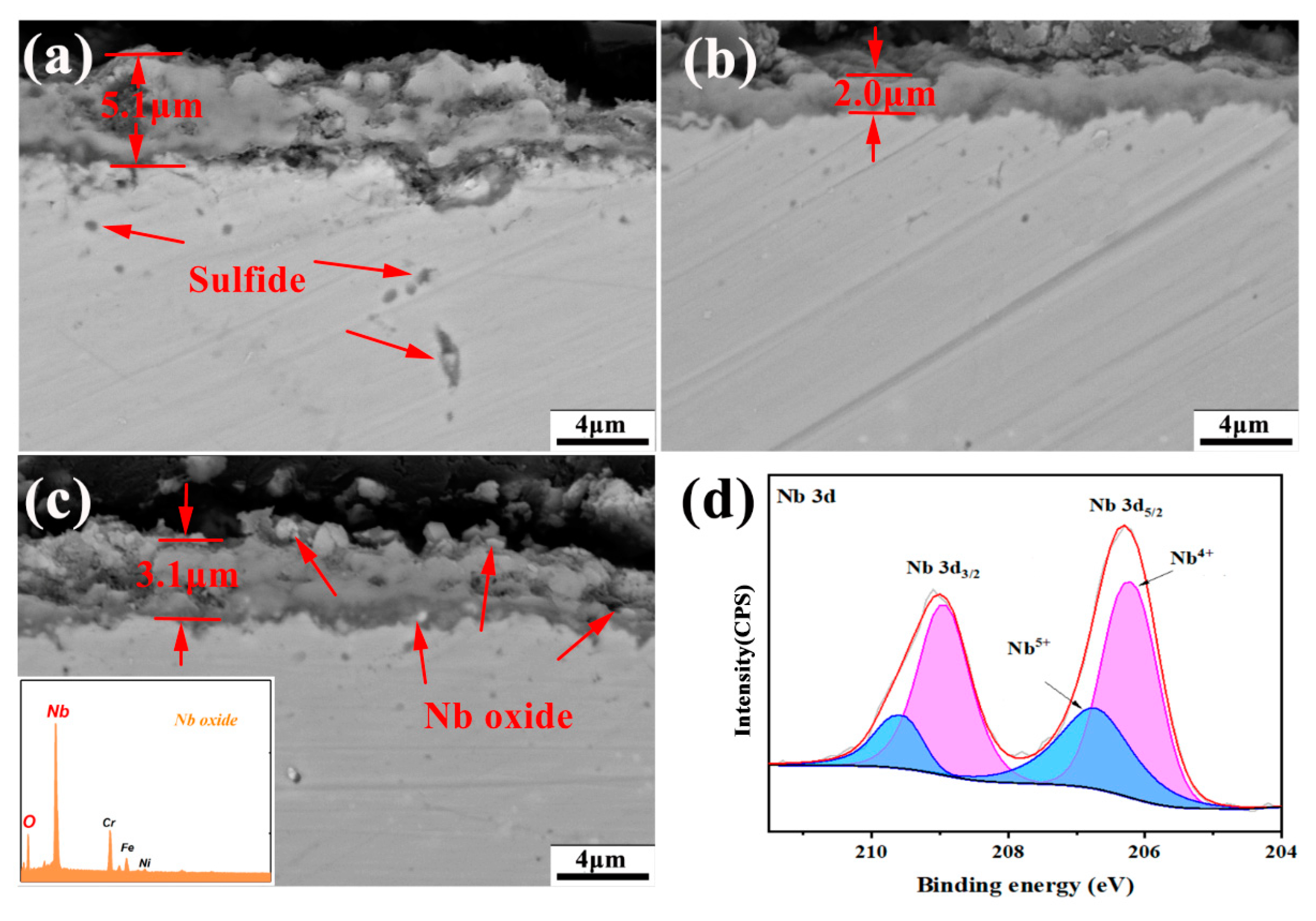
3.3. Hot Corrosion Resistance
4. Discussion
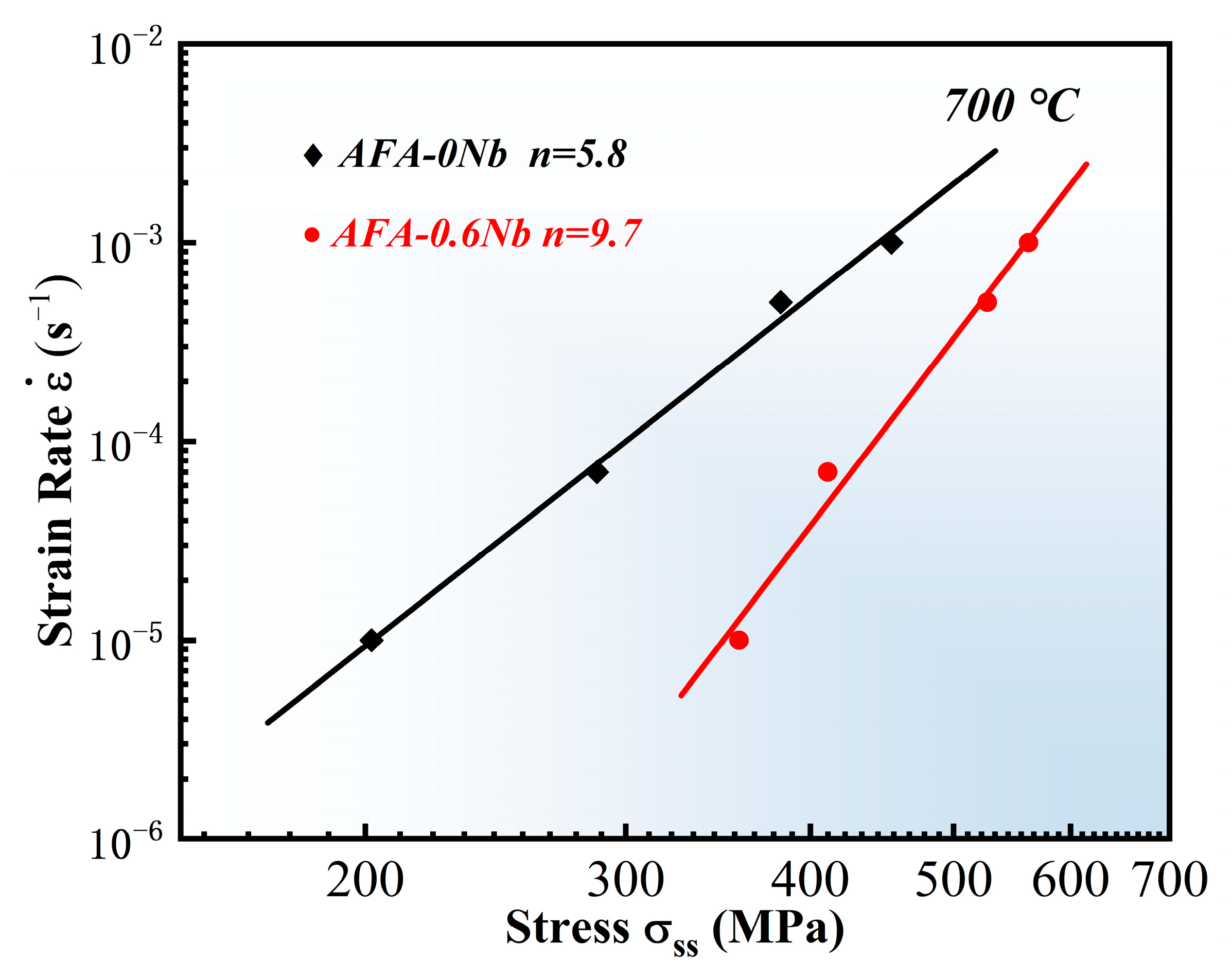
4.1. Effect of Nb on Creep Resistance
4.2. Effect of Nb on Hot Corrosion Resistance
5. Conclusions
- (1)
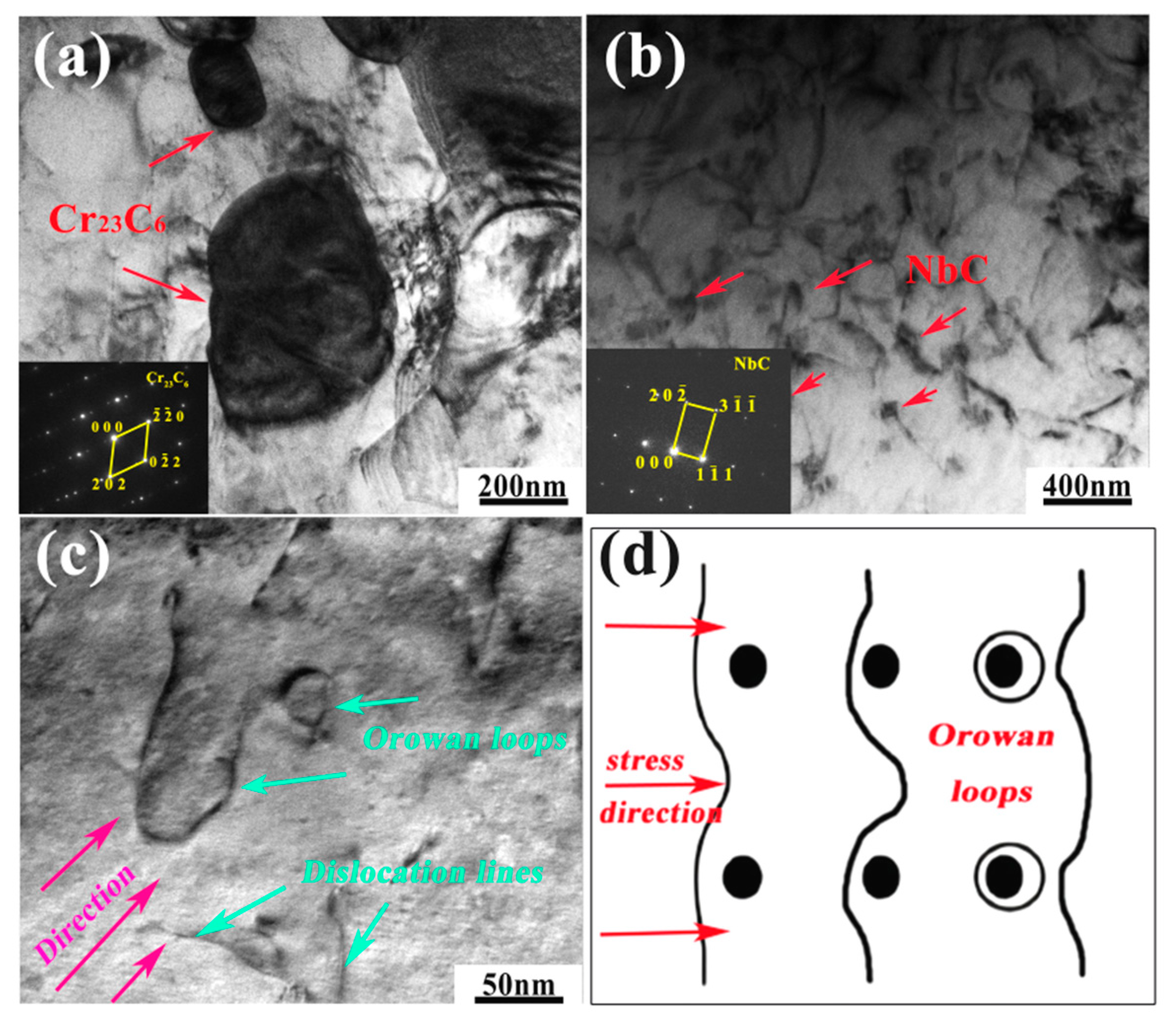
- The precipitates observed in AFA-0Nb steel after creep testing were identified as Cr23C6, NiAl, and the σ phase. In contrast, the precipitates in AFA-0.6Nb steel after creep testing included NiAl, Cr23C6, NbC, γ′-Ni3(Al, Nb), and σ phase. The addition of Nb was found to promote the precipitation of both NbC and γ′-Ni3(Al, Nb) phases, each exhibiting very low coarsening rate constants, with the value of Kγ′ = 6.06 × 10−29 m3/s and KNbC = 1.25 × 10−29 m3/s.
- (2)
- The influence of precipitates on the creep performance of AFA steel was investigated. The results indicated that the creep life of AFA-0Nb steel was 331 h with a minimum creep rate of 2.8 × 10−4 h−1. Conversely, AFA-0.6Nb steel demonstrated a significantly extended creep life of 5687 h and a reduced minimum creep rate of 4.5 × 10−6 h−1. The addition of Nb markedly improved the creep performance of the AFA steels. The apparent stress exponent values for AFA-0Nb and AFA-0.6Nb steels were determined to be 5.8 and 9.7, respectively. The creep process in AFA-0Nb steel was dislocation-controlled, while the AFA-0.6Nb steel was governed by precipitate-controlled deformation. The precipitates NbC and γ′-Ni3(Al, Nb) precipitates effectively hindered dislocation migration.
- (3)
- The corrosion of AFA steels in Na2SO4-25%K2SO4 was characterized as low-temperature hot corrosion, primarily governed by an “oxidation-sulfidation” mechanism, resulting in relatively mild surface corrosion. The addition of Nb, serving as a third element, facilitated the formation of protective Cr2O3 and Al2O3 films. Furthermore, Nb enhanced the activities of Al and Cr, ensuring rapid replenishment of these alloy elements during corrosion. However, when the Nb content reached 1.2 wt%, the formation Nb2O5 was found to compromise the compactness of the oxide film, which adversely affected the corrosion resistance.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Somova, E.V.; Tugov, A.N.; Tumanovskii, A.G. Modern Coal-Fired Power Units for Ultra-Supercritical Steam Conditions (Review). Therm. Eng. 2023, 70, 81–96. [Google Scholar] [CrossRef]
- Di Gianfrancesco, A. Materials for Ultra-Supercritical and Advanced Ultra-Supercritical Power Plants; Elsevier: Amsterdam, The Netherlands, 2016; pp. 423–468. [Google Scholar]
- Bugge, J.; Kjær, S.; Blum, R. High-efficiency coal-fired power plants development and perspectives. Energy 2006, 31, 1437–1445. [Google Scholar] [CrossRef]
- Viswanathan, R.; Bakker, W. Materials for ultrasupercritical coal power plants—Boiler materials: Part 1. J. Mater. Eng. Perform. 2001, 10, 81–95. [Google Scholar] [CrossRef]
- Abe, F. Research and Development of Heat-Resistant Materials for Advanced USC Power Plants with Steam Temperatures of 700 °C and Above. Engineering 2015, 1, 211–224. [Google Scholar] [CrossRef]
- Zhong, Z.H.; Gu, Y.F.; Yuan, Y.; Shi, Z. A new wrought Ni-Fe-base superalloy for advanced ultra-supercritical power plant applications beyond 700 °C. Mater. Lett. 2013, 109, 38–41. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Brady, M.P.; Santella, M.L.; Bei, H.; Maziasz, P.J.; Pint, B.A. Overview of Strategies for High-Temperature Creep and Oxidation Resistance of Alumina-Forming Austenitic Stainless Steels. Metall. Mater. Trans. A 2011, 42, 922–931. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Brady, M.P.; Lu, Z.P.; Maziasz, P.J.; Liu, C.T.; Pint, B.A.; More, K.L.; Meyer, H.M.; Payzant, E.A. Creep-resistant, Al2O3-forming austenitic stainless steels. Science 2007, 316, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Brady, M.P.; Muralidharan, G.; Yamamoto, Y.; Pint, B.A. Development of 1100 °C Capable Alumina-Forming Austenitic Alloys. Oxid. Met. 2017, 87, 1–10. [Google Scholar] [CrossRef]
- Brady, M.P.; Unocic, K.A.; Lance, M.J.; Santella, M.L.; Yamamoto, Y.; Walker, L.R. Increasing the Upper Temperature Oxidation Limit of Alumina Forming Austenitic Stainless Steels in Air with Water Vapor. Oxid. Met. 2011, 75, 337–357. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, X.; Chen, G.; Lu, Z. Improvement of high-temperature oxidation resistance and strength in alumina-forming austenitic stainless steels. Mater. Lett. 2011, 65, 3285–3288. [Google Scholar] [CrossRef]
- Gao, Q.; Liu, Z.; Sun, L.; Ma, Q.; Zhang, H.; Bai, J.; Lin, X.; Yu, L.; Li, H. Review on precipitates and high-temperature properties of alumina-forming austenitic stainless steel. J. Mater. Res. Technol. 2023, 25, 5372–5393. [Google Scholar] [CrossRef]
- Wen, D.H.; Li, Z.; Jiang, B.B.; Wang, Q.; Chen, G.Q.; Tang, R.; Zhang, R.Q.; Dong, C.; Liaw, P.K. Effects of Nb/Ti/V/Ta on phase precipitation and oxidation resistance at 1073 K in alumina-forming austenitic stainless steels. Mater. Charact. 2018, 144, 86–98. [Google Scholar] [CrossRef]
- Baker, I.; Afonina, N.; Wang, Z.; Wu, M. Preliminary creep testing of the alumina-forming austenitic stainless steel Fe-20Cr-30Ni-2Nb-5Al. Mater. Sci. Eng. A 2018, 718, 492–498. [Google Scholar] [CrossRef]
- Zhao, W.X.; Zhou, D.Q.; Jiang, S.H.; Wang, H.; Wu, Y.; Liu, X.J.; Wang, X.Z.; Lu, Z.P. Ultrahigh stability and strong precipitation strengthening of nanosized NbC in alumina-forming austenitic stainless steels subjecting to long-term high-temperature exposure. Mater. Sci. Eng. A 2018, 738, 295–307. [Google Scholar] [CrossRef]
- Zhou, D.Q.; Zhao, W.X.; Mao, H.H.; Hu, Y.X.; Xu, X.Q.; Sun, X.Y.; Lu, Z.P. Precipitate characteristics and their effects on the high-temperature creep resistance of alumina-forming austenitic stainless steels. Mater. Sci. Eng. A 2015, 622, 91–100. [Google Scholar] [CrossRef]
- Zhou, D.Q.; Xu, X.Q.; Mao, H.H.; Yan, Y.F.; Nieh, T.G.; Lu, Z.P. Plastic flow behaviour in an alumina-forming austenitic stainless steel at elevated temperatures. Mater. Sci. Eng. A 2014, 594, 246–252. [Google Scholar] [CrossRef]
- Hu, B.; Trotter, G.; Baker, I.; Miller, M.K.; Yao, L.; Chen, S.; Cai, Z. The Effects of Cold Work on the Microstructure and Mechanical Properties of Intermetallic Strengthened Alumina-Forming Austenitic Stainless Steels. Metall. Mater. Trans. A 2015, 46A, 3773–3785. [Google Scholar] [CrossRef]
- Moon, J.; Lee, T.; Heo, Y.; Han, Y.; Kang, J.; Ha, H.; Suh, D. Precipitation sequence and its effect on age hardening of alumina-forming austenitic stainless steel. Mater. Sci. Eng. A 2015, 645, 72–81. [Google Scholar] [CrossRef]
- Hu, B.; Baker, I. High temperature deformation of Laves phase precipitates in alumina-forming austenitic stainless steels. Mater. Lett. 2017, 195, 108–111. [Google Scholar] [CrossRef]
- Trotter, G.; Hu, B.; Sun, A.Y.; Harder, R.; Miller, M.K.; Yao, L.; Baker, I. Precipitation kinetics during aging of an alumina-forming austenitic stainless steel. Mater. Sci. Eng. A 2016, 667, 147–155. [Google Scholar] [CrossRef]
- Wang, Z.N.; Tian, L.; Wei, X.W.; Du, A.B.; Gao, M.; Ma, Y.C.; Liu, K. σ-Phase Precipitation Mechanism of 15Cr-15Ni Titanium- Modified Austenitic Stainless Steel During Long-Term Thermal Exposure. Acta Metall. Sin. 2018, 31, 281–289. [Google Scholar] [CrossRef]
- Bei, H.; Yamamoto, Y.; Brady, M.P.; Santella, M.L. Aging effects on the mechanical properties of alumina-forming austenitic stainless steels. Mater. Sci. Eng. A 2010, 527, 2079–2086. [Google Scholar] [CrossRef]
- Facco, A.; Couvrat, M.; Magné, D.; Roussel, M.; Guillet, A.; Pareige, C. Microstructure influence on creep properties of heat-resistant austenitic alloys with high aluminum content. Mater. Sci. Eng. A 2020, 783, 139276. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Li, Z.; Wang, Q.; Dong, C.; Xu, F.; Sun, L.; Liaw, P.K. Effect of Ti/Nb/Ta addition on the γ/γ′ coherent microstructure in low-density and high-strength Co-Al-W-Mo-based superalloys. J. Mater. Sci. Technol. 2024, 186, 174–187. [Google Scholar] [CrossRef]
- Zhang, P.; Yuan, Y.; Zhong, L.; Gu, Y.F.; Yan, J.B.; Lu, J.T. Yang. Microstructural stability and tensile properties of a new γ′-hardened Ni-Fe-base superalloy. Materialia 2021, 16, 101061. [Google Scholar] [CrossRef]
- Xu, K.; Jiang, H.; Yan, J.B.; Zhang, P.; Liu, P.; Cao, G.H.; Yuan, Y. Tensile properties and deformation mechanisms of a solution treated Ni–Fe-based alloy at high temperatures. Mater. Sci. Eng. A 2023, 881, 145418. [Google Scholar] [CrossRef]
- Wu, Y.; Li, C.; Li, Y.; Wu, J.; Xia, X.; Liu, Y. Effects of heat treatment on the microstructure and mechanical properties of Ni3Al-based superalloys: A review. Int. J. Miner. Metall. Mater. 2021, 28, 553–566. [Google Scholar] [CrossRef]
- Wang, Z.; Ning, Y.; Yu, H.; Zhang, B.; Xie, B.; Huang, S.; Zhang, W.; Zhang, B. Mechanical degradation behavior and γ′ coarsening mechanism of a Ni-based superalloy during long-term high-temperature thermal exposure. J. Mater. Res. Technol. 2024, 30, 9510–9520. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Muralidharan, G.; Brady, M.P. Development of L12-ordered Ni3(Al,Ti)-strengthened alumina-forming austenitic stainless steel alloys. Scr. Mater. 2013, 69, 816–819. [Google Scholar] [CrossRef]
- Chen, G.; Du, S.; Zhou, Z. The Effect of Replacing Ni with Mn on the Microstructure and Properties of Al2O3-Forming Austenitic Stainless Steels: A Review. Materials 2024, 17, 19. [Google Scholar] [CrossRef]
- Meng, H.; Wang, J.; Wang, L.; Fang, X.; Dong, N.; Zhang, C.; Han, P. The precipitation control in aged alumina-forming austenitic stainless steels Fe-15Cr-25Ni-3Al-NbWCu by W addition and its effect on the mechanical properties. Mater. Charact. 2020, 163, 110233. [Google Scholar] [CrossRef]
- Brady, M.P.; Yamamoto, Y.; Santella, M.L.; Maziasz, P.J.; Pint, B.A.; Liu, C.T.; Lu, Z.P.; Bei, H. The development of alumina-forming austenitic stainless steels for high-temperature structural use. JOM 2008, 60, 12–18. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Brady, M.P.; Lu, Z.P.; Liu, C.T.; Takeyama, M.; Maziasz, P.J.; Pint, B.A. Alumina-Forming Austenitic Stainless Steels Strengthened by Laves Phase and MC Carbide Precipitates. Metall. Mater. Trans. A 2007, 38, 2737–2746. [Google Scholar] [CrossRef]
- Nicol, K. Status of Advanced Ultra-Supercritical Pulverised Coal Technology; IEA Clean Coal Centre: Paris, France, 2013. [Google Scholar]
- Yan, Y.F.; Xu, X.Q.; Zhou, D.Q.; Wang, H.; Wu, Y.; Liu, X.J.; Lu, Z.P. Hot corrosion behaviour and its mechanism of a new alumina-forming austenitic stainless steel in molten sodium sulphate. Corros. Sci. 2013, 77, 202–209. [Google Scholar] [CrossRef]
- Zhou, Y.B.; Wang, H.; Mo, C.G.; Zhang, L.N. Effect of Minor Nb on Isothermal-Oxidation and Hot Corrosion Behavior of Two Nickel-Based Superalloys Under Na2SO4/Na2SO4-NaCl. Corrosion-Us 2021, 77, 949–960. [Google Scholar] [CrossRef] [PubMed]
- Weng, F.; Yu, H.; Wan, K.; Chen, C. The influence of Nb on hot corrosion behavior of Ni-based superalloy at 800 °C in a mixture of Na2SO4–NaCl. J. Mater. Res. 2014, 29, 2596–2603. [Google Scholar] [CrossRef]
- Zeng, K.; Jin, Z.; Huang, P. Effect of Nb on the hot corrosion behavior of Ni-base superalloy. Cheminform 1989, 80, 129–134. [Google Scholar] [CrossRef]
- Elger, R.; Pettersson, R. Effect of Addition of 4 % Al on the High Temperature Oxidation and Nitridation of a 20Cr-25Ni Austenitic Stainless Steel. Oxid. Met. 2014, 82, 469–490. [Google Scholar] [CrossRef]
- Wang, M.; Sun, H.; Phaniraj, M.P.; Han, H.N.; Jang, J.; Zhou, Z. Evolution of microstructure and tensile properties of Fe-18Ni-12Cr based AFA steel during aging at 700 °C. Mater. Sci. Eng. A 2016, 672, 23–31. [Google Scholar] [CrossRef]
- Brady, M.P.; Yamamoto, Y.; Santella, M.L.; Walker, L.R. Composition, Microstructure, and Water Vapor Effects on Internal/External Oxidation of Alumina-Forming Austenitic Stainless Steels. Oxid. Met. 2009, 72, 311–333. [Google Scholar] [CrossRef]
- Cermak, J.; Gazda, A.; Rothova, V. Interdiffusion in ternary Ni3Al/Ni3Al-X diffusion couples with X=Cr, Fe, Nb and Ti. Intermetallics 2003, 11, 939–946. [Google Scholar] [CrossRef]
- Zhao, Y.-H.; Huang, Z.-W.; Li, A.-H.; Mu, Y.-Q.; Yang, M.-H.; Hou, H.; Han, P.-D.; Zhang, S.-Y. First principles study on substitution behavior and alloying effects of Nb in Ni3Al*. Acta Phys. Sin.-Ch. Ed. 2011, 60, 564–570. [Google Scholar]
- Chen, L.; Wang, M.; Wang, Q.; Gao, Y.; Dong, H.; Che, H.; Zhou, Z. Microstructure and mechanical property evolution of an AFA alloy with simple composition design during ageing at 700 °C. Mater. Sci. Eng. A 2020, 779, 139157. [Google Scholar] [CrossRef]
- Kuehmann, C.J.; Voorhees, P.W. Ostwald ripening in ternary alloys. Metall. Mater. Trans. A 1996, 27, 937–943. [Google Scholar] [CrossRef]
- Lu, F.; Antonov, S.; Lu, S.; Zhang, J.; Li, L.; Wang, D.; Zhang, J.; Feng, Q. Unveiling the Re effect on long-term coarsening behaviors of γ′ precipitates in Ni-based single crystal superalloys. Acta Mater. 2022, 233, 117979. [Google Scholar] [CrossRef]
- Antonov, S.; Prithiv, T.S.; Zhou, X.; Peterson, A.; Gault, B.; Baker, I. Phase transformations in the alumina-forming austenitic stainless-steel Fe-20Cr-30Ni-2Nb-5Al during creep at 750 °C 45 MPa. Materialia 2023, 30, 101849. [Google Scholar] [CrossRef]
- Solenthaler, C.; Ramesh, M.; Uggowitzer, P.J.; Spolenak, R. Precipitation strengthening of Nb-stabilized TP347 austenitic steel by a dispersion of secondary Nb(C,N) formed upon a short-term hardening heat treatment. Mater. Sci. Engineering. A Struct. Mater. Prop. Microstruct. Process. 2015, 647, 294–302. [Google Scholar] [CrossRef]
- Sherby, O.D.; Burke, P.M. Mechanical behavior of crystalline solids at elevated temperature. Prog. Mater. Sci 1968, 13, 323–390. [Google Scholar] [CrossRef]
- Weertman, J. Steady-State Creep of Crystals. J. Appl. Phys. 1957, 28, 1185–1189. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, D.B. Hot Corrosion of Inconel 740 Alloys in Na2SO4-NaCl Salts. Appl. Mech. Mater. 2015, 719–720, 106–109. [Google Scholar] [CrossRef]
- Zeng, C.; Zhang, T. Electrochemical impedance study of corrosion of B-1900 alloy in the presence of a solid Na2SO4 and a liquid 25 wt.% NaCl-75 wt.% Na2SO4 film at 800 °C in air. Electrochim. Acta 2004, 49, 1429–1433. [Google Scholar]
- Kuo, Y.L.; Hayashi, S.; Kakehi, K. The Effects of Nb Addition on the Oxidation Behavior of Ni-Fe-Cr Alloys at 800 °C. Oxid. Met. 2021, 95, 189–202. [Google Scholar] [CrossRef]
- Shi, H.; Fetzer, R.; Tang, C.C.; Szabó, D.V.; Schlabach, S.; Heinzel, A.; Weisenburger, A.; Jianu, A.; Müller, G. The influence of Y and Nb addition on the corrosion resistance of Fe-Cr-Al-Ni model alloys exposed to oxygen-containing molten Pb. Corros. Sci. 2021, 179, 109152. [Google Scholar] [CrossRef]
- Taniguchi, S.; Uesaki, K.; Zhu, Y.C.; Zhang, H.X.; Shibata, T. Influence of niobium ion implantation on the oxidation behaviour of TiAl under thermal cycle conditions. Mater. Sci. Eng. A 1998, 249, 223–232. [Google Scholar] [CrossRef]
- Shuan, L.M.; Ming, Z.Y. A Review on Effect of Reactive Elements on Oxidation of Metals. Corrsion Sci. Technol. Prot. 2001, 13, 333–337. [Google Scholar]
- Chen, G.; Wang, H.; Sun, H.; Zhang, Y.; Cao, P.; Wang, J. Effects of Nb-doping on the mechanical properties and high-temperature steam oxidation of annealing FeCrAl fuel cladding alloys. Mater. Sci. Eng. A 2020, 803, 140500. [Google Scholar] [CrossRef]
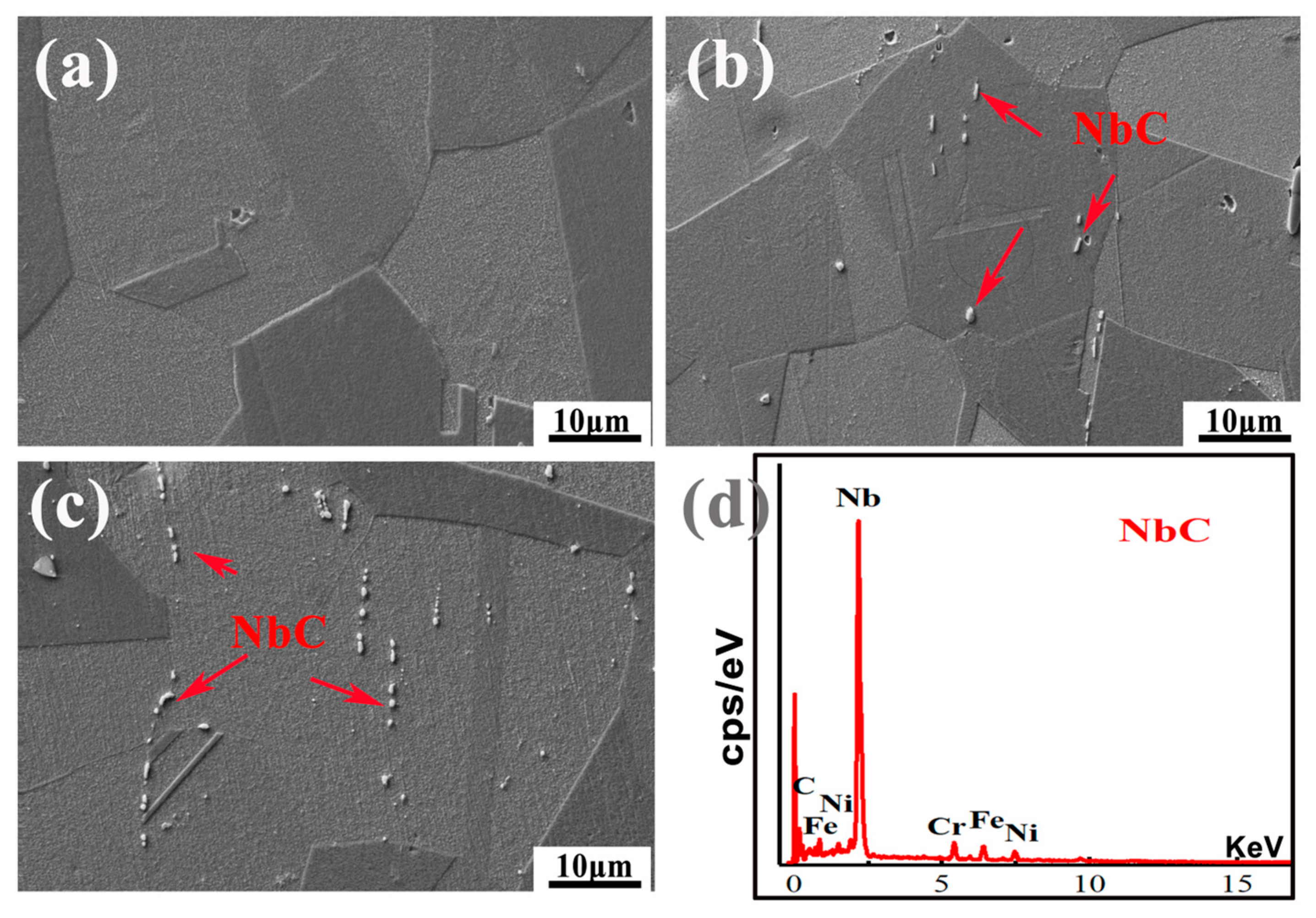
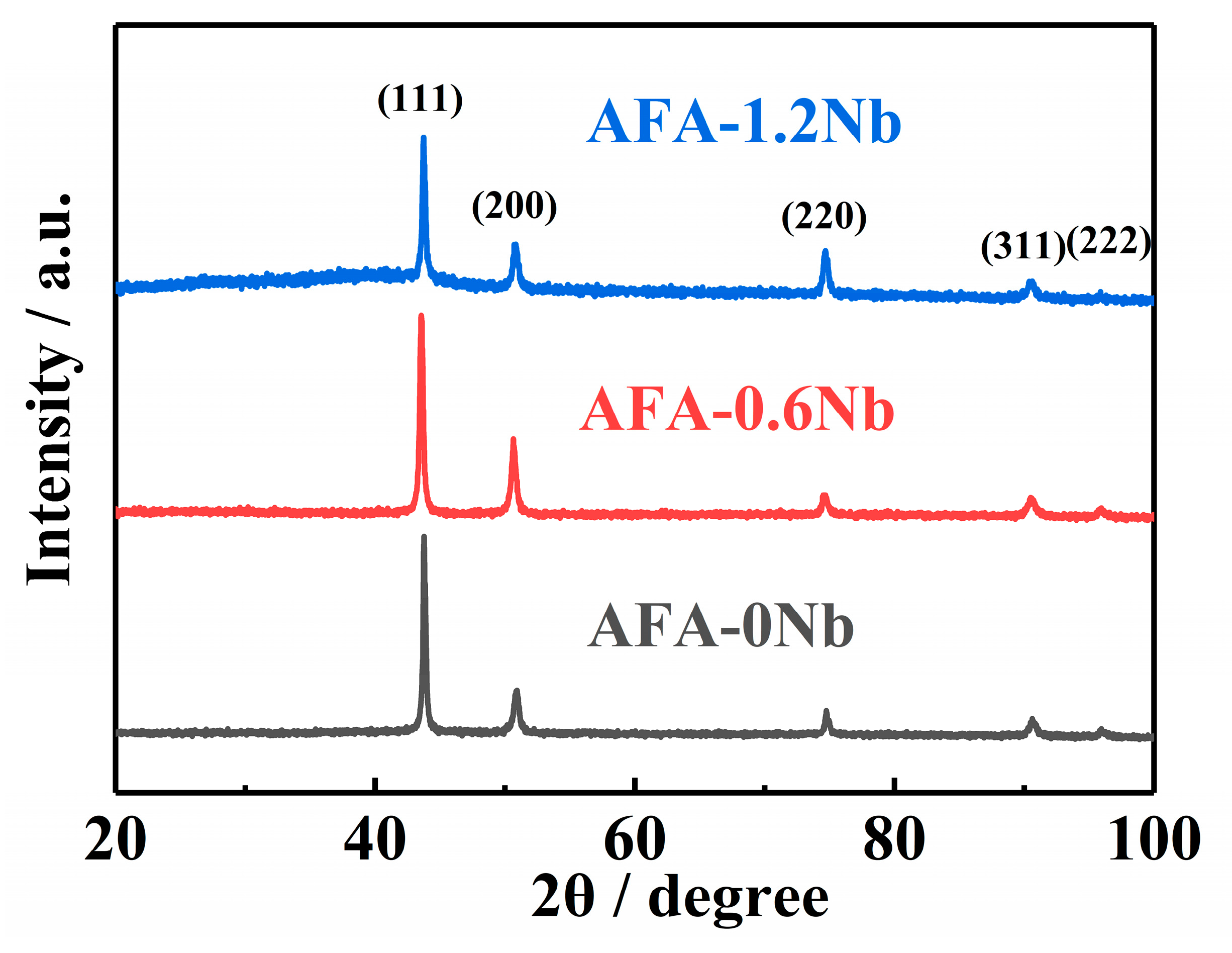
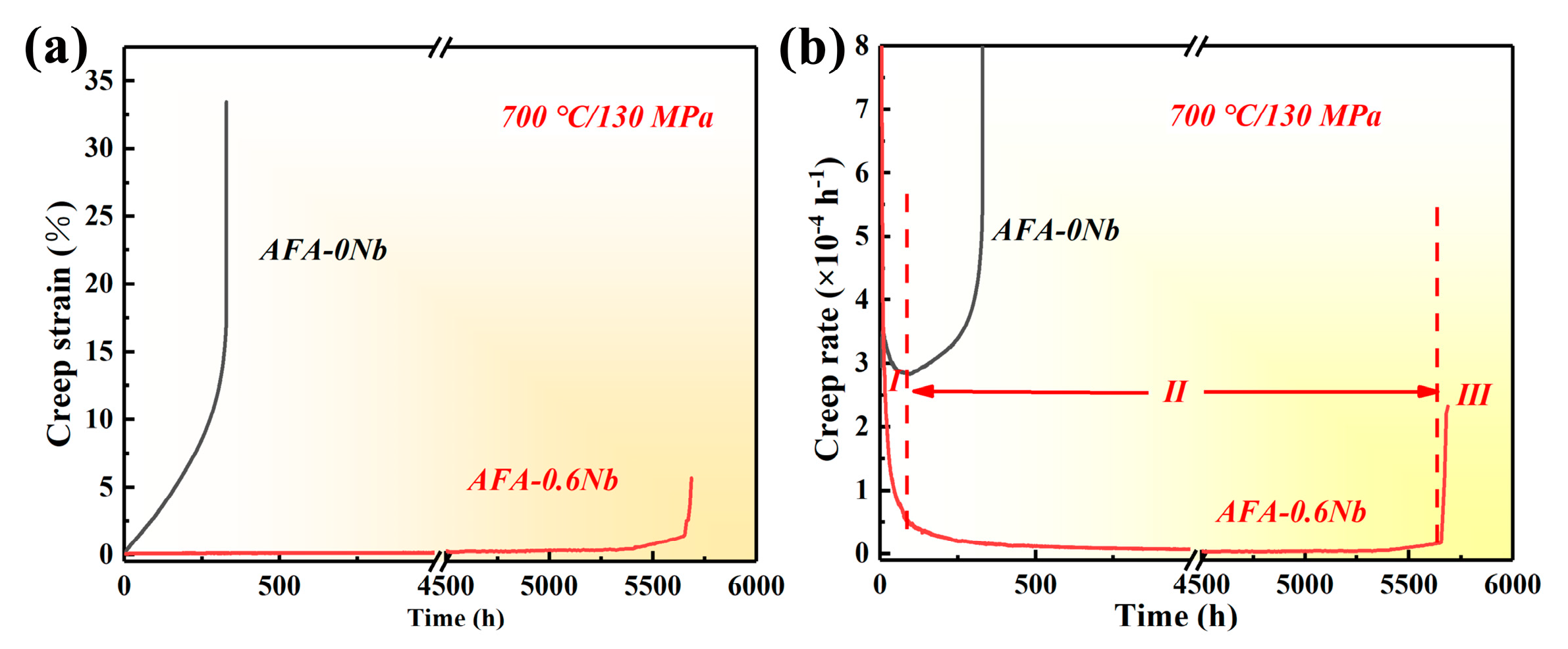
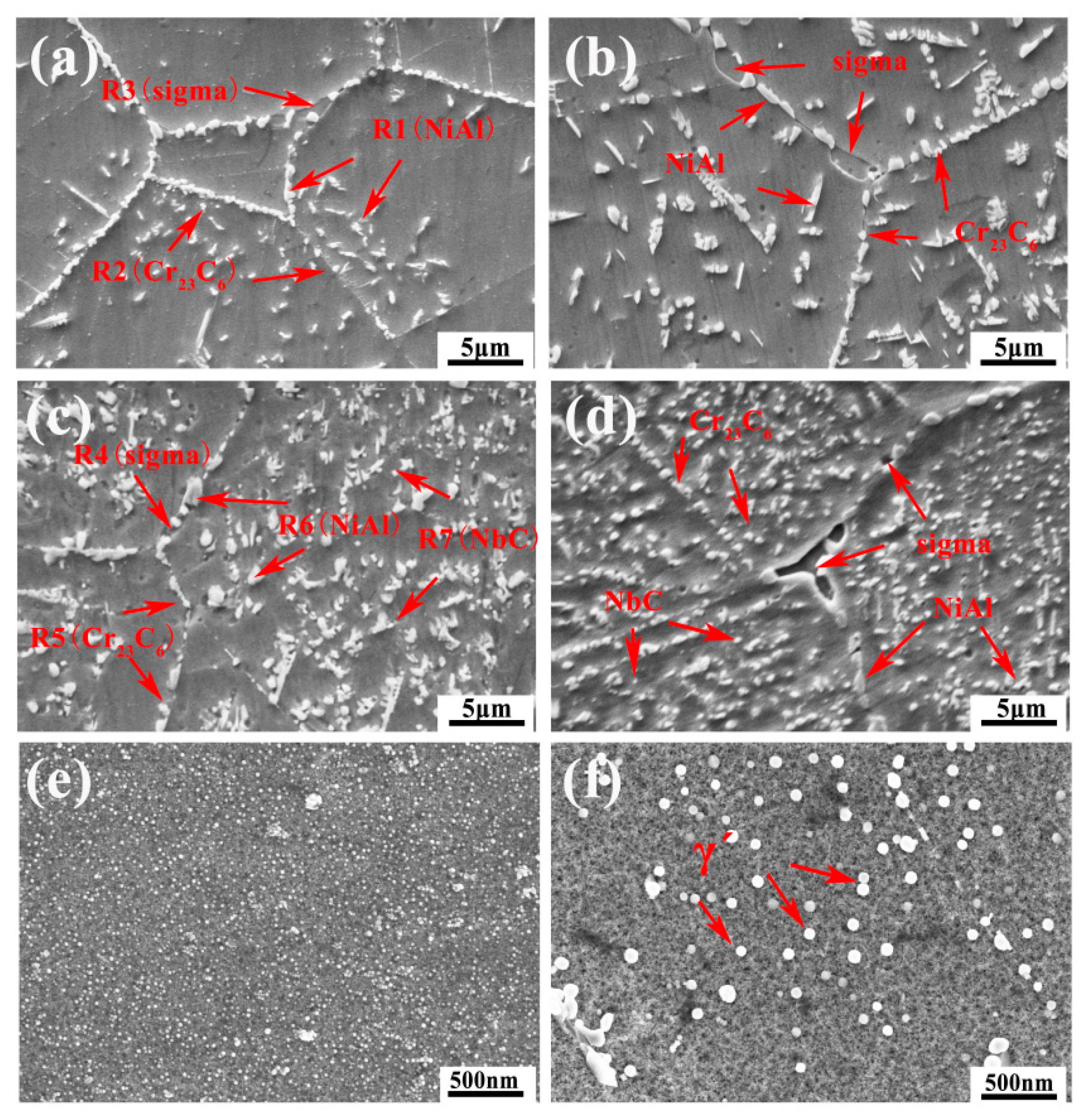
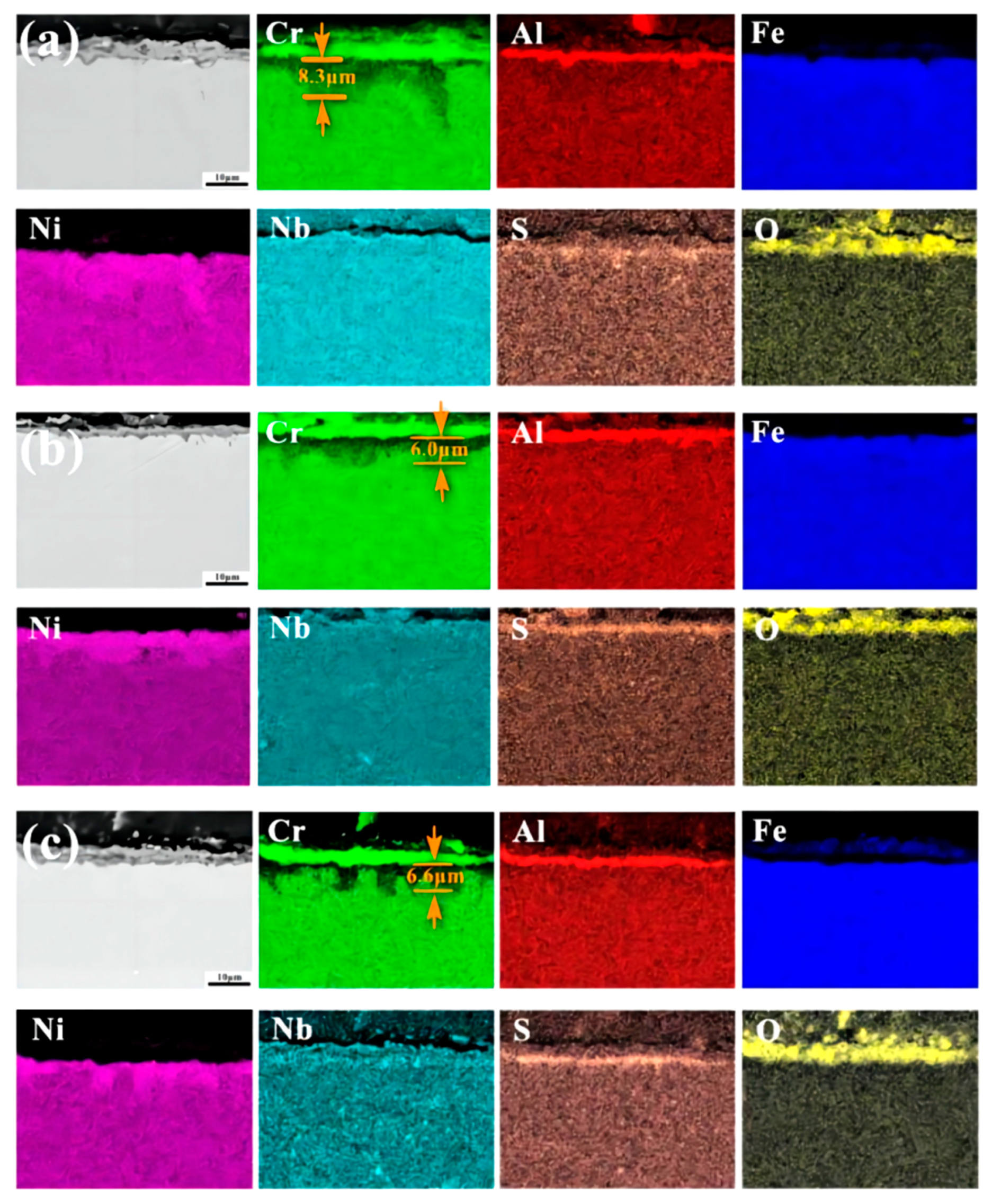



| Position | Composition (wt.%) | |||||
|---|---|---|---|---|---|---|
| C | Cr | Al | Fe | Ni | Nb | |
| R1 | 0.14 | 7.29 | 26.98 | 12.43 | 53.16 | - |
| R2 | 3.35 | 63.12 | 0.45 | 18.65 | 14.43 | |
| R3 | 1.56 | 46.39 | 1.33 | 43.40 | 7.32 | - |
| R4 | 0.32 | 41.87 | 0.82 | 51.27 | 5.72 | - |
| R5 | 4.65 | 59.45 | - | 12.32 | 23.58 | |
| R6 | 0.34 | 13.65 | 28.97 | 10.47 | 46.57 | |
| R7 | 4.17 | 9.76 | 3.53 | 13.94 | 10.68 | 57.92 |
| Reaction | ∆G700°C/kJ/mol |
|---|---|
| 4/3Al + O2 (g) = 2/3Al2O3 | −913.3 |
| 4/3Cr + O2 (g) = 2/3Cr2O3 | −585.4 |
| 2Ni + O2 (g) = 2NiO | −301.8 |
| 4/3Fe + O2 (g) = 2/3Fe2O3 | −377.3 |
| 4/5Nb + O2 (g) = 2/5Nb2O5 | −589.5 |
| 2Al + Na2SO4 = Al2O3 + Na2O + S(g) | −496.9 |
| 2Cr + Na2SO4 = Cr2O3 + Na2O + S(g) | −5.01 |
| 3Ni + Na2SO4 = 3NiO + Na2O + S(g) | 420.4 |
| 2Fe + Na2SO4 = Fe2O3 + Na2O + S(g) | 307.1 |
| 2/3Al + S = 1/3Al2S3 | −196.9 |
| Cr + S = CrS | −138.6 |
| 1/2Fe +S =1/2 FeS2 | −58.4 |
| 3/2Ni + S = 1/2Ni3S2 | −95.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Jia, G.; Pan, J.; Wang, Z.; Li, J.; Xiao, X. Effects of Nb on Creep Properties and Hot Corrosion Resistance of New Alumina-Forming Austenitic Steels at 700 °C. Metals 2024, 14, 870. https://doi.org/10.3390/met14080870
Xu W, Jia G, Pan J, Wang Z, Li J, Xiao X. Effects of Nb on Creep Properties and Hot Corrosion Resistance of New Alumina-Forming Austenitic Steels at 700 °C. Metals. 2024; 14(8):870. https://doi.org/10.3390/met14080870
Chicago/Turabian StyleXu, Wanjian, Guodong Jia, Jie Pan, Zixie Wang, Jun Li, and Xueshan Xiao. 2024. "Effects of Nb on Creep Properties and Hot Corrosion Resistance of New Alumina-Forming Austenitic Steels at 700 °C" Metals 14, no. 8: 870. https://doi.org/10.3390/met14080870




