Abstract
The corrosion behaviors of three bridge steels in a real tropical marine environment for 2 years were studied. One weathering steel (WS) was designed with higher levels of nickel, copper, and molybdenum compared to the other. These two kinds of WSs and one kind of ordinary high-strength low-alloy steel (Q345qe) were compared under two conditions (marine atmospheric zone and marine immersion zone at Sanya Marine Environmental Test Station). The morphology, corrosion rate, and corrosion product analysis of the steels were performed through SEM, XPS, FTIR and other characterization methods. The results demonstrated that weathering steels facilitate the densification of the corrosion product layer due to the addition of alloying elements Cr, Ni, and Cu, promoting rust nucleation and enhancing the compactness of the protective layer. However, in an immersion environment, the extensive erosion by chloride ions renders the benefits of WS ineffective.
1. Introduction
The use of weathering steels in marine environments has gained significant attention due to their exceptional resistance to atmospheric corrosion [1,2,3,4]. These steels are engineered to form a protective rust layer when exposed to atmospheric conditions, providing durable and cost-effective solutions for various structural applications and significantly reducing the requirement for painting and other surface treatments. However, despite their renowned corrosion resistance, weathering steels can still experience degradation when subjected to aggressive marine atmospheric and immersion conditions [5,6]. Understanding the corrosion behaviors of weathering steels in actual marine environments is crucial for assessing their long-term performance and optimizing their applications in marine structures.
Over the recent few decades, extensive research has been conducted to investigate the factors influencing the corrosion of weathering steels in marine atmospheric and immersion zones [6,7,8,9,10,11]. The corrosion mechanisms of weathering steels in marine environments involve complex electrochemical processes influenced by various factors, such as temperature, humidity, chloride concentration, and exposure duration. Characterized by high levels of salinity, constant humidity, and periodic immersion in seawater, these conditions are particularly aggressive toward metal structures. The protective layer that forms on weathering steels in terrestrial environments may not develop as effectively in marine settings. Saltwater, in particular, can compromise the integrity of the layer, leading to pitting and crevice corrosion. In essence, the presence of high levels of chloride ions can negatively affect the corrosion resistance of a material by interfering with the formation of a key protective compound. Since nickel ferrite forms a protective inner film on the metal, high concentrations of chloride ions prevent its formation, resulting in the loss of the electronegative effect that this film provides [12]. Consequently, the underlying metal becomes more susceptible to corrosion because the protective barrier is compromised. Yang et al. proposed a corrosion failure chain model. These authors suggested that the rust layer tends to crack longitudinally, increasing the accumulation of Cl− in thick corrosion products. Large amounts of β-FeOOH and γ-FeOOH form and penetrate deeply into these thick corrosion products. The significant stresses in the corrosion product weaken its adhesion, leading to cracking or exfoliation [13,14,15].
Obviously, weathering steels perform well in less aggressive atmospheric conditions [16,17,18] and are less effective in marine environments [19,20,21,22]. To address this issue, the addition of various alloying elements has been extensively attempted [23,24,25]. Sun et al. reported that the chromium in weathering steels significantly promotes the transformation of α-FeOOH. Chromium forms Cr2O3 and Cr(OH)3, both of which have excellent corrosion resistance [26]. Assumpção et al. evaluated the behavior of three weathering steels with different chemical compositions under dry/wet cycling corrosion tests [27], suggesting that Si influences corrosion rates. Yue et al. found that the addition of rare earth (RE) can improve the corrosion resistance of weathering steels because the original strip-shaped MnS inclusions and Al2O3 inclusions with sharp angles are modified into fine spherical RE inclusions [5]. Clearly, enhancing the corrosion resistance of weathering steels is crucial for delaying the deterioration of engineering structures in marine environments. Therefore, investigating the corrosion damage processes of weathering steels in tropical marine environments, which can provide a foundation for the development and application of new weathering steels, is essential.
In this work, the corrosion behaviors of three bridge steels in a real tropical marine environment for 2 years were studied. One type of weathering steel (WS) was designed with higher levels of nickel, copper, and molybdenum than the other. These two kinds of WSs and one kind of ordinary high-strength low-alloy steel (Q345qe) were compared through various characterization methods under two conditions (a marine atmospheric zone and a marine immersion zone at the Sanya Marine Environmental Test Station). The morphology, corrosion rate, and corrosion product analysis of the steels were performed. Finally, the corrosion behavior of the three steels was evaluated in terms of alloy composition.
2. Materials and Methods
2.1. Materials and Sample Preparation
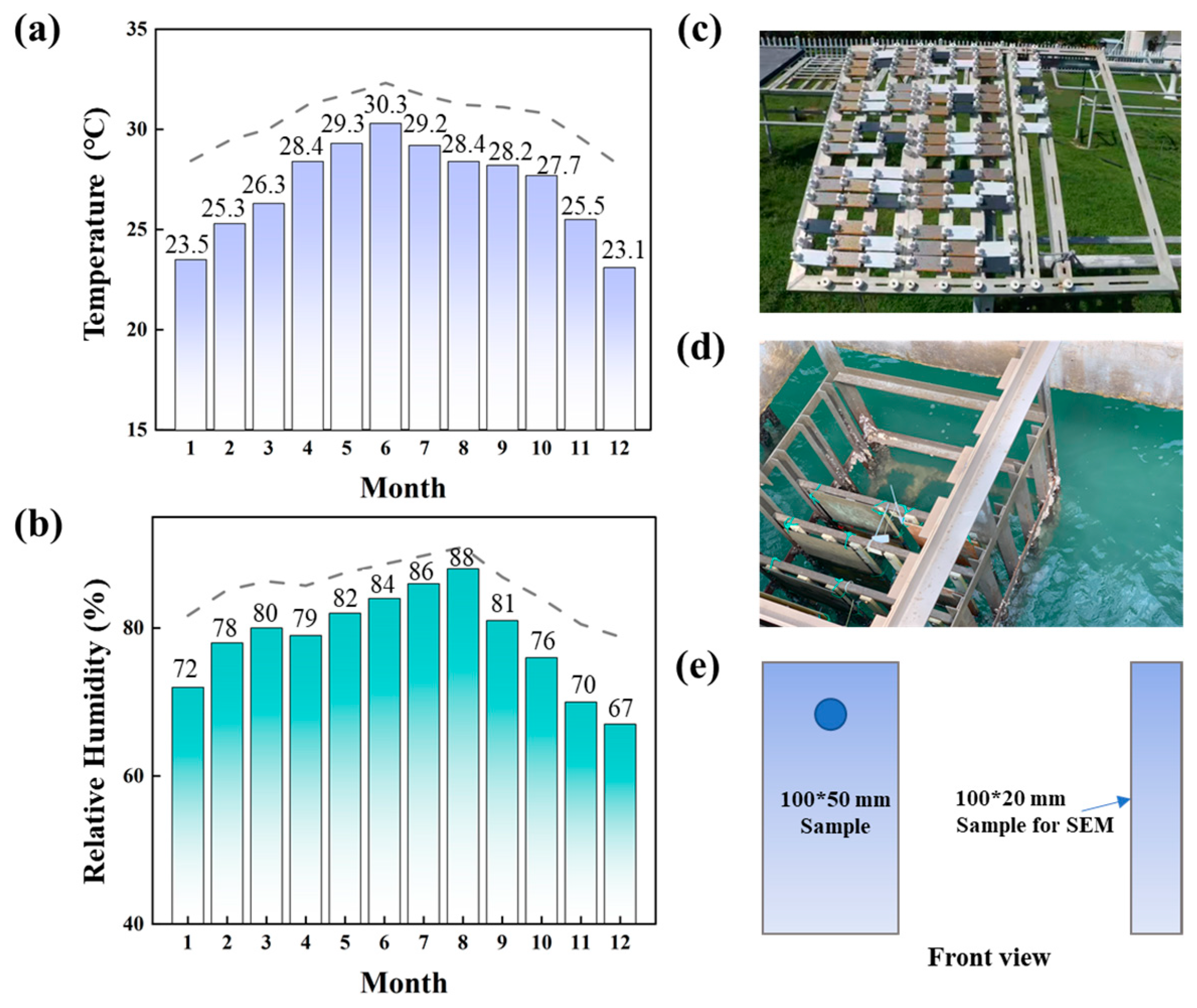
Three steels were used in this study. The ordinary high-strength low-alloy steel Q345qe was selected as the control subject and defined as 1#. One ordinary WS was defined as 2#, while the newly designed WS was defined as 3# in this paper. Their chemical compositions are shown in Table 1. The three steels went through thermo-mechanical control process (TMCP) rolling, and then 2# and 3# steels were subjected to low-temperature tempering. These weathered steel samples were cut into 100 mm × 20 mm × 3 mm pieces for SEM observation and 100 mm × 50 mm × 3 mm pieces for other testing (Figure 1e). All the samples were ground to 2000 mesh and cleaned with acetone and alcohol. The samples were then dried and weighed in preparation for use in actual marine environments.

Table 1.
Chemical compositions of the weathering steels (wt.%).
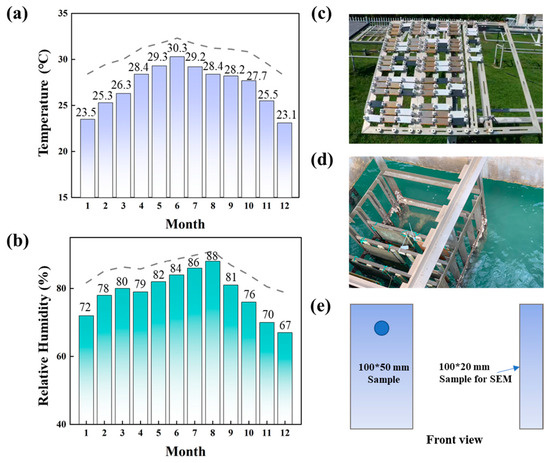
Figure 1.
Climatic data during the exposure period at Sanya station: (a) the average temperature for each month; (b) the average relative humidity for each month. Photos of the corrosion test equipment and samples in the (c) marine atmospheric zone and (d) marine immersion zone. (e) Schematic diagram of the size of the samples.
2.2. Atmospheric Zone and Immersion Zone Exposure Tests in the Actual Marine Environment
All the samples were placed in a real tropical marine environment for 2 years. The exposure tests were performed at the Sanya Marine Environmental Test Station, which is a typical tropical marine environment on Hainan Island, China. Figure 1a,b show the temperature and relative humidity of the station. The annual average temperature is 26.7 °C, and the annual average humidity is 79%. The Cl− concentration is approximately 0.1–0.4 mg/100 cm2·d−1. At the atmospheric zone test site (Figure 1c), the samples were fixed on the shelf at an angle of 45° to the horizontal, which ensures that the sample surface was more evenly exposed to atmospheric conditions, including sunlight, rain, dust, and other factors. This enables a more realistic simulation of the corrosion process in a natural environment. The 45° angle also allows rainwater and other liquid contaminants to flow smoothly off the sample surface, preventing the accumulation of water on the surface. At the immersion zone test site (Figure 1d), the samples were fixed on the test rack and immersed in seawater. The parallel samples were taken out at time points of 0.5, 1, 1.5 and 2 years. Further testing was conducted in the laboratory.
2.3. Corrosion Analysis and Characterization
The mass of each sample before and after service was measured via a microbalance (MC5, Sartorius, Göttingen, Germany). The mass change was calculated on the basis of the obtained data. The final corrosion rate result was determined by averaging the values from three parallel samples via the following equation:
where V is the average corrosion rate, mm/a; ΔW is the mass change before and after corrosion, mg; A is the surface area of the sample, mm2; t is the corrosion time, a (1 year); and D is the material density, g/mm3. To remove the corrosion products from the surface of the sample, derusting solution (500 mL, 38 wt.% HCl, 3.5 g C6H12N4 and 500 mL H2O) was utilized at room temperature. After the corrosion products were completely removed, the samples were washed with alcohol, dried, and weighed.
The surface states of the samples after service were observed by stereomicroscopy and scanning electron microscopy (SEM, JEOL, JSM-7001F, Akishima, Japan). The corresponding elemental compositions and distributions were determined using energy-dispersive spectroscopy (EDS). Afterward, the rust layers were scraped with a knife to obtain the dried rust powders, which were analyzed by X-ray diffraction (XRD). A Bruker D8 XRD analyzer with a Co target and a Bruker D2 XRD analyzer (Billerica, MA, USA) with a Cu target were used to perform the measurements. The scanning angle ranged from 5° to 45° with a step rate of 4°/min. The software Jade 6.0 was used to analyze the spectra. FTIR was used to analyze the rust layers. A VERTEX70 infrared spectrometer (Bruker Instruments, Mannheim, Germany) was used to analyze the samples. Potassium bromide was selected as the diluent for the tablet method. During the test, the corrosion products on the surface of the substrate were first scraped with a scraper, and the corrosion products were evenly mixed with analytically pure KBr at a mass ratio of 3:100. After grinding, the thickness of each slice was approximately 1 mm. During the test, the scanning range was 400–4000 cm−1, the resolution was 4 cm−1, 64 scans were performed without gain, and the test results were analyzed using Omnic software (V8.2).
3. Results and Discussion
3.1. Metallographic Structure
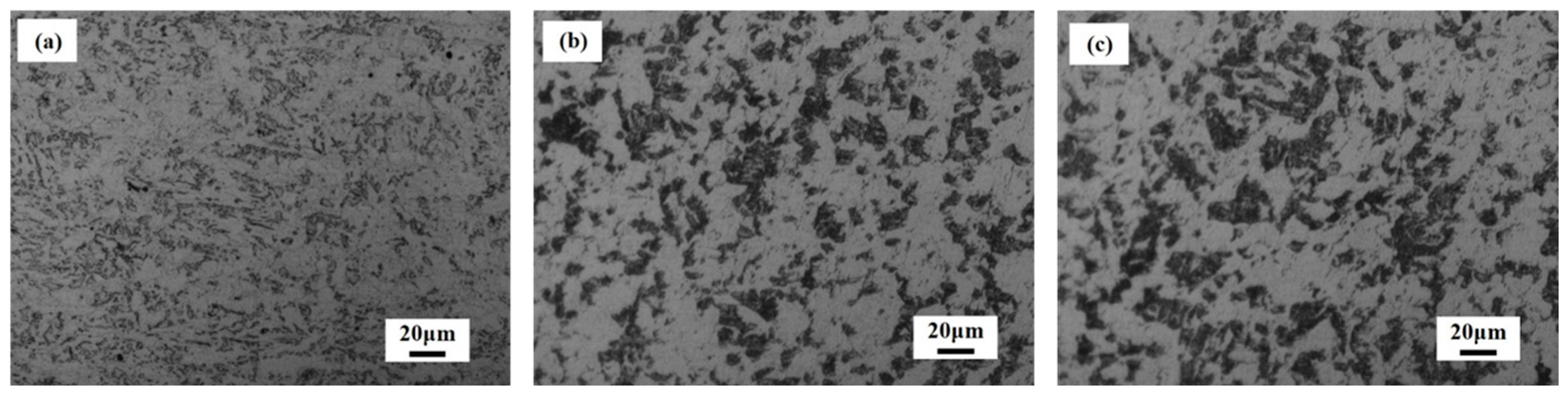
The metallographic structures of the three steels are shown in Figure 2. The metallographic structure of the three steels is mainly ferrite and a flake pearlite structure, and inclusions are distributed in them. At the same magnification, the morphology of the pearlite in steels #2 and #3 is greater than that in #1 steel.

Figure 2.
Metallographic structure of bridge steels: (a) #1 steel; (b) #2 steel; and (c) #3 steel.
3.2. Corrosion Weight Loss and Local Corrosion Depth
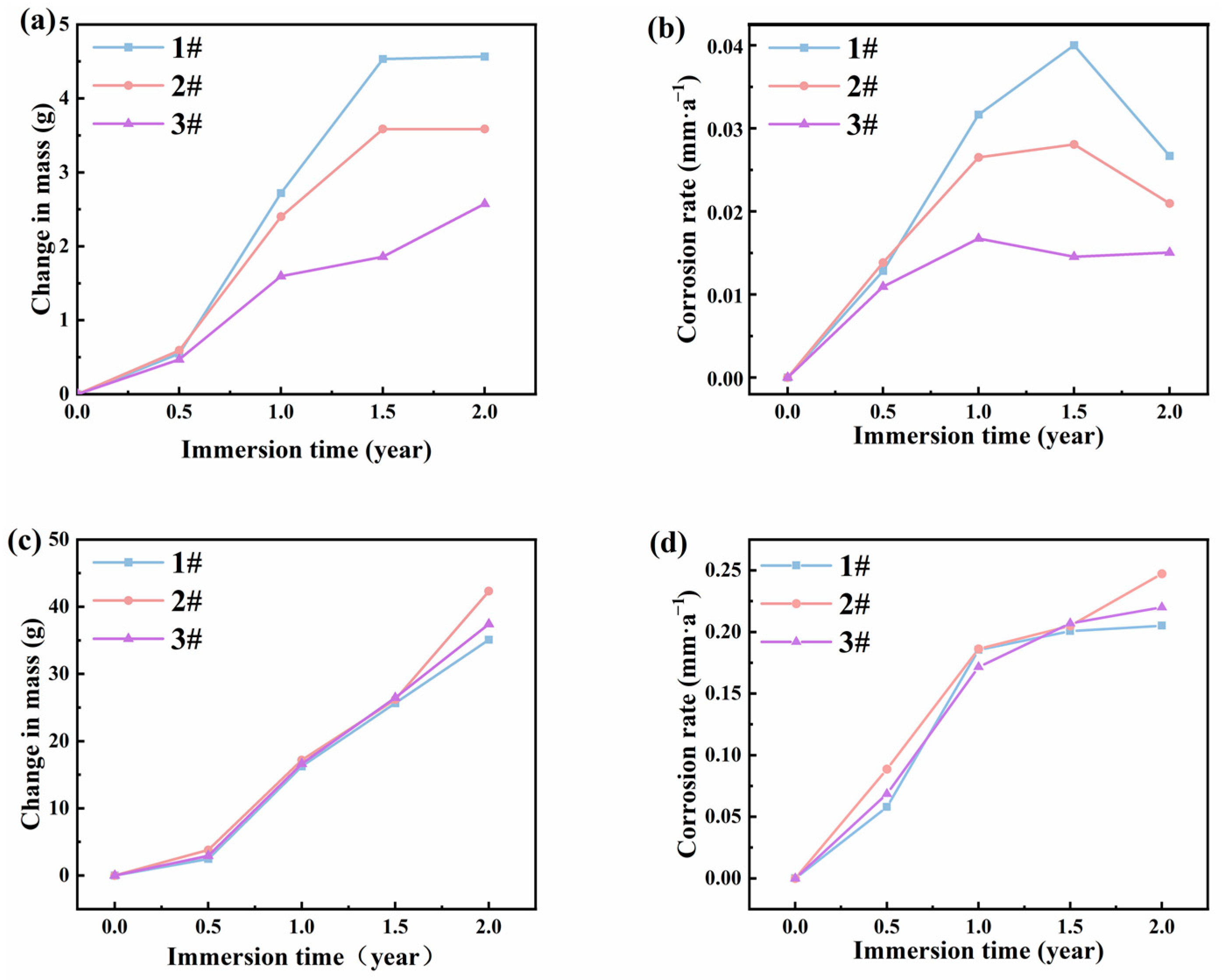
The mass changes and corrosion rates of the three steels in the atmospheric zones of actual marine environments are shown in Figure 3a,b. The results indicated that during corrosion cycles, the mass change of steel 1# and steel 2# exhibited a rapid increase and finally stabilization as the exposure time extended, while the mass change of steel 3# showed a consistent slow increase over time. The corrosion rate showed a trend of rapid increase and then slow increase for steel 1# and steel 2#, and then eventually rapidly decrease. In contrast, the corrosion rate of steel 3# demonstrated a trend of initial increase and stabilization with the lowest values. At 0.5 years, there was minimal difference in corrosion rates among the three steels. However, by 1 year, more significant differences emerged, with corrosion rates approximately at 0.032 mm/a, 0.027 mm/a, and 0.017 mm/a for steels 1#, 2#, and 3#, respectively. At 1.5 years, steels 1# and 2# experienced the highest corrosion rates at approximately 0.04 mm/a and 0.028 mm/a. However, the corrosion rate of steel 3# saw a slight decrease to about 0.015 mm/a. At 2 years, the corrosion rates of steels 1# and 2# decreased sharply to approximately 0.027 mm/a and 0.021 mm/a, respectively, while the corrosion rate of steel 3# remained relatively unchanged, indicating that all bridge steels may form protective rust layers. From the data above, steel 3# showed the least corrosion rates, which may due to the formation of strongly protective corrosion products by the addition of Ni collaborates with Mo.
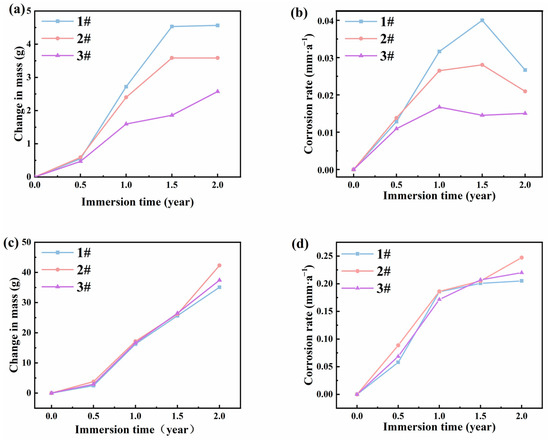
Figure 3.
Mass changes and corrosion rates of the three steels at different time points for (a,b) atmospheric zone and (c,d) immersion zone.
For the results in immersion zones, the weight loss and corrosion rates of three steels were similar. The weight losses of the three types of steels increased rapidly over time, and the corrosion rates still increased with immersion time. Relatively high corrosion rates of the three steels suggest that a protective layer of corrosion products may not have formed on the steel surface after 2 years. The three types of steels experienced severe corrosion in the immersion zone. It is unsuitable to use steels without a protective coating in immersion conditions.
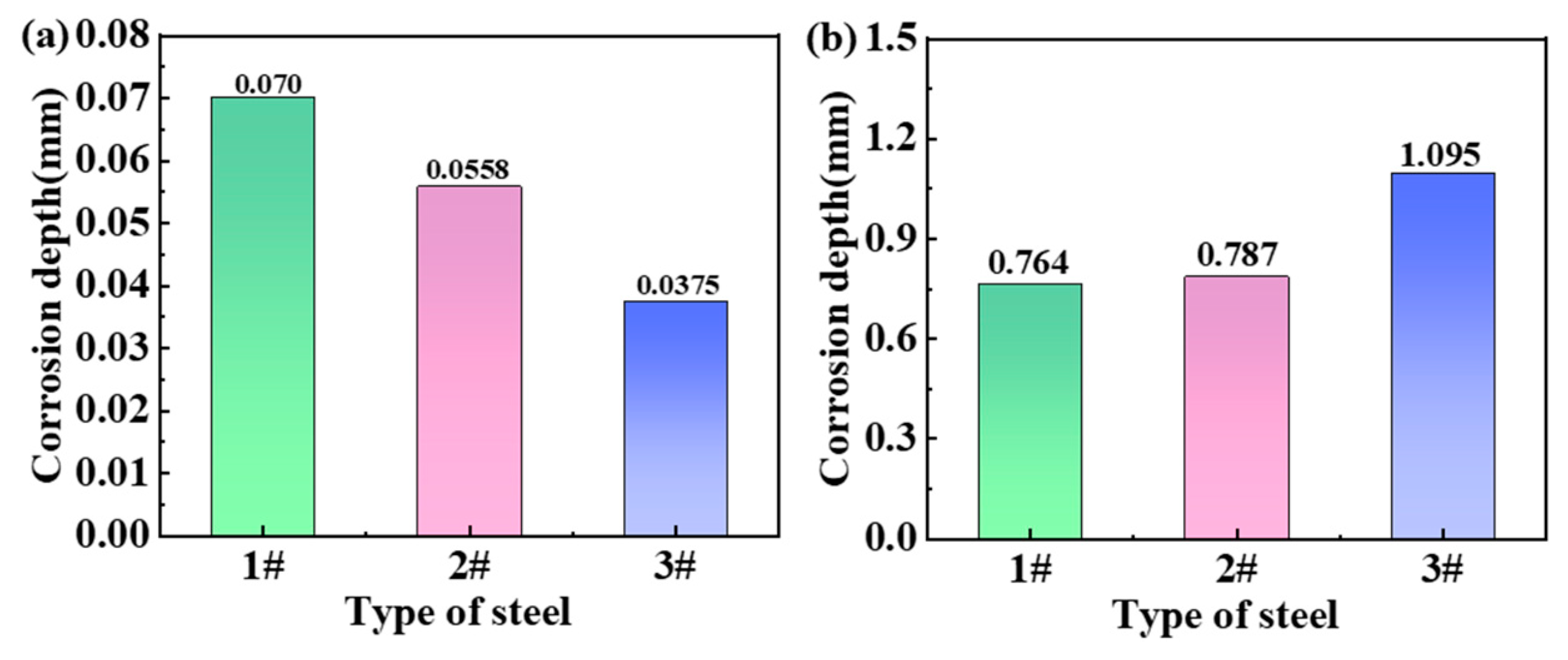
The local corrosion depths of the three steels after two years are shown in Figure 4, which is the result based on the statistics of six pitting corrosion sites on each sample. The results show that the local corrosion depth of the steel in the marine atmosphere is small (Figure 4a). The corrosion depth of steel 1# had the greatest value of 0.07 mm. Steel 3# was relatively shallow, indicating the lowest degree of corrosion of 0.0375 mm. This reflects that steel 3# is corrosion-resistant, whereas steel 1# is highly susceptible to corrosion in the marine atmospheric environment. However, the situation changed in the immersed zone, as shown in Figure 4b. The results indicated that the local corrosion depth of steel 3# was the greatest. Considering the absolutely high corrosion depths, none of the three steels demonstrate good corrosion resistance when exposed to seawater. Therefore, the differences in corrosion depth among the three steels are not meaningful.
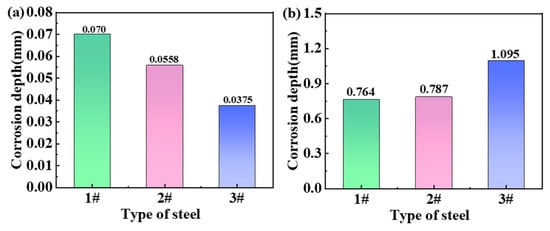
Figure 4.
Corrosion depths of the three steels after two years for (a) atmospheric zone and (b) immersion zone.
3.3. Macroscopic Corrosion Morphology
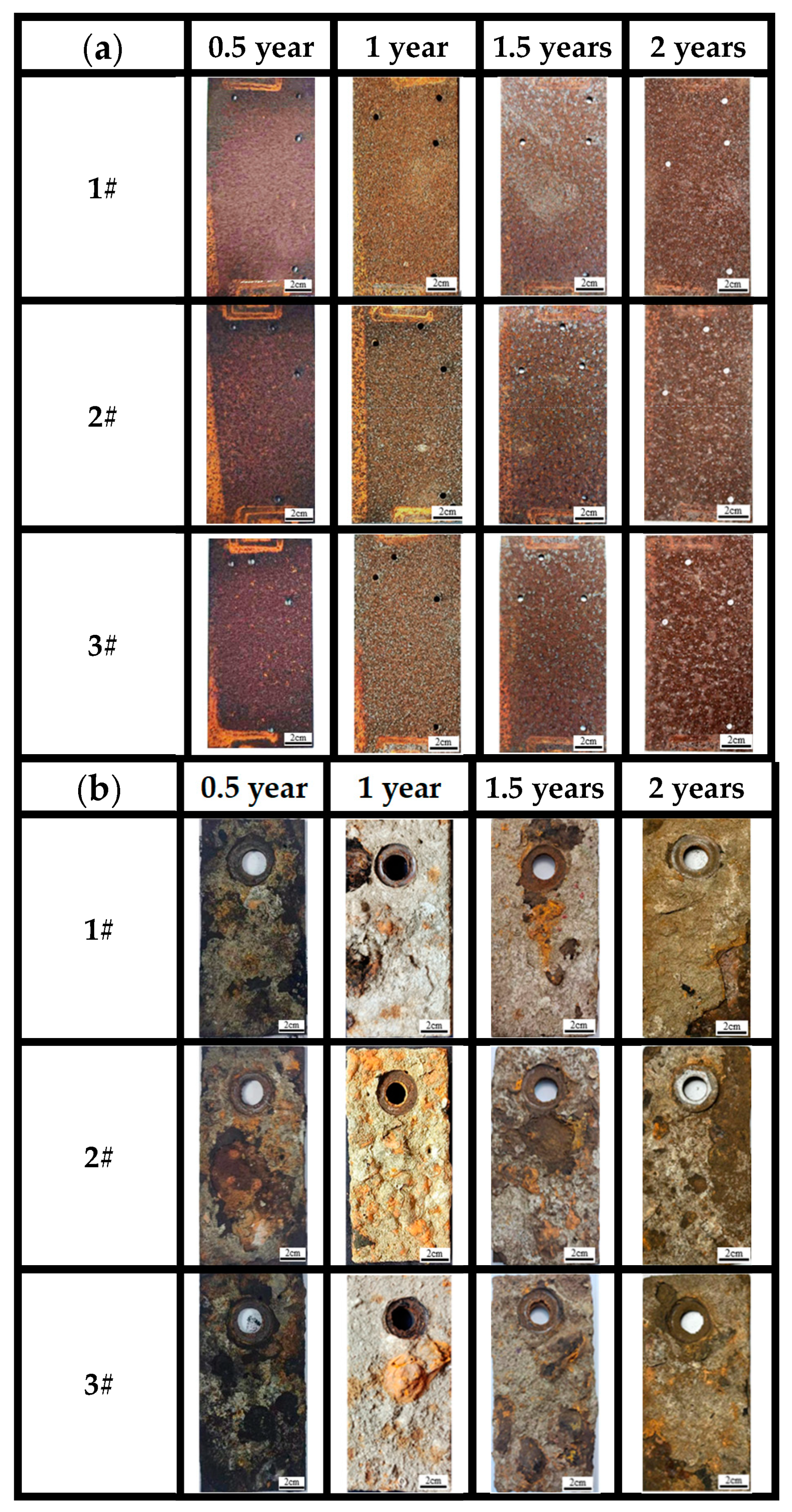
Figure 5a shows the macroscopic surface morphologies of the three steels in the atmospheric zones of actual marine environments for 0.5, 1, 1.5 and 2 years. Observations showed that in the 0.5-year period, the surfaces of the three steels were covered with dense rust. As time elapses, the color of the surface rust layer changes from reddish brown to dark red. From the macroscopic color change and surface condition, a preliminary judgment can be made, which qualitatively suggests that a uniform, dense rust layer formed on the surface of all three bridge steels. The macroscopic morphology of the three steels shows almost no difference at this time.
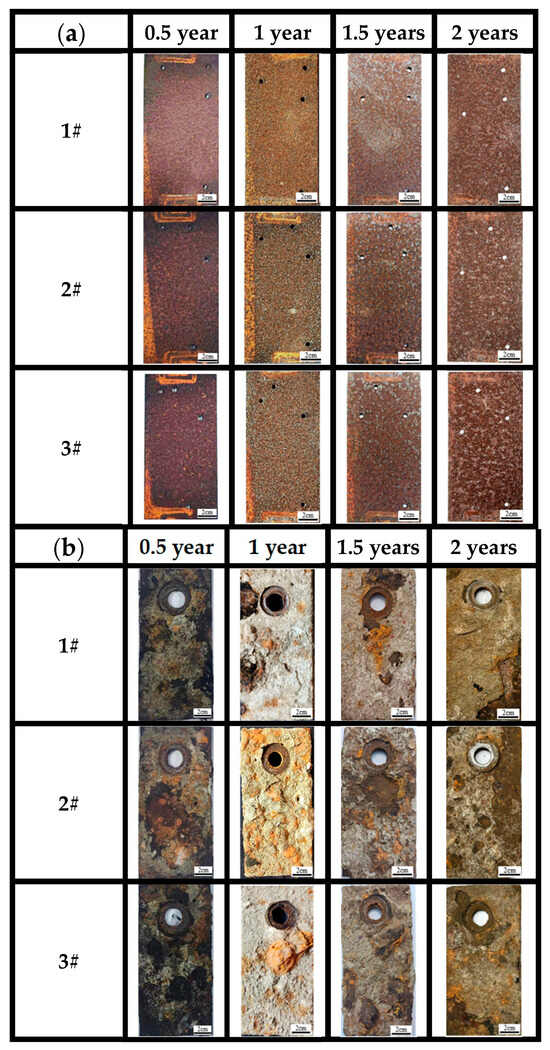
Figure 5.
Macroscopic corrosion morphology of three kinds of steels at different time points for (a) atmospheric zone and (b) immersion zone.
After 1 year of corrosion, the surface rust layer gradually became uniform. After 1.5 years of corrosion, the surface rust layer started to darken. After 2 years of corrosion, the surface rust layer gradually became rougher, with an increasing number of surface pits, and the surface color turned to dark brown. Based on the nature of the corrosion products [13], the brown corrosion products observed after 0.5 years are likely the intermediate amorphous oxides formed during the transformation from γ-FeOOH to α-FeOOH, which are brown in color. The dark brown corrosion products observed after 1 year, 1.5 years, and 2 years could be α-FeOOH, amorphous oxides, or a mixture of both.
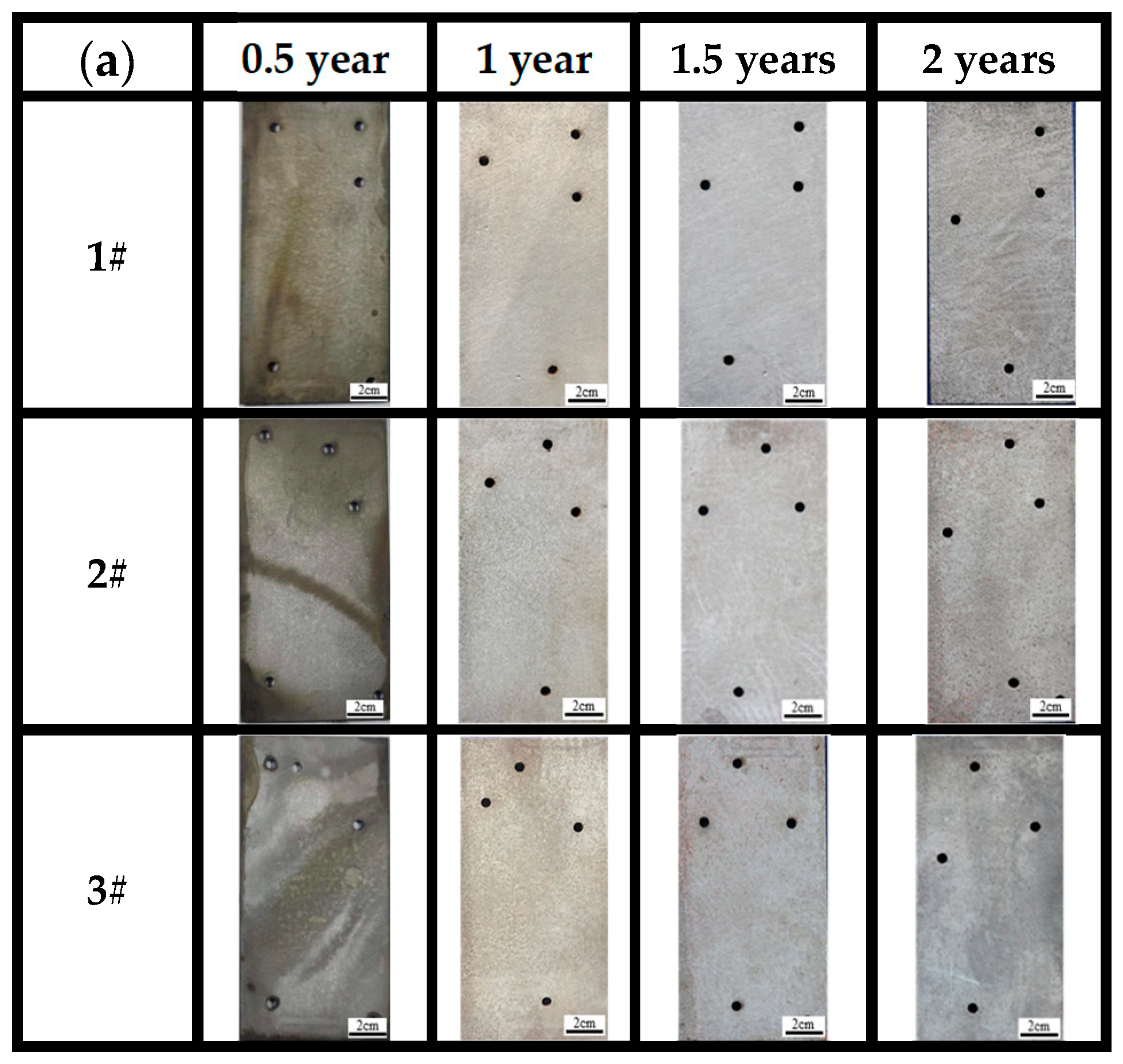
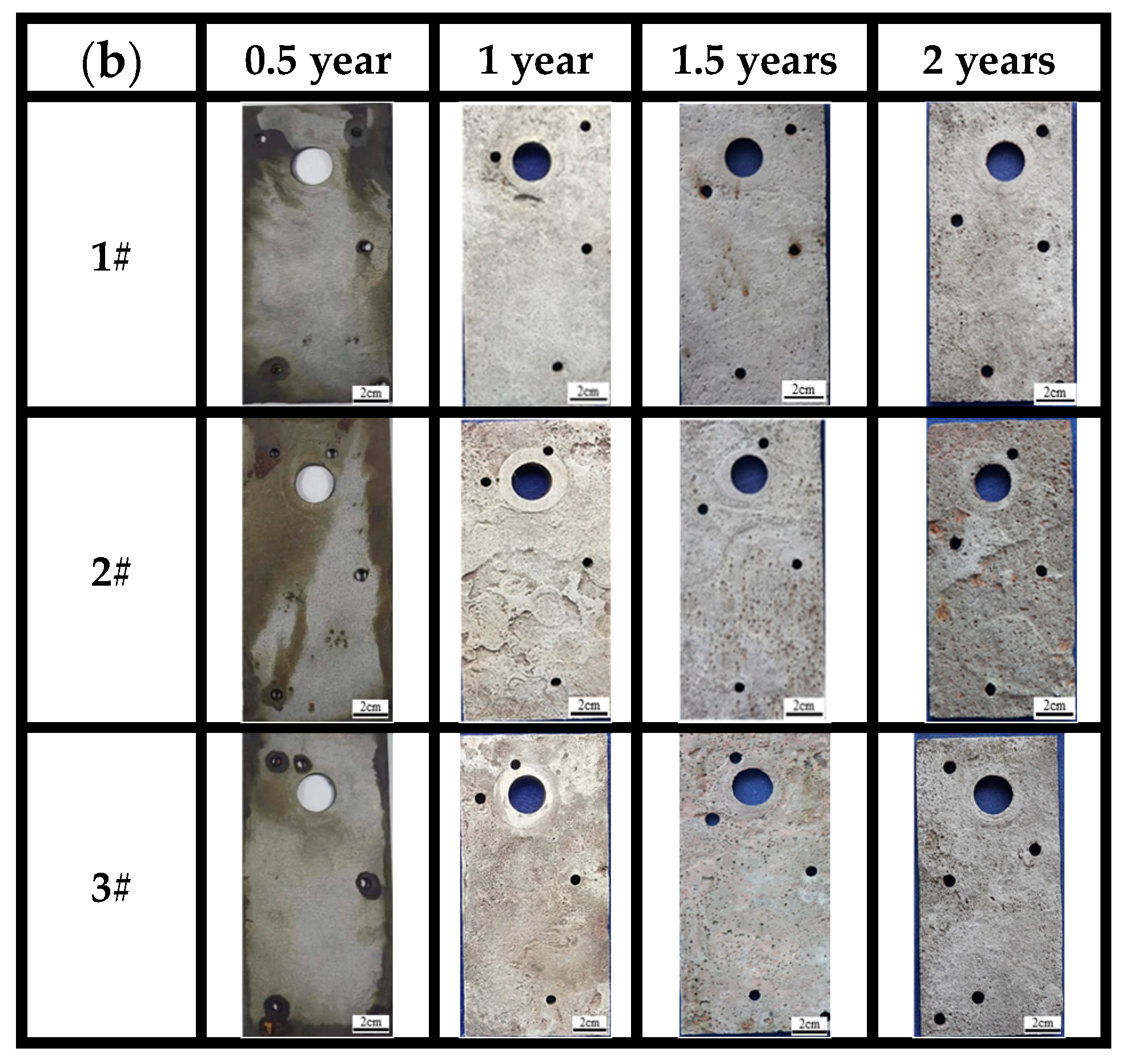
After completely removing the corrosion products (Figure 6a), it can be observed that the three bridge steels exhibit relatively light corrosion in the marine atmospheric environment. During the corrosion periods of 0.5 years, 1 year, and 1.5 years, no visible corrosion pits are on the sample surfaces, and the surfaces are relatively smooth. The appearances of steels at the same time point cannot be directly distinguished with the naked eye. After 2 years of corrosion, however, numerous localized corrosion pits can be observed on the surface of steel 1# compared to steels 2# and 3#, suggesting that steel 1# is less corrosion-resistant. The surface of the steel 2# substrate is relatively rough, whereas the surface of the steel 3# substrate is comparatively smooth. The results indicate that higher contents of Ni, Cu, and Mo have a significant influence on corrosion resistance compared to general low-alloy steels and weathering steels in tropical marine atmospheric environments.
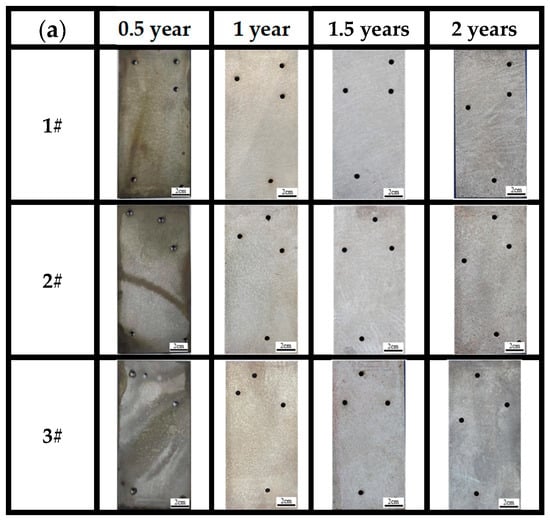
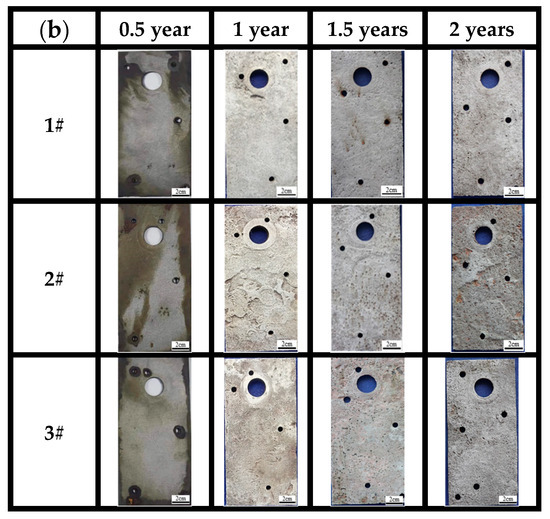
Figure 6.
Morphology of substrates after complete removal of rust for (a) atmospheric zone and (b) immersion zone.
As for the macro morphology of the sample in marine immersion zone (Figure 5b), the results indicate that the surfaces of the three types of steels developed a loose outer rust layer and a dense inner rust layer, since the outer layer can be easily peeled off from the substrate with fingers. The corrosion degree of the steels also cannot be judged by the naked eye alone but should be analyzed with the corrosion weight loss, microscopic corrosion morphology, and phase results of corrosion products. According to the photos, a thin outer rust layer and a black inner rust layer can be seen on the surface, with the outer corrosion products peeling off at 0.5 years, especially for steel 2#. At 1 year and 1.5 years, the outer rust layer uniformly covers the surface of the substrate and gradually thickens, with the formation of rust nodules mainly concentrated in the middle and lower parts of the samples. As time progresses, the area of black-brown regions in the inner rust layer significantly decreases, while the area of yellow-brown regions increases, and the outer rust layer becomes increasingly loose. After 2 years, the peeling of the outer rust layer at the edges of the samples significantly increases.
On the other hand, the macroscopic morphology of the surfaces after removal of corrosion products indicated that the substrates have no significant changes until 1 year of immersion. After 1 year of corrosion, the surfaces of steels became rough. Large pits were found on the surface of steel 2#. After 2 years, pitting was almost uniformly distributed across the entire surface of the three steels, but no perforations were found. This indicates that the corrosion state of 3# weathering steel in the immersion environment is localized corrosion. There is not much difference compared to the other two steels. All the steels have lost their corrosion resistance in the immersion zone.
3.4. Microscopic Corrosion Morphology
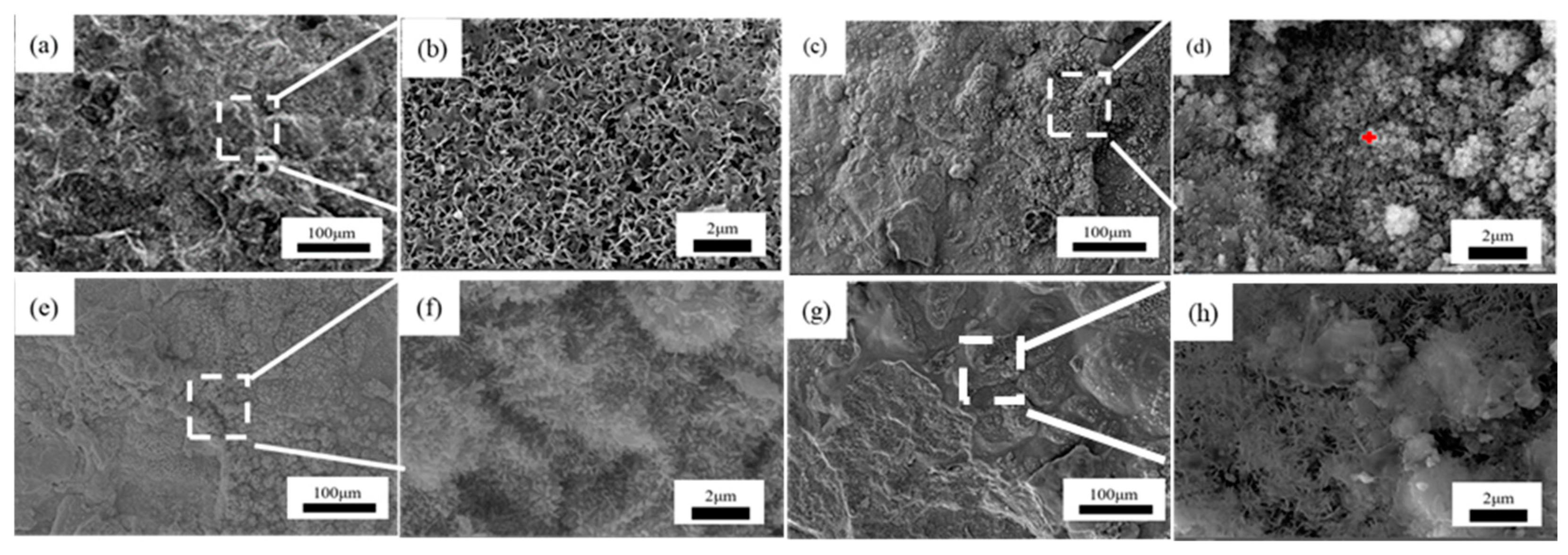
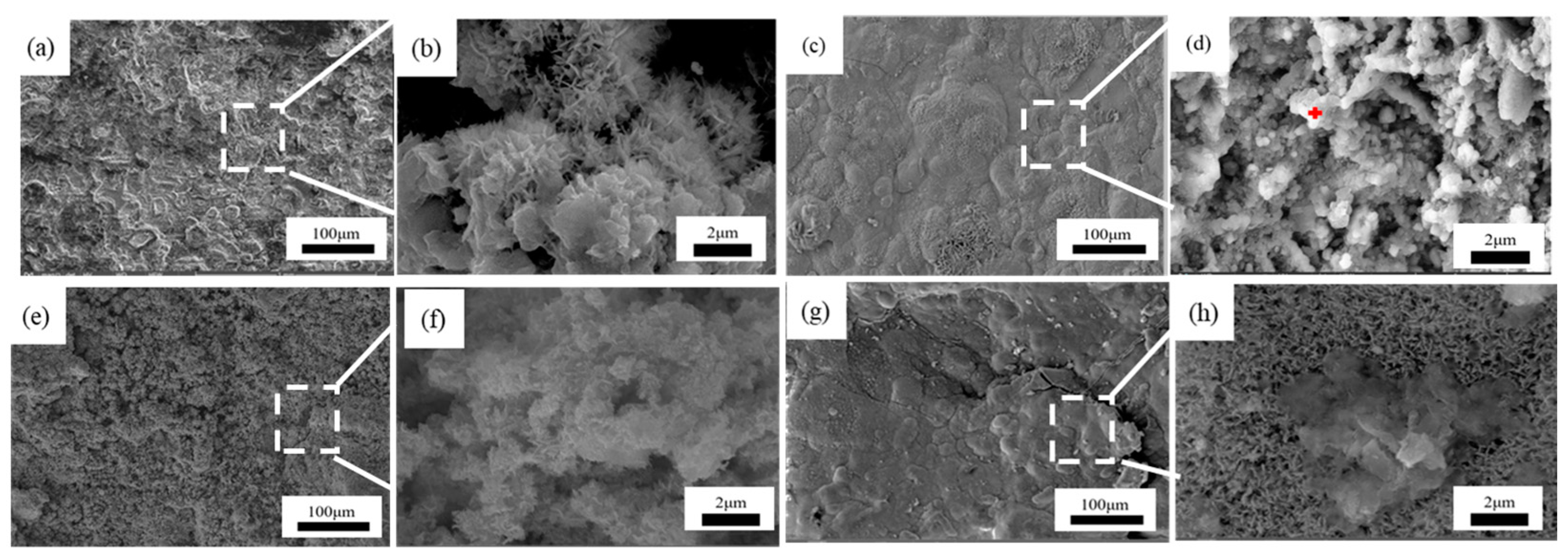
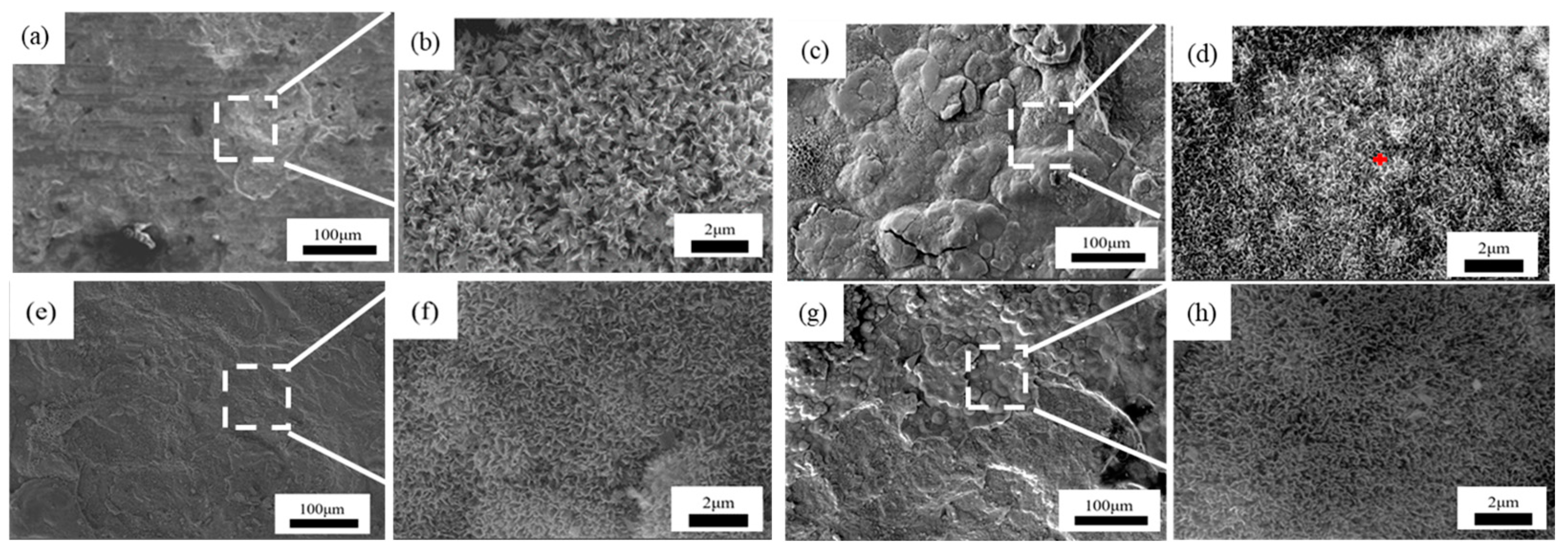
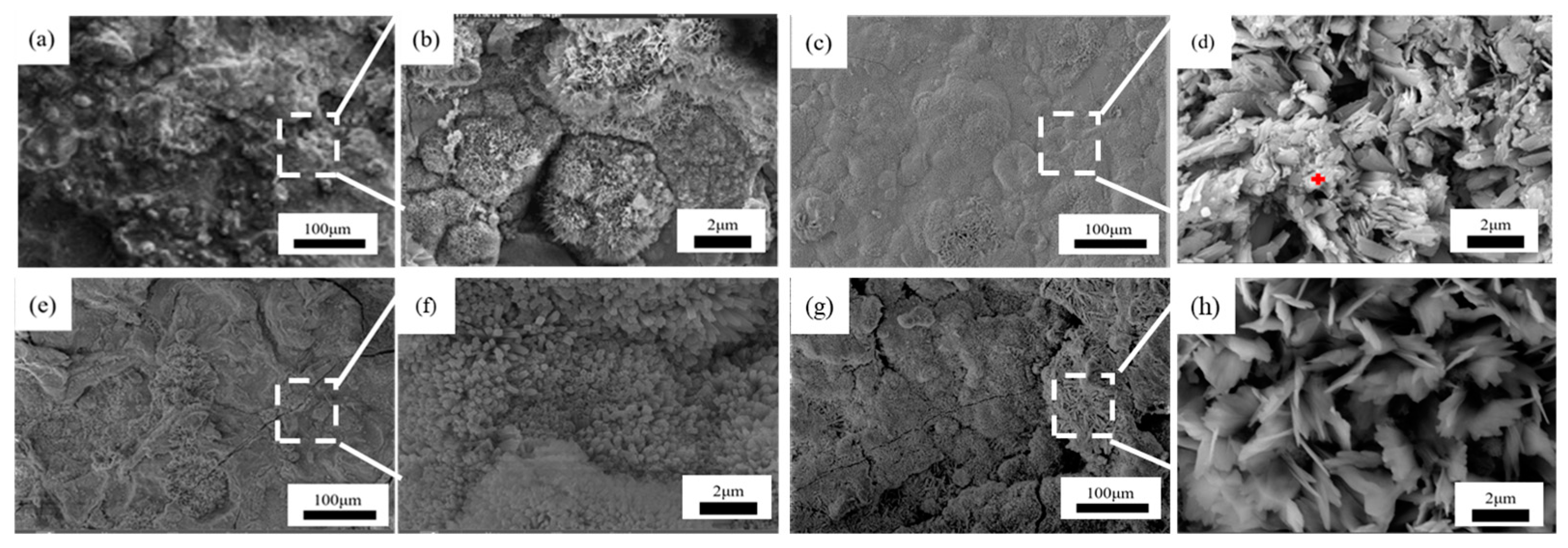
Figure 7, Figure 8 and Figure 9 showed the surface micromorphology of 1–3# steels after 0.5, 1, 1.5, and 2 years in the marine atmosphere zone. For 1# steel, needle-like corrosion products can be observed after 0.5 years in service. At 1 year of exposure, clustered corrosion products appear on the surface (Figure 7d). After 1.5 years of exposure, the entire surface shows a uniform distribution, and the high magnification micrograph reveals large areas of clustered corrosion products. After 2 years, the corrosion products begin to peel off from the surface, indicating delamination into an outer rust layer and an inner rust layer, with localized observations of needle-like and blocky corrosion products. For steel 2#, the surface morphology development trend is similar to that of steel 1#. However, the corrosion products on steel 3# are noticeably more compact and intact. After 2 years of exposure, delamination also occurs on the surface of steel 3#, but the surface is smoother compared to steel 1# and steel 2#. Upon closer examination, the corrosion products are mainly needle-like. Generally, finer corrosion products are beneficial to the protection of the rust layer. The results show that corrosion products on 3# steel containing more alloy elements have stronger corrosion resistance in an atmospheric environment.
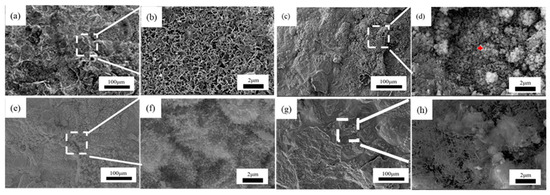
Figure 7.
Microscopic corrosion morphology of 1# steel in atmospheric zone at different times: (a,b) 0.5 years and its high magnification micrograph; (c,d) 1 year and its high magnification micrograph with the feature of clustered corrosion products (red plus sign); (e,f) 1.5 years and its high magnification micrograph; (g,h) 2 years and its high magnification micrograph.
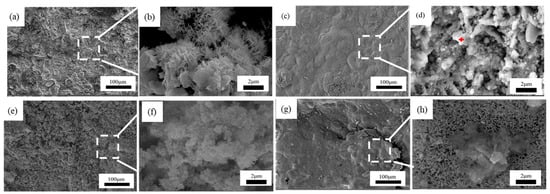
Figure 8.
Microscopic corrosion morphology of 2# steel in atmospheric zone at different times: (a,b) 0.5 years and its high magnification micrograph; (c,d) 1 year and its high magnification micrograph with the feature of rod clustered corrosion products (red plus sign); (e,f) 1.5 years and its high magnification micrograph; (g,h) 2 years and its high magnification micrograph.
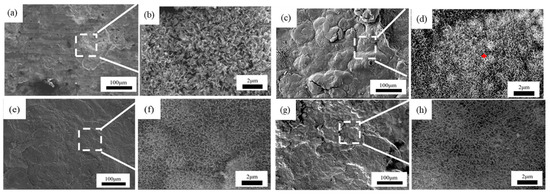
Figure 9.
Microscopic corrosion morphology of 3# steel in atmospheric zone at different times: (a,b) 0.5 years and its high magnification micrograph; (c,d) 1 year and its high magnification micrograph with the feature of needle-like corrosion products (red plus sign); (e,f) 1.5 years and its high magnification micrograph; (g,h) 2 years and its high magnification micrograph.
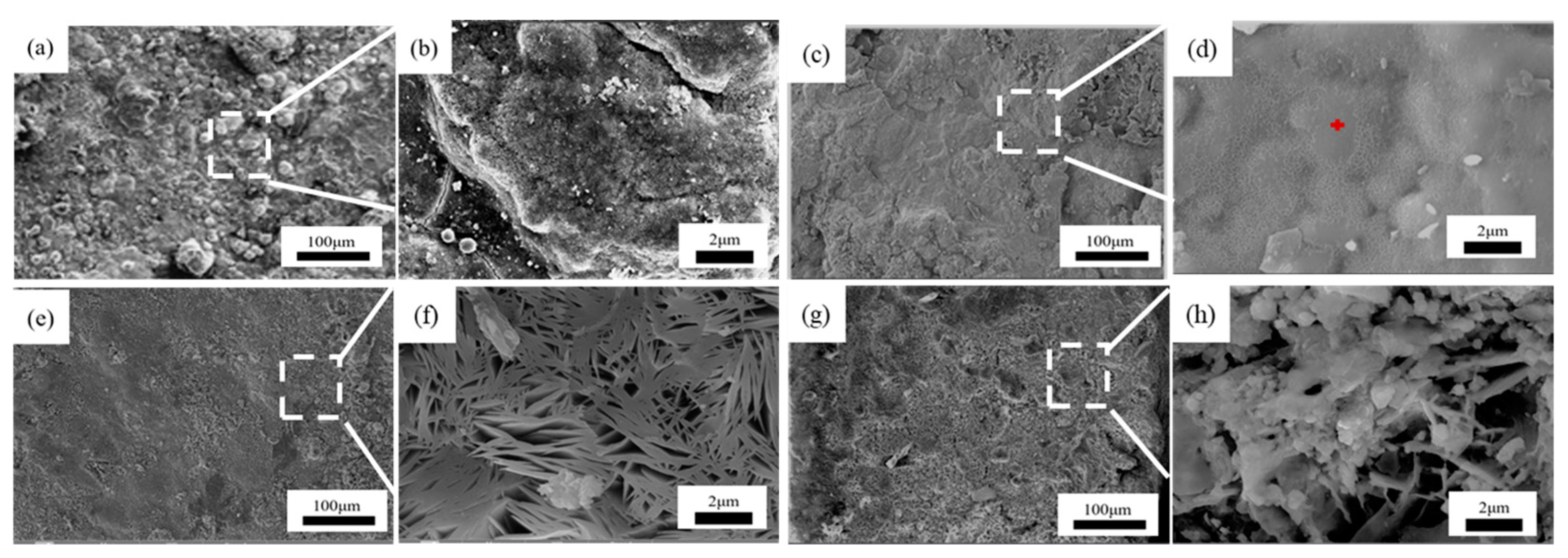
Figure 10, Figure 11 and Figure 12 present the surface micromorphology of 1–3# steels in the marine immersion zone. For steel 1#, after 0.5 years of immersion, a large amount of corrosion products could be observed on the surface, with blocky regions appearing under magnification. After 1 year, the surface was covered with a large area of spherical corrosion products and became rough. After 1.5 years, the surface became uneven and rough, with columnar corrosion products appearing in some areas. After 2 years of corrosion, many prominent rust blisters were found on the surface, and most areas formed columnar corrosion products. Dense columnar corrosion products could be observed under magnification. For steel 2#, after 2 years of corrosion, the surface showed significant cracking, with small corrosion products appearing in most areas. These corrosion products appeared as fine needles under magnification. For steel 3#, after 2 years of immersion, the surface of the corrosion products was uneven and rough with pits of varying degrees. Blocky and strip-like corrosion products were observed under magnification.
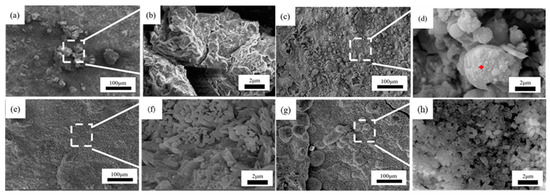
Figure 10.
Microscopic corrosion morphology of 1# steel in immersion zone at different times: (a,b) 0.5 years and its high magnification micrograph; (c,d) 1 year and its high magnification micrograph with the feature of spherical corrosion products (red plus sign); (e,f) 1.5 years and its high magnification micrograph; (g,h) 2 years and its high magnification micrograph.
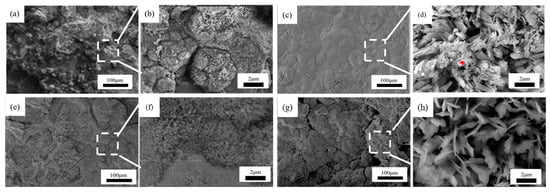
Figure 11.
Microscopic corrosion morphology of 2# steel in immersion zone at different times: (a,b) 0.5 years and its high magnification micrograph; (c,d) 1 year and its high magnification micrograph with the feature of fine needles corrosion products (red plus sign); (e,f) 1.5 years and its high magnification micrograph; (g,h) 2 years and its high magnification micrograph.

Figure 12.
Microscopic corrosion morphology of 3# steel in immersion zone at different times: (a,b) 0.5 years and its high magnification micrograph; (c,d) 1 year and its high magnification micrograph with the feature of blocky and strip-like corrosion products (red plus sign); (e,f) 1.5 years and its high magnification micrograph; (g,h) 2 years and its high magnification micrograph.
In summary, the corrosion extent of the three steels in the marine immersion zone is more severe than in the marine atmospheric zone. However, the corrosion degree of the three steels in the immersion marine zone cannot be entirely judged based on the microscopic morphology.
3.5. Composition of the Rust Layer
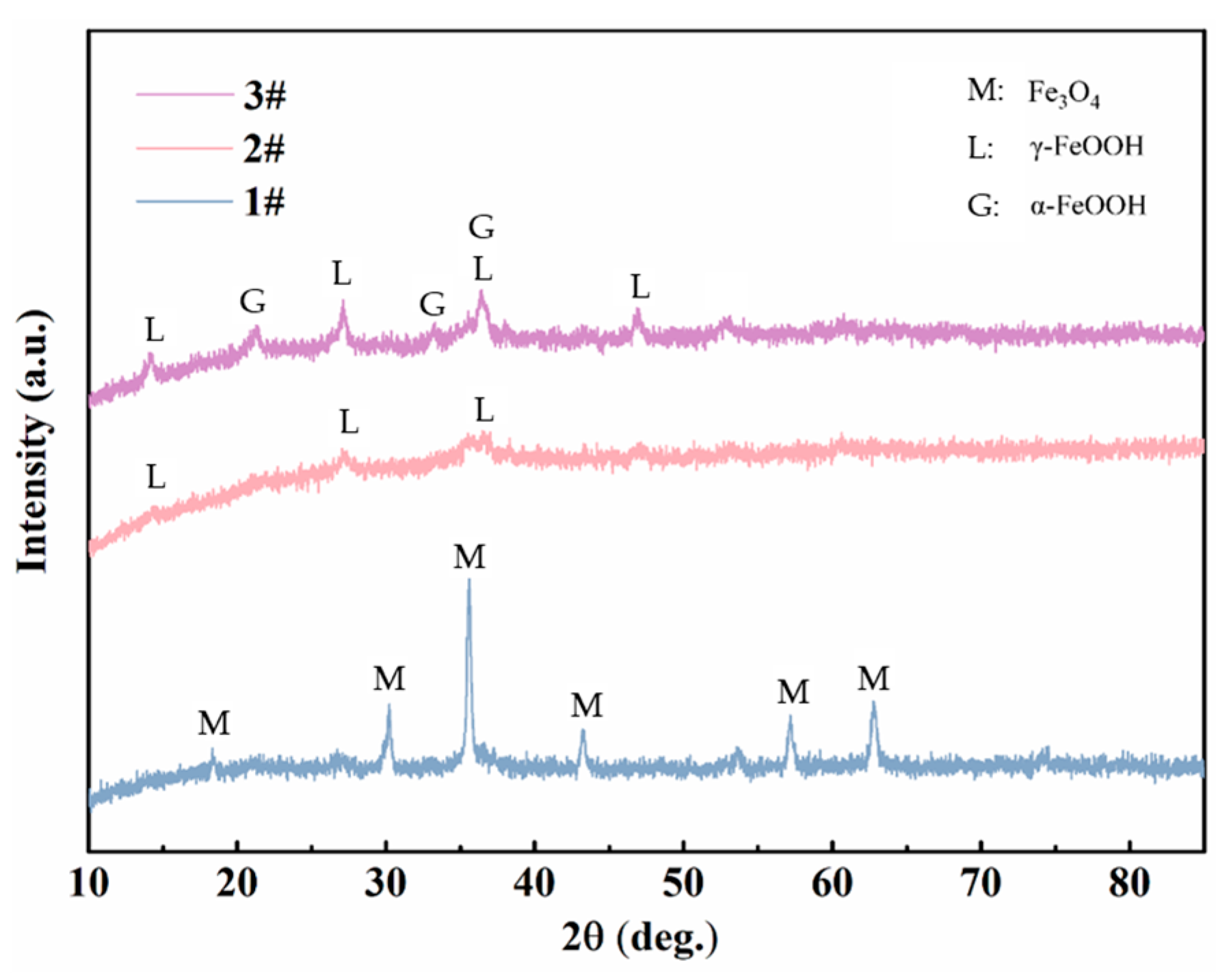
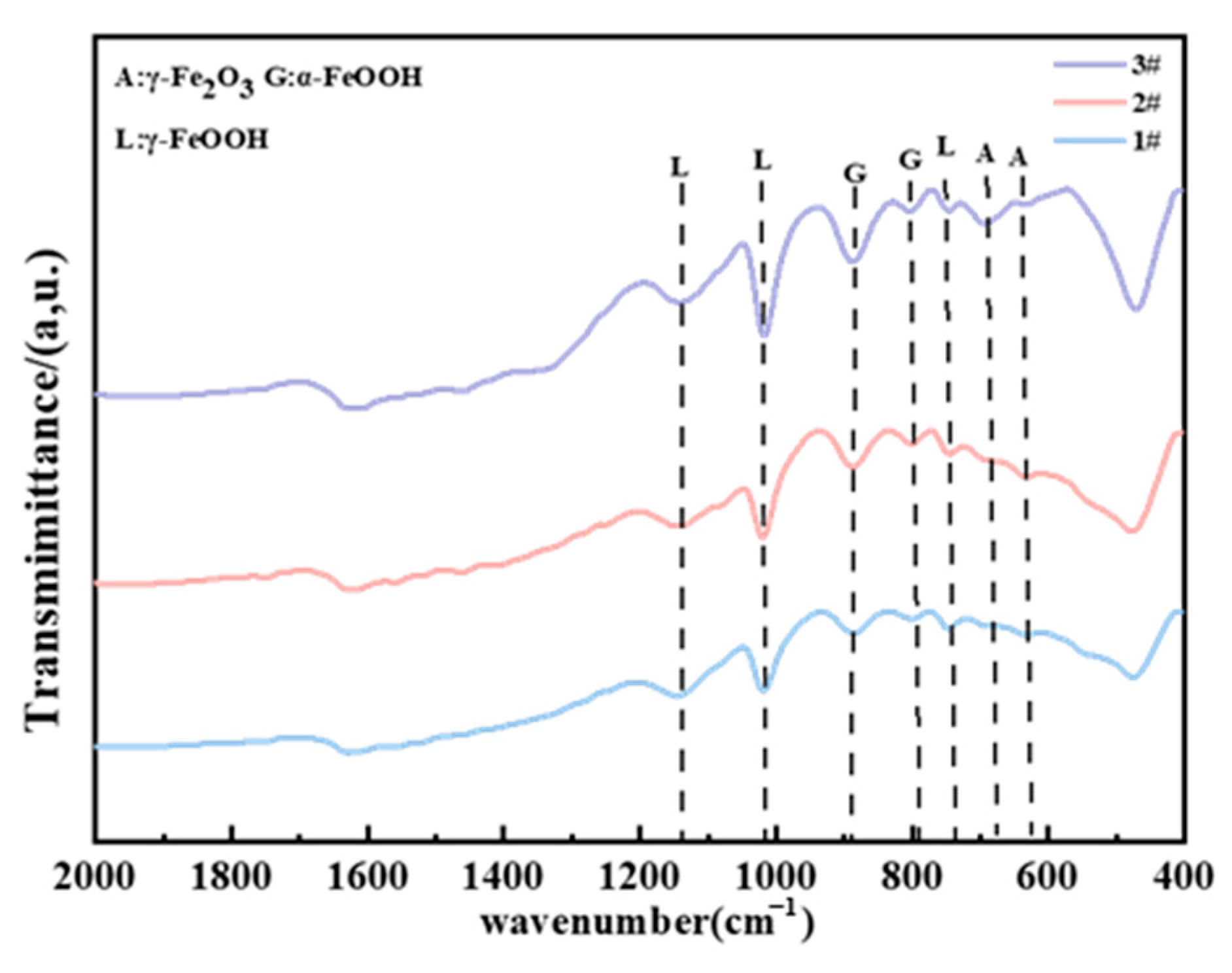
XRD and FTIR were utilized to analyze the composition of the rust layers. The XRD spectra of the corrosion products of three steels exposed to the marine atmospheric environment for 0.5 years are shown in Figure 13. It can be observed that, in the early stages of exposure, due to the limited amount of collectible corrosion products and the presence of amorphous phases, the phase results in the XRD spectra are not very distinct. Nevertheless, Fe3O4 was clearly detected in steel 1#, while the rust layer products of steel 2# mainly comprised γ-FeOOH. In steel 3#, both α-FeOOH and γ-FeOOH were present. It is generally believed that lepidocrocite (γ-FeOOH) tends to first be formed on the steel surface at the early stage of corrosion [16,23]. The formation of lepidocrocite occurs in low Cl⁻ concentration environments, where iron (Fe) first dissolves to form ferrous ions (Fe2⁺), which are subsequently oxidized and precipitated. As we know, γ-FeOOH has poor thermodynamic stability. According to the theory proposed by Misawa et al. [28], γ-FeOOH may gradually transition to α-FeOOH in a low Cl− concentration environment. α-FeOOH exhibits a high stability and almost has no further phase transformation [14,29]. Therefore, in the early stages of exposure, when the Cl− concentration is relatively low, the two types of weathering steels tend to form more compact and protective corrosion products, with both γ-FeOOH and α-FeOOH having small and similar molar volumes. In contrast, the Fe₃O₄ detected in steel 1# is likely transformed from the initial γ-FeOOH. The phase transformation from γ-FeOOH to Fe3O4 involves a large change in molar volume, expanding by 98.9% [13,30]. Consequently, the compactness of its corrosion product layer is relatively poor, which is why its corrosion rate exceeds that of the two weathering steels. In the FTIR results of different steels in the atmospheric zone after 2 years (Figure 14), Fe2O3 was detected in all three steels, which should be transformed from Fe₃O₄ through a further solid oxidation process [30]. The decrease in the intensity of the peaks associated with amorphous substances indicates that the corrosion products gradually stabilize over time.
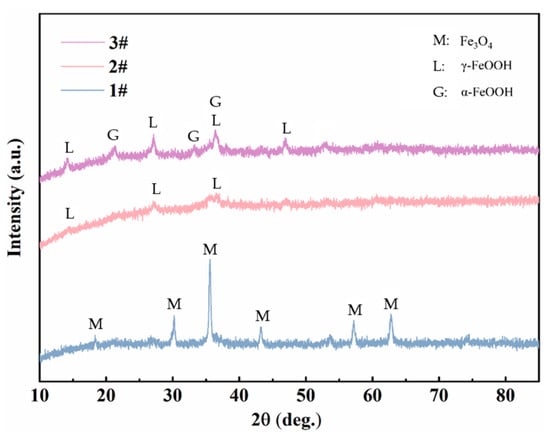
Figure 13.
XRD results of the corrosion products of steels exposed to the marine atmospheric environment for 0.5 years.
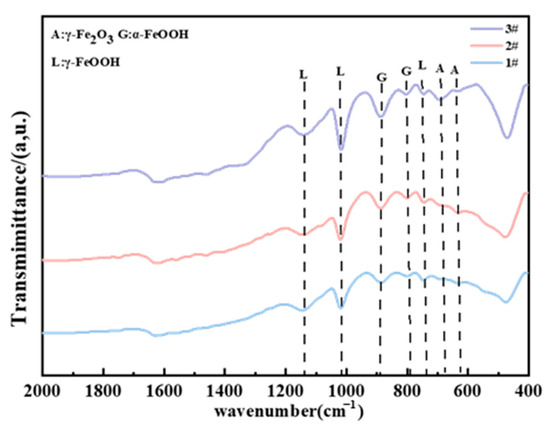
Figure 14.
FTIR results of different steels in the atmospheric zone after 2 years.
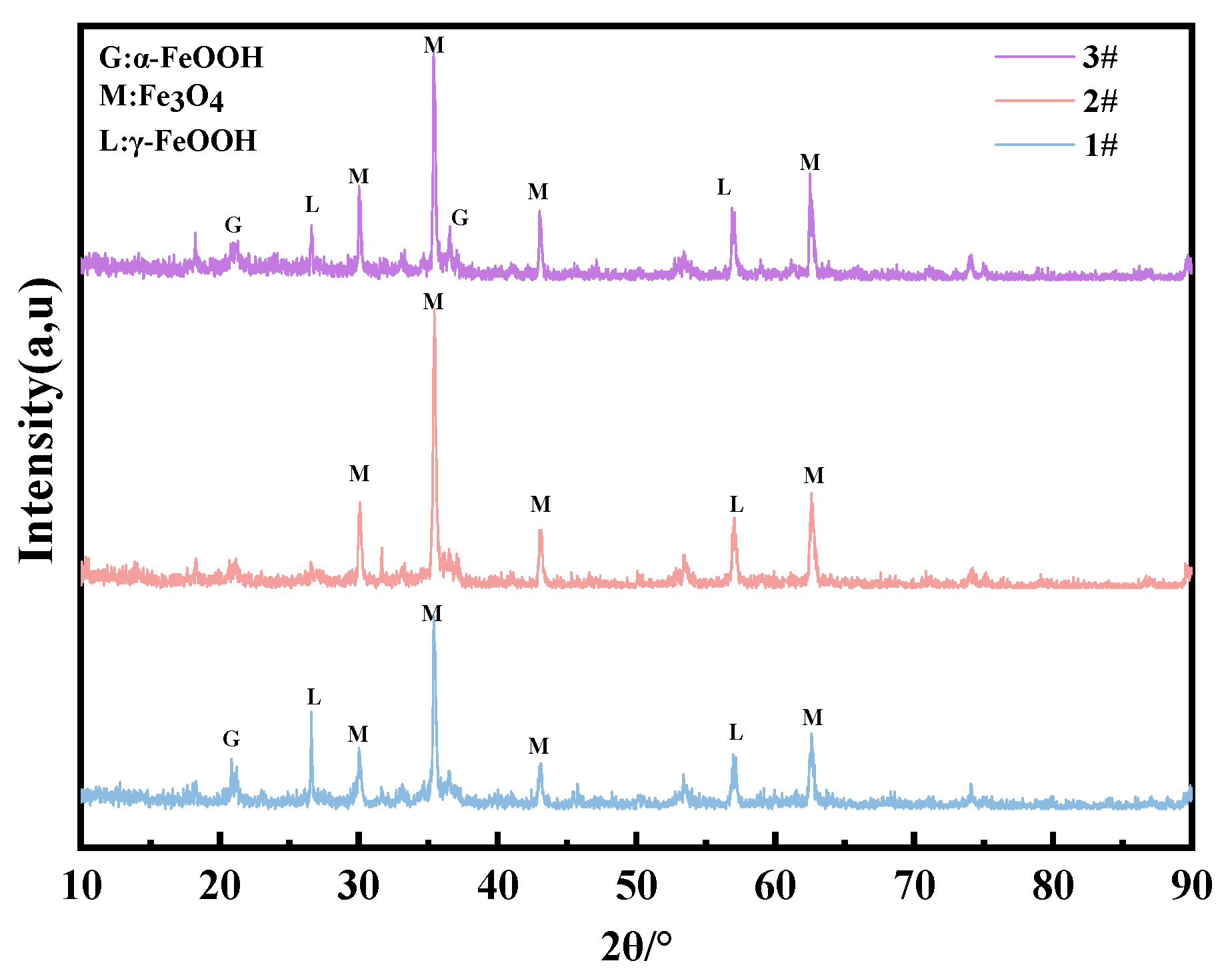
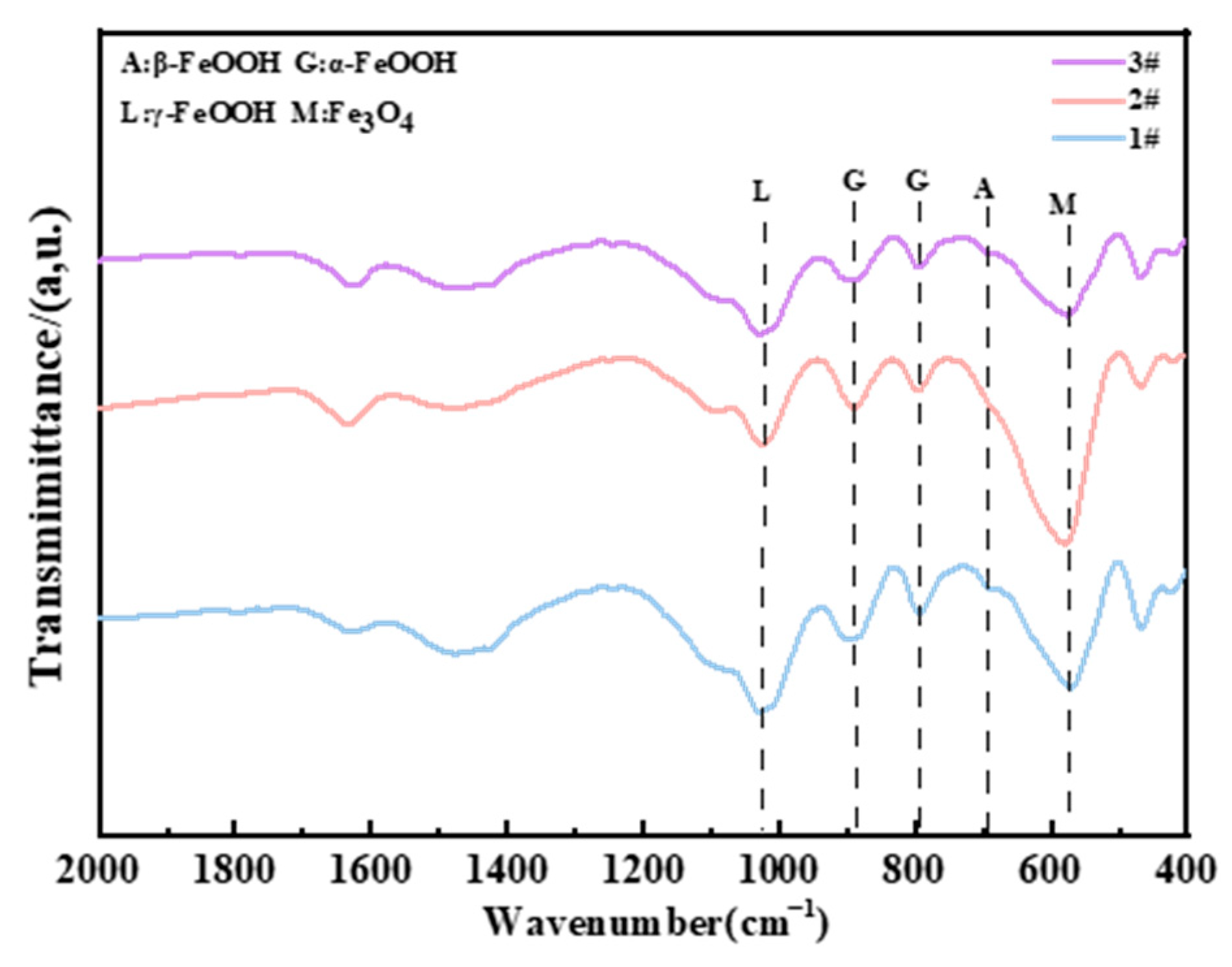
For the composition of the corrosion products in the immersion zone, Figure 15 shows that the main corrosion products of three steels are Fe3O4, γ-FeOOH, and α-FeOOH. γ-FeOOH tends to transform to Fe3O4 since the steels directly make contact with chlorinated seawater. The significant change in molar volume brought about by Fe3O4 prevents the formation of a compact corrosion product layer under immersion conditions (as shown in Figure 5b, Figure 10, Figure 11 and Figure 12), which explains why none of the steel types exhibit corrosion resistance in the immersion zone. Additionally, due to the lack of O2 in the immersion environment, Fe3O4 is detected in the infrared spectrum instead of Fe2O3 (Figure 16).
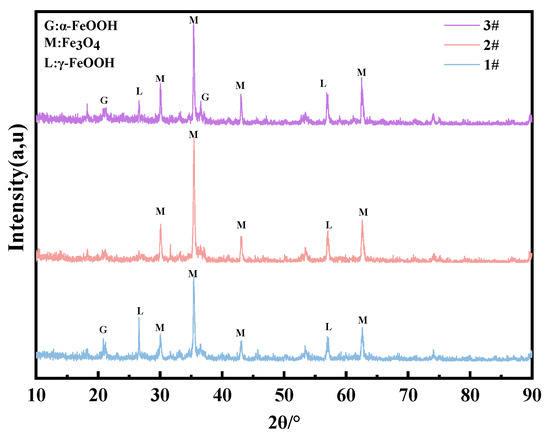
Figure 15.
XRD results of the corrosion products of steels immersed in the marine immersion zone for 0.5 years.
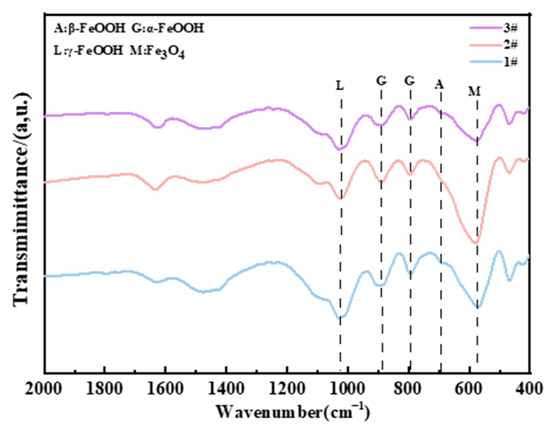
Figure 16.
FTIR results of different steels in the immersion zone after 2 years.
In summary, the corrosion analysis of the various steels is as follows. Due to the ‘autocatalytic effect’ of Cl− ions under tropical marine atmospheric conditions [31,32], the corrosion of the steel substrate progresses rapidly in local areas, leading to the formation of a significant amount of γ-FeOOH. Steel 1#, a conventional low-alloy high-strength steel, lacks any key alloying elements, resulting in Fe3O4 being the predominant corrosion product. It can be anticipated that the initial formation and evolution of a large amount of Fe3O4 occurs within the corrosion product layer, ultimately promoting the cracking and disintegration of the rust layer’s overall structure; hence, it does not exhibit good corrosion resistance. For steel 2#, the added alloying elements Cr, Ni, and Cu form their respective oxides, which facilitate the heterogeneous nucleation of rust during aggregation, promoting the densification of the corrosion product layer and thereby providing better corrosion resistance [13,33]. Compared with ordinary weathering steels, steel 3# substantially increases the additions of Ni and introduces a small amount of Mo. Beyond the electronegative spinel oxides (NiFe2O4) and Mo oxides further promoting rust nucleation and enhancing the compactness of the protective layer, it is reported that high Ni content enhances the exhalation effect of Cl− ions from the corrosion product layer [1,2,13,23], making the protective layer more compact and less prone to cracking. However, in an immersion environment, the extensive erosion by chloride ions renders these benefits ineffective, with the corrosion process becoming fundamentally indistinguishable from that of other steels; hence, none exhibit good corrosion resistance.
Based on the analysis above, the potential corrosion mitigation strategies can be summarized in the following two points. The addition of Cr, Ni, Cu, and Mo can significantly enhance the corrosion resistance of weathering steels in tropical marine environments. Consequently, in practical applications, further optimization and customization of the alloy’s composition, particularly the ratios of Ni and Mo, can effectively improve its protective performance. In addition, in severe corrosive environments, even weathering steels may require additional protective measures. The application of high-performance anti-corrosion coatings, such as polymer coatings, ceramic coatings, or metal coatings, can significantly slow down the corrosion rate.
4. Conclusions
- A high Cl− concentration in the tropical marine atmospheric environment promotes the corrosion of the steel substrate due to the autocatalytic effect. γ-FeOOH and Fe3O4 occur within the corrosion product layer, ultimately promoting the cracking of the rust layer. Weathering steels facilitate the densification of the corrosion product layer due to the addition of alloying elements Cr, Ni, and Cu, thereby providing better corrosion resistance.
- In the marine immersion environment, metal dissolution intensifies at a high Cl− concentration due to its strong depolarization effect, resulting in local defects and corrosion pits. The corrosion products are loose and mixed. The corrosion process of steel 3# becomes fundamentally indistinguishable from that of other steels; hence, none exhibit good corrosion resistance.
- Steel 3# optimizes the alloying elements Ni, Cu, and Mo. The addition of Ni forms electronegative spinel oxides (NiFe2O4), further promoting rust nucleation and enhancing the compactness of the protective layer in the tropical marine atmospheric environment.
Author Contributions
Conceptualization, Y.Y. and T.L.; methodology, M.S. and G.W.; validation, T.L., Y.Y. and G.W.; investigation, F.M.; resources, Y.Y. and Y.W.; writing—original draft preparation, Y.W. and M.S.; writing—review and editing, F.M.; visualization, F.W.; supervision, F.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number U20A20233.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
Authors Ying Yang, Tianzi Lin, Yubo Wang were employed by the company Anshan Iron and Steel Group. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Zhang, B.; Liu, W.; Sun, Y.; Yang, W.; Chen, L.; Xie, J.; Li, W. Corrosion behavior of the 3 wt.% Ni weathering steel with replacing 1 wt.% Cr in the simulated tropical marine atmospheric environment. J. Phys. Chem. Solids 2023, 175, 111221. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, W.; Sun, Z.; Li, H.; Zhang, B.; Yang, W.; Chen, L.; Dong, B.; Zhang, T. Optimizing microstructure for enhanced atmospheric corrosion resistance of a novel Ni–Mo weathering steel: A field exposure study in Thailand’s tropical marine environment. Vacuum 2024, 221, 112876. [Google Scholar] [CrossRef]
- Melchers, R.E. A new interpretation of the corrosion loss processes for weathering steels in marine atmospheres. Corros. Sci. 2008, 50, 3446–3454. [Google Scholar] [CrossRef]
- Jia, J.H.; Wu, W.; Cheng, X.Q.; Zhao, J.B. Ni-advanced weathering steels in Maldives for two years: Corrosion results of tropical marine field test. Constr. Build. Mater. 2020, 245, 118463. [Google Scholar] [CrossRef]
- Yue, L.J.; Meng, Y.S.; Han, J.S.; Xie, K.; Sun, Y.P.; Tan, Y.L. Pitting corrosion behavior of Cu-P-RE weathering steels. J. Rare Earths 2023, 41, 321–330. [Google Scholar] [CrossRef]
- Melchers, R.E. Long-term corrosion of steels exposed to marine environments. Eur. J. Environ. Civ. Eng. 2009, 13, 527–546. [Google Scholar] [CrossRef]
- Zhou, M.; Liao, J.C.; An, L. Effect of Multiple Environmental Factors on the Adhesion and Diffusion Behaviors of Chlorides in a Bridge with Coastal Exposure: Long-Term Experimental Study. J. Bridge Eng. 2020, 25, 04020081. [Google Scholar] [CrossRef]
- Artigas, A.; Monsalve, A.; Sipos, K.; Bustos, O.; Mena, J.; Seco, R.; Garza-Montes-de-Oca, N. Development of accelerated wet–dry cycle corrosion test in marine environment for weathering steels. Corros. Eng. Sci. Technol. 2015, 50, 628–632. [Google Scholar] [CrossRef]
- Morcillo, M.; Alcántara, J.; Díaz, I.; Chico, B.; Simancas, J.; De la Fuente, D. Marine atmospheric corrosion of carbon steels. Rev. Metal. 2015, 51, e045. [Google Scholar] [CrossRef]
- Díaz, I.; Cano, H.; Lopesino, P.; de la Fuente, D.; Chico, B.; Jiménez, J.A.; Medina, S.F.; Morcillo, M. Five-year atmospheric corrosion of Cu, Cr and Ni weathering steels in a wide range of environments. Corros. Sci. 2018, 141, 146–157. [Google Scholar] [CrossRef]
- Wu, W.; Cheng, X.; Zhao, J.; Li, X. Benefit of the corrosion product film formed on a new weathering steel containing 3% nickel under marine atmosphere in Maldives. Corros. Sci. 2020, 165, 108416. [Google Scholar] [CrossRef]
- Zhang, T.Y.; Liu, W.; Chen, L.J.; Dong, B.J.; Yang, W.J.; Fan, Y.M.; Zhao, Y.G. On how the corrosion behavior and the functions of Cu, Ni and Mo of the weathering steel in environments with different NaCl concentrations. Corros. Sci. 2021, 192, 109851. [Google Scholar] [CrossRef]
- Hu, Q.; Yang, S.; Zhang, W.; Da, G.; Xu, X.; Wang, X. Corrosion failure analysis of engineering structural steels in tropical marine atmospheres: A comparative study of ordinary and new weathering steels. Eng. Fail. Anal. 2024, 156, 107830. [Google Scholar] [CrossRef]
- Morcillo, M.; Chico, B.; de la Fuente, D.; Aicantara, J.; Wallinder, I.O.; Leygraf, C. On the Mechanism of Rust Exfoliation in Marine Environments. J. Electrochem. Soc. 2017, 164, C8–C16. [Google Scholar] [CrossRef]
- Tamura, H. The role of rusts in corrosion and corrosion protection of iron and steel. Corros. Sci. 2008, 50, 1872–1883. [Google Scholar] [CrossRef]
- Morcillo, M.; Díaz, I.; Chico, B.; Cano, H.; de la Fuente, D. Weathering steels: From empirical development to scientific design. A review. Corros. Sci. 2014, 83, 6–31. [Google Scholar] [CrossRef]
- Morcillo, M.; Díaz, I.; Cano, H.; Chico, B.; de la Fuente, D. Atmospheric corrosion of weathering steels. Overview for engineers. Part II: Testing, inspection, maintenance. Constr. Build. Mater. 2019, 222, 750–765. [Google Scholar] [CrossRef]
- Feng, J.; Mikihito, H.; Kazuki, O.; Jia, L. An analysis on spatial properties of corroded surface of weathering steel by different environmental conditions. Corros. Eng. Sci. Technol. 2023, 58, 577–587. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Y.; Wan, H.X.; Deng, J.H.; Li, W.H.; Liu, F.Q. Actual Xisha marine atmospheric corrosion behavior of 30CrMnSiA steel in different parts of the aircraft. Eng. Fail. Anal. 2023, 154, 107684. [Google Scholar] [CrossRef]
- Miura, S.; Murase, M.; Okamoto, T.; Tung, D.D.; Iwasaki, E. Corrosion Behavior and Applicability of Weathering Steel in Vietnam. J. Mater. Civ. Eng. 2017, 29, 04016264. [Google Scholar]
- Vuong, V.D.; Nguyen, D.B.; Pham, N.T.; Tran, A.T.; Kawai, M.; Vu, A.Q.; Thao, N.D.; Le, T.V. Five-Year Field Exposure for Visualized Corrosion of STK400 Graded Steel Pile in Brackish Environment of Phu My Industrial Port (Southern Vietnam). J. Mater. Eng. Perform. 2022, 31, 2801–2809. [Google Scholar] [CrossRef]
- Jaén, J.A.; Guzmán, K.; Iglesias, J.; Manrique, G.C. Ten years outdoor exposure of steel in an urban and coastal tropical atmosphere. Corros. Eng. Sci. Technol. 2021, 56, 522–529. [Google Scholar] [CrossRef]
- Cano, H.; Neff, D.; Morcillo, M.; Dillmann, P.; Diaz, I.; de la Fuente, D. Characterization of corrosion products formed on Ni 2.4 wt%–Cu 0.5 wt%–Cr 0.5 wt% weathering steel exposed in marine atmospheres. Corros. Sci. 2014, 87, 438–451. [Google Scholar] [CrossRef]
- Wu, W.; Dai, Z.; Liu, Z.; Liu, C.; Li, X. Synergy of Cu and Sb to enhance the resistance of 3%Ni weathering steel to marine atmospheric corrosion. Corros. Sci. 2021, 183, 109353. [Google Scholar] [CrossRef]
- Nam, N.D.; Kim, M.J.; Jang, Y.W.; Kim, J.G. Effect of tin on the corrosion behavior of low-alloy steel in an acid chloride solution. Corros. Sci. 2010, 52, 14–20. [Google Scholar] [CrossRef]
- Sun, M.H.; Du, C.W.; Liu, Z.Y.; Liu, C.; Li, X.G.; Wu, Y.M. Fundamental understanding on the effect of Cr on corrosion resistance of weathering steel in simulated tropical marine atmosphere. Corros. Sci. 2021, 186, 109427. [Google Scholar] [CrossRef]
- Assumpção, R.F.; Silva, A.P.; Lins, V.F.C.; Sicupira, D.C. Corrosion Performance of New-Type Si-Based Weathering Steel in Marine Environment. J. Mater. Eng. Perform. 2022, 32, 8541–8548. [Google Scholar] [CrossRef]
- Misawa, T.; Hashimoto, K.; Shimodaira, S. The mechanism of formation of iron oxide and oxyhydroxides in aqueous solutions at room temperature. Corros. Sci. 1974, 14, 131–149. [Google Scholar] [CrossRef]
- Lair, V.; Antony, H.; Legrand, L.; Chaussé, A. Electrochemical reduction of ferric corrosion products and evaluation of galvanic coupling with iron. Corros. Sci. 2006, 48, 2050–2063. [Google Scholar] [CrossRef]
- Tanaka, H.; Mishima, R.; Hatanaka, N.; Ishikawa, T.; Nakayama, T. Formation of magnetite rust particles by reacting iron powder with artificial α-, β- and γ-FeOOH in aqueous media. Corros. Sci. 2014, 78, 384–387. [Google Scholar] [CrossRef]
- Martin, U.; Bastidas, D.M. Stress corrosion cracking failure analysis of AISI 1018 carbon steel reinforcing bars in carbonated and chloride contaminated environment. Eng. Fail. Anal. 2023, 147, 107159. [Google Scholar] [CrossRef]
- Alcántara, J.; Fuente, D.d.l.; Chico, B.; Simancas, J.; Díaz, I.; Morcillo, M. Marine Atmospheric Corrosion of Carbon Steel: A Review. Materials 2017, 10, 406. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Cheng, X.; Hou, H.; Liu, B.; Li, X. Insight into the product film formed on Ni-advanced weathering steel in a tropical marine atmosphere. Appl. Surf. Sci. 2018, 436, 80–89. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).