An Efficient and Stable MXene-Immobilized, Cobalt-Based Catalyst for Hydrogen Evolution Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
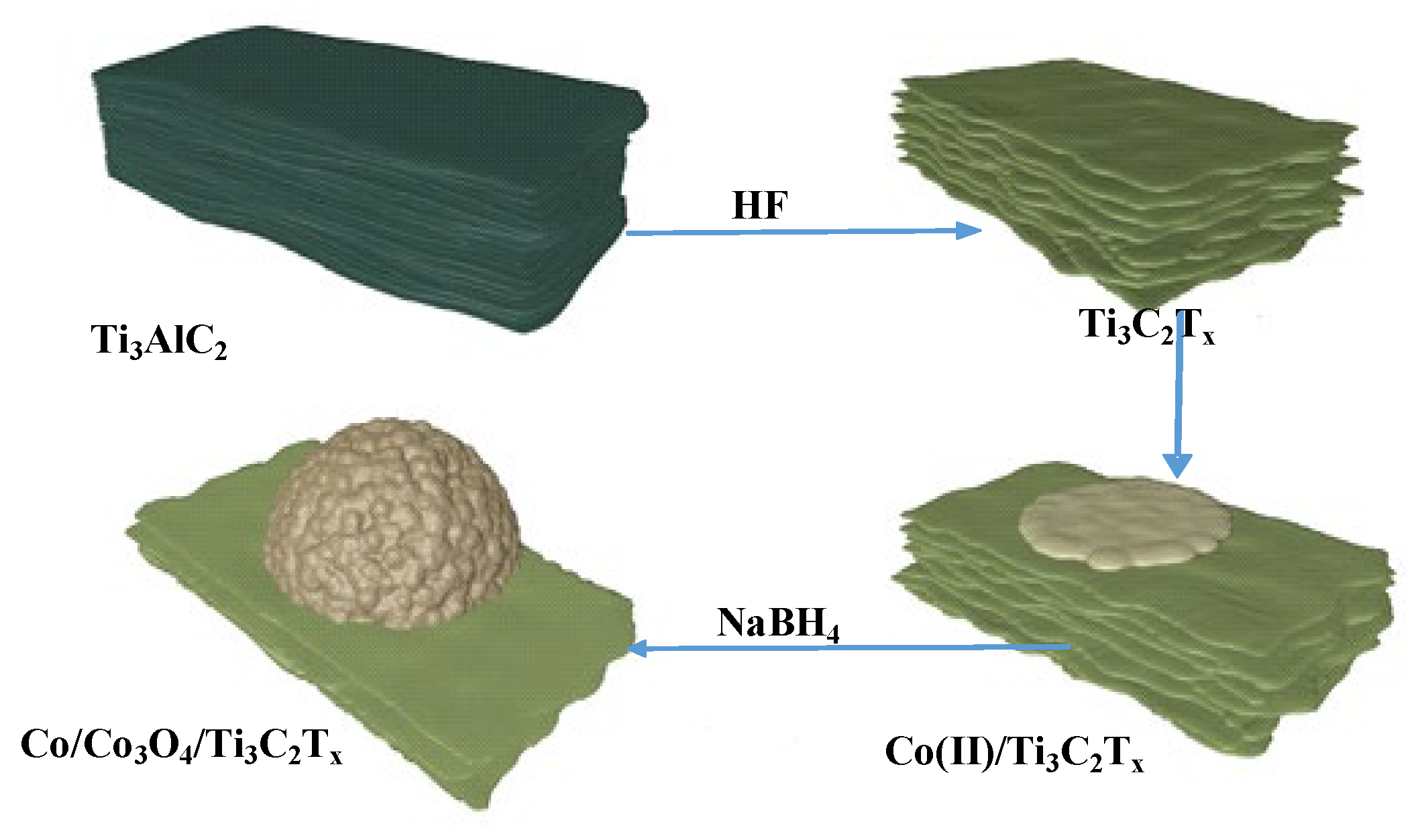
2.2. Synthesis of Co/Co3O4/Ti3C2Tx Catalyst
2.3. Catalyst Characterization
2.4. Electrocatalytic Hydrogen Evolution Test of Catalyst
3. Results
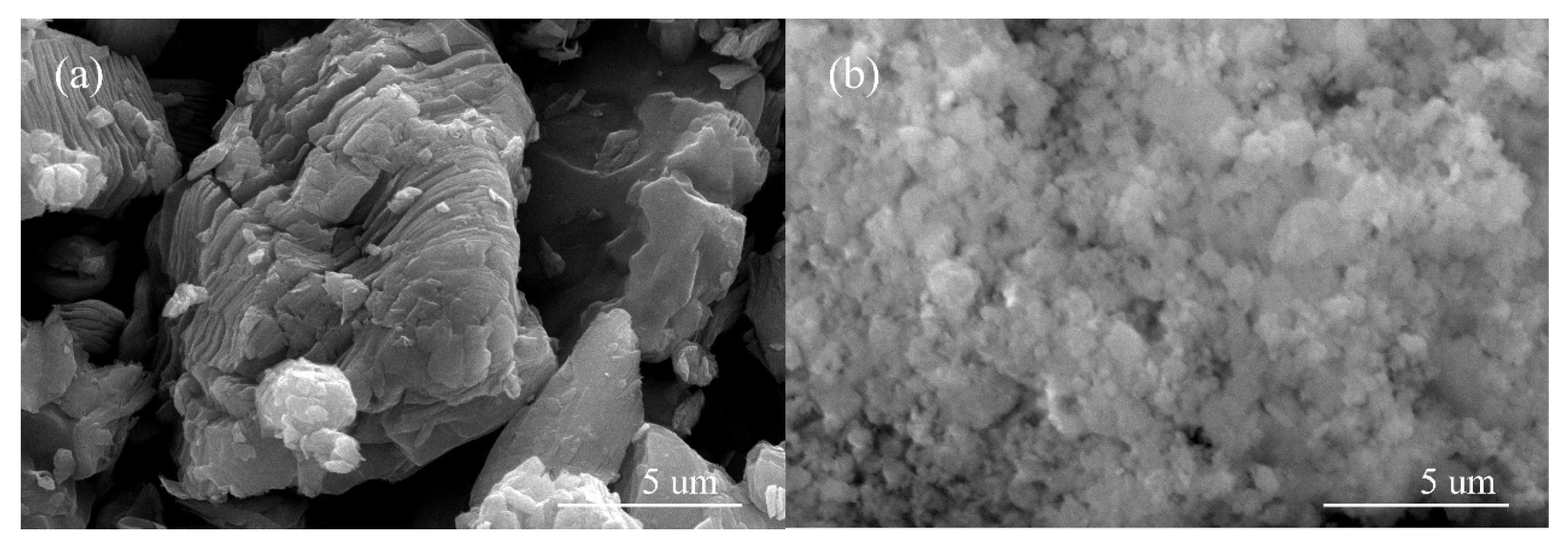
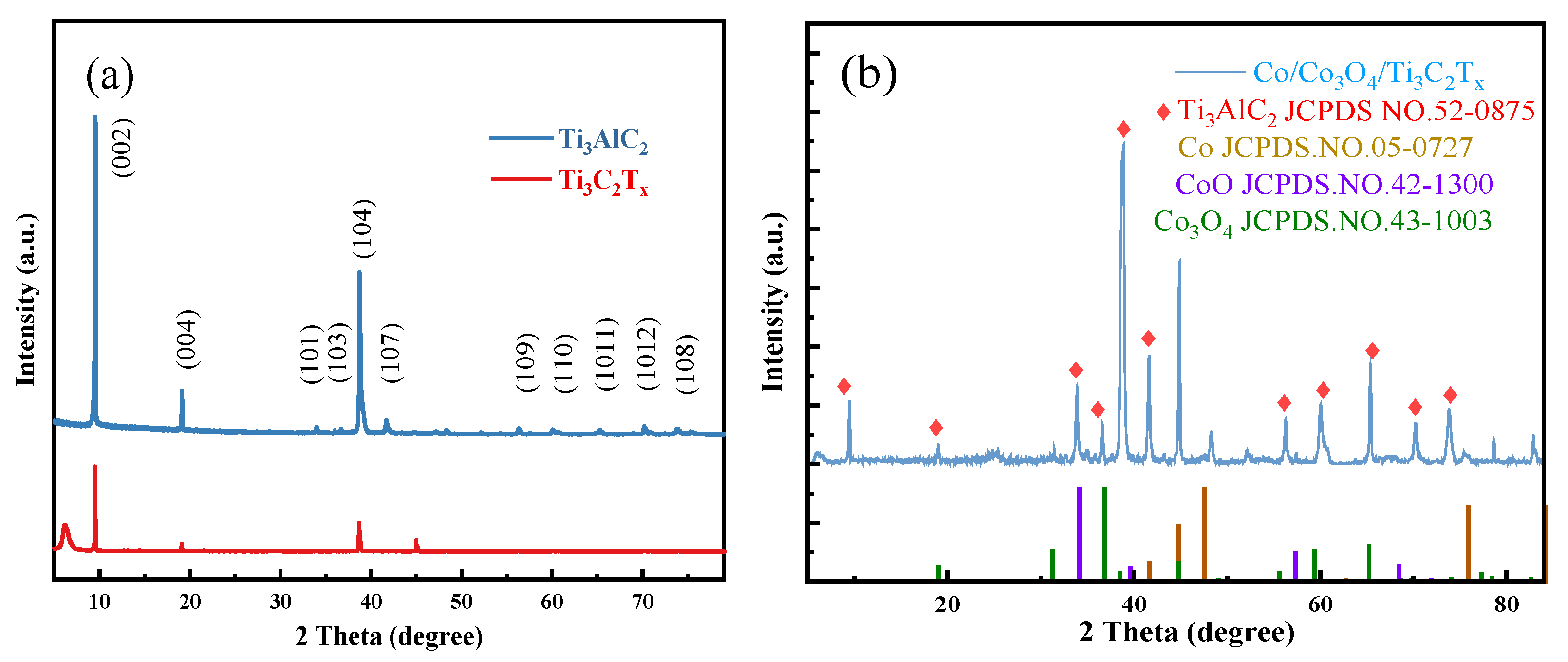
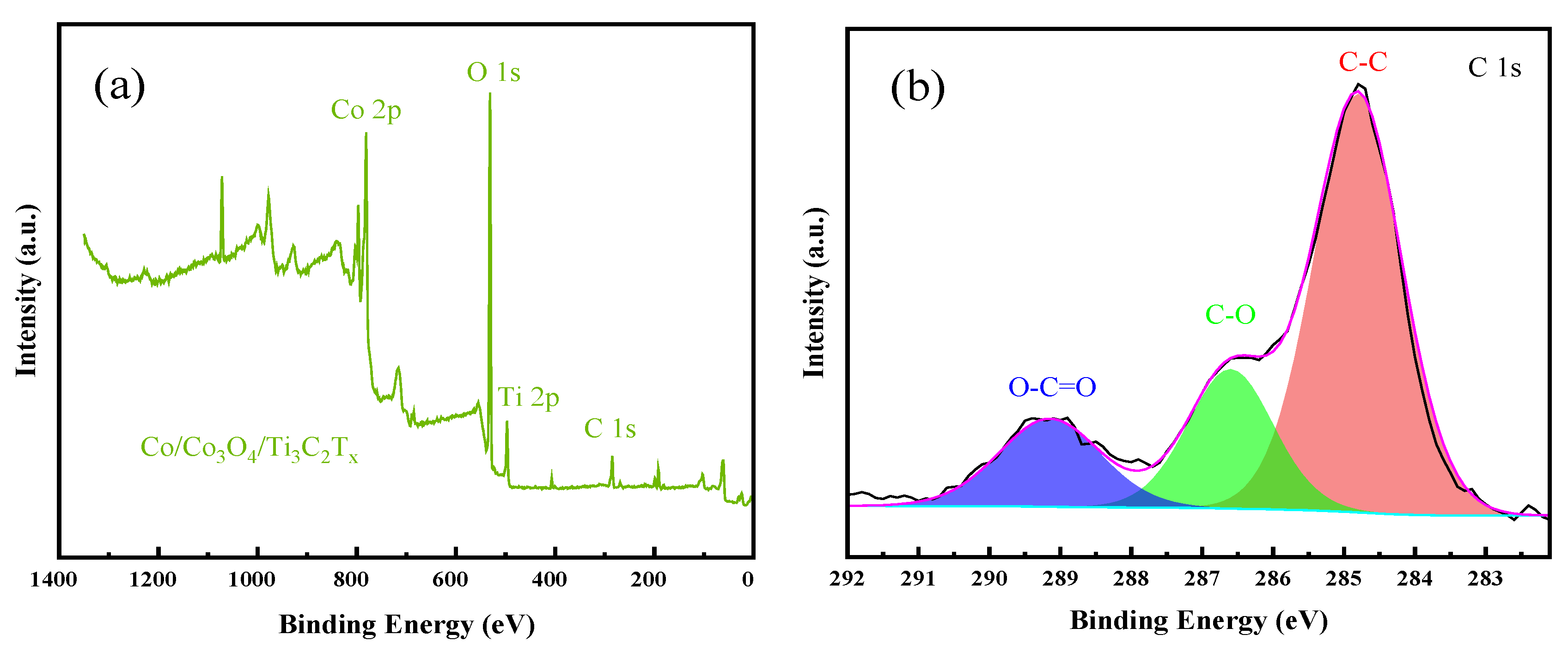
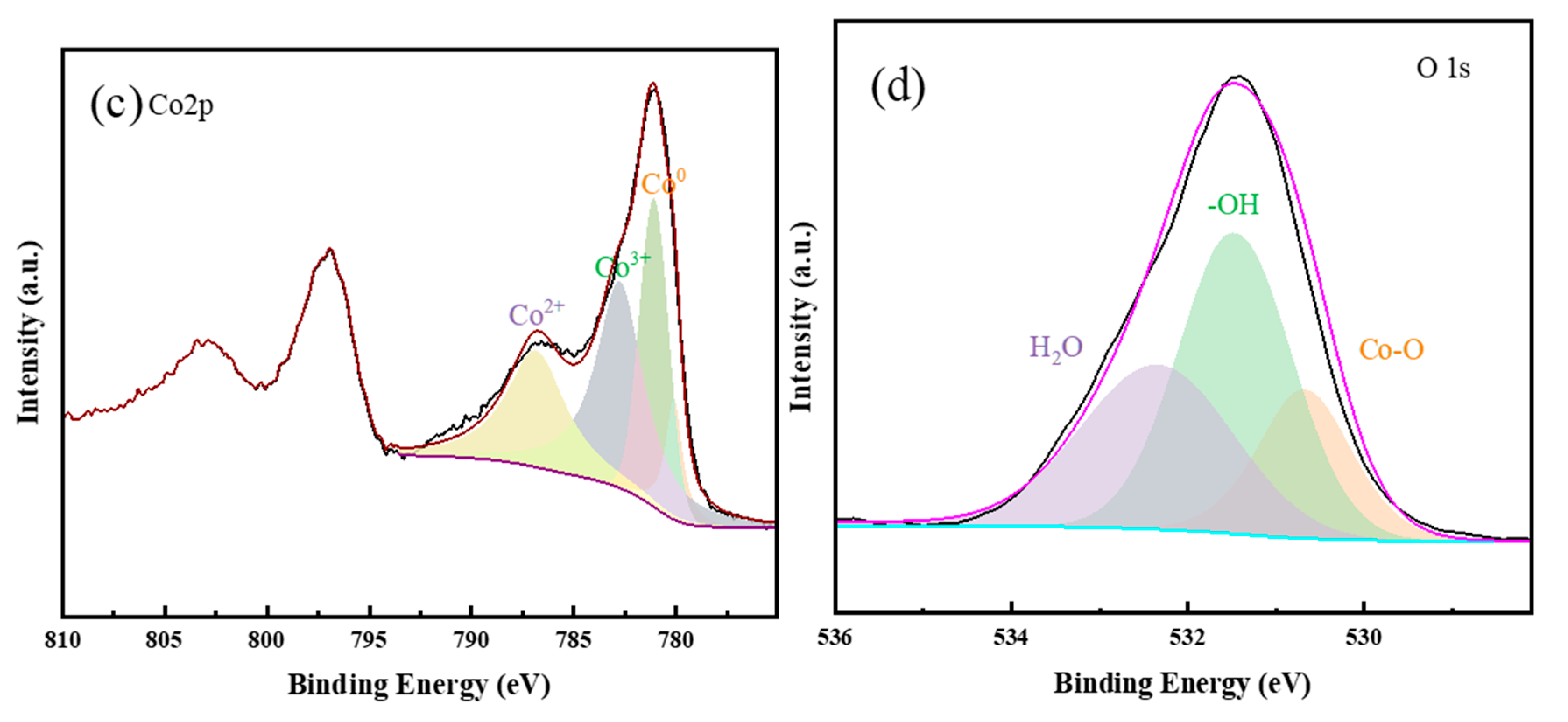
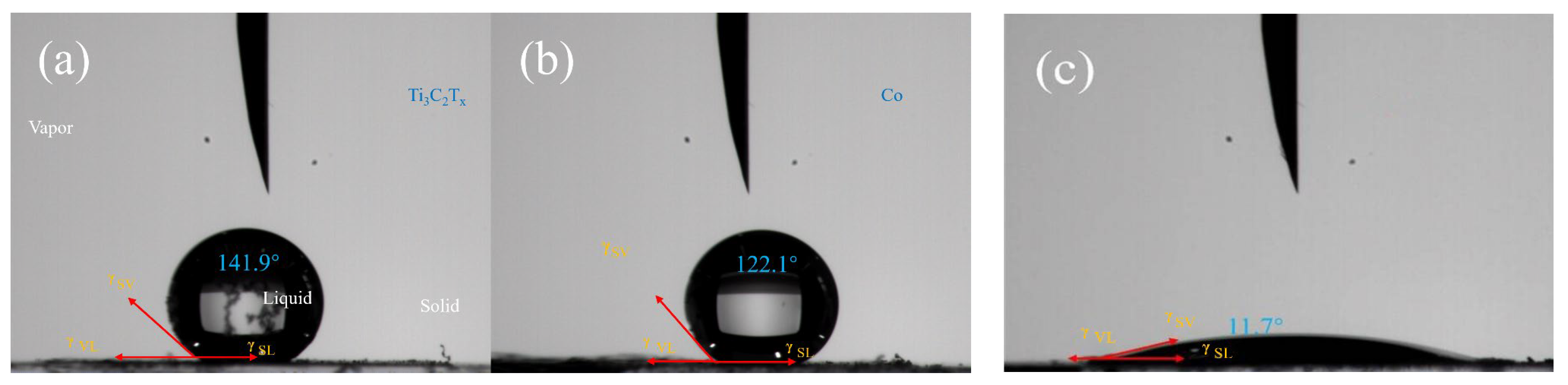
3.1. Characterization of Catalyst
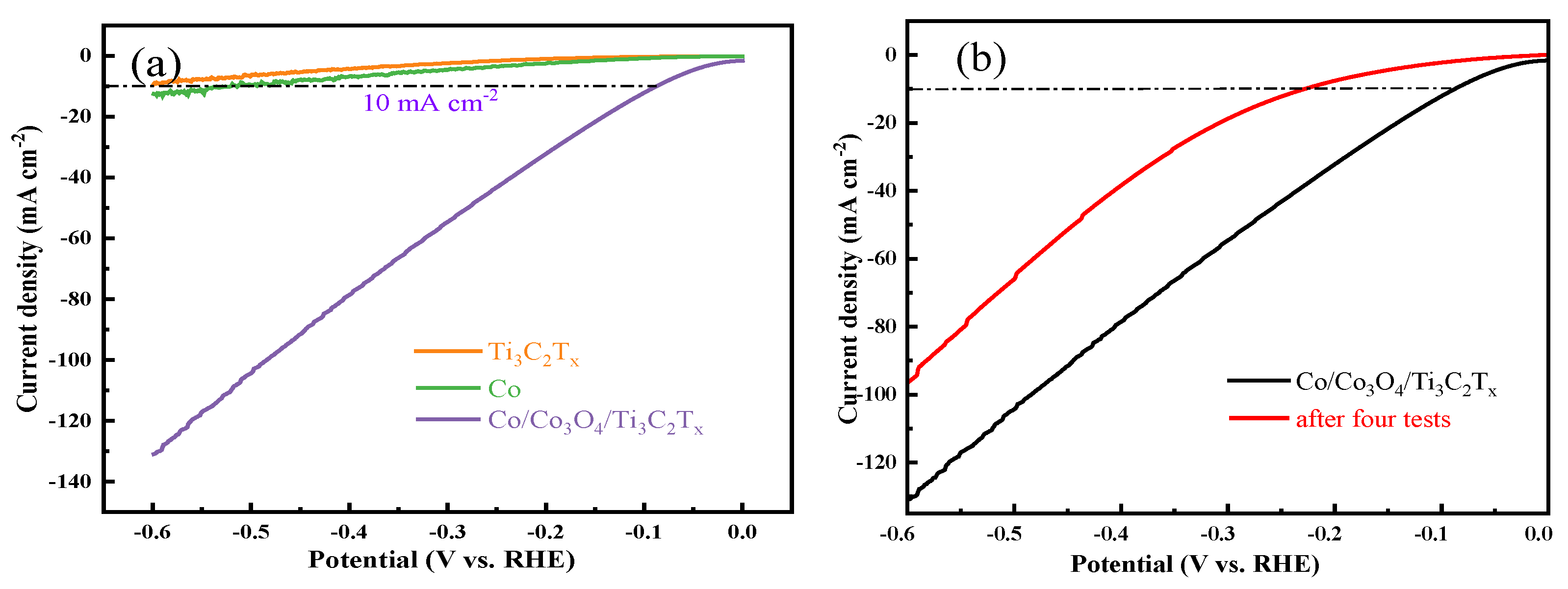
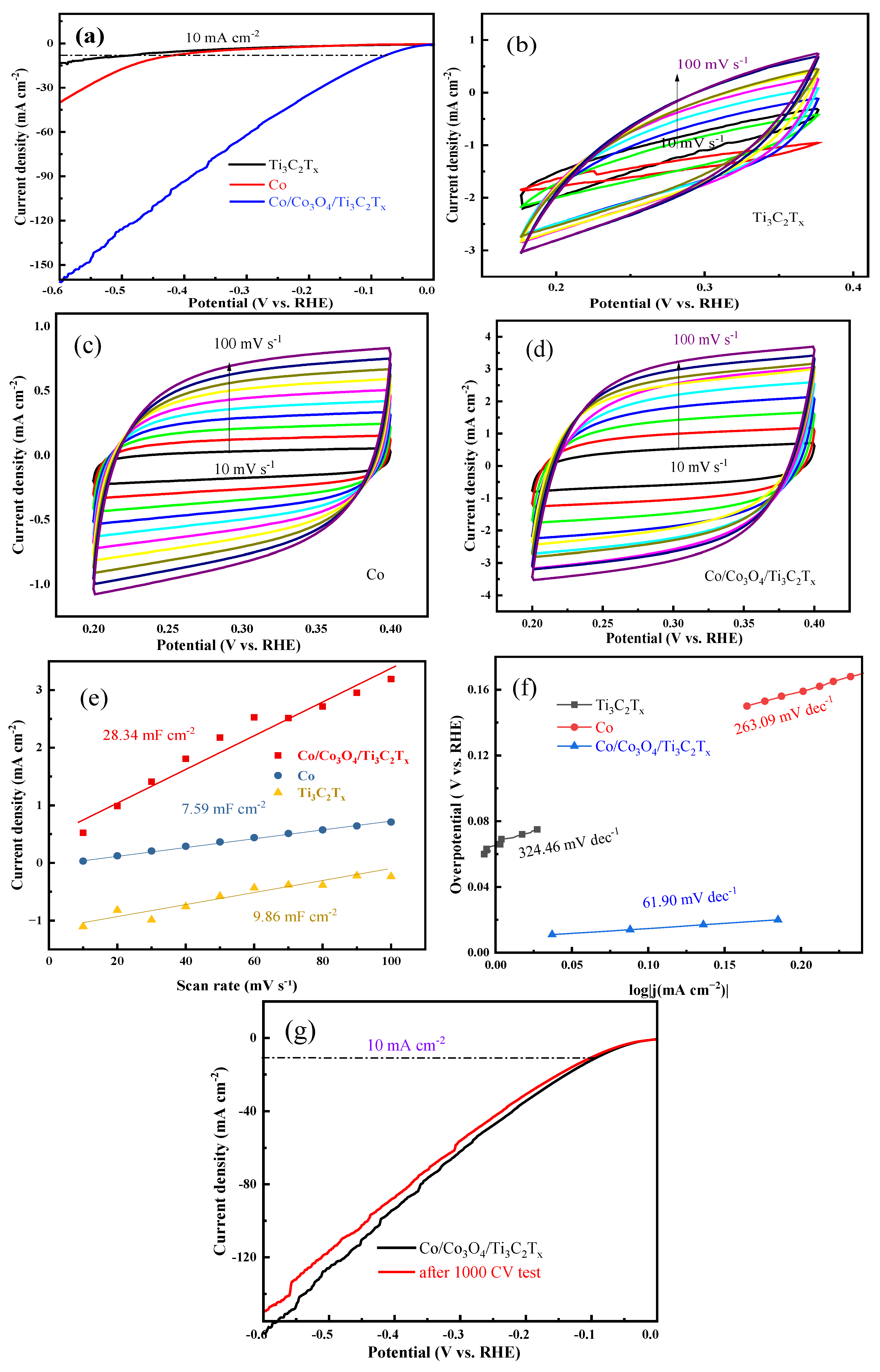
3.2. Electrocatalytic HER Performance Test in Acidic Electrolyte
3.3. Electrocatalytic HER Performance Test in Alkaline Electrolyte
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Song, H.; Luo, S.; Huang, H.; Deng, B.; Ye, J. Solar-driven hydrogen production: Recent advances, challenges, and future perspectives. ACS. Energy. Lett. 2022, 7, 1043–1065. [Google Scholar] [CrossRef]
- Cheng, R.; Min, Y.; Li, H.; Fu, C. Electronic structure regulation in the design of low-cost efficient electrocatalysts: From theory to applications. Nano Energy 2023, 115, 108718. [Google Scholar] [CrossRef]
- Yusuf, B.A.; Yaseen, W.; Xie, M.; Zayyan, R.S.; Muhammad, A.I.; Nankya, R.; Xie, J.; Xu, Y. Recent advances in understanding and design of efficient hydrogen evolution electrocatalysts for water splitting: A comprehensive review. Adv. Colloid Int. Sci. 2023, 311, 102811. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wu, R.; Wang, J.; Li, Z.; Wei, X.; Chen, J.S.; Chen, Y. Synthesis of noble metal-based intermetallic electrocatalysts by space-confined pyrolysis: Recent progress and future perspective. J. Energy Chem. 2021, 60, 61–74. [Google Scholar] [CrossRef]
- Wu, H.; Alkhatami, A.G.; Farhan, Z.A.; AbdalSalam, A.G.; Hamadan, R.; Aldarrji, M.Q.; Izzat, S.E.; Yosif, A.A.; Hadrawi, S.K.; Riyahi, Y.; et al. Recent developments in the production of hydrogen: Efficiency comparison of different techniques, economic dimensions, challenges and environmental impacts. Fuel Process. Technol. 2023, 248, 107819. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, D.; Hankari, S.E.; Zou, Y.; Wang, S. Recent progress on layered double hydroxides and their derivatives for electrocatalytic water splitting. Adv. Sci. 2018, 5, 1800064. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, Y.; Gu, C.; Zhang, L.; Wang, X.; Tu, J. Active Co@CoO core/shell nanowire arrays as efficient electrocatalysts for hydrogen evolution reaction. Chem. Eng. J. 2022, 429, 132226. [Google Scholar] [CrossRef]
- Liu, H.; He, P.; Wang, S.; Gao, J.; Zhou, L.; Li, C.; Zhang, Y.; Yang, D.; He, M.; Jia, L.; et al. Facile one-step fabrication of bimetallic Co–Ni–P hollow nanospheres anchored on reduced graphene oxide as highly efficient electrocatalyst for hydrogen evolution reaction. Int. J. Hydrogen. Energy 2019, 44, 24140–24150. [Google Scholar] [CrossRef]
- Li, T.; Niu, K.; Yang, M.; Shrestha, N.K.; Gao, Z.; Song, Y. Ultrathin CoS2 shells anchored on Co3O4 nanoneedles for efficient hydrogen evolution electrocatalysis. J. Power Sources 2017, 356, 89–96. [Google Scholar] [CrossRef]
- Zhang, X.; Bi, Y.; Wang, Y.; Liu, Q.; Zhang, Y.; Wang, J.; Chen, L. S co-doped graphene coupled with Ni2P/CoP-loaded carbon nanospheres: A novel catalyst for hydrogen evolution reaction. Mater. Today Sustain. 2024, 25, 100677. [Google Scholar] [CrossRef]
- Lin, B.; Chen, J.; Yang, R.; Mao, S.; Qin, M.; Wang, Y. Multi-hierarchical cobalt-based electrocatalyst towards high rate H2 production. Appl. Catal. B Environ. 2022, 316, 121666. [Google Scholar] [CrossRef]
- Hanan, A.; Lakhan, M.N.; Bibi, F.; Khan, A.; Soomro, I.A.; Hussain, A.; Aftab, U. MOFs coupled transition metals, graphene, and MXenes: Emerging electrocatalysts for hydrogen evolution reaction. Chem. Eng. J. 2024, 482, 148776. [Google Scholar] [CrossRef]
- Ali, S.A.; Sadiq, I.; Ahmad, T. Superlative porous organic polymers for photochemical and electrochemical CO2 reduction applications: From synthesis to functionality. Langmuir 2024, 40, 10414–10432. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.A.; Ahmad, T. MBenes for energy conversion: Advances, bottlenecks, and prospects. Langmuir 2024, 40, 10835–10846. [Google Scholar] [CrossRef]
- Karthikeyan, S.C.; Sidra, S.; Ramakrishnan, S.; Kim, D.H.; Sagayaraj, P.J.; Sekar, K.; Yoo, D.J. Heterostructured NiO/IrO2 synergistic pair as durable trifunctional electrocatalysts towards water splitting and rechargeable zinc-air batteries: An experimental and theoretical study. Appl. Catal. B Environ. Energy 2024, 355, 124196. [Google Scholar] [CrossRef]
- Shaikh, Z.A.; Laghari, A.A.; Litvishko, O.; Litvishko, V.; Kalmykova, T.; Meynkhard, A. Liquid-phase deposition synthesis of ZIF-67-derived synthesis of Co3O4@TiO2 composite for efficient electrochemical water splitting. Metals 2021, 11, 420. [Google Scholar] [CrossRef]
- Sheng, H.; Ye, K.; Gao, Y.; Zhu, K.; Yan, J.; Wang, G.; Cao, D. Simultaneously boosting hydrogen production and ethanol upgrading using a highly-efficient hollow needle-like copper cobalt sulfide as a bifunctional electrocatalyst. J. Colloid Interface Sci. 2021, 602, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liu, A.; Wang, C.; Liu, F.; He, J.; Li, S.; Wang, J.; You, R.; Yan, X.; Sun, P.; et al. Improvement of gas and humidity sensing properties of organ-like MXene by alkaline treatment. ACS Sens. 2019, 4, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Deng, T.; Hu, X.; Shi, X.; Wang, H.; Qin, T.; Zhang, X.; Qi, J.; Zhang, W.; Zheng, W. Increasing surface active Co2+ sites of MOF-derived Co3O4 for enhanced supercapacitive performance via NaBH4 reduction. Electrochim. Acta 2018, 289, 319–323. [Google Scholar] [CrossRef]
- Cao, S.; Shen, B.; Tong, T.; Fu, J. 2D/2D heterojunction of ultrathin MXene/Bi2WO6 nanosheets for improved photocatalytic CO2 reduction. Adv. Funct. Mater. 2018, 28, 1800136. [Google Scholar] [CrossRef]
- Feng, A.; Yu, Y.; Wang, F.; Jiang, Y.; Yu, L.; Mi, L.; Song, L. Two-dimensional MXene Ti3C2 produced by exfoliation of Ti3AlC2. Mater. Design. 2017, 114, 161–166. [Google Scholar] [CrossRef]
- Ma, Y.Y.; Lang, Z.L.; Yan, L.K.; Wang, Y.H.; Tan, H.Q.; Feng, K.; Xia, Y.J.; Zhong, J.; Liu, Y.; Kang, Z.H.; et al. Highly efficient hydrogen evolution triggered by a multi-interfacial Ni/WC hybrid electrocatalyst. Energy Environ. Sci. 2018, 11, 2114–2123. [Google Scholar] [CrossRef]
- Eid, K.; Lu, Q.; Abdel-Azeim, S.; Soliman, A.; Abdullah, A.M.; Abdelgwad, A.M.; Forbes, R.P.; Ozoemena, K.I.; Varma, R.S.; Shibl, M.F. Highly exfoliated Ti3C2Tx MXene nanosheets atomically doped with Cu for efficient electrochemical CO2 reduction: An experimental and theoretical study. J. Mater. Chem. A 2022, 10, 1965–1975. [Google Scholar] [CrossRef]
- Islam, M.M.; Faisal, S.N.; Akhter, T.; Roy, A.K.; Minett, A.I.; Konstantinov, K.; Shi, X.D. Liquid-crystal-mediated 3D macrostructured composite of Co/Co3O4 embedded in graphene: Free-standing electrode for efficient water splitting. Part. Part. Syst. Character. 2017, 34, 1600386. [Google Scholar] [CrossRef]
- Ding, D.; Shen, K.; Chen, X.; Chen, H.; Chen, J.; Fan, T.; Wu, R.; Li, Y. Multi-level architecture optimization of MOF-templated Co-based nanoparticles embedded in hollow N-doped carbon polyhedra for efficient OER and ORR. ACS Catal. 2018, 8, 7879–7888. [Google Scholar] [CrossRef]
- McCrum, I.T.; Koper, M.T.M. The role of adsorbed hydroxide in hydrogen evolution reaction kinetics on modified platinum. Nat. Energy 2020, 5, 891–899. [Google Scholar] [CrossRef]
- Wu, J.; Fan, J.; Zhao, Y.; Wang, D.; Wang, H.; Liu, L.; Gu, Q.; Zhang, L.; Zheng, X.; Cui, D.J.; et al. Atomically dispersed MoOx on rhodium metallene boosts electrocatalyzed alkaline hydrogen evolution. Angew. Chem. Int. Edit. 2022, 61, 202207512. [Google Scholar]
- Do, H.H.; Tekalgne, M.A.; Tran, V.A.; Le, Q.V.; Cho, J.H.; Ahn, S.H.; Kim, S.Y. MOF-derived Co/Co3O4/C hollow structural composite as an efficient electrocatalyst for hydrogen evolution reaction. Fuel 2022, 329, 125468. [Google Scholar] [CrossRef]
- Iqbal, M.F.; Gao, W.; Mao, Z.; Hu, E.; Gao, X.; Zhang, J.; Chen, Z. Strategies to enhance the electrocatalytic behavior of metal selenides for hydrogen evolution reaction: A review. Int. J. Hydrogen. Energy 2023, 48, 36722–36749. [Google Scholar] [CrossRef]
- Ma, C.; He, H.; Qin, J.; Luo, L.; Lan, Y.; Zhang, J.; Yang, L.; Jiang, Q.; Huang, H. The marriage of hydrazone-linked covalent organic frameworks and MXene enables efficient electrocatalytic hydrogen evolution. Small Struct. 2024, 5, 2300279. [Google Scholar] [CrossRef]
- Ma, C.; He, H.; Qin, J.; Hao, L.; Jia, L.; Yang, L.; Huang, H. Combining MXene nanosheets with iron-based metal-organic frameworks for enhanced electrocatalytic hydrogen evolution reaction. Mater. Today Chem. 2023, 30, 101531. [Google Scholar] [CrossRef]
- Seh, Z.W.; Fredrickson, K.D.; Anasori, B.; Kibsgaard, J.; Strickler, A.L.; Lukatskaya, M.R.; Gogotsi, Y.; Jaramillo, T.F.; Vojvodic, A. Two-dimensional molybdenum carbide (MXene) as an efficient electrocatalyst for hydrogen evolution. ACS Energy Lett. 2016, 1, 589–594. [Google Scholar] [CrossRef]
- Wu, X.; Wang, Z.; Yu, M.; Xiu, L.; Qiu, J. Stabilizing the MXenes by carbon nanoplating for developing hierarchical nanohybrids with efficient lithium storage and hydrogen evolution capability. Adv. Mater. 2017, 29, 1607017. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Ding, C.; Liu, J.; Chen, T.; Peng, W.; Li, Y.; Zhang, F.; Fan, X. Heterostructure engineering of Co-doped MoS2 coupled with Mo2CTx MXene for enhanced hydrogen evolution in alkaline media. Nanoscale 2019, 11, 10992–11000. [Google Scholar] [CrossRef] [PubMed]



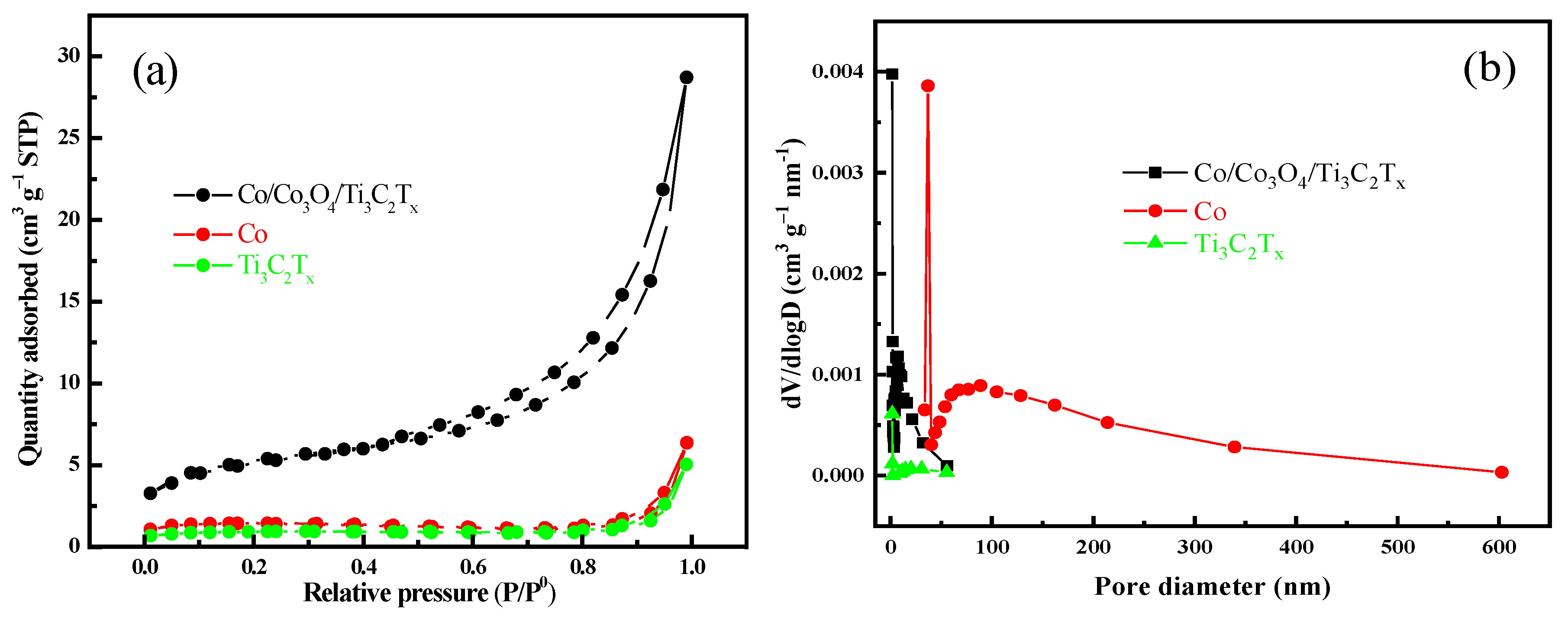
| Catalyst | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|
| Ti3C2Tx | 2.94 | 0.0076 | 10.71 |
| Co | 64.02 | 0.30 | 186.79 |
| Co/Co3O4/Ti3C2Tx | 17.64 | 0.044 | 10.07 |
| Type of Electrocatalyst | Electrolyte | Electrochemical Performance | Refs. |
|---|---|---|---|
| COF/Ti3C2Tx | 0.5 M H2SO4 | A very low onset potential of 19 mV and a small Tafel slope of 50 mV dec−1 | [30] |
| MIL/Ti3C2Tx | 0.5 M H2SO4 | An operating overpotential of 107 mV at 10 mA/cm2 | [31] |
| Mo2CTx | 0.5 M H2SO4 | A low overpotential of 283 mV at 10 mA cm−2 and an average TOF of −0.02 H2 s−1 at 200 mV | [32] |
| MoS2/Ti3C2/C | 0.5 M H2SO4 | A low overpotential of 135 mV at 10 mA cm−2 and a Tafel slope of 45 mV dec−1 | [33] |
| Co-MoS2/Mo2CTx | 1 M KOH | A low overpotential of 112 mV at 10 mA cm−2 and a Tafel slope of 82 mV dec−1 | [34] |
| Co/Co3O4/Ti3C2Tx | 1 M KOH | A low overpotential of 87 mV at 10 mA cm−2 and a Tafel slope of 105.17 mV dec−1 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, W.; Wang, B.; Shu, Q. An Efficient and Stable MXene-Immobilized, Cobalt-Based Catalyst for Hydrogen Evolution Reaction. Metals 2024, 14, 922. https://doi.org/10.3390/met14080922
Guo W, Wang B, Shu Q. An Efficient and Stable MXene-Immobilized, Cobalt-Based Catalyst for Hydrogen Evolution Reaction. Metals. 2024; 14(8):922. https://doi.org/10.3390/met14080922
Chicago/Turabian StyleGuo, Wei, Buxiang Wang, and Qing Shu. 2024. "An Efficient and Stable MXene-Immobilized, Cobalt-Based Catalyst for Hydrogen Evolution Reaction" Metals 14, no. 8: 922. https://doi.org/10.3390/met14080922





