Effect of Heat Treatment on Microstructures and Mechanical Properties of Graphene/Aluminum Composites: Insights from Atomic Simulations
Abstract
:1. Introduction
2. Simulation Methods
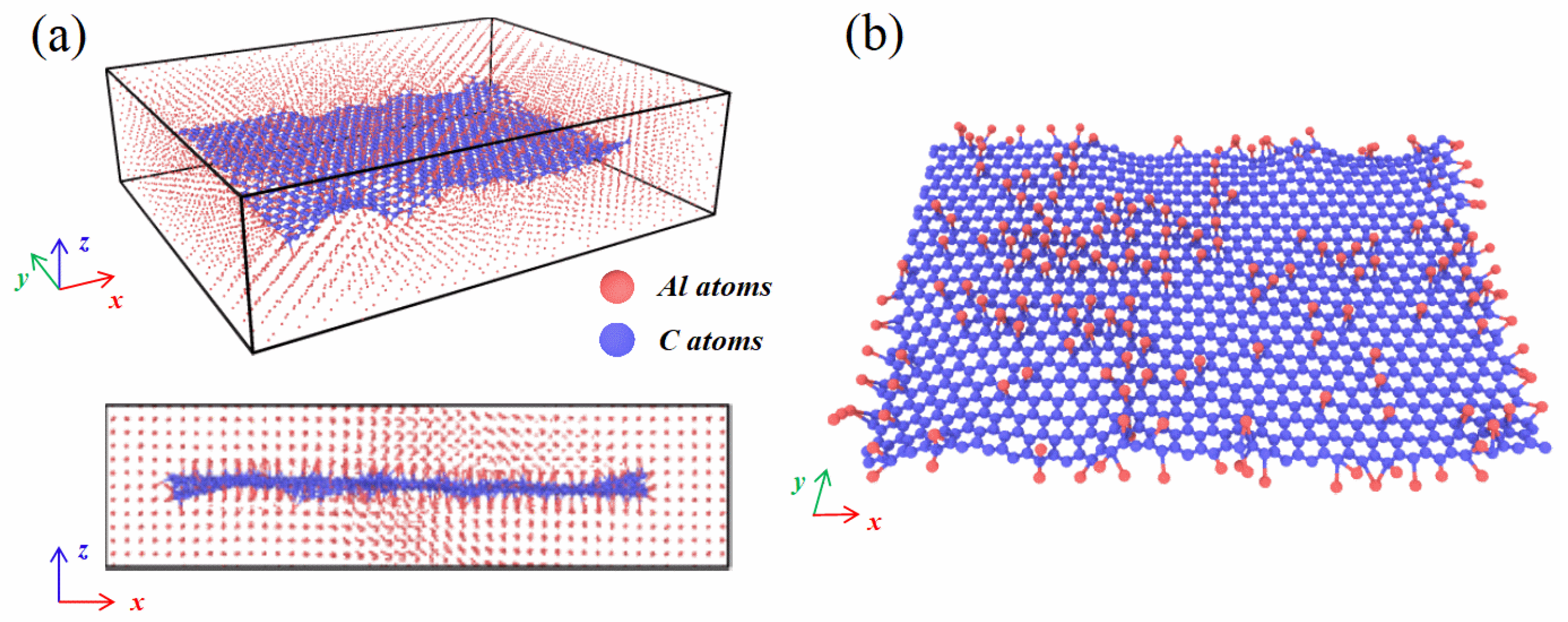
2.1. Atomistic Models and Molecular Dynamics Simulations
2.2. Interatomic Potential
3. Results and Discussion
3.1. Role of Heat Treatment Temperature
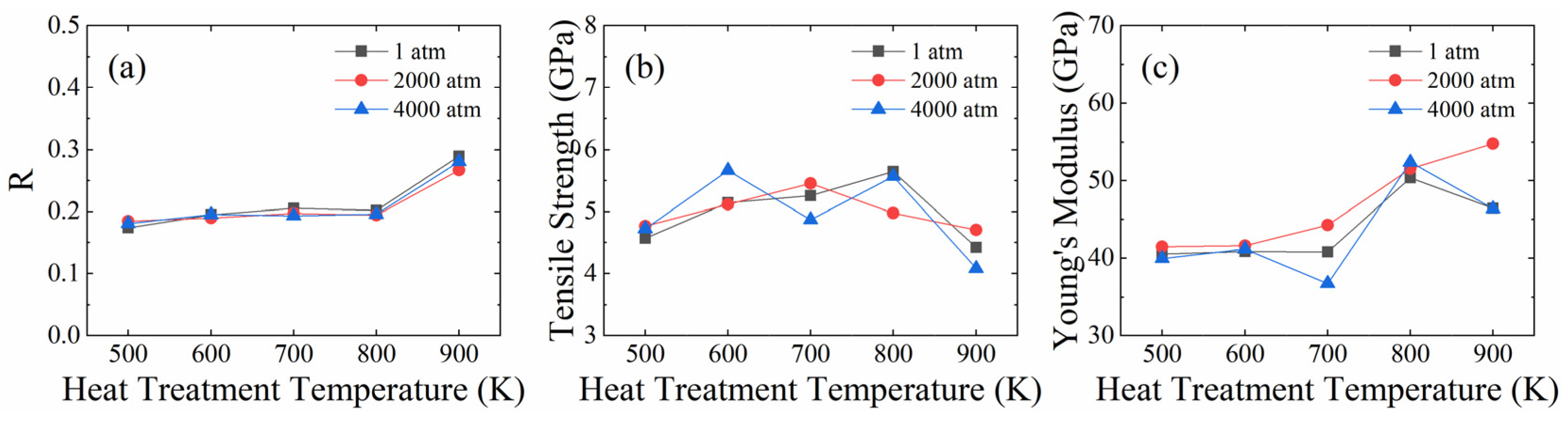
3.2. Effect of Heat Treatment Pressure
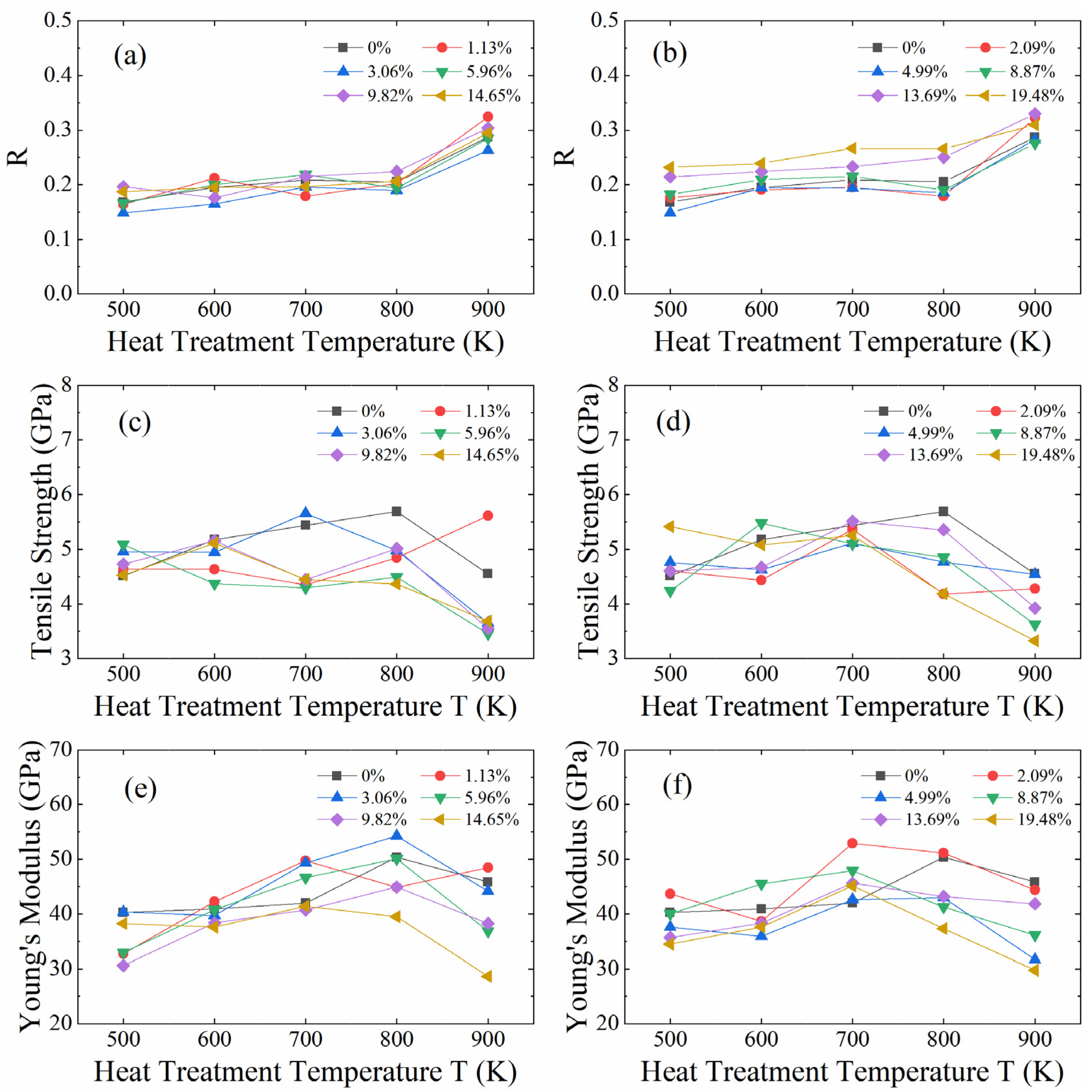
3.3. Impact of Defective GNPs
4. Conclusions
- The heat treatment temperature and pressure have notable effects on the microstructure (crystal structure, dislocation, position and orientation of GNPs, interface bondings) and mechanical properties of GNPs/AlMCs. The results showed that the number of interfacial bonds increased significantly when the heat treatment temperature reached 900 K, below which it stays at a low level. In the heat treatment temperature range of 500 K ≤ T ≤ 800 K, an increased number of interface bondings improved the tensile strength, Young’s modulus, and ductility of GNPs/AlMCs in general.
- The concentration and structure of GNPs defects play interesting roles in modifying the mechanical properties of GNPs/AlMCs via the formation of bonding structures at defect sites without a notable loss of intrinsic strength of GNP. This offers a plausible strategy for improving the mechanical properties of GNPs/AlMCs.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Salvetat, J.P.; Bonard, J.M.; Thomson, N.H.; Kulik, A.J.; Forro, L.; Benoit, W.; Zuppiroli, L. Mechanical properties of carbon nanotubes. Appl. Phys. A 1999, 69, 255–260. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R. Mechanical properties of graphene and graphene-based nanocomposites. Prog. Mater. Sci. 2017, 90, 75–127. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Chen, C.F.; Guo, W.L. Magnetoelectric effect in graphene nanoribbons on substrates via electric bias control of exchange splitting. Phys. Rev. Lett. 2009, 103, 187204. [Google Scholar] [CrossRef] [PubMed]
- Jariwala, D.; Sangwan, V.K.; Lauhon, L.J.; Marks, T.J.; Hersam, M.C. Carbon nanomaterials for electronics, optoelectronics, photovoltaics, and sensing. Chem. Soc. Rev. 2013, 42, 2824–2860. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.F.; Liu, R.M.; Tang, C.; Wang, L.F. Dynamic tuning of moiré superlattice morphology by out-of-plane deformation. Appl. Phys. Lett. 2024, 124, 173508. [Google Scholar] [CrossRef]
- Popov, V.N. Carbon nanotubes: Properties and application. Mater. Sci. Eng. R Rep. 2004, 43, 61–102. [Google Scholar] [CrossRef]
- Ren, S.; Rong, P.; Yu, Q. Preparations, properties and applications of graphene in functional devices: A concise review. Ceram. Int. 2018, 44, 11940–11955. [Google Scholar] [CrossRef]
- Luo, S.H.; Samad, Y.A.; Chan, V.; Liao, K. Cellular graphene: Fabrication, mechanical properties, and strain-sensing applications. Matterials 2019, 1, 1148–1202. [Google Scholar] [CrossRef]
- Merneedi, A.; Natrayan, L.; Kaliappan, S.; Veeman, D.; Angalaeswari, S. Experimental investigation on mechanical properties of carbon nanotube-reinforced epoxy composites for automobile application. J. Nanomater. 2021, 2021, 4937059. [Google Scholar] [CrossRef]
- Srinivasan, V.; Kunjiappan, S.; Palanisamy, P. A brief review of carbon nanotube reinforced metal matrix composites for aerospace and defense applications. Int. Nano Lett. 2021, 11, 321–345. [Google Scholar] [CrossRef]
- Krasley, A.T.; Li, E.; Galeana, J.M.; Bulumulla, C.; Beyene, A.G.; Demirer, G.S. Carbon Nanomaterial Fluorescent Probes and Their Biological Applications. Chem. Rev. 2024, 124, 3085–3185. [Google Scholar] [CrossRef]
- Kinloch, I.A.; Suhr, J.; Lou, J.; Young, R.J.; Ajayan, P.M. Composites with carbon nanotubes and graphene: An outlook. Science 2018, 362, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Ahmad, F.; Yusoff, P.S.M.M.; Muhamad, N.; Onate, E.; Raza, M.R.; Malik, K. A review of graphene reinforced Cu matrix composites for thermal management of smart electronics. Compos. Part A Appl. Sci. Manuf. 2021, 144, 106357. [Google Scholar] [CrossRef]
- Moustafa, E.B.; Abdel Aziz, S.S.; Taha, M.A.; Saber, A. Influence of graphene and silver addition on aluminum’s thermal conductivity and mechanical properties produced by the powder metallurgy technique. Metals 2023, 13, 836. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, S.; Ren, W.; Yang, Y.; Shi, L.; Zhou, Q.; Liu, M. Uniformly dispersing GNPs for fabricating graphene-reinforced pure Ti matrix composites with enhanced strength and ductility. J. Alloys Compd. 2021, 888, 161527. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, N.; Yang, H.; Wu, C. Application of carbon nanotubes from waste plastics as filler to epoxy resin composite. ACS Sustain. Chem. Eng. 2022, 10, 2204–2213. [Google Scholar] [CrossRef] [PubMed]
- Srivyas, P.D.; Charoo, M.S. Application of hybrid aluminum matrix composite in automotive industry. Mater. Today Proc. 2019, 18, 3189–3200. [Google Scholar] [CrossRef]
- Fereiduni, E.; Ghasemi, A.; Elbestawi, M. Selective laser melting of aluminum and titanium matrix composites: Recent progress and potential applications in the aerospace industry. Aerospace 2020, 7, 77. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, S.Q.; Xia, J.H.; Su, Q.; Ma, B.C.; Wu, J.H.; Zhou, J.X.; Wang, X.T.; Hu, L.X. Microstructural evolution, mechanical and physical properties of graphene reinforced aluminum composites fabricated via powder metallurgy. Mater. Sci. Eng. A 2021, 802, 140669. [Google Scholar] [CrossRef]
- Hasan, M.S.; Wong, T.; Rohatgi, P.K.; Nosonovsky, M. Analysis of the friction and wear of graphene reinforced aluminum metal matrix composites using machine learning models. Tribol. Int. 2022, 170, 107527. [Google Scholar] [CrossRef]
- Alwahib, A.A.; Muttlak, W.H.; Mahdi, B.S.; Mohammed, A.Z. Corrosion resistance enhancement by laser and reduced graphene oxide-based nano-silver for 1050 aluminum alloy. Surf. Interfaces 2020, 20, 100557. [Google Scholar] [CrossRef]
- Shirvanimoghaddam, K.; Hamim, S.U.; Akbari, M.K.; Fakhrhoseini, S.M.; Khayyam, H.; Pakseresht, A.H.; Ghasali, E.; Zabet, M.; Munir, K.S.; Jia, S.; et al. Carbon fiber reinforced metal matrix composites: Fabrication processes and properties. Compos. Part A Appl. Sci. Manuf. 2017, 92, 70–96. [Google Scholar] [CrossRef]
- Zhao, Z.; Bai, P.; Du, W.; Liu, B.; Pan, D.; Das, R.; Liu, C.; Guo, Z. An overview of graphene and its derivatives reinforced metal matrix composites: Preparation, properties and applications. Carbon 2020, 170, 302–326. [Google Scholar] [CrossRef]
- Wang, J.; Li, Z.; Fan, G.; Pan, H.; Chen, Z.; Zhang, D. Reinforcement with graphene nanosheets in aluminum matrix composites. Scr. Mater. 2012, 66, 594–597. [Google Scholar] [CrossRef]
- Xie, Y.; Meng, X.; Chang, Y.; Mao, D.; Yang, Y.; Xu, Y.; Wan, L.; Huang, Y. Ameliorating strength-ductility efficiency of graphene nanoplatelet-reinforced aluminum composites via deformation-driven metallurgy. Compos. Sci. Technol. 2022, 219, 109225. [Google Scholar] [CrossRef]
- Sadoun, A.M.; Najjar, I.M.R.; Wagih, A. Electroless-plating of Ag nanoparticles on Al2O3 and graphene Nano sheets (GNs) for improved wettability and properties of Al–Al2O3/GNs nanocomposites. Ceram. Int. 2021, 47, 10855–10865. [Google Scholar] [CrossRef]
- Chu, K.; Wang, F.; Wang, X.; Li, Y.; Geng, Z.; Huang, D.; Zhang, H. Interface design of graphene/copper composites by matrix alloying with titanium. Mater. Des. 2018, 144, 290–303. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, C.; Liu, E.; Zhao, N.; He, C. Effect of interface structure on the mechanical properties of graphene nanosheets reinforced copper matrix composites. ACS Appl. Mater. Interfaces 2018, 10, 37586–37601. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Wang, L.H.; Zan, Y.N.; Wang, W.G.; Xiao, B.L.; Wang, D.; Wang, Q.Z.; Ni, D.R.; Ma, Z.Y. Enhancing strengthening efficiency of graphene nano-sheets in aluminum matrix composite by improving interface bonding. Compos. Part B Eng. 2020, 199, 108268. [Google Scholar] [CrossRef]
- Liu, P.; Xie, J.; Wang, A.; Ma, D.; Mao, Z. First-principles prediction of enhancing graphene/Al interface bonding strength by graphene doping strategy. Appl. Surf. Sci. 2020, 517, 146040. [Google Scholar] [CrossRef]
- Guo, B.; Chen, B.; Zhang, X.; Cen, X.; Wang, X.; Song, M.; Ni, S.; Yi, J.; Shen, T.; Du, Y. Exploring the size effects of Al4C3 on the mechanical properties and thermal behaviors of Al-based composites reinforced by SiC and carbon nanotubes. Carbon 2018, 135, 224–235. [Google Scholar] [CrossRef]
- Trujillo-Vázquez, E.; Pech-Canul, M.I.; Guía-Tello, J.C.; Pech-Canul, M.A. Surface chemistry modification for elimination of hydrophilic Al4C3 in B4C/Al composites. Mater. Des. 2016, 89, 94–101. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Geng, L. Effect of heat treatment on interfacial bonding and strengthening efficiency of graphene in GNP/Al composites. Compos. Part A Appl. Sci. Manuf. 2019, 121, 487–498. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, R.; Tan, Z.; Ji, G.; Fan, G.; Li, Z.; Xiong, D.B.; Guo, Q.; Li, Z.; Zhang, D. Interface-induced strain hardening of graphene nanosheet/aluminum composites. Carbon 2019, 146, 17–27. [Google Scholar] [CrossRef]
- Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO–the Open Visualization Tool. Model. Simul. Mater. Sci. Eng. 2009, 18, 015012. [Google Scholar] [CrossRef]
- Rong, Y.; He, H.P.; Zhang, L.; Li, N.; Zhu, Y.C. Molecular dynamics studies on the strengthening mechanism of Al matrix composites reinforced by graphene nanoplatelets. Comput. Mater. Sci. 2018, 153, 48–56. [Google Scholar] [CrossRef]
- Lee, W.; Jang, S.; Kim, M.J.; Myoung, J.M. Interfacial interactions and dispersion relations in carbon–aluminium nanocomposite systems. Nanotechnology 2008, 19, 285701. [Google Scholar] [CrossRef]
- Wu, L.; Liu, L.; Liu, J.; Zhang, R. Effects of high pressure heat treatment on microstructure and micro-mechanical properties of Cu77.96Al22.04 alloy. Mater. Trans. 2012, 53, 504–507. [Google Scholar] [CrossRef]
- Du, P.; Sun, H.; Kong, L.; Wang, Z.; Zhang, J.; Liu, W.; Xue, X.; He, Y. A study on recrystallization behavior and recrystallization texture of high pressure heat-treated Al–Mg alloy. J. Mater. Sci. 2023, 58, 2876–2892. [Google Scholar] [CrossRef]
- Van Duin, A.C.T.; Dasgupta, S.; Lorant, F.; Goddard, W.A. ReaxFF: A reactive force field for hydrocarbons. J. Phys. Chem. A 2001, 105, 9396–9409. [Google Scholar] [CrossRef]
- Senftle, T.P.; Hong, S.; Islam, M.M.; Kylasa, S.B.; Zheng, Y.; Shin, Y.K.; Junkermeier, C. The ReaxFF reactive force-field: Development, applications and future directions. npj Comput. Mater. 2016, 2, 15011. [Google Scholar] [CrossRef]
- Hong, S.; Van Duin, A.C.T. Atomistic-scale analysis of carbon coating and its effect on the oxidation of aluminum nanoparticles by ReaxFF-molecular dynamics simulations. J. Phys. Chem. C 2016, 120, 9464–9474. [Google Scholar] [CrossRef]
- Mahata, A.; Zaeem, M.A.; Baskes, M.I. Understanding homogeneous nucleation in solidification of aluminum by molecular dynamics simulations. Model. Simul. Mater. Sci. Eng. 2018, 26, 025007. [Google Scholar] [CrossRef]
- Zhu, Q.; Liu, X.; Yang, Q.S. Dislocation-blocking mechanism for the strengthening and toughening of laminated graphene/Al composites. Comput. Mater. Sci. 2019, 160, 72–81. [Google Scholar] [CrossRef]

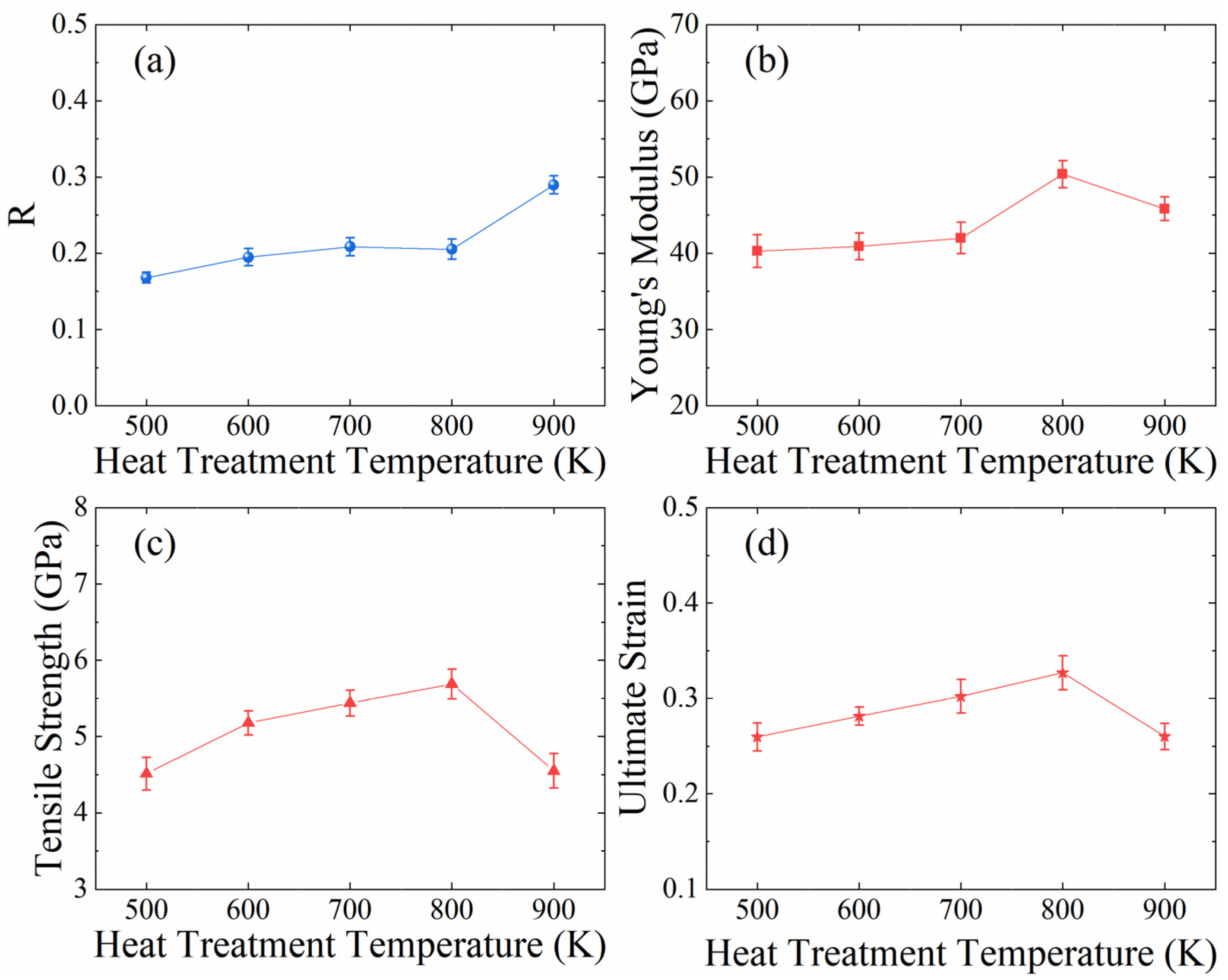

| Temperature (K) | Increase in Tensile Strength | Increase in Young’s Modulus | Increase in Ultimate Strain |
|---|---|---|---|
| 500 | 39.0% | 12.5% | 8.6% |
| 600 | 56.0% | 11.7% | 21.5% |
| 700 | 59.3% | 13.4% | 29.8% |
| 800 | 71.2% | 39.9% | 36.3% |
| 900 | 34.0% | 29.0% | 3.3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Z.; Chen, L.; Wang, L.; Xia, J.; Wang, R.; Tang, C. Effect of Heat Treatment on Microstructures and Mechanical Properties of Graphene/Aluminum Composites: Insights from Atomic Simulations. Metals 2024, 14, 923. https://doi.org/10.3390/met14080923
Shen Z, Chen L, Wang L, Xia J, Wang R, Tang C. Effect of Heat Treatment on Microstructures and Mechanical Properties of Graphene/Aluminum Composites: Insights from Atomic Simulations. Metals. 2024; 14(8):923. https://doi.org/10.3390/met14080923
Chicago/Turabian StyleShen, Zixuan, Lei Chen, Liya Wang, Jun Xia, Ruijie Wang, and Chun Tang. 2024. "Effect of Heat Treatment on Microstructures and Mechanical Properties of Graphene/Aluminum Composites: Insights from Atomic Simulations" Metals 14, no. 8: 923. https://doi.org/10.3390/met14080923





