Yttrium Separation from Phosphorite Extract Using Liquid Extraction with Room Temperature Ionic Liquids Followed by Electrochemical Reduction
Abstract
:1. Introduction
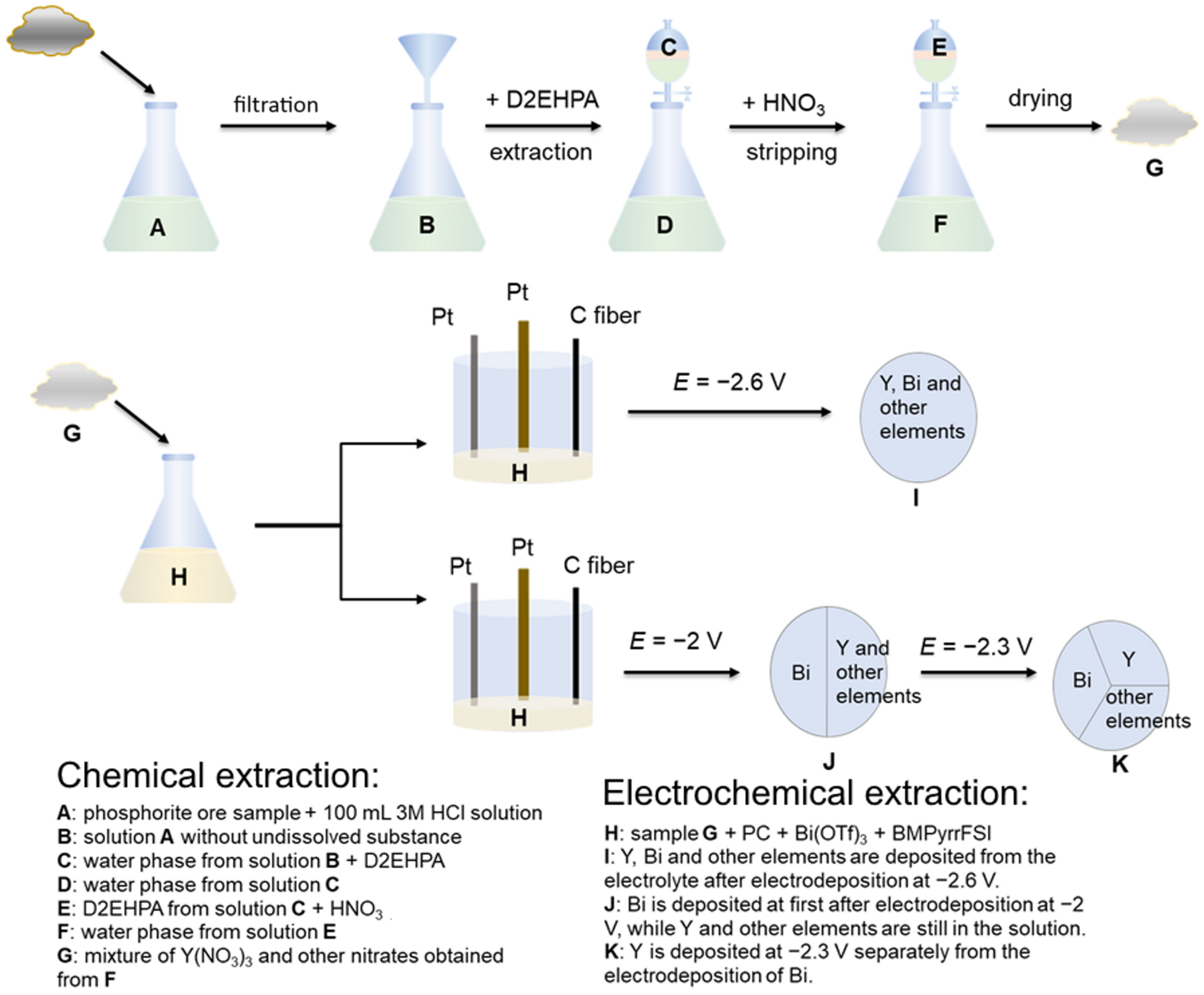
2. Experimental Section
2.1. Raw Materials and Chemicals
2.2. Feed Solution
2.3. Equipment
2.4. Experimental Conditions
2.5. Quantitative Analysis of REEs Extraction
3. Results and Discussion
3.1. Results of Extraction and Stripping Experiment
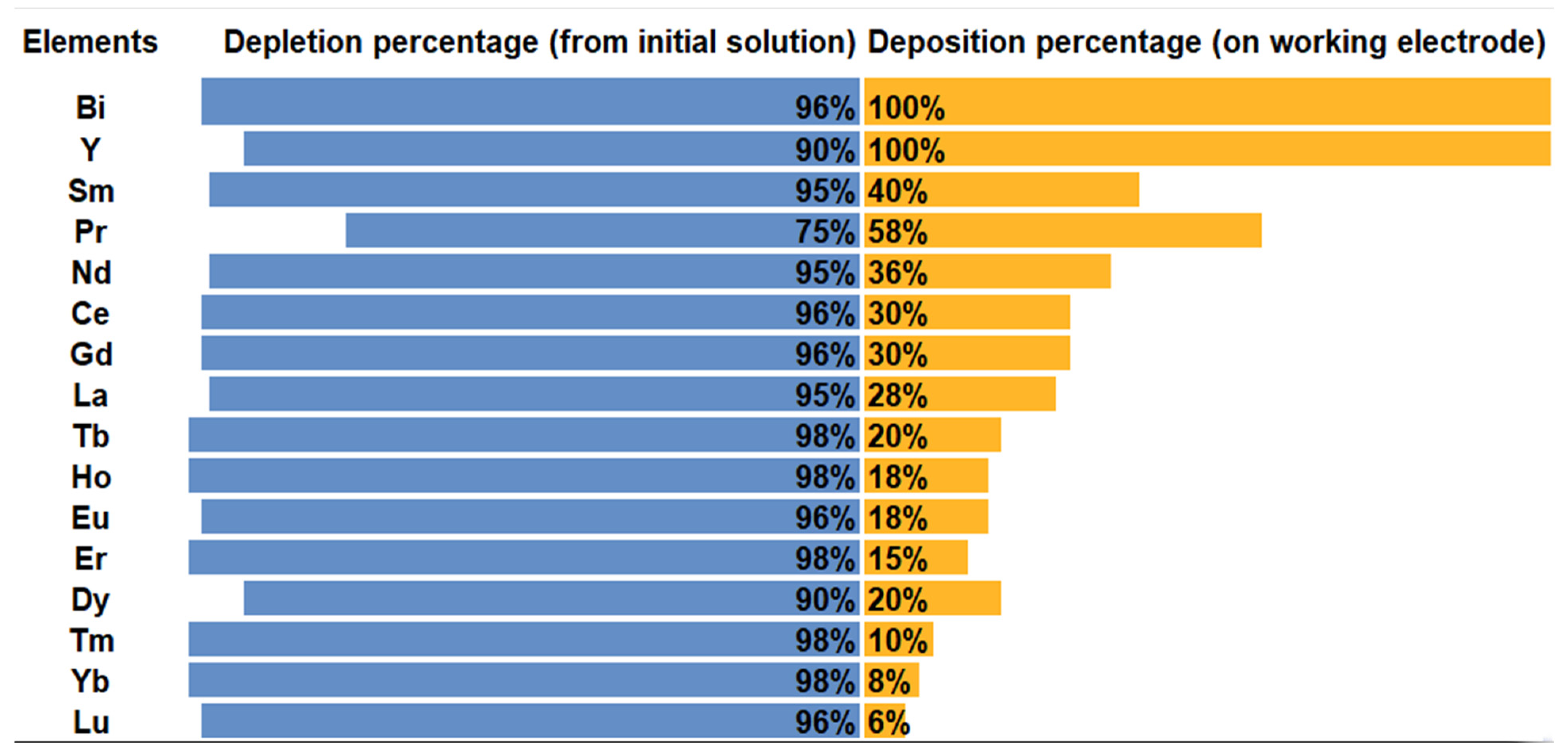
3.1.1. Extraction and Stripping of Y and Rare Earth Elements
3.1.2. Extraction and Stripping of Other Elements than Y and REE
3.2. Results of Electrochemical Experiments
3.2.1. Cyclic Voltammetry Results for Concentrated Y and REEs Solution
3.2.2. Chronoamperometry (i vs. Time t) Results
3.3. Post-Experiment: Dissolution of Deposited Metals from Pt(Bi) Electrode Surface with Acid Solution
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guzik, K.; Galos, K.; Kot-Niewiadomska, A.; Eerola, T.; Eilu, P.; Carvalho, J.; Fernandez-Naranjo, F.J.; Arvidsson, R.; Arvanitidis, N.; Raaness, A. Potential benefits and constraints of development of critical raw materials’ production in the EU: Analysis of selected case studies. Resources 2021, 10, 67. [Google Scholar] [CrossRef]
- Balaram, V. Potential Future Alternative Resources for Rare Earth Elements: Opportunities and Challenges. Minerals 2023, 13, 425. [Google Scholar] [CrossRef]
- Gao, W.; Wen, D.; Ho, J.C.; Qu, Y. Incorporation of rare earth elements with transition metal–based materials for electrocatalysis: A review for recent progress. Mater. Today Chem. 2019, 12, 266–281. [Google Scholar] [CrossRef]
- Tian, S.; Zhang, L.; Xie, R.; Lu, A.; Huang, Y.; Xing, H.; Chen, X. The electronic, magnetic and optical properties of GaN monolayer doped with rare-earth elements. Solid State Commun. 2023, 371, 115292. [Google Scholar] [CrossRef]
- Long, P.; Qiuhui, Y.; Zhang, H.; Guangliang, X.U.; Zhang, M.; Jingdong, W. Rare earth permanent magnets Sm2 (Co, Fe, Cu, Zr) 17 for high temperature applications. J. Rare Earths 2008, 26, 378–382. [Google Scholar]
- Qiao, S.; Zhou, Q.; Ma, M.; Liu, H.K.; Dou, S.X.; Chong, S. Advanced Anode Materials for Rechargeable Sodium-Ion Batteries. ACS Nano 2023, 17, 11220–11252. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Xia, J.; Yin, D.; Luo, M.; Yan, C.; Du, Y. Rare earth incorporated electrode materials for advanced energy storage. Coord. Chem. Rev. 2019, 390, 32–49. [Google Scholar] [CrossRef]
- Ringuedé, A.; Hubert, S.; Atwi, L.; Lair, V. Prospects of hydrogen and its derivative as energy vector for electricity production at high temperature: Fuel cells and electrolysers. Curr. Opin. Electrochem. 2023, 42, 101401. [Google Scholar] [CrossRef]
- Vestli, M.; Lust, E.; Nurk, G. Characterization of Terbium and Samarium Co-Doped Ceria Films Prepared Using Ultrasonic Spray Pyrolysis. J. Electrochem. Soc. 2015, 162, F812–F820. [Google Scholar] [CrossRef]
- Lust, E.; Möller, P.; Kivi, I.; Nurk, G.; Kallip, S.; Nigu, P.; Lust, K. Optimization of the cathode composition for the intermediate-temperature SOFC. J. Electrochem. Soc. 2005, 152, A2306–A2308. [Google Scholar] [CrossRef]
- Ryszko, U.; Rusek, P.; Kołodyńska, D. Quality of Phosphate Rocks from Various Deposits Used in Wet Phosphoric Acid and P-Fertilizer Production. Materials 2023, 16, 793. [Google Scholar] [CrossRef] [PubMed]
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Van Gerven, T.; Pontikes, Y. Towards zero-waste valorisation of rare-earth-containing industrial process residues: A critical review. J. Clean. Prod. 2015, 99, 17–38. [Google Scholar] [CrossRef]
- Riaño, S.; Binnemans, K. Extraction and separation of neodymium and dysprosium from used NdFeB magnets: An application of ionic liquids in solvent extraction towards the recycling of magnets. Green Chem. 2015, 17, 2931–2942. [Google Scholar] [CrossRef]
- Alguacil, F.J.; Robla, J.I. Recent Work on the Recovery of Rare Earths Using Ionic Liquids and Deep Eutectic Solvents. Minerals 2023, 13, 1288. [Google Scholar] [CrossRef]
- Komasawa, I.; Hisada, K.; Miyamura, M. Extraction and separation of rare-earth elements by tri-n-octylmethylammonium nitrate. J. Chem. Eng. Jpn. 1990, 23, 308–315. [Google Scholar] [CrossRef]
- Rout, A.; Binnemans, K. Separation of rare earths from transition metals by liquid–liquid extraction from a molten salt hydrate to an ionic liquid phase. Dalton Trans. 2014, 43, 3186–3195. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Sahu, K.K.; Sahu, S.K. Solvent Extraction and Separation of Nd, Pr and Dy from Leach Liquor of Waste NdFeB Magnet Using the Nitrate Form of Mextral® 336At in the Presence of Aquo-Complexing Agent EDTA. Metals 2019, 9, 269. [Google Scholar] [CrossRef]
- Larsson, K.; Binnemans, K. Separation of Rare Earths by Solvent Extraction with an Undiluted Nitrate Ionic Liquid. J. Sustain. Metall. 2017, 3, 73–78. [Google Scholar] [CrossRef]
- Liang, H.; Zhang, P.; Jin, Z.; DePaoli, D. Rare Earth and Phosphorus Leaching from a Flotation Tailings of Florida Phosphate Rock. Minerals 2018, 8, 416. [Google Scholar] [CrossRef]
- Skorovarov, J.I.; Kosynkin, V.D.; Moiseev, S.D.; Rura, N.N. Recovery of rare earth elements from phosphorites in the USSR. J. Alloys Compd. 1992, 180, 71–76. [Google Scholar] [CrossRef]
- Raudsep, R. Estonian georesources in the European context. Est. J. Earth Sci. 2008, 57, 80. [Google Scholar] [CrossRef]
- Yin, S.-H.; Li, S.-W.; Wu, W.-Y.; Bian, X.; Peng, J.-H.; Zhang, L.-B. Extraction and separation of Ce(III) and Pr(III) in the system containing two complexing agents with di- (2-ethylhexyl) phosphoric acid. RSC Adv. 2014, 4, 59997–60001. [Google Scholar] [CrossRef]
- Rout, S.; Abhilash; Meshram, P.; Zhang, P. A Comprehensive Review on Occurrence and Processing of Phosphate Rock Based Resources- Focus on REEs. Miner. Process. Extr. Metall. Rev. 2024, 45, 368–388. [Google Scholar] [CrossRef]
- Wu, S.; Wang, L.; Zhang, P.; El-Shall, H.; Moudgil, B.; Huang, X.; Zhao, L.; Zhang, L.; Feng, Z. Simultaneous recovery of rare earths and uranium from wet process phosphoric acid using solvent extraction with D2EHPA. Hydrometallurgy 2018, 175, 109–116. [Google Scholar] [CrossRef]
- Wu, S.; Wang, L.; Zhao, L.; Zhang, P.; El-Shall, H.; Moudgil, B.; Huang, X.; Zhang, L. Recovery of rare earth elements from phosphate rock by hydrometallurgical processes—A critical review. Chem. Eng. J. 2018, 335, 774–800. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, F.; Wang, H.; Zhou, S.; Yan, C. The Occurrence States of Rare Earth Elements Bearing Phosphorite Ores and Rare Earth Enrichment Through the Selective Reverse Flotation. Minerals 2019, 9, 698. [Google Scholar] [CrossRef]
- Jürjo, S.; Siinor, L.; Siimenson, C.; Paiste, P.; Lust, E. Two-Step Solvent Extraction of Radioactive Elements and Rare Earths from Estonian Phosphorite Ore Using Nitrated Aliquat 336 and Bis(2-ethylhexyl) Phosphate. Minerals 2021, 11, 388. [Google Scholar] [CrossRef]
- Jürjo, S.; Oll, O.; Paiste, P.; Külaviir, M.; Zhao, J.; Lust, E. Electrochemical co-reduction of praseodymium and bismuth from 1-butyl-1-methylpyrrolidinium bis (fluorosulfonyl) imide ionic liquid. Electrochem. Commun. 2022, 138, 107285. [Google Scholar] [CrossRef]
- Ni, S.; Gao, Y.; Yu, G.; Zhang, S.; Zeng, Z.; Sun, X. Tailored ternary hydrophobic deep eutectic solvents for synergistic separation of yttrium from heavy rare earth elements. Green Chem. 2022, 24, 7148–7161. [Google Scholar] [CrossRef]
- Su, H.; Ni, S.; Bie, C.; Wu, S.; Sun, X. Efficient and sustainable separation of yttrium from heavy rare earth using functionalized ionic liquid [N1888][NDA]. Sep. Purif. Technol. 2022, 285, 120302. [Google Scholar] [CrossRef]
- Agarwal, V.; Safarzadeh, M.S.; Galvin, J. Solvent extraction and separation of Y (III) from sulfate, nitrate and chloride solutions using PC88A diluted in kerosene. Miner. Process. Extr. Metall. Rev. 2018, 39, 258–265. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, H.; Wang, Y.; Li, F.; Sun, X. Separation of high-purity yttrium from ion-absorbed rare earth concentrate using (2, 6-dimethylheptyl) phenoxy acetic/propanoic acid. Sep. Purif. Technol. 2017, 184, 280–287. [Google Scholar] [CrossRef]
- Miaomiao, T.; Qiong, J.I.A.; Wuping, L. Studies on synergistic solvent extraction of rare earth elements from nitrate medium by mixtures of 8-hydroxyquinoline with Cyanex 301 or Cyanex 302. J. Rare Earths 2013, 31, 604–608. [Google Scholar]
- Batchu, N.K.; Dewulf, B.; Riaño, S.; Binnemans, K. Development of a solvometallurgical process for the separation of yttrium and europium by Cyanex 923 from ethylene glycol solutions. Sep. Purif. Technol. 2020, 235, 116193. [Google Scholar] [CrossRef]
- Sapsford, D.J.; Bowell, R.J.; Geroni, J.N.; Penman, K.M.; Dey, M. Factors influencing the release rate of uranium, thorium, yttrium and rare earth elements from a low grade ore. Miner. Eng. 2012, 39, 165–172. [Google Scholar] [CrossRef]
- Vukojević, V.; Đurđić, S.; Stefanović, V.; Trifković, J.; Čakmak, D.; Perović, V.; Mutić, J. Scandium, yttrium, and lanthanide contents in soil from Serbia and their accumulation in the mushroom Macrolepiota procera (Scop.) Singer. Environ. Sci. Pollut. Res. 2019, 26, 5422–5434. [Google Scholar] [CrossRef]
- Chen, H.; Chen, L.; Zhang, L.; Guo, M. Spatial Heterogeneity of Rare Earth Elements: Implications for the Topsoil of Regional Ion-Adsorption Type Rare Earth Deposit Areas in Southern China. Minerals 2023, 13, 784. [Google Scholar] [CrossRef]
- Kovarikova, M.; Tomaskova, I.; Soudek, P. Rare earth elements in plants. Biol. Plant. 2019, 63, 20–32. [Google Scholar] [CrossRef]
- Yuan, Y.; Gaugler, J.; Fan, J.; Thapaliya, B.P.; Luo, H.; Atifi, A.; Diaz, L.A.; Dai, S. Facile Room-Temperature Electrodeposition of Rare Earth Metals in a Fluorine-Free Task-Specific Electrolyte. ACS Sustain. Chem. Eng. 2023, 11, 12532–12540. [Google Scholar] [CrossRef]
- Li, J.; Gong, A.; Li, F.; Qiu, L.; Zhang, W.; Gao, G.; Liu, Y.; Li, J. Synthesis and characterization of magnetic mesoporous Fe3O4 @mSiO2–DODGA nanoparticles for adsorption of 16 rare earth elements. RSC Adv. 2018, 8, 39149–39161. [Google Scholar] [CrossRef]
- Basuki, K.T.; Rohmaniyyah, A.; Pusparini, W.R.; Saputra, A. Extraction development for the separation of gadolinium from yttrium and dysprosium concentrate in nitric acid using cyanex 572. Int. J. Technol. 2020, 11, 450–460. [Google Scholar] [CrossRef]
- Puzikov, E.A.; Zilberman, B.Y.; Goletskii, N.D.; Kudinov, A.S. Description of the Extraction of Rare Earth Element Nitrates from Weakly Acidic Solutions with Concentrated Tributyl Phosphate Solutions. Radiochemistry 2019, 61, 447–458. [Google Scholar] [CrossRef]
- Evangelista, L.R.; Lenzi, E.K.; Barbero, G.; Macdonald, J.R. On the equivalence between specific adsorption and kinetic equation descriptions of the admittance response in electrolytic cells. J. Chem. Phys. 2013, 138, 114702. [Google Scholar] [CrossRef] [PubMed]
- Bagri, P.; Luo, H.; Popovs, I.; Thapaliya, B.P.; Dehaudt, J.; Dai, S. Trimethyl phosphate based neutral ligand room temperature ionic liquids for electrodeposition of rare earth elements. Electrochem. Commun. 2018, 96, 88–92. [Google Scholar] [CrossRef]
- Romann, T. Preparation and Surface Modification of Bismuth Thin Film, Porous, and Microelectrodes. Ph.D. Thesis, University of Tartu, Tartu, Estonia, 2010. [Google Scholar]


| Element | Cw Initial, ppb | Corg, ppb | Extraction Efficiency |
|---|---|---|---|
| Y | 36471 | 22371 | 61.3% |
| La | 16751 | 0 | 0.0% |
| Ce | 39128 | 0 | 0.0% |
| Pr | 4916 | 0 | 0.0% |
| Nd | 21518 | 229 | 1.1% |
| Sm | 4479 | 0 | 0.0% |
| Eu | 1037 | 36 | 3.5% |
| Gd | 5847 | 0 | 0.0% |
| Tb | 852 | 136 | 16.0% |
| Dy | 5132 | 1647 | 32.1% |
| Ho | 1008 | 487 | 48.3% |
| Er | 2612 | 1751 | 67.0% |
| Tm | 296 | 262 | 88.5% |
| Yb | 1441 | 1410 | 97.8% |
| Lu | 177 | 176 | 99.4% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jürjo, S.; Oll, O.; Lust, E. Yttrium Separation from Phosphorite Extract Using Liquid Extraction with Room Temperature Ionic Liquids Followed by Electrochemical Reduction. Metals 2024, 14, 927. https://doi.org/10.3390/met14080927
Jürjo S, Oll O, Lust E. Yttrium Separation from Phosphorite Extract Using Liquid Extraction with Room Temperature Ionic Liquids Followed by Electrochemical Reduction. Metals. 2024; 14(8):927. https://doi.org/10.3390/met14080927
Chicago/Turabian StyleJürjo, Silvester, Ove Oll, and Enn Lust. 2024. "Yttrium Separation from Phosphorite Extract Using Liquid Extraction with Room Temperature Ionic Liquids Followed by Electrochemical Reduction" Metals 14, no. 8: 927. https://doi.org/10.3390/met14080927








