Effect of Etching Condition on Nanoporous Structure and Methyl Orange Decomposition of Fe-Si-B Metallic Glass
Abstract
:1. Introduction
2. Materials and Experiments
2.1. Materials and Reagents
2.2. Preparation and Structural Characterization
2.3. Batch Experiments for Degradation of Azo Dyes
3. Results and Discussion
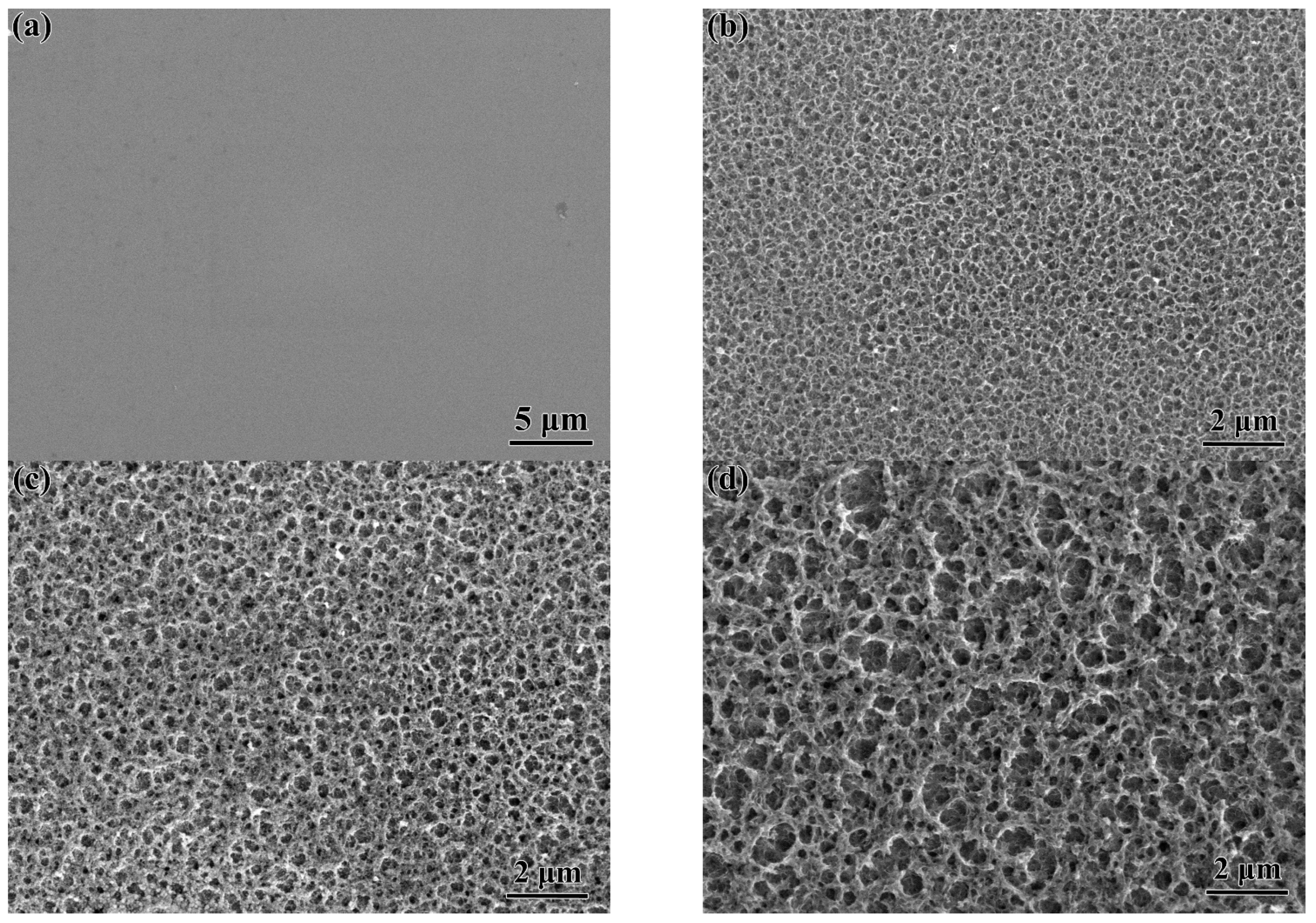
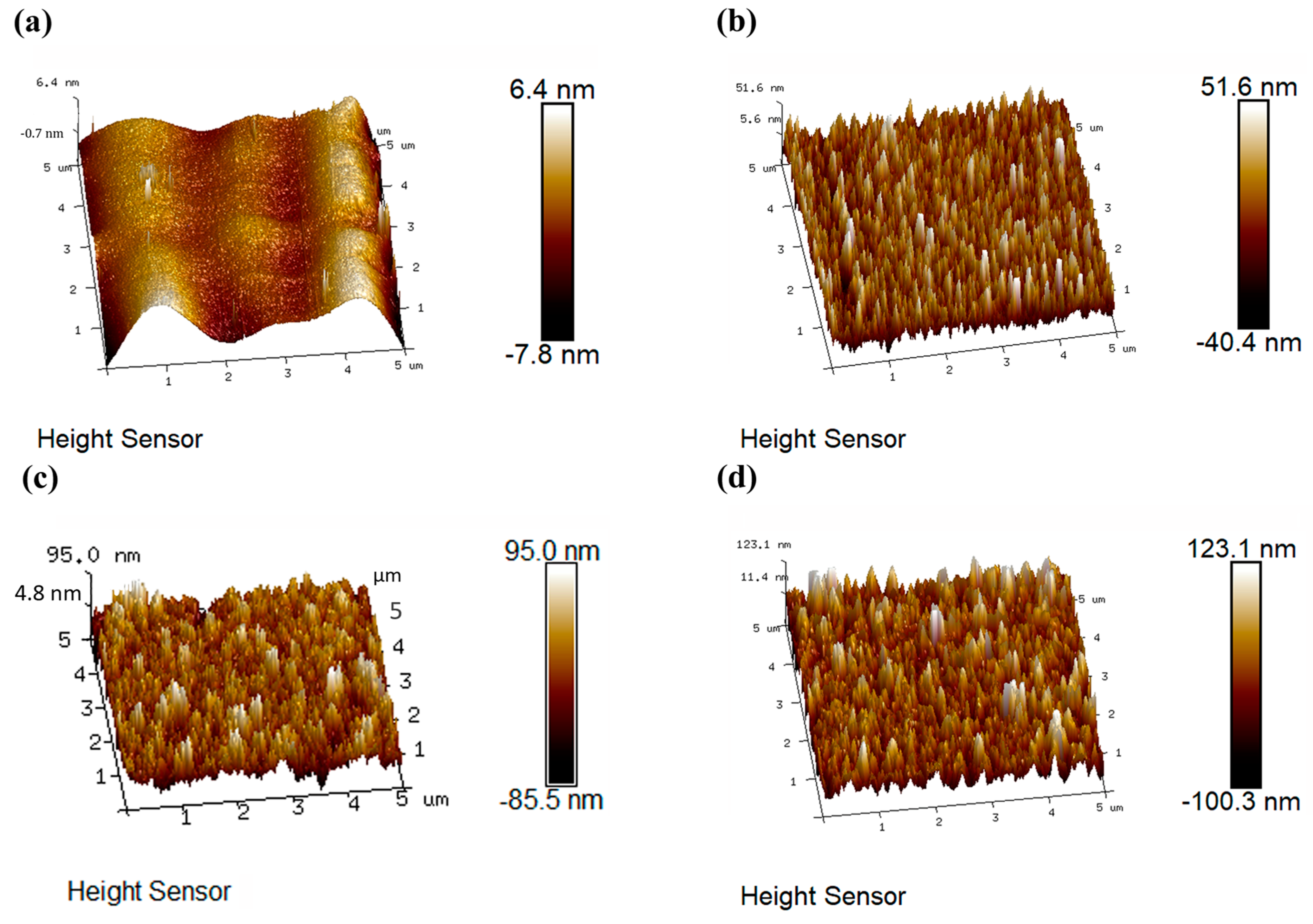
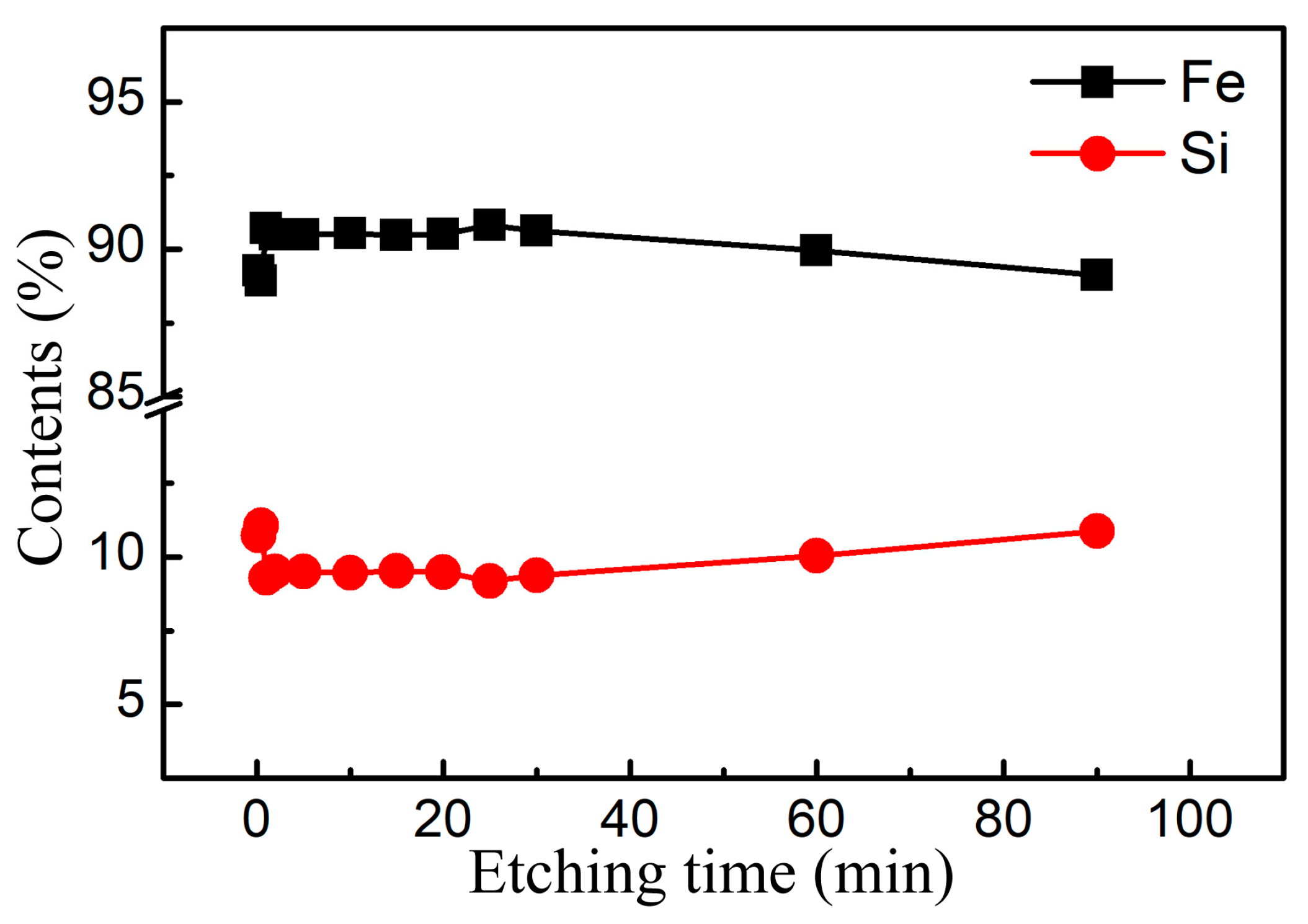
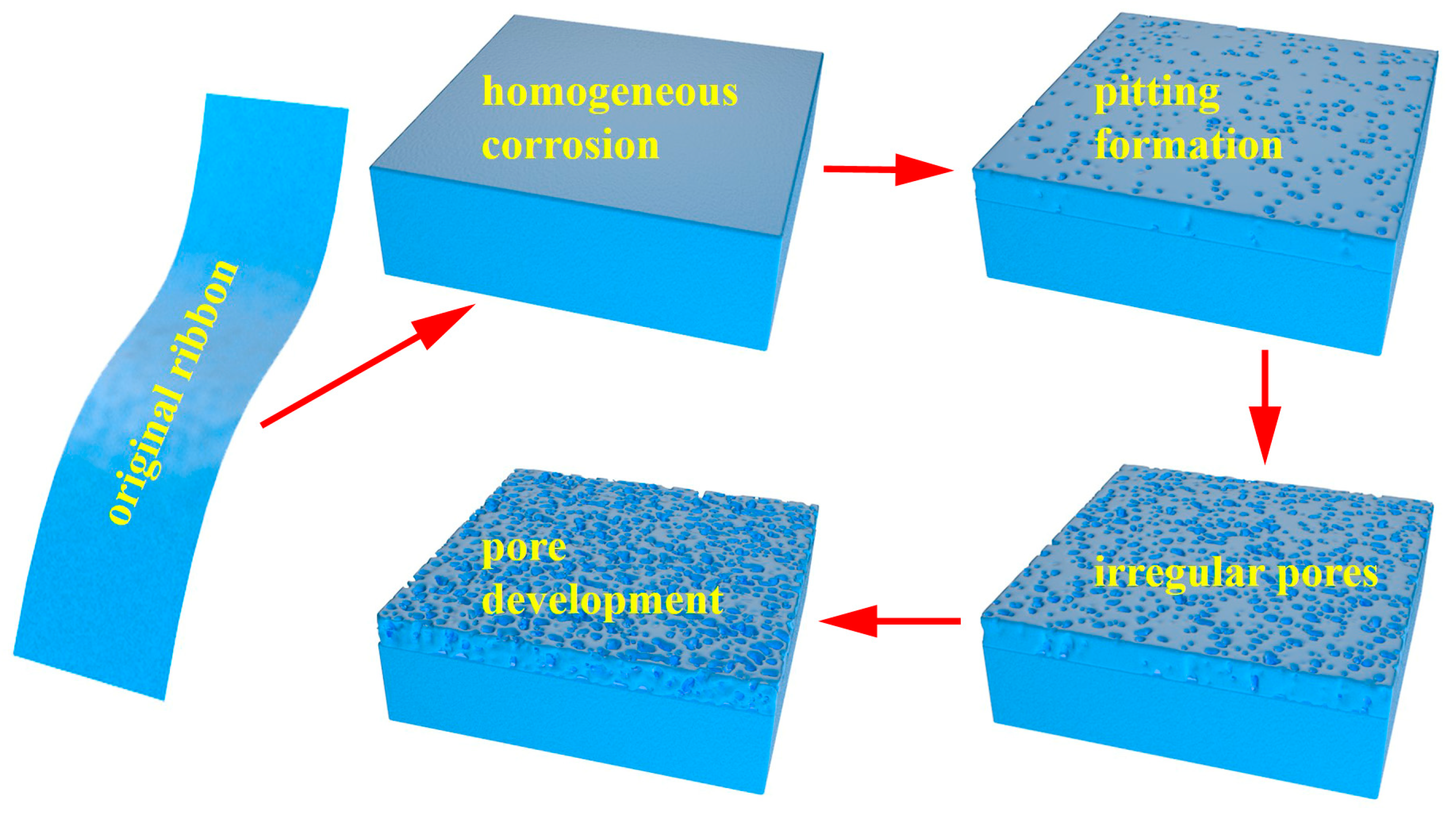
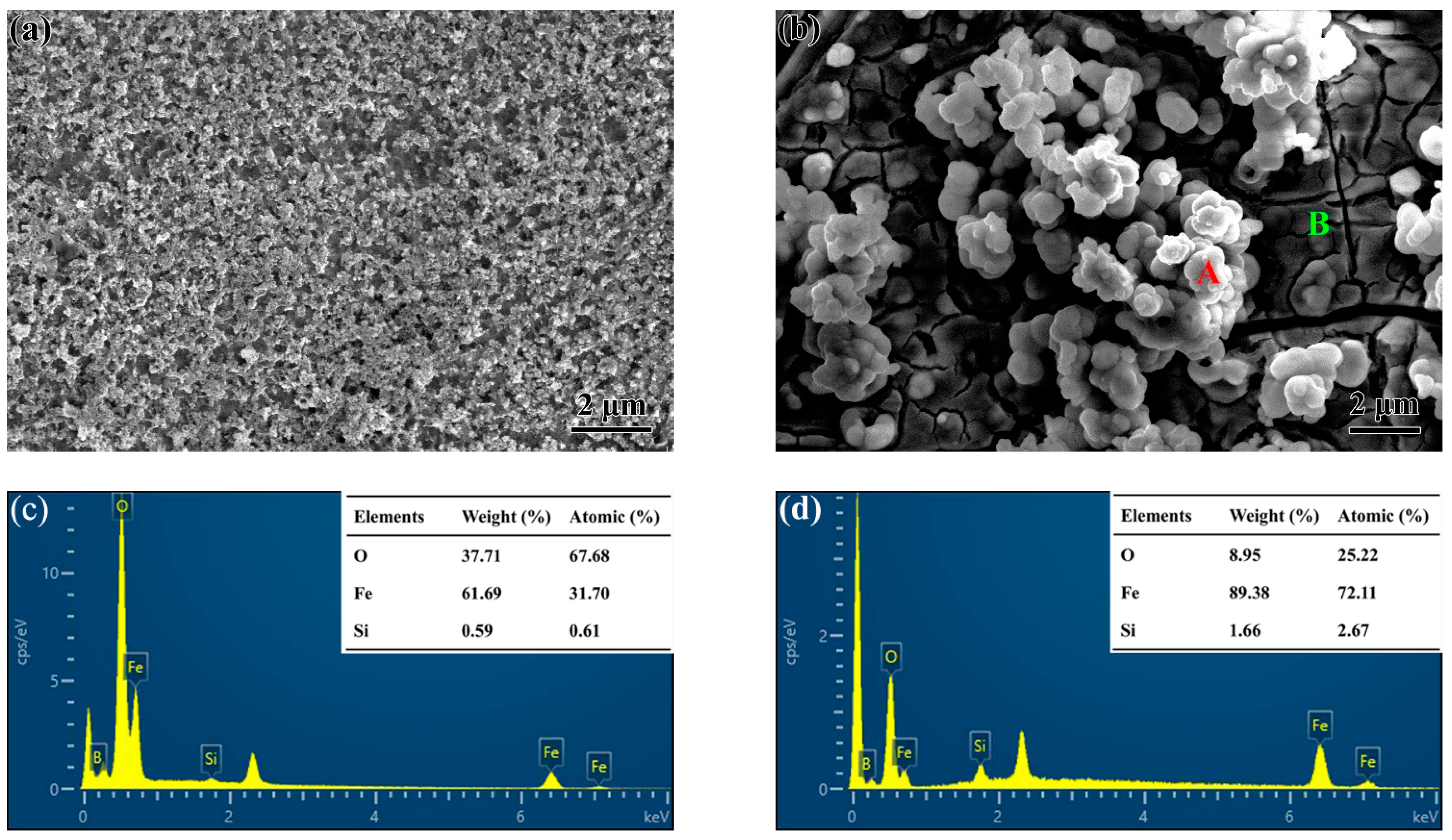
3.1. Structural Characterization and Forming Mechanism of the Surface Porous Structures
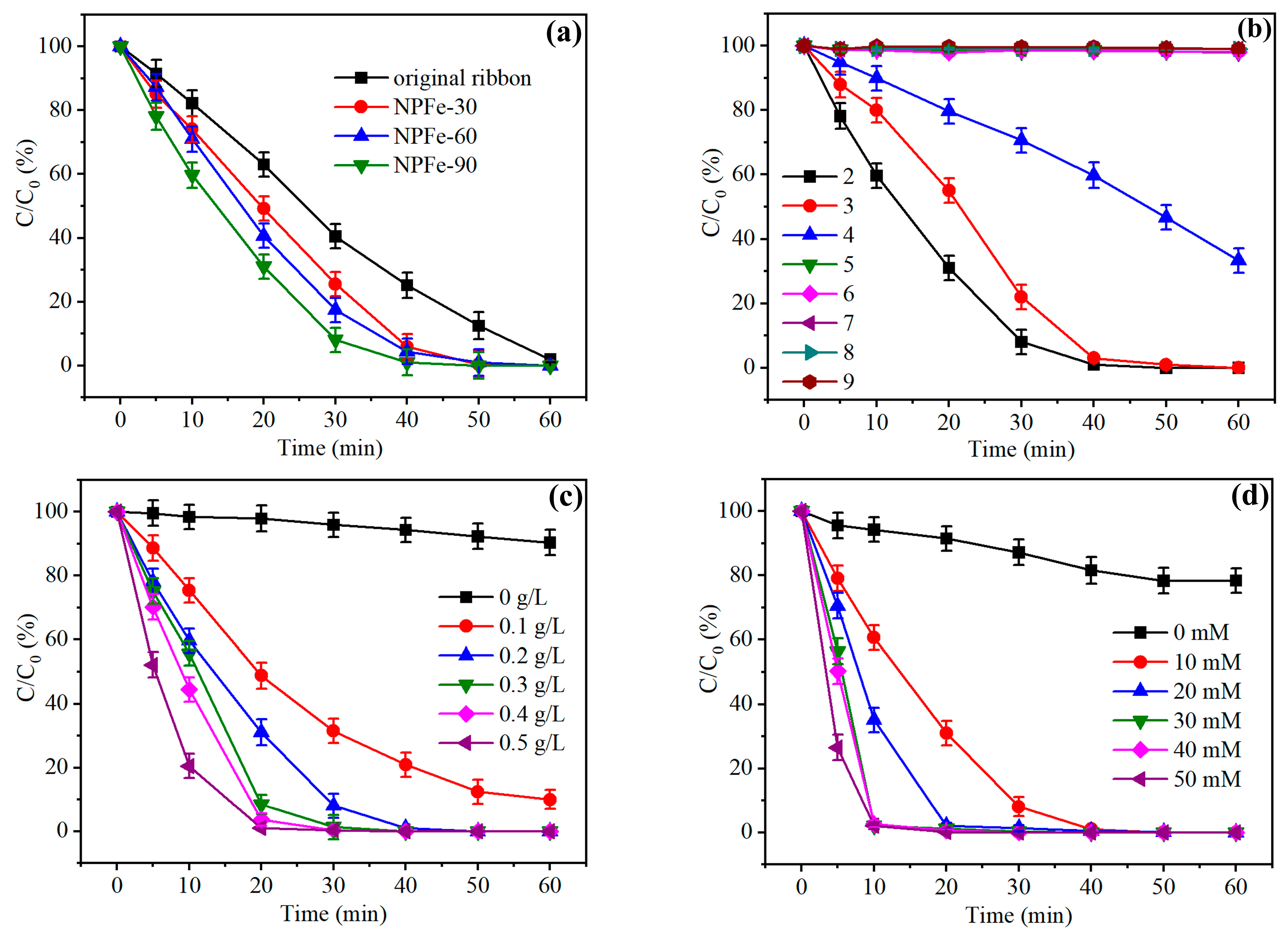
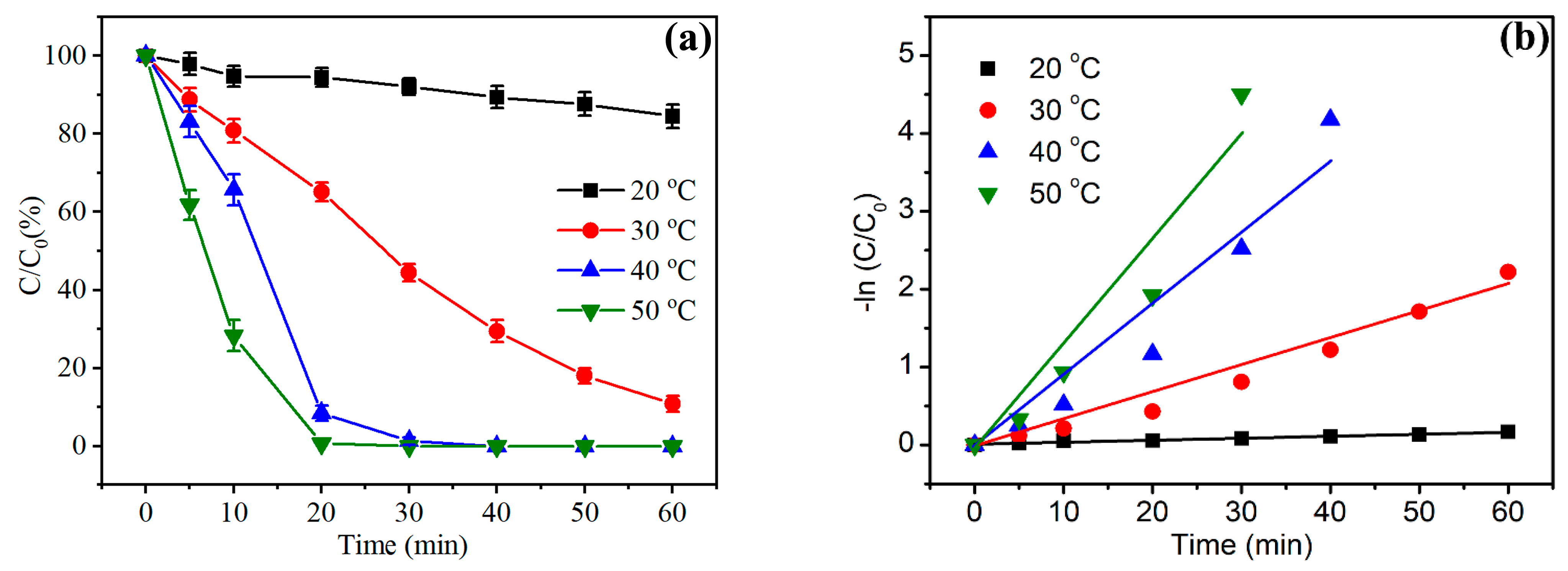
3.2. Dye Degradation Properties of NPFe Catalyst
3.3. Dye Degradation Mechanism of NPFe Catalyst
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, H.B.; Wang, F.; Lu, Q.M.; Qiu, Y.B.; Liu, W.Q.; Chen, J.W.; Yi, X.F.; Yue, M. Nd-Fe-B: From sludge waste to powders via purification and modified Ca-reduction reaction process. J. Magn. Magn. Mater. 2022, 543, 168606. [Google Scholar] [CrossRef]
- Zhao, B.W.; Liu, Y.L.; Zhao, H.; Li, S.T.; Ma, G.F.; Zhang, S.M.; Zhang, H.F.; Zhu, Z.W. In-situ activation of Fe78Si9B13 metallic glasses for electro-Fenton degradation of industrial oily wastewater. Sustain. Mater. Technol. 2023, 35, e00539. [Google Scholar] [CrossRef]
- Lin, H.M.; Chen, K.L.; Zheng, S.J.; Zeng, R.P.; Lin, Y.C.; Jian, R.K.; Bai, W.B.; Xu, Y.L. Facile fabrication of natural superhydrophobic eleostearic acid-SiO2@cotton fabric for efficient separation of oil/water mixtures and emulsions. Sustain. Mater. Technol. 2022, 32, e00418. [Google Scholar] [CrossRef]
- Wang, S.S.; Liu, L. Fabrication of novel nanoporous copper powder catalyst by dealloying of ZrCuNiAl amorphous powders for the application of wastewater treatments. J. Hazard. Mater. 2017, 340, 445–453. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. A review on Fenton and improvements to the Fenton process for wastewater treatment. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar] [CrossRef]
- Zuo, M.Q.; Yi, S.H.; Choi, J.H. Excellent dye degradation performance of FeSiBP amorphous alloys by Fenton-like process. J. Environ. Sci. 2021, 105, 116–127. [Google Scholar] [CrossRef]
- Pignatello, J.J.; Oliveros, E.; Mackay, A. Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Crane, R.A.; Scott, T.B. Nanoscale zero-valent iron: Future prospects for an emerging water treatment technology. J. Hazard. Mater. 2012, 211, 112–125. [Google Scholar] [CrossRef]
- Fu, F.; Dionysiou, D.D.; Liu, H. The use of zero-valent iron for groundwater remediation and wastewater treatment: A review. J. Hazard. Mater. 2014, 267, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Qiu, L.; Zhu, Q.; Liang, X.; Huang, J.; Yang, M.; Zhang, Z.; Ma, J.; Shen, J. Insight into efficient degradation of 3,5-dichlorosalicylic acid by Fe-Si-B amorphous ribbon under neutral condition. Appl. Catal. B 2021, 294, 120258. [Google Scholar] [CrossRef]
- Zhang, P.; Tan, J.; Tian, Y.; Yan, H.; Yu, Z. Research progress on selective laser melting (SLM) of bulk metallic glasses (BMGs): A review. Int. J. Adv. Manuf. Technol. 2022, 118, 2017–2057. [Google Scholar] [CrossRef]
- Li, H.F.; Zheng, Y.F. Recent advances in bulk metallic glasses for biomedical applications. Acta. Biomater. 2016, 36, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, C.; Chen, Z.; Li, Y.; Yan, W.; Yu, H.; Liu, L. Three-dimensional hierarchical porous structures of metallic glass/copper composite catalysts by 3D Printing for efficient wastewater treatments. ACS Appl. Mater. Interfaces 2021, 13, 7227–7237. [Google Scholar] [CrossRef] [PubMed]
- Weng, N.; Wang, F.; Qin, F.; Tang, W.; Dan, Z. Enhanced Azo-Dyes Degradation Performance of Fe-Si-B-P Nanoporous Architecture. Materials 2017, 10, 1001. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Shao, Y.; Chen, N.; Liu, X.; Chen, S.Q.; Yao, K.F. Insight into the high reactivity of commercial Fe–Si–B amorphous zero-valent iron in degrading azo dye solutions. RSC Adv. 2015, 5, 34032–34039. [Google Scholar] [CrossRef]
- Wang, X.; Pan, Y.; Zhu, Z.; Wu, J. Efficient degradation of rhodamine B using Fe-based metallic glass catalyst by Fenton-like process. Chemosphere 2014, 117, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Kang, J.; Zhang, W.C. Surface aging behaviour of Fe-based amorphous alloys as catalysts during heterogeneous photo Fenton-like process for water treatment. Appl. Catal. B Environ. 2017, 204, 537–547. [Google Scholar] [CrossRef]
- Liang, S.X.; Salamon, S.; Zerebecki, S.; Zhang, L.C.; Jia, Z.; Wende, H.; Reichenberger, S.; Barcikowski, S. A laser-based synthesis route for magnetic metallic glass nanoparticles. Scr. Mater. 2021, 203, 114094. [Google Scholar] [CrossRef]
- Si, J.J.; Gu, J.L.; Luan, H.W. Porous composite architecture bestows Fe-based glassy alloy with high and ultra-durable degradation activity in decomposing azo dye. J. Hazard. Mater. 2020, 388, 122043. [Google Scholar] [CrossRef]
- Wang, J.Q.; Liu, Y.H.; Chen, M.W.; Xie, G.Q. Rapid Degradation of Azo Dye by Fe-Based Metallic Glass Powder. Adv. Funct. Mater. 2012, 22, 2567–2570. [Google Scholar] [CrossRef]
- Wang, H.; Wang, B.; Liu, Z.Q.; Zhao, J.F.; Zhang, H.M. Microstructure and mechanical properties of NiTi nanoporous structures fabricated with dealloying process. J. Alloys Compd. 2023, 933, 167804. [Google Scholar] [CrossRef]
- Paschalidou, E.M.; Celegato, F.; Scaglione, F. The mechanism of generating nanoporous Au by dealloying amorphous alloys. Acta. Mater. 2016, 119, 177–183. [Google Scholar] [CrossRef]
- Li, R.; Liu, X.J.; Wang, H.; Wu, Y.; Chu, X.M.; Lu, Z.P. Nanoporous silver with tunable pore characteristics and superior surface enhanced Raman scattering. Corros. Sci. 2014, 84, 159–164. [Google Scholar] [CrossRef]
- Gu, L.Y.; Wang, S.S.; Hui, X.D.; Li, F.D.; Lin, H.F.; Wu, K.M. Degradation performance and mechanism toward methyl orange via nanoporous copper powders fabricated by dealloying of ZrCuNiAl metallic glassy precursors. Nanotechnology 2022, 33, 135713. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Q.; Li, M.; Ji, Q.M.; Chen, X.; Lan, S.; Feng, T.; Yao, K.F. Functional 3D nanoporous Fe-based alloy from metallic glass for high-efficiency water splitting and wastewater treatment. J. Non-Cryst. Solids 2021, 571, 121070. [Google Scholar] [CrossRef]
- Zhang, X.H.; Zeng, Y.Q.; Yin, L. Formation of micro/nano pits with high catalytic activity on Fe80B20 amorphous alloy. Corros. Sci. 2018, 141, 109–116. [Google Scholar] [CrossRef]
- Liotta, L.F.; Gruttadauria, M.; Carlo, G.D. Heterogeneous catalytic degradation of phenolic substrates: Catalysts activity. J. Hazard. Mater. 2009, 162, 588–606. [Google Scholar] [CrossRef] [PubMed]
- Si, J.J.; Yang, X.L.; Luan, H.W.; Shao, Y.; Yao, K.F. Cheap, fast and durable degradation of azo dye wastewater by zero-valent iron structural composites. J. Environ. Chem. Eng. 2021, 9, 106314. [Google Scholar] [CrossRef]
- Laat, J.D.; Gallard, H. Catalytic decomposition of hydrogen peroxide by Fe(III) in homogeneous aqueous solution: Mechanism and kinetic modeling. Environ. Sci. Technol. 1999, 33, 2726–2732. [Google Scholar] [CrossRef]
- Devi, L.G.; Kumar, S.G.; Reddy, K.M.; Munikrishnappa, C. Photo degradation of methyl orange an azo dye by advanced Fenton process using zero valent metallic iron: Influence of various reaction parameters and its degradation mechanism. J. Hazard. Mater. 2009, 164, 459–467. [Google Scholar] [CrossRef]
- Jia, Z.; Wang, Q.; Sun, L.; Wang, Q.; Zhang, L.C.; Wu, G.; Luan, J.H.; Jiao, Z.B.; Wang, A.D.; Liang, S.X. Attractive in situ self-reconstructed hierarchical gradient structure of metallic glass for high efficiency and remarkable stability in catalytic performance. Adv. Funct. Mater. 2019, 29, 1807857. [Google Scholar] [CrossRef]
- Noorjahan, M.; Kumari, V.D.; Subrahmanyam, M.; Panda, L. Immobilized Fe(III)-HY: An efficient and stable photo-Fenton catalyst. Appl. Catal. B Environ. 2005, 57, 291–298. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Duan, Z.; Guo, Y.; Gu, L.; Wu, K. Effect of Etching Condition on Nanoporous Structure and Methyl Orange Decomposition of Fe-Si-B Metallic Glass. Metals 2024, 14, 936. https://doi.org/10.3390/met14080936
Wang S, Duan Z, Guo Y, Gu L, Wu K. Effect of Etching Condition on Nanoporous Structure and Methyl Orange Decomposition of Fe-Si-B Metallic Glass. Metals. 2024; 14(8):936. https://doi.org/10.3390/met14080936
Chicago/Turabian StyleWang, Shushen, Zhiwei Duan, Yongzhi Guo, Lingyu Gu, and Kaiming Wu. 2024. "Effect of Etching Condition on Nanoporous Structure and Methyl Orange Decomposition of Fe-Si-B Metallic Glass" Metals 14, no. 8: 936. https://doi.org/10.3390/met14080936



