Recovery of Silver and Lead from Jarosite Residues by Roasting and Reducing Pyrometallurgical Processes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
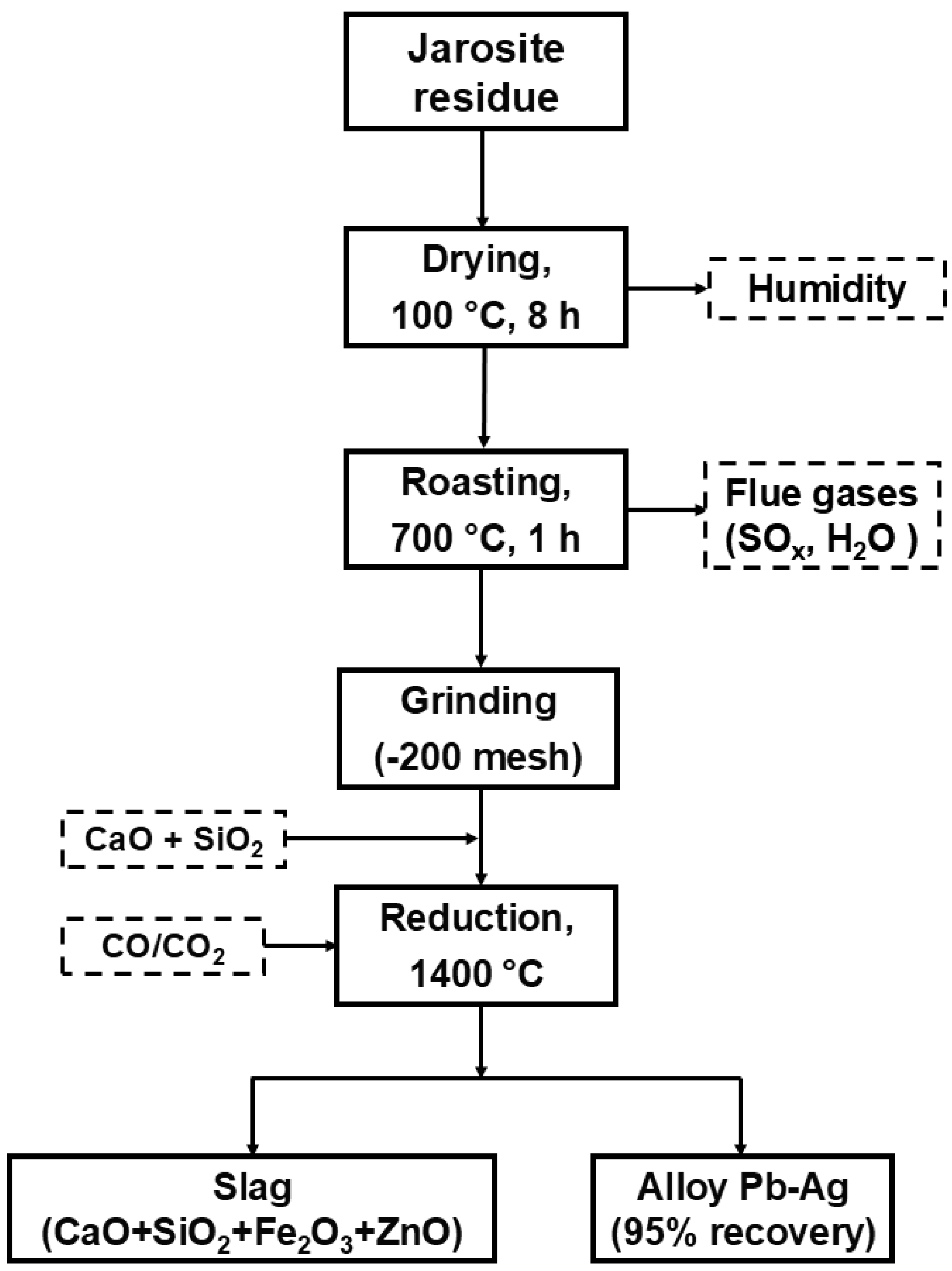
2.2. Methods
2.3. Characterization
2.4. Thermodynamic Modeling
2.5. Leaching
3. Results and Discussion
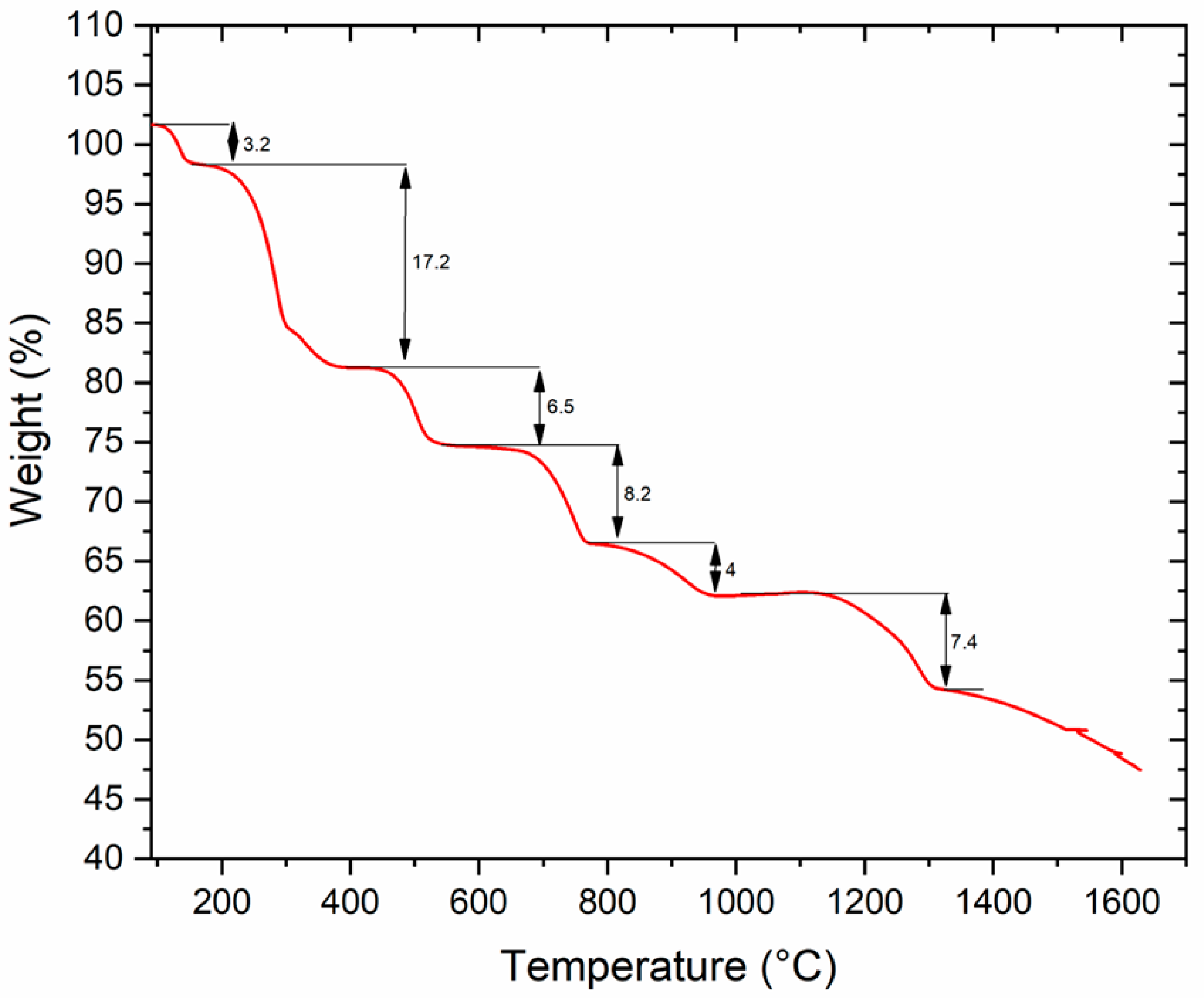
3.1. TGA Results
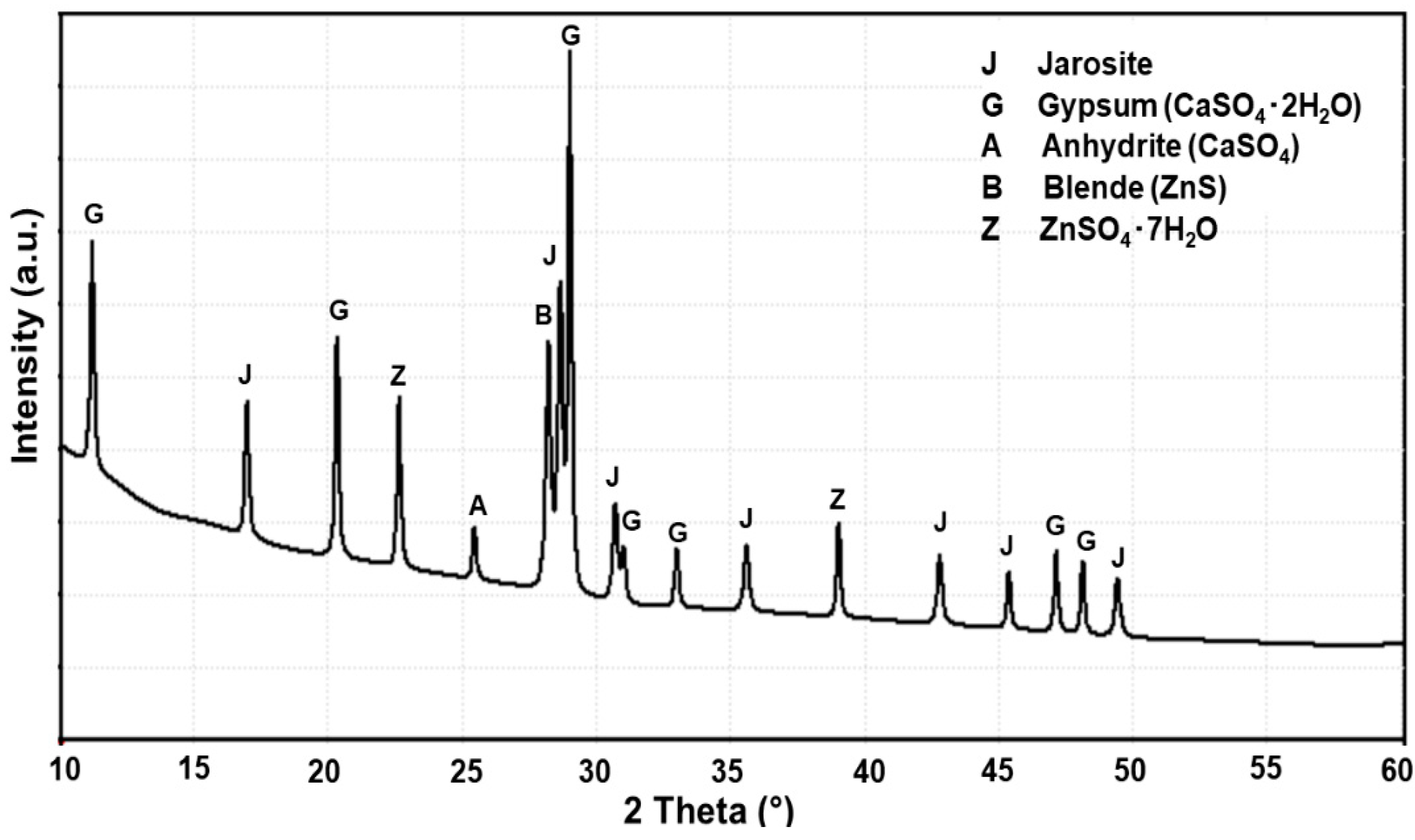
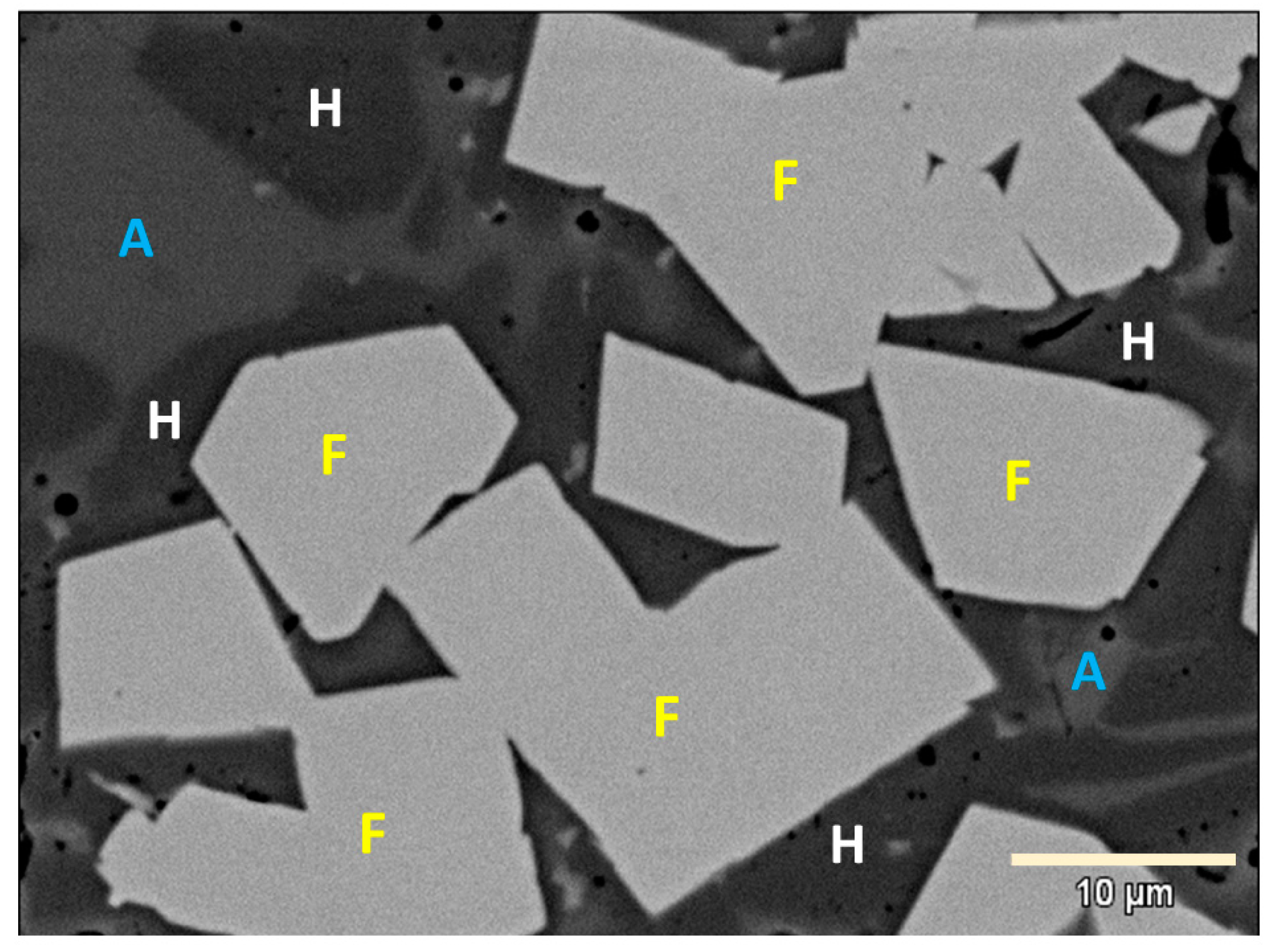
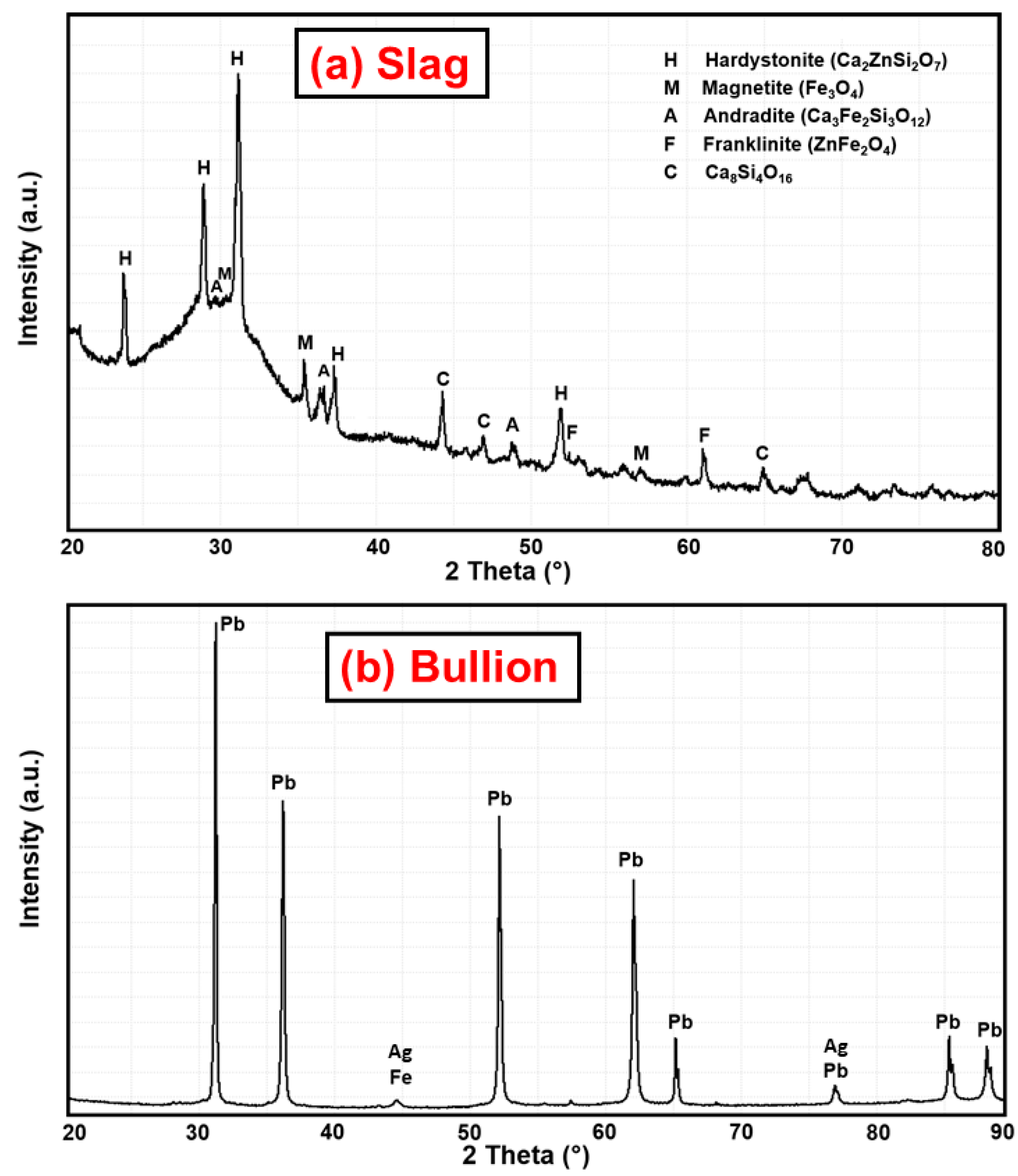
3.2. XRD Results at High Temperature
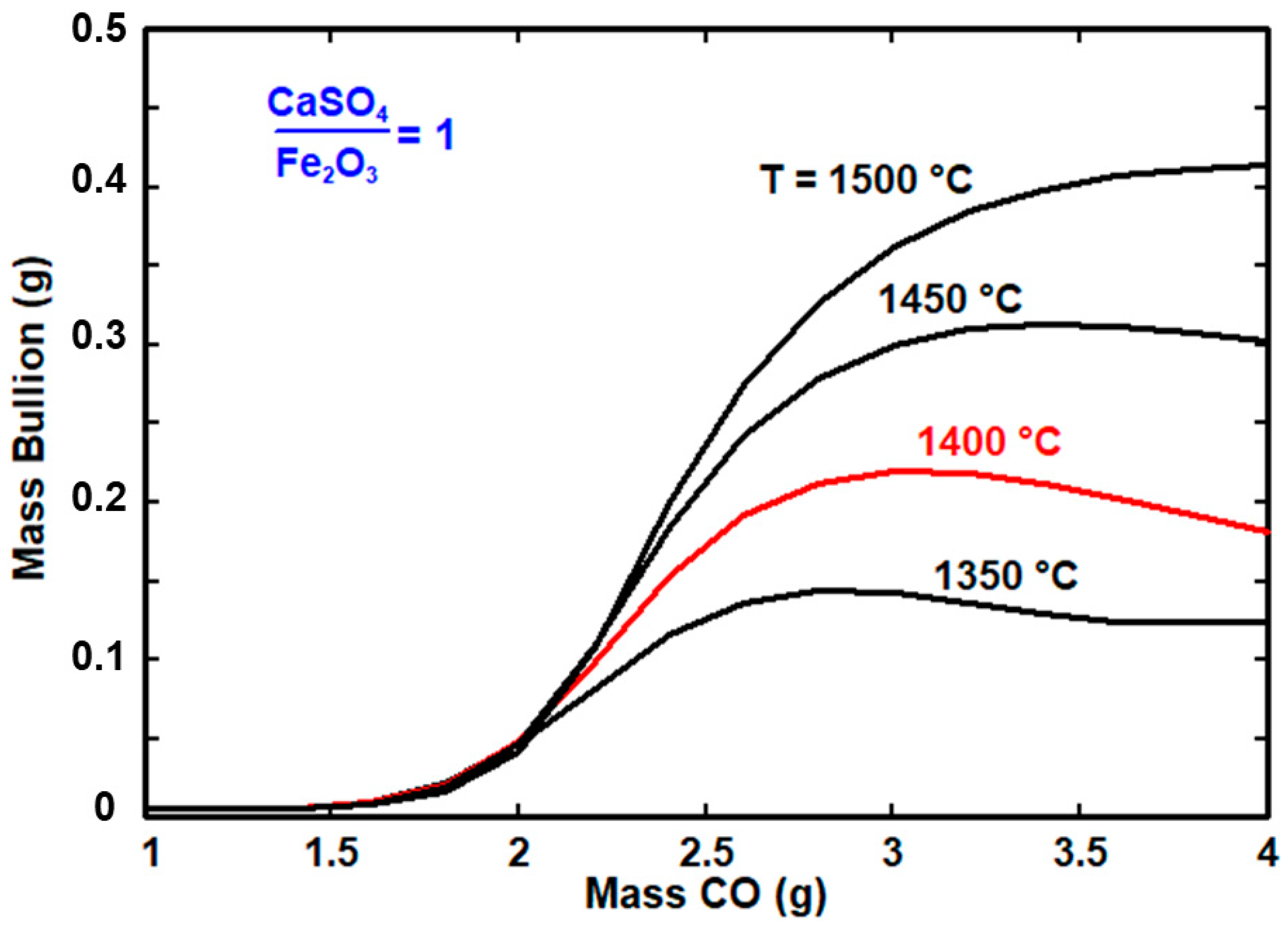
3.3. Reduction Experiments
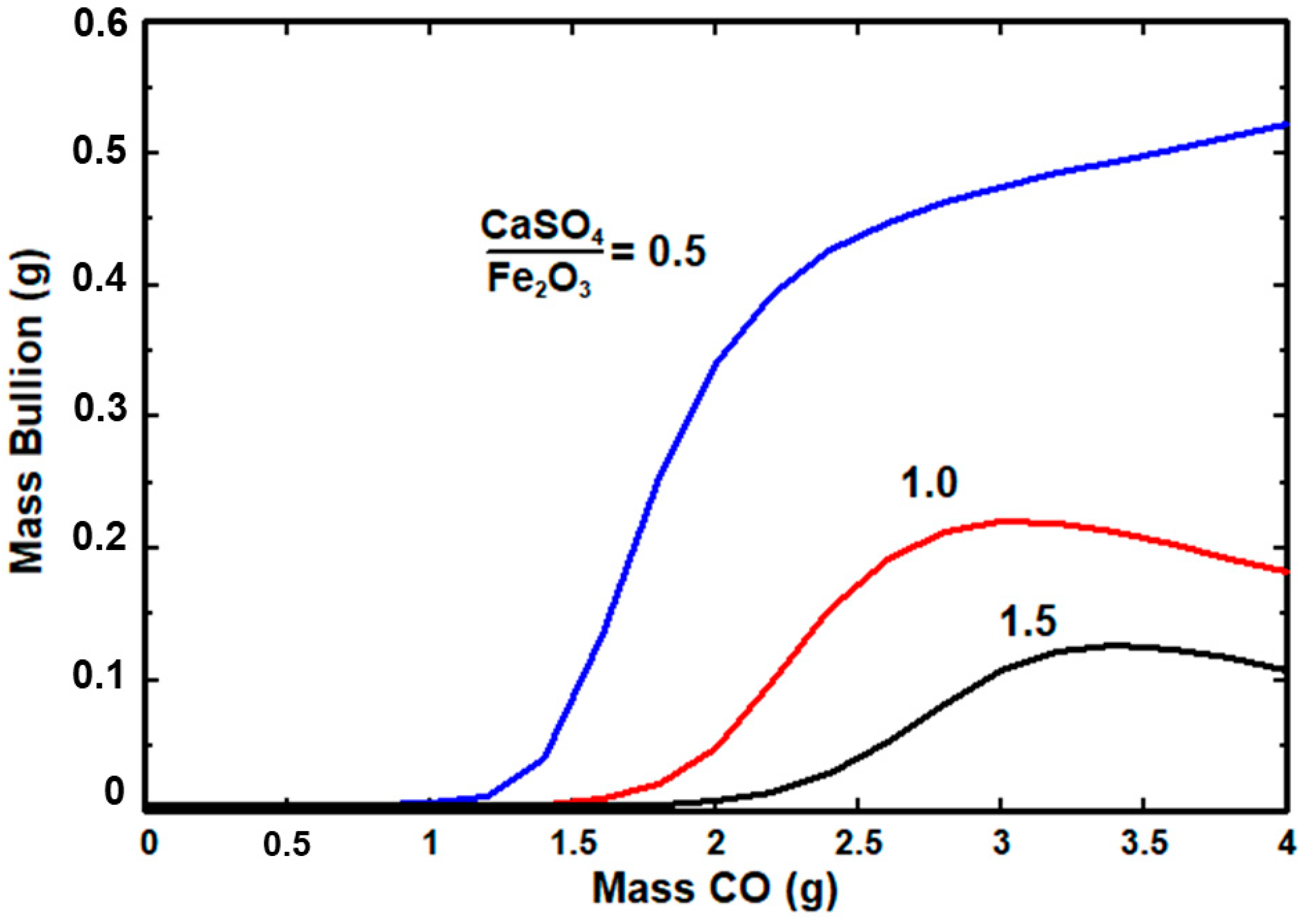
3.4. Thermodynamic Analysis
- (a)
- The metallic phase consists mainly of molten lead with small amounts of silver, iron, and zinc.
- (b)
- Most of the zinc and iron remain in the slag phase, which consists mainly of silicate species and magnetite.
- (c)
- The CaSO4 content in the roasted jarosite considerably affects the formation of the lead-rich metallic phase (bullion). The increase in the CaSO4 content led to a decrease in the metallic phase.
- (d)
- The high CaSO4 content also required an increased use of reducing agent gas to form the metallic phase.
3.5. Results of the Reduction Experiments
3.6. Leaching Results
4. Conclusions
- ▪
- The jarosite residues were decomposed at 700 °C into Fe2O3, Fe3O4 and CaSO4.
- ▪
- A mixture of 48 mass percent CaO and 52 mass percent SiO2 was used as a flux for the roasted residue to obtain a completely liquid system. In addition to the fluxes, a reducing gas with a molar ratio of 70/30 CO/CO2 was introduced into the molten system to obtain a slag and a lead-rich metallic phase.
- ▪
- Lead and silver were almost completely recovered in this process, while zinc and iron remained in the slag.
- ▪
- The presence of CaSO4 in the system influences the amount of the lead-rich metallic phase. A high CaSO4 content requires the addition of a larger amount of reducing agent to produce the metallic phase.
- ▪
- The slag resulting from the reduction process meets environmental specifications and could be used as a raw material in other industries.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eftekhari, N.; Kargar, M.; Rokhbakhsh Zamin, F.; Rastakhiz, N.; Manafi, Z. A review on various aspects of jarosite and its utilization potentials. Ann. Chim.-Sci. Mat. 2020, 44, 43–52. [Google Scholar] [CrossRef]
- Jha, M.K.; Kumar, V.; Singh, R.J. Review of hydrometallurgical recovery of zinc from industrial wastes. Resour. Conserv. Recycl. 2001, 33, 1–22. [Google Scholar] [CrossRef]
- Dutrizac, J.E. The effectiveness of jarosite species for precipitating sodium jarosite. JOM 1999, 51, 30–32. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, H.; Zhang, W.; Song, R.; Jiang, B. Study on recovery of lead, zinc, iron from jarosite residues and simultaneous sulfur fixation by direct reduction. Physicochem. Probl. Miner. Process. 2018, 54, 517–526. [Google Scholar] [CrossRef]
- Ju, S.; Zhang, Y.; Zhang, Y.; Xue, P.; Wang, Y. Clean hydrometallurgical route to recover zinc, silver, lead, copper, cadmium and iron from hazardous jarosite residues produced during zinc hydrometallurgy. J Hazard. Mater. 2011, 192, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, A.M.; Pons, M.N.; Ricoux, Q.; Issa, S.; Goettmann, F.; Lapicque, F. New pathway for utilization of jarosite, an industrial waste of zinc hydrometallurgy. Miner. Eng. 2021, 170, 107030. [Google Scholar] [CrossRef]
- Picazo-Rodríguez, N.G.; Soria-Aguilar, M.J.; Chaidez, J.; Flores, M.; Almaguer-Guzmán, I.; Carrillo-Pedroza, F.R. Analysis of the behavior of As and Pb during the pretreatments applied to a jarosite residue for the recovery of gold and silver. Metals 2023, 13, 138. [Google Scholar] [CrossRef]
- Jiang, G.M.; Bing, P.E.; Liang, Y.J.; Chai, L.Y.; Wang, Q.W.; Li, Q.Z.; Ming, H.U. Recovery of valuable metals from zinc leaching residue by sulfate roasting and water leaching. Trans. Nonferrous Met. Soc. China 2017, 27, 1180–1187. [Google Scholar] [CrossRef]
- Ju, S.H.; Zhang, L.; Peng, J.; Shi, Z.; Guou, S.; Liu, B.; Wang, Y. Thermodynamics of leaching roasted jarosite residue from zinc hydrometallurgy in NH4Cl system. Trans. Nonferrous Met. Soc. China 2013, 23, 1179–1183. [Google Scholar] [CrossRef]
- Steinlechner, S.; Antrekowitsch, J. Thermodynamic considerations for a pyrometallurgical extraction of indium and silver from a residue. Metals 2018, 8, 335. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Yang, H.F.; Jiang, B.; Song, R.L.; Zhang, W.H. Comprehensive recovery of lead, zinc, and iron from hazardous jarosite residues using direct reduction followed by magnetic separation. Int. J. Miner. Metall. Mater. 2018, 25, 123–130. [Google Scholar] [CrossRef]
- Zhu, D.; Yang, C.; Pan, J.; Guo, Z.; Li, S. New pyrometallurgical route for separation and recovery of Fe, Zn, In, Ga and S from jarosite residues. J. Clean. Prod. 2018, 205, 781–788. [Google Scholar] [CrossRef]
- Han, H.; Sun, W.; Hu, Y.; Jia, B.; Tang, H. Anglesite and silver recovery from jarosite residues through roasting and sulfidization-flotation in zinc hydrometallurgy. J. Hazard. Mater. 2014, 278, 49–54. [Google Scholar] [CrossRef]
- Mombelli, D.; Mapelli, C.; Di Cecca, C.; Barella, S.; Gruttadauria, A.; Ragona, M.; Pisu, M.; Viola, A. Characterization of cast iron and slag produced by jarosite sludges reduction via Arc Transferred Plasma (ATP) reactor. J. Environ. Chem. Eng. 2018, 6, 773–783. [Google Scholar] [CrossRef]
- Ge, H.; Pan, Z.; Xie, F.; Lu, D.; Wang, W.; Wu, S. Recovery of valuable metals by roasting of jarosite in cement kiln. Metals 2023, 13, 250. [Google Scholar] [CrossRef]
- Rämä, M.; Nurmi, S.; Jokilaakso, A.; Klemettinen, L.; Taskinen, P.; Salminen, J. Thermal processing of jarosite leach residue for a safe disposable slag and valuable metals recovery. Metals 2018, 8, 744. [Google Scholar] [CrossRef]
- Chaidez-Felix, J.; Romero-Serrano, A.; Hernandez-Ramirez, A.; Perez-Labra, M.; Almaguer-Guzman, I.; Benavides-Perez, R.; Flores-Favela, M. Effect of copper, sulfur, arsenic and antimony on silver distribution in phases of lead blast furnace. Trans. Nonferrous Met. Soc. China 2014, 24, 1202–1209. [Google Scholar] [CrossRef]
- Mexican Official Norms. NOM-053-ECOL. 1993. Available online: http://www.Semarnat.gob.mx (accessed on 5 March 2024).
- ASTM C702/C702M-18, Standard Practice for Reducing Samples of Aggregate to Testing Size. Available online: https://compass.astm.org/document/?contentCode=ASTM%7CC0702_C0702M-18%7Cen-US&proxycl=https%3A%2F%2Fsecure.astm.org&fromLogin=true (accessed on 9 February 2024).
- Das, G.K.; Anand, S.; Acharya, S.; Das, R.P. Preparation and decomposition of ammoniojarosite at elevated temperatures in H2O (NH4)2SO4 H2SO4 media. Hydrometallurgy 1995, 38, 263–276. [Google Scholar] [CrossRef]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Gheribi, A.E.; Hack, K.; Jung, I.H.; Kang, Y.B.; Melançon, J.; et al. FactSage Thermochemical Software and Databases, 2010–2016. Calphad 2016, 54, 35–53. [Google Scholar] [CrossRef]
- Ristic, M.; Music, S.; Orehovec, Z. Thermal decomposition of synthetic ammonium jarosite. J. Mol. Struct. 2005; 744–747, 295–300. [Google Scholar] [CrossRef]
- Frost, R.L.; Wills, R.A.; Kloprogge, J.T.; Martens, W. Thermal decomposition. J. Therm. Anal. Calorim. 2006, 84, 489–496. [Google Scholar] [CrossRef]
- Muan, A.; Osborn, E.F. Phase Equilibria among Oxides in Steelmaking; Addison-Wesley Pub.: Reading, MA, USA, 1965. [Google Scholar]
- Park, J.; Kim, H.J.; Park, J.H. Effect of slag composition on the distribution and separation behavior of arsenic between CaO-based slag and liquid copper. J. Hazard. Mater. 2023, 436, 129154. [Google Scholar] [CrossRef] [PubMed]


| Element | O | Ca | Zn | Fe | S | Si | Na | Mg | Pb | As | Ag (ppm) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| mass% | 47.75 | 12.65 | 4.19 | 11.05 | 17.2 | 1.05 | 0.93 | 0.18 | 3.57 | 0.23 | 144 |
| Test Number | Reduction Time (min) | Temperature (°C) | Reduction Gas Flow (mL/min) |
|---|---|---|---|
| 1 | 40 | 1400 | 150 |
| 2 | 60 | 1400 | 150 |
| 3 | 80 | 1400 | 150 |
| 4 | 100 | 1400 | 150 |
| Test Number | Mass Bullion (g) | %Ag | %Fe | %Zn |
|---|---|---|---|---|
| 1 | 0.31 | 0.376 | 0.064 | 0.053 |
| 2 | 0.43 | 0.382 | 0.072 | 0.065 |
| 3 | 0.51 | 0.403 | 0.088 | 0.092 |
| 4 | 0.51 | 0.410 | 0.089 | 0.091 |
| Ag, mg/L | Pb, mg/L | Zn, mg/L | |
|---|---|---|---|
| NOM-053-SEMARNAT-1993 | 5 max. | 5 max. | – |
| Jarosite residue | 7.4 | 16.7 | 11,300 |
| Slag (test number 3) | 0.04 | 3.2 | 26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Lugos, C.; Flores-Favela, M.; Romero-Serrano, A.; Hernández-Ramírez, A.; López-Rodríguez, J.; Cuéllar-Herrera, L.; Colin-García, E. Recovery of Silver and Lead from Jarosite Residues by Roasting and Reducing Pyrometallurgical Processes. Metals 2024, 14, 954. https://doi.org/10.3390/met14080954
Jiménez-Lugos C, Flores-Favela M, Romero-Serrano A, Hernández-Ramírez A, López-Rodríguez J, Cuéllar-Herrera L, Colin-García E. Recovery of Silver and Lead from Jarosite Residues by Roasting and Reducing Pyrometallurgical Processes. Metals. 2024; 14(8):954. https://doi.org/10.3390/met14080954
Chicago/Turabian StyleJiménez-Lugos, Cancio, Manuel Flores-Favela, Antonio Romero-Serrano, Aurelio Hernández-Ramírez, Josué López-Rodríguez, Lorena Cuéllar-Herrera, and Eduardo Colin-García. 2024. "Recovery of Silver and Lead from Jarosite Residues by Roasting and Reducing Pyrometallurgical Processes" Metals 14, no. 8: 954. https://doi.org/10.3390/met14080954
APA StyleJiménez-Lugos, C., Flores-Favela, M., Romero-Serrano, A., Hernández-Ramírez, A., López-Rodríguez, J., Cuéllar-Herrera, L., & Colin-García, E. (2024). Recovery of Silver and Lead from Jarosite Residues by Roasting and Reducing Pyrometallurgical Processes. Metals, 14(8), 954. https://doi.org/10.3390/met14080954







