Recent Progress on Atmospheric Corrosion of Field-Exposed Magnesium Alloys
Abstract
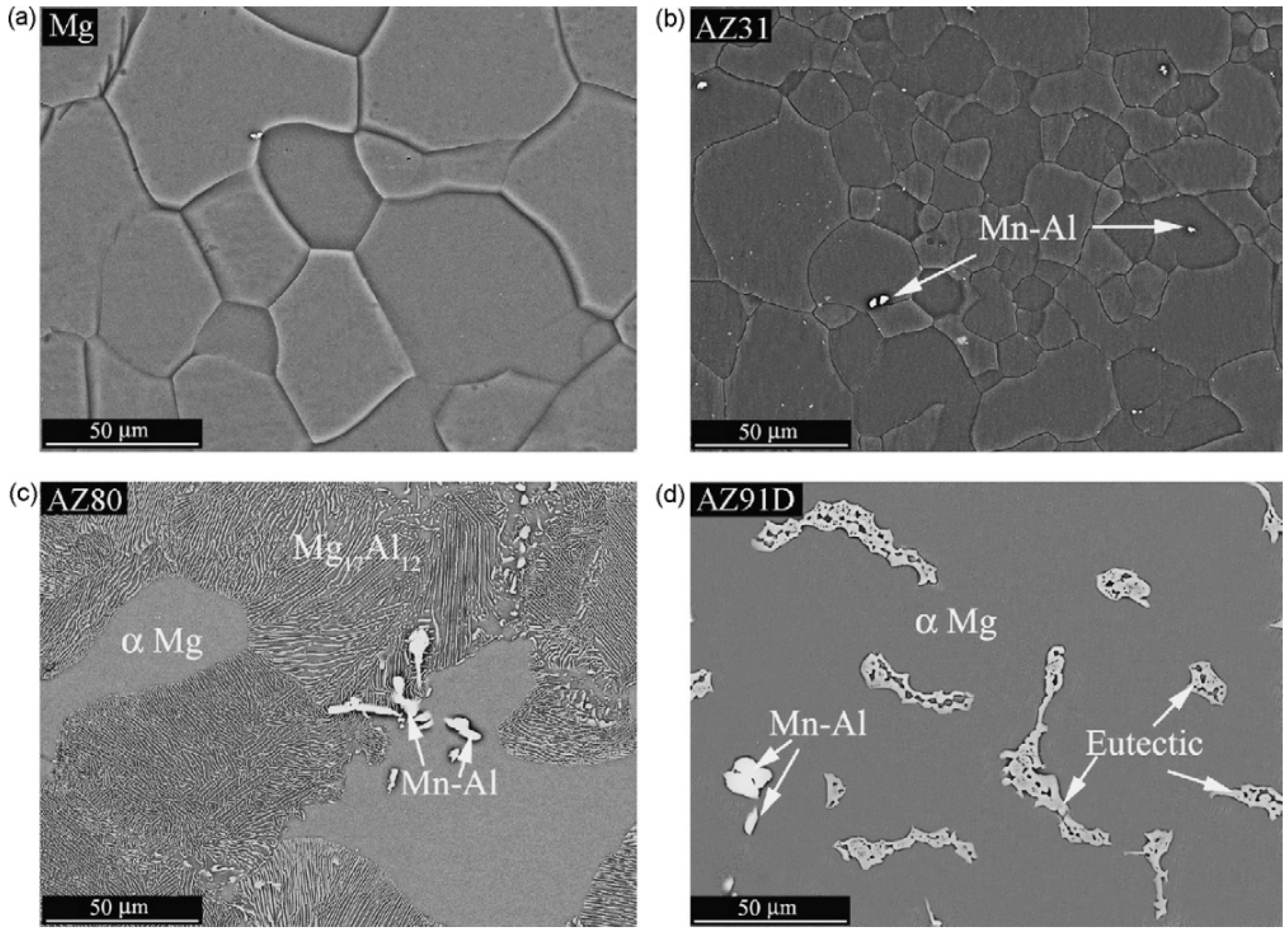
1. Introduction
1.1. Magnesium Alloys Advantages and Prospects
1.2. Limitations and Improvements in Magnesium Alloys
2. Corrosion Behavior of Magnesium Alloys in Different Atmospheres
2.1. Corrosion Behavior of Magnesium Alloys in Marine Atmospheric Environments
2.2. Corrosion Behavior of Magnesium Alloys in Industrial Atmospheres
3. Conclusions
- (1)
- The effects of temperature, relative humidity, and chloride concentration on the corrosion of magnesium alloys are more significant in marine atmospheric environments. Temperature increases the kinetics of the chemical reaction, and the corrosion intensity increases as the temperature rises, and as the relative humidity increases, the thicker the molecular layer of liquid film on the surface of the magnesium alloys, the less protective the layer of corrosion products becomes. The effect of relative humidity is more significant than that of temperature. Chlorides usually cause localized corrosion and pitting on the surface of magnesium alloys. In coastal areas, large amounts of chloride ions migrate and settle on the surface of magnesium alloys, providing high opportunity for electrochemical reactions. However, CO2 can neutralize the cathodic zone, leading to a decrease in cathodic activity, inhibiting the long-term atmospheric corrosion behavior of magnesium alloys.
- (2)
- Compared with static atmospheric exposure experiments, magnesium alloys suffer the most severe corrosion in dynamic marine atmospheric environments. The extreme harsh environment of sea navigation, including periodic dry/wet alternation, and the large amount of chloride ions carried by sea winds and waves, cause serious damage to magnesium alloys. However, there are only a few studies on the corrosion behavior of magnesium alloys in dynamic marine atmospheric environments and more dynamic atmospheric corrosion research is needed.
- (3)
- In industrial atmospheres, SO2 and dust particles affect the corrosion behavior of magnesium alloys. SO2 increases the corrosion process and has a synergistic effect with soluble salts on atmospheric corrosion of magnesium alloys. Dust particles can accelerate the corrosion process of Mg by reducing the localized critical relative humidity on dust-contaminated surfaces. However, there is relatively little research on the industrial or urban atmospheric environment, and the research prospects are relatively broad.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kojima, Y. Project of platform science and technology for advanced magnesium alloys. Mater. Trans. 2001, 42, 1154–1159. [Google Scholar] [CrossRef]
- Dargusch, M.S.; Shi, Z.; Zhu, H.; Atrens, A.; Song, G.L. Microstructure modification and corrosion resistance enhancement of die-cast Mg-Al-Re alloy by Sr alloying. J. Magnes. Alloys 2020, 9, 950–963. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, Z.; Xue, J. Electrochemical and corrosion behaviours of the wrought Mg-Y-Zn based alloys with high Y/Zn mole ratios. J. Magnes. Alloys 2021, 9, 1419–1427. [Google Scholar] [CrossRef]
- Du, Y.; Wang, X.; Liu, D.; Sun, W.; Jiang, B. Corrosion behaviour of a Mg-Zn-Ca-La alloy in 3.5 wt.% NaCl solution. J. Magnes. Alloys 2022, 10, 527–539. [Google Scholar] [CrossRef]
- Gerashi, E.; Alizadeh, R.; Langdon, T.G. Effect of crystallographic texture and twinning on the corrosion behavior of Mg alloys: A review. J. Magnes. Alloys 2021, 10, 313–325. [Google Scholar] [CrossRef]
- Song, J.; Chen, J.; Xiong, X.; Peng, X.; Chen, D.; Pan, F. Research advances of magnesium and magnesium alloys worldwide in 2021. J. Magnes. Alloys 2022, 10, 863–898. [Google Scholar] [CrossRef]
- Feng, Y.J.; Li, Q.; Zhao, T.L.; Pan, F.S. A quasi-passivated film formed on as-solutionized Mg-Sm-Zn-Zr alloy in NaCl solution. Corros. Sci. 2022, 198, 110136. [Google Scholar] [CrossRef]
- Liu, H.G.; Cao, F.Y.; Song, G.L.; Zheng, D.J.; Shi, Z.M.; Dargusch, M.S.; Atren, A. Review of the atmospheric corrosion of magnesium alloys. J. Mater. Sci. Technol. 2019, 35, 2003–2016. [Google Scholar] [CrossRef]
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Bribilis, N.; Frankel, G.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L. Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Atrens, A.; Chen, X.; Shi, Z. Mg Corrosion-Recent Progress. Corros. Mater. Degrad. 2022, 3, 566–597. [Google Scholar] [CrossRef]
- Feng, J.W.; Zhang, H.; Zhang, L.; Zou, G.D.; Wang, J.; Peng, Q.M. Microstructure and corrosion properties for ultrahigh-pressure Mg-Li alloys. Corros. Sci. 2022, 206, 110519. [Google Scholar] [CrossRef]
- Bexiga, N.M.; Alves, M.M.; Taryba, M.G.; Pinto, S.N.; Montemor, M. Early biomimetic degradation of Mg-2Ca alloy reveals the impact of β-phases at the interface of this biomaterial on a micro-scale level. Corros. Sci. 2022, 207, 110526. [Google Scholar] [CrossRef]
- Kousis, C.; Keil, P.; Hamilton, N.M.; Williams, G. The kinetics and mechanism of filiform corrosion affecting organic coated Mg alloy surfaces. Corros. Sci. 2022, 206, 110477. [Google Scholar] [CrossRef]
- Li, M.; Cucinotta, C.S.; Horsfield, A.P. The influence of surface Fe on the corrosion of Mg. J. Phys. Chem. Solids. 2022, 170, 110936. [Google Scholar] [CrossRef]
- Yang, L.H.; Jiang, Q.T.; Zheng, M.; Hou, B.R.; Li, Y.T. Corrosion behavior of Mg-8Li-3Zn-Al alloy in neutral 3.5% NaCl solution. J. Magnes. Alloys 2016, 4, 22–26. [Google Scholar] [CrossRef]
- Liao, J.S.; Hotta, M. Atmospheric corrosion behavior of field-exposed magnesium alloys: Influences of chemical composition and microstructure. Corros. Sci. 2015, 100, 353–364. [Google Scholar] [CrossRef]
- Feliu, S.; Pardo, A.; Merino, M.C.; Coy, A.E.; Viejo, F.; Arrabal, R. Correlation between the surface chemistry and the atmospheric corrosion of AZ31, AZ80 and AZ91D magnesium alloys. Appl. Surf. Sci. 2009, 255, 4102–4108. [Google Scholar] [CrossRef]
- Song, G.; Atrens, A. Recent insights into the mechanism of magnesium corrosion and research suggestions. Adv. Eng. Mater. 2007, 9, 177–183. [Google Scholar] [CrossRef]
- Li, J.Y.; Hao, J.M.; Chen, Y.N.; Chen, H. Stripping Process of the Magnesium Alloy Micro-Arc Oxidation Coating. Surf. Technol. 2015, 44, 27–32,45. [Google Scholar]
- Song, G.L.; Liu, M.H. The effect of surface pretreatment on the corrosion performance of Electroless E-coating coated AZ31. Corros. Sci. 2012, 62, 61–72. [Google Scholar] [CrossRef]
- Meira, G.R.; Andrade, C.; Alonso, C.; Padaratz, I.J.; Borba, J.C. Modelling sea-salt transport and deposition in marine atmosphere zone—A tool for corrosion studies. Corros. Sci. 2008, 50, 2724–2731. [Google Scholar] [CrossRef]
- Meira, G.R.; Andrade, C.; Alonso, C.; Padaratz, I.J.; Borba, J.C. Salinity of marine aerosols in a Brazilian coastal area—Influence of wind regime. Atmos. Environ. 2007, 41, 8431–8441. [Google Scholar] [CrossRef]
- Cui, L.Y.; Liu, Z.Y.; Hu, P.; Shao, J.M.; Li, X.G.; Du, C.W.; Jiang, B. The Corrosion Behavior of AZ91D Magnesium Alloy in Simulated Haze Aqueous Solution. Materials 2018, 11, 970. [Google Scholar] [CrossRef]
- Xu, W.; Ma, Y.; Lv, W.; Chen, T.; Li, Y.; Hao, Y. Atmosphere corrosion behavior of thixoformed AZ91D magnesium alloy at Lanzhou. Corros. Sci. Prot. Technol. 2007, 19, 31–34. [Google Scholar]
- Mikhailovskii, Y.; Skurikhin, A.; Czerny, M.; Wellesz, R.; Zaydel, M. Atmospheric corrosion of metallic systems III. Corrosion behaviour of aluminium and magnesium alloys in various atmospheric conditions. Protect. Met. 1979, 15, 419–426. [Google Scholar]
- Pearlstein, F.; Teitell, L. Corrosion of aluminium and magnesium in the tropics. Mater. Perform. 1974, 13, 22–27. [Google Scholar]
- Cui, Z.Y.; Li, X.G.; Xiao, K.; Dong, C.F. Atmospheric corrosion of field-exposed AZ31 magnesium in a tropical marine environment. Corros. Sci. 2013, 76, 243–256. [Google Scholar] [CrossRef]
- Jiang, Q.T.; Lu, D.Z.; Wang, N.; Wang, X.T.; Zhang, J.; Duan, J.Z.; Hou, B.R. The corrosion behavior of Mg-Nd binary alloys in the harsh marine environment. J. Magnes. Alloys 2021, 9, 292–304. [Google Scholar] [CrossRef]
- Yu, R.H.; Cao, F.Y.; Zhao, C.; Yao, J.H.; Wang, J.J.; Wang, Z.M.; Zou, Z.W.; Zheng, D.J.; Cai, J.L.; Song, G.L. The marine atmospheric corrosion of pure Mg and Mg alloys in field exposure and lab simulation. Corros. Eng. Sci. Technol. 2020, 55, 609–621. [Google Scholar] [CrossRef]
- Shi, X.Z.; Cui, Z.Y.; Li, J.; Hu, B.C.; An, Y.Q.; Wang, X.; Cui, H.Z. Atmospheric Corrosion of AZ31B Magnesium Alloy in the Antarctic Low-Temperature Environment. Acta Metall. Sin.-Engl. Lett. 2023, 36, 1421–1432. [Google Scholar] [CrossRef]
- Liao, J.S.; Hotta, M.; Motoda, S.; Shinohara, T. Atmospheric corrosion of two field-exposed AZ31B magnesium alloys with different grain size. Corros. Sci. 2013, 71, 53–61. [Google Scholar] [CrossRef]
- Nakatsugawa, I.; Yamada, K.; Yamashita, T.; Nishikawa, T.; Taniguchi, Y.; Chino, Y. Effect of Al and Ca Elements on the Atmospheric Corrosion of Field-Exposed Mg-Al-Zn-Ca-Mn Magnesium Alloys. J. Electrochem. Soc. 2023, 170, 091502. [Google Scholar] [CrossRef]
- Jönsson, M.; Persson, D.; Leygraf, C.J.C.S. Atmospheric corrosion of field-exposed magnesium alloy AZ91D. Corros. Sci. 2008, 50, 1406–1413. [Google Scholar] [CrossRef]
- Southwell, C.R.; Alexander, A.L.; Hummer, C.W. Corrosion of metals in tropical environments-aluminium and magnesium. Mater. Perform. 1965, 4, 30–35. [Google Scholar]
- Wang, Y.; Xu, W.C.; Wang, X.T.; Jiang, Q.T.; Li, Y.T.; Huang, Y.L.; Yang, L.H. Research on Dynamic Marine Atmospheric Corrosion Behavior of AZ31 Magnesium Alloy. Metals 2022, 12, 1886. [Google Scholar] [CrossRef]
- Yang, L.; Liu, C.; Wang, Y.; Wang, X.; Gao, H. Dynamic Marine Atmospheric Corrosion Behavior of AZ91 Mg Alloy Sailing from Yellow Sea to Western Pacific Ocean. Metals 2024, 17, 2294. [Google Scholar] [CrossRef]
- Jiang, Q.T.; Lu, D.Z.; Cheng, L.R.; Liu, N.Z.; Yang, L.H.; Hou, B.R. The corrosion behavior of EW75 magnesium alloy in the research vessel KEXUE during the ocean voyage. NPJ Mater. Degrad. 2022, 6, 28. [Google Scholar] [CrossRef]
- LeBozec, N.; Jönsson, M.; Thierry, D. Atmospheric corrosion of magnesium alloys: Influence of temperature, relative humidity, and chloride deposition. Corrosion 2004, 60, 356–361. [Google Scholar] [CrossRef]
- Merino, M.C.; Pardo, A.; Arrabal, R.; Merino, S.; Casajús, P.; Mohedano, M. Influence of chloride ion concentration and temperature on the corrosion of Mg-Al alloys in salt fog. Corros. Sci. 2010, 52, 1696–1704. [Google Scholar] [CrossRef]
- Song, G.L.; Hapugoda, S.; St John, D. Degradation of the surface appearance of magnesium and its alloys in simulated atmospheric environments. Corros. Sci. 2007, 49, 1245–1265. [Google Scholar] [CrossRef]
- Lin, Q. Preliminary Corrosion Behavior of AE44/MS Bolt Structure in Marine Atmosphere with Different Humidity. Mater. Prot. 2023, 56, 91–96. [Google Scholar]
- Jönsson, M.; Persson, D.; Thierry, D. Corrosion product formation during NaCl induced atmospheric corrosion of magnesium alloy AZ91D. Corros. Sci. 2007, 49, 1540–1558. [Google Scholar] [CrossRef]
- Lin, C.; Li, X.G.; Li, M.; Wang, F.P. Corrosion behavior of AZ91D magnesium alloy in city atmosphere. Acta Metall. Sin. 2004, 40, 191–196. [Google Scholar]
- Esmaily, M.; Shahabi-Navid, M.; Svensson, J.E.; Halvarsson, M.; Nyborg, L.; Cao, Y.; Johansson, L.G. Influence of temperature on the atmospheric corrosion of the Mg-Al alloy AM50. Corros. Sci. 2015, 90, 420–433. [Google Scholar] [CrossRef]
- Esmaily, M.; Svensson, J.E.; Johansson, L.G. Corrosion of Magnesium-Aluminum (Mg-Al) Alloys—An Interplay between Al Content and CO2. Magnes. Technol. 2017, 2017, 397–403. [Google Scholar]
- Song, Y.W.; Dai, J.M.; Sun, S. A comparative study on the corrosion behavior of AZ80 and EW75 Mg alloys in industrial atmospheric environment. Mater. Today Commun. 2024, 38, 108263. [Google Scholar] [CrossRef]
- Sun, S.; Dai, J.M.; Song, Y.W.; Ai, C.J. Corrosion Behavior of Extruded EW75 Mg-alloy in Shenyang Industrial Atmosphere. J. Chin. Soc. Corros. Prot. 2024, 44, 141–150. [Google Scholar]
- Li, Y.G.; Wei, Y.H.; Hou, L.F.; Han, P.J. Atmospheric corrosion of AM60 Mg alloys in an industrial city environment. Corros. Sci. 2013, 69, 67–76. [Google Scholar] [CrossRef]
- Gong, P.; Cao, X.J.; Ning, S.Q.; Zhu, L.P. Influence of Different Environments on Atmospheric Corrosion of AZ61 Magnesium Alloy. China Surf. Eng. 2015, 28, 123–128. [Google Scholar]
- Wang, F.P.; Li, X.G.; Lin, C.; Jiao, Q.Z.; Li, J.L. Atmospheric corrosion behavior of AZ91D magnesium alloy in Beijing area atmospheric corrosion behavior of AZ91D magnesium alloy in Beijing area. J. Chin. Soc. Corros. Prot. 2004, 24, 345–349. [Google Scholar]
- Wan, Y.; Yan, C.W. Synergetic effect of soluble salts and SO2 on atmospheric corrosion of diecast AZ91D magnesium alloy. Chin. J. Nonferrous Met. 2006, 16, 176–182. [Google Scholar]
- Esmaily, M.; Blücher, D.B.; Lindström, R.W.; Svensson, J.E.; Johansson, L.G. The Influence of SO2 on the Corrosion of Mg and Mg-Al Alloys. J. Electrochem. Soc. 2015, 162, C260–C269. [Google Scholar] [CrossRef]
- Lin, C.; Liang, J.N. Corrosion Behavior of AZ91D Magnesium Alloy in Atmosphere Containing SO2. Rare Metal Mat. Eng. 2011, 40, 499–502. [Google Scholar]
- Yang, L.J.; Li, Y.F.; Wei, Y.H.; Hou, L.F.; Li, Y.G.; Tian, Y. Atmospheric corrosion of field-exposed AZ91D Mg alloys in a polluted environment. Corros. Sci. 2010, 52, 2188–2196. [Google Scholar] [CrossRef]
- Liu, F.; Song, Y.W.; Shan, D.Y.; Han, E.H. Corrosion behavior of AZ31 magnesium alloy in simulated acid rain solution. Corros. Sci. 2010, 52, 2188–2196. [Google Scholar] [CrossRef]
- Hu, T.T.; Xiang, B.; Liao, S.G.; Huang, W.Z. Corrosion of AM60B magnesium alloy in simulated acid rain. Anti-Corros. Methods Mater. 2010, 57, 244–248. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J.Q.; Han, E.H.; Ke, W. Effects of dust and salt particles on the formation and spreading of micro-droplets on AZ91 magnesium alloy. Corros. Sci. 2008, 50, 1449–1459. [Google Scholar] [CrossRef]
- Zhao, C.; Cao, F.Y.; Song, G.L. Corrosivity of haze constituents to pure Mg. J. Magnes. Alloys 2020, 8, 150–162. [Google Scholar] [CrossRef]



| Exposed Locations | Magnesium Alloy Types | Exposure Time | Environmental Parameters | Corrosion Depth | Corrosion Rate |
|---|---|---|---|---|---|
| Xisha (China) [27] | AZ31 | 2 years | Temp: 27 °C RH: 77% Cl−: 64.39 mg/m2 d pH: 6.5 | − | 17.66 μm/a |
| Nansha (China) [28] | Mg-1.5Nd | 2 years | Temp: 28~30 °C Rainfall: 2800 mm/a | 103.4 μm | 51.70 μm/a |
| Mg-1.0Nd | 138.6 μm | 69.30 μm/a | |||
| Mg-0.5Nd | 167.4 μm | 83.70 μm/a | |||
| Xiamen (China) [29] | AZ91D | 6 months | Temp: 14~28 °C RH: 64~80% | − | 6.55 μm/a |
| AM60 | − | 6.93 μm/a | |||
| ZE41 | − | 6.61 μm/a | |||
| Zhongshan station (Antarctica) [30] | AZ31B | 2 years | Temp: −9.9 °C RH: 62.5% Lowest temp: −36.4 °C | 24.3 μm | 11.13 μm/a |
| Shimizu (Japan) [16,31] | AZ31B | 3 years | Temp: 15~27 °C Temp: 8~29 °C RH: 58~84% Cl−: 4.2 mg/m2·d : 0.7 mg/m2·d | − | 46.82 μm/a |
| Choshi (Japan) [32] | AZ91D | 5 years | Temp: 15.1 °C RH: 79% Cl−: 17.2 mg/m2·d SO2: 2.1 mg/m2·d | 43.6 μm | 3.16 μm/a |
| Miyakojima (Japan) [32] | AZ91D | 5 years | Temp: 23.8 °C RH: 80% Cl−: 40 mg/m2·d SO2: 1.2 mg/m2·d | 63.6 μm | 2.11 μm/a |
| Brest (France) [33] | AZ91D | 12 months | Temp: 12.5 °C RH: 84% Cl−: 42.5 mg/L pH: 6.1 | − | 4.20 μm/a |
| AM50 | − | 8.80 μm/a | |||
| Texas (United States) [34] | AZ91D | − | − | − | 19.2 μm/a |
| Research Vessel KEXUE [35,36,37] | AZ31 | 2 years | Temp: −0.9~33.1 °C RH: 18~97% Cl−: 64~1130 mg/m2·d Wind speed: 5.2 m/s | Deepest: 276.3 μm | 52.23 μm/a |
| AZ91 | 2 years | Temp: 0~31 °C RH: 34~94% Cl−: 110~530 mg/m2·d | Deepest: 196.9 μm | 32.50 μm/a | |
| EW75 | 3 months | Temp: 25.9 °C RH: 77.7% Cl−: 413.65 mg/m2·d Wind speed: 5.23 m/s | 150.3 μm | 90.30 μm/a |
| Exposed Locations | Magnesium Alloy Types | Exposure Time | Environmental Parameters | Corrosion Depth | Corrosion Rate |
|---|---|---|---|---|---|
| Shenyang (China) [46] | AZ80 | 12 months | Temp: 9.18 °C RH: 62.96% Rainfall: 916.2 mm/a | − | 2.95 μm/a |
| EW75 | − | 11 μm/a | |||
| Shenyang (China) [47] | EW75 | 12 months | Temp: 9.18 °C RH: 62.96% Rainfall: 916.2 mm/a | − | 15 μm/a |
| Taiyuan (China) [48] | AM60 | 12 months | RH: 55% SO2: 18~106 μg/m3 NO2: 25~39 μg/m3 PM10: 73~113 μg/m3 | 8.5–28.6 μm * | 0.8 μm/a |
| Jiangjin (China) [49] | AZ61 | 2 months | SO2: 256.5 μg/m3 NO2: 380 μg/m3 H2S: 21.20 mg/m2·d SO3: 93.83 mg/m2·d pH: 4.77 | 1.41 μm | 8.5 μm/a |
| Beijing (China) [50] | AZ91D | 85 days | Temp: 11.6 °C RH: 57% Rainfall: 586.0 mm/a SO2: 48~80 mg/m3 CO2: 45~49 mg/m3 NO2: 28~43 mg/m3 | − | 10 μm/a |
| Stockholm (Sweden) [33] | AZ91D | 12 months | Temp: 8.3 °C RH: 76% Cl−: 2.2 mg/L pH: 5.2 | − | 1.8 μm/a |
| Osaka City (Japan) [31] | AZ31B | 12 months | Temp: 5~29 °C RH: 54~72% Cl−: 1.1 mg/m2·d : 3.4 mg/m2·d | − | 24.2 μm/a |
| Component | Concentration/(mg·L−1) |
|---|---|
| Sulfuric acid (96%) | 31.85 |
| Nitric acid (70%) | 15.75 |
| Sodium nitrate | 21.25 |
| Ammonium sulfate | 46.20 |
| Sodium sulfate | 31.95 |
| Sodium chloride | 84.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Yang, L.; Liu, H.; Wang, X.; Li, Y.; Huang, Y. Recent Progress on Atmospheric Corrosion of Field-Exposed Magnesium Alloys. Metals 2024, 14, 1000. https://doi.org/10.3390/met14091000
Wang M, Yang L, Liu H, Wang X, Li Y, Huang Y. Recent Progress on Atmospheric Corrosion of Field-Exposed Magnesium Alloys. Metals. 2024; 14(9):1000. https://doi.org/10.3390/met14091000
Chicago/Turabian StyleWang, Mengqi, Lihui Yang, Hao Liu, Xiutong Wang, Yantao Li, and Yanliang Huang. 2024. "Recent Progress on Atmospheric Corrosion of Field-Exposed Magnesium Alloys" Metals 14, no. 9: 1000. https://doi.org/10.3390/met14091000
APA StyleWang, M., Yang, L., Liu, H., Wang, X., Li, Y., & Huang, Y. (2024). Recent Progress on Atmospheric Corrosion of Field-Exposed Magnesium Alloys. Metals, 14(9), 1000. https://doi.org/10.3390/met14091000






