Influence of Partial Er Substitution for Sc on the Microstructure, Mechanical Properties and Corrosion Resistance of Short-Processed Al-4.7Mg-0.6Mn-0.3Zr-0.3Sc Sheets
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
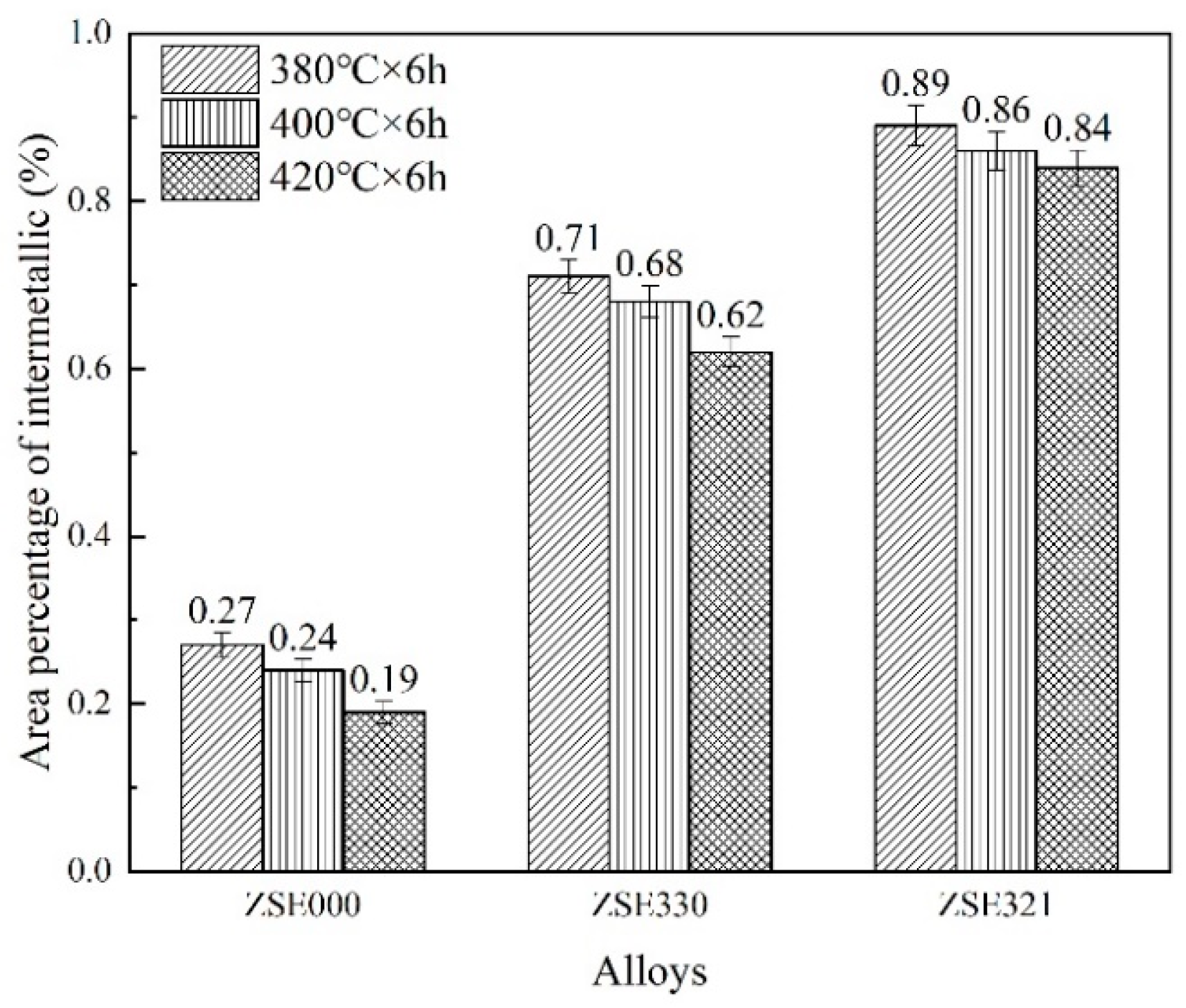
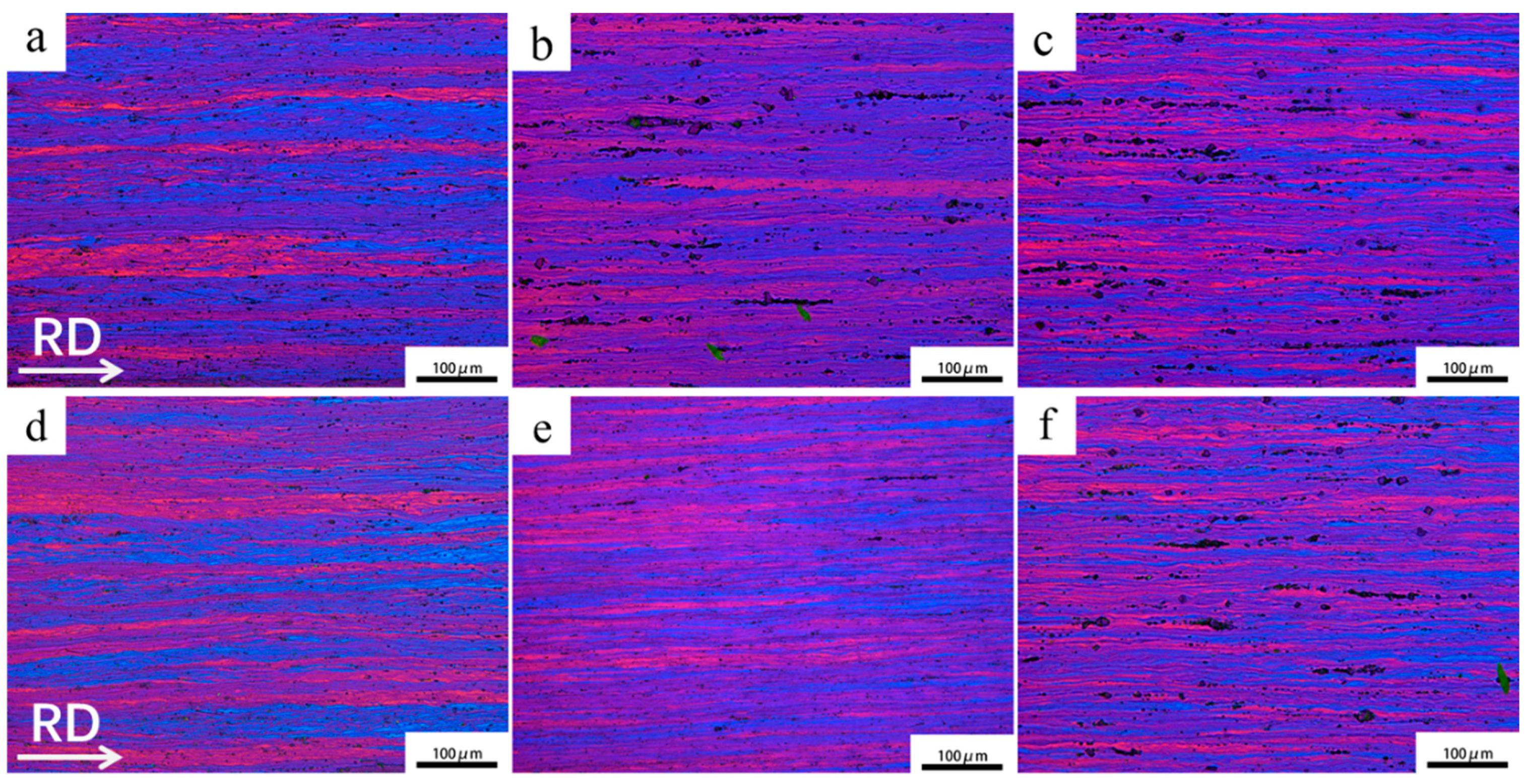
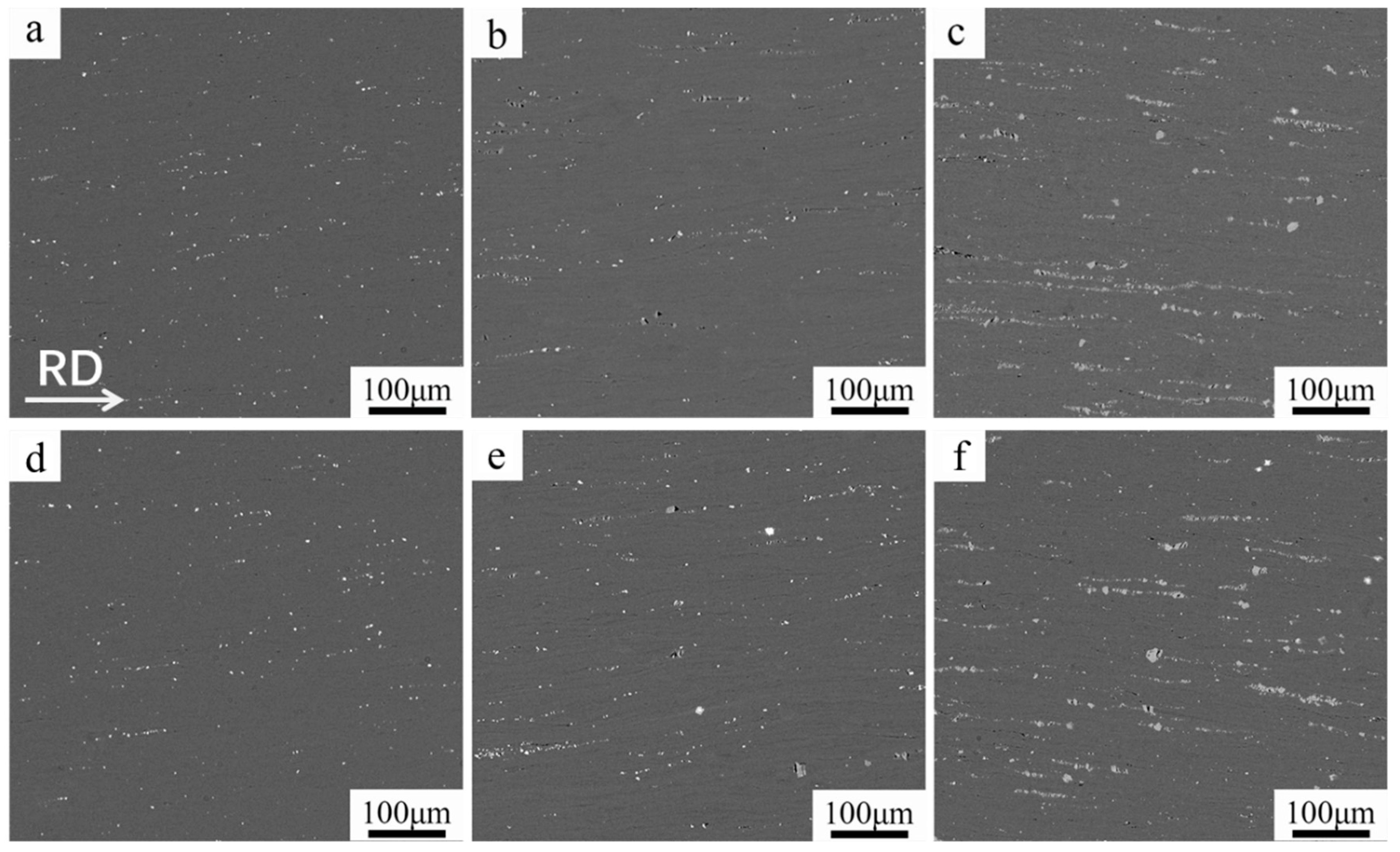
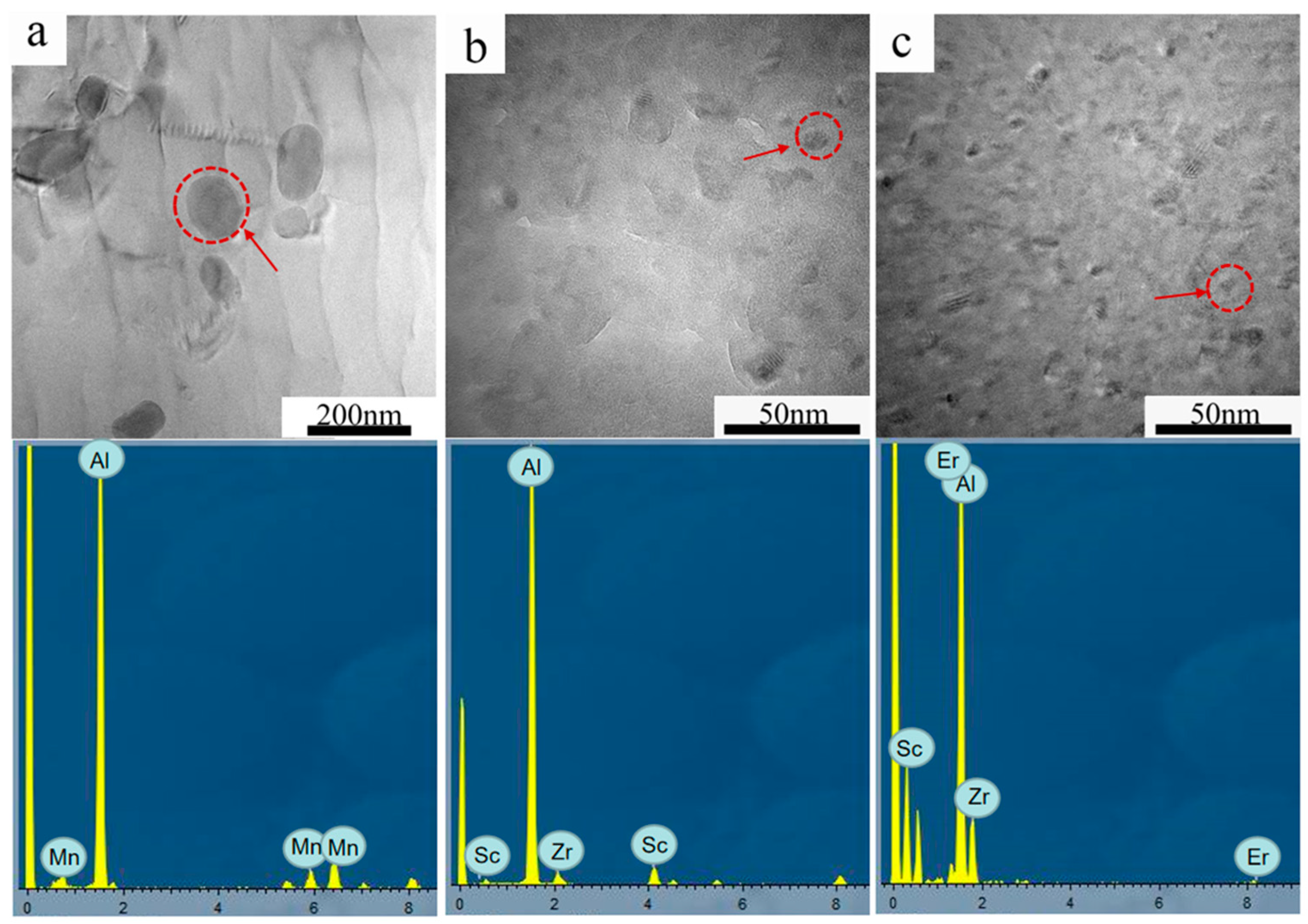
3.1. Microstructural Evolution
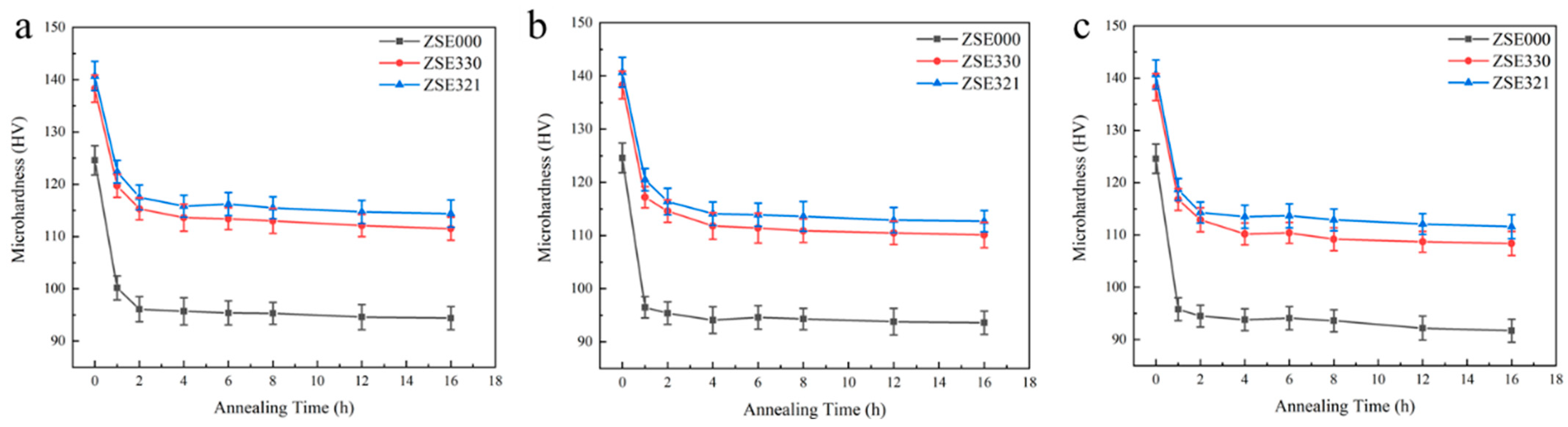
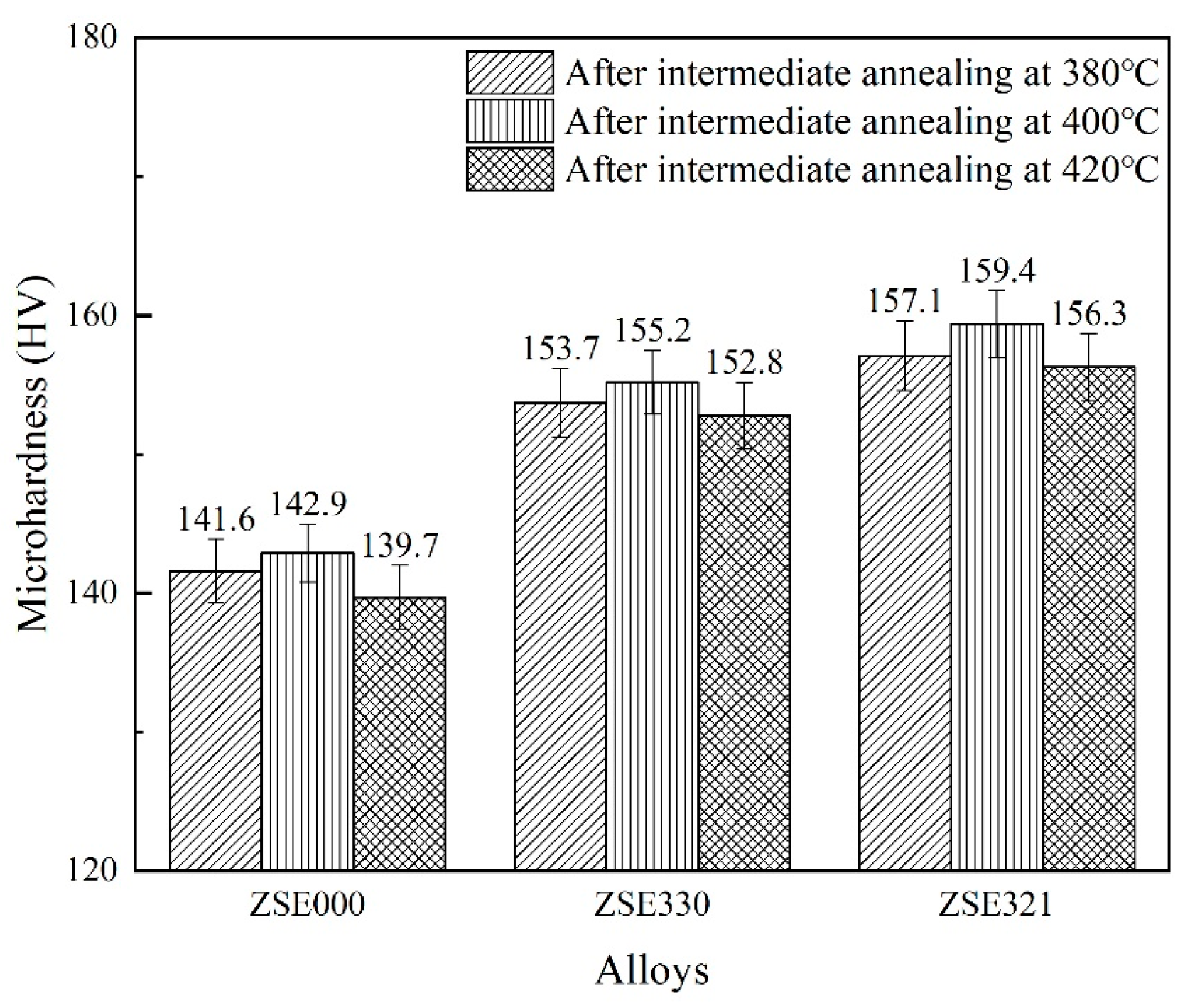
3.2. Microhardness
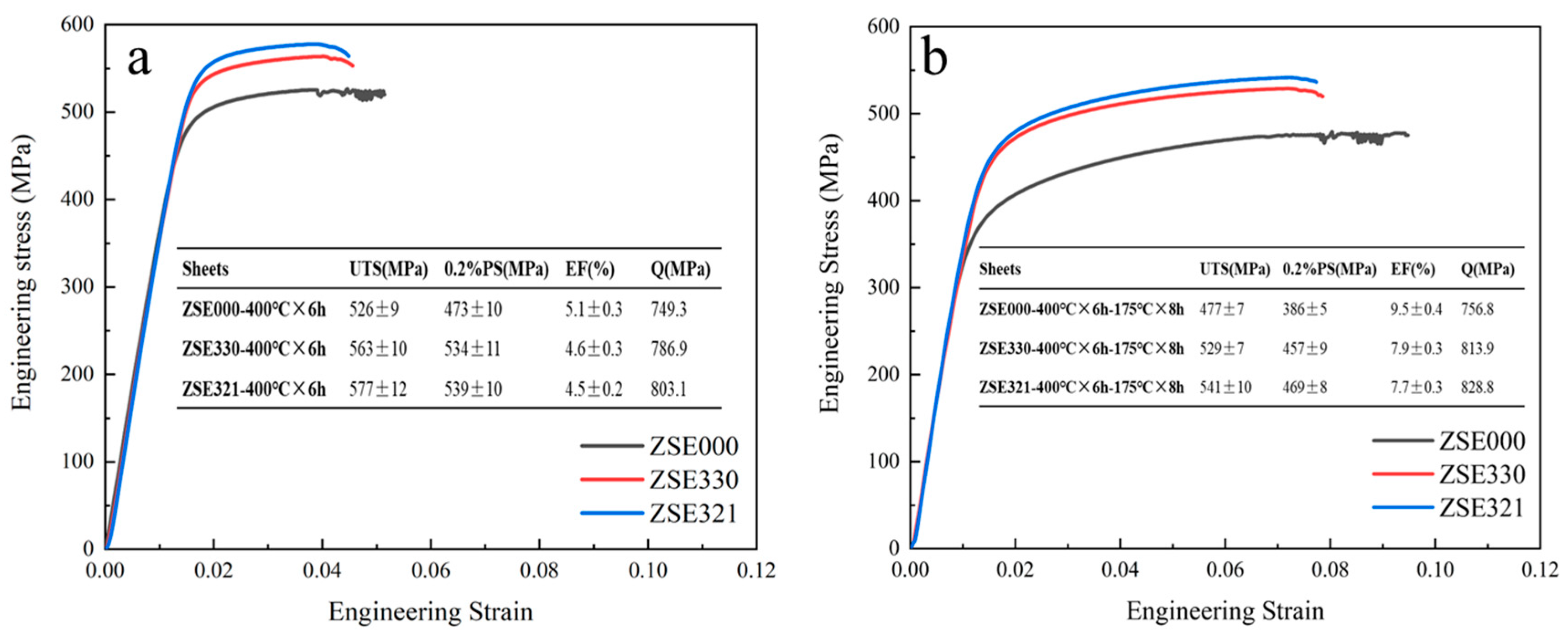
3.3. Tensile Properties
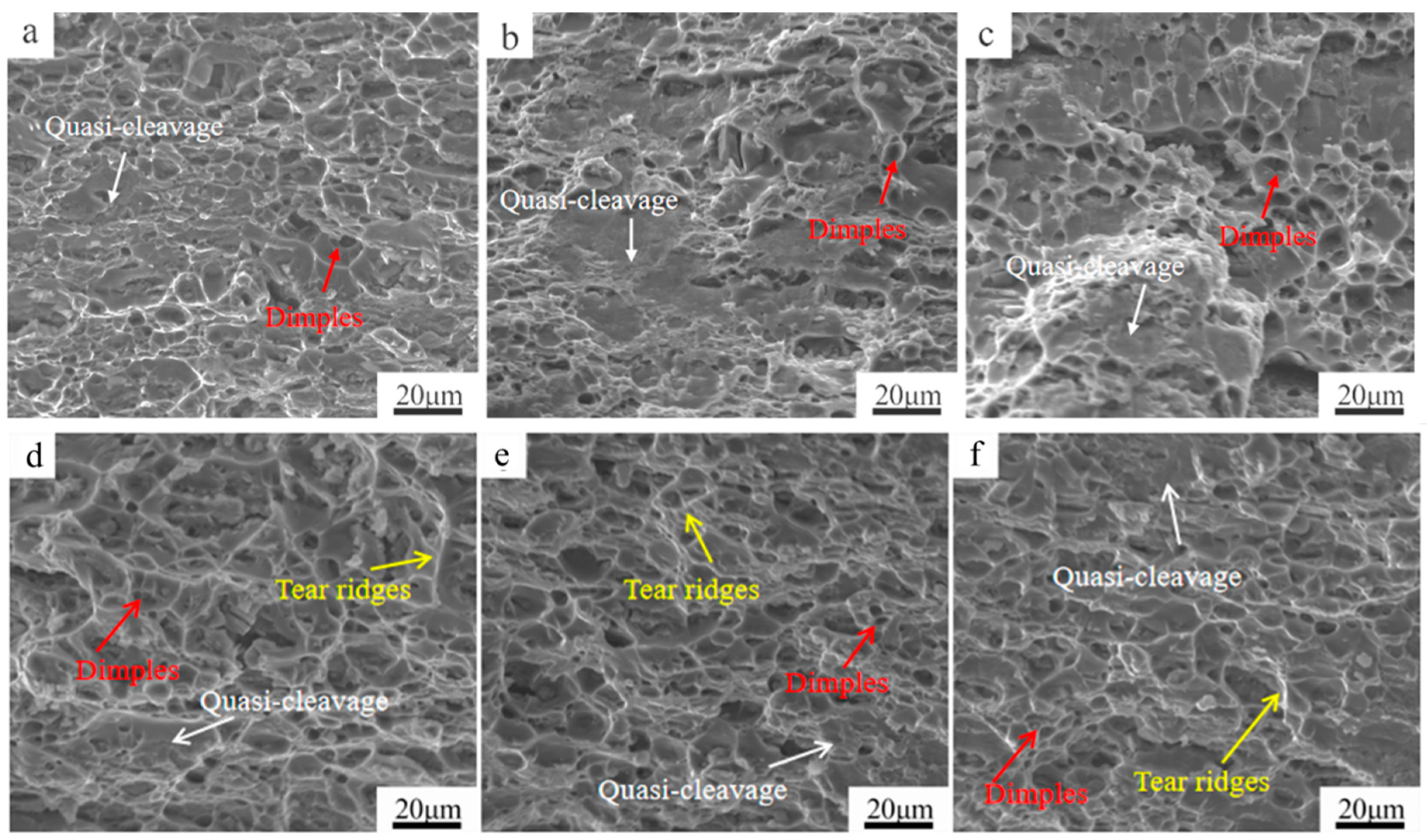
3.4. Fractography
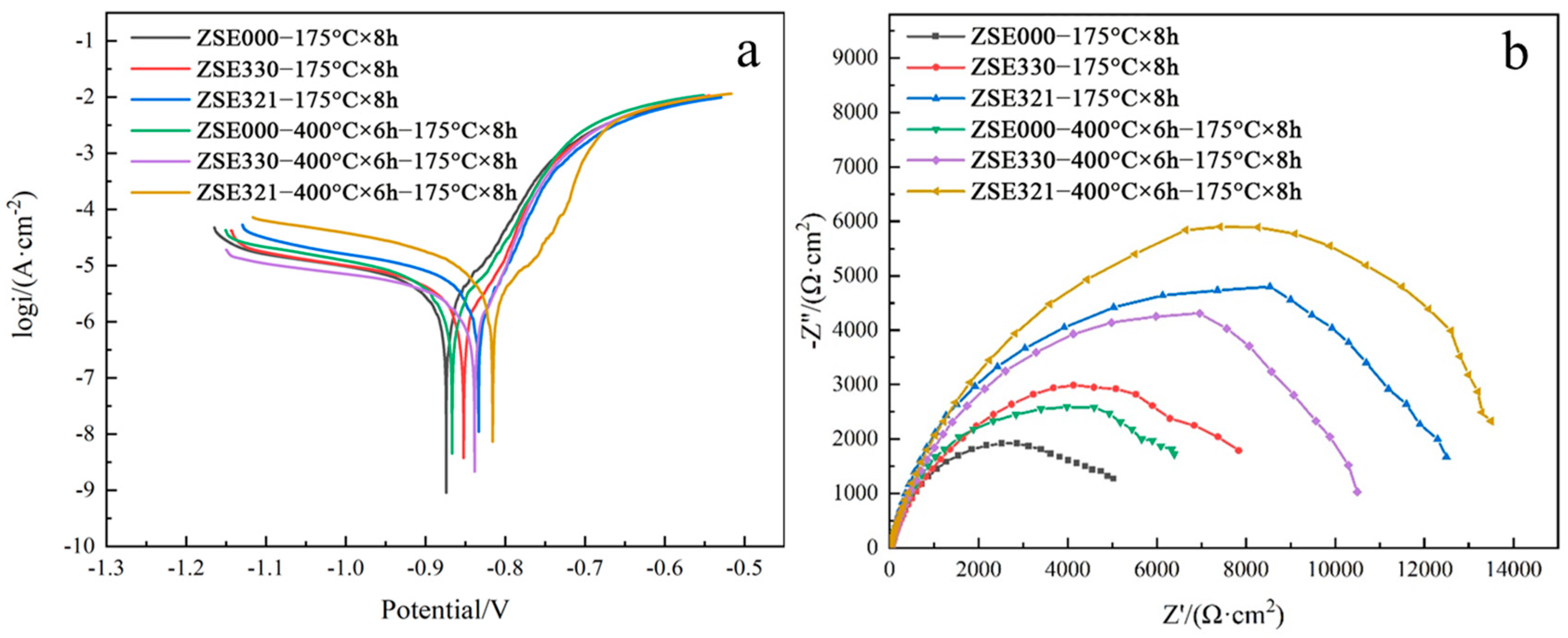
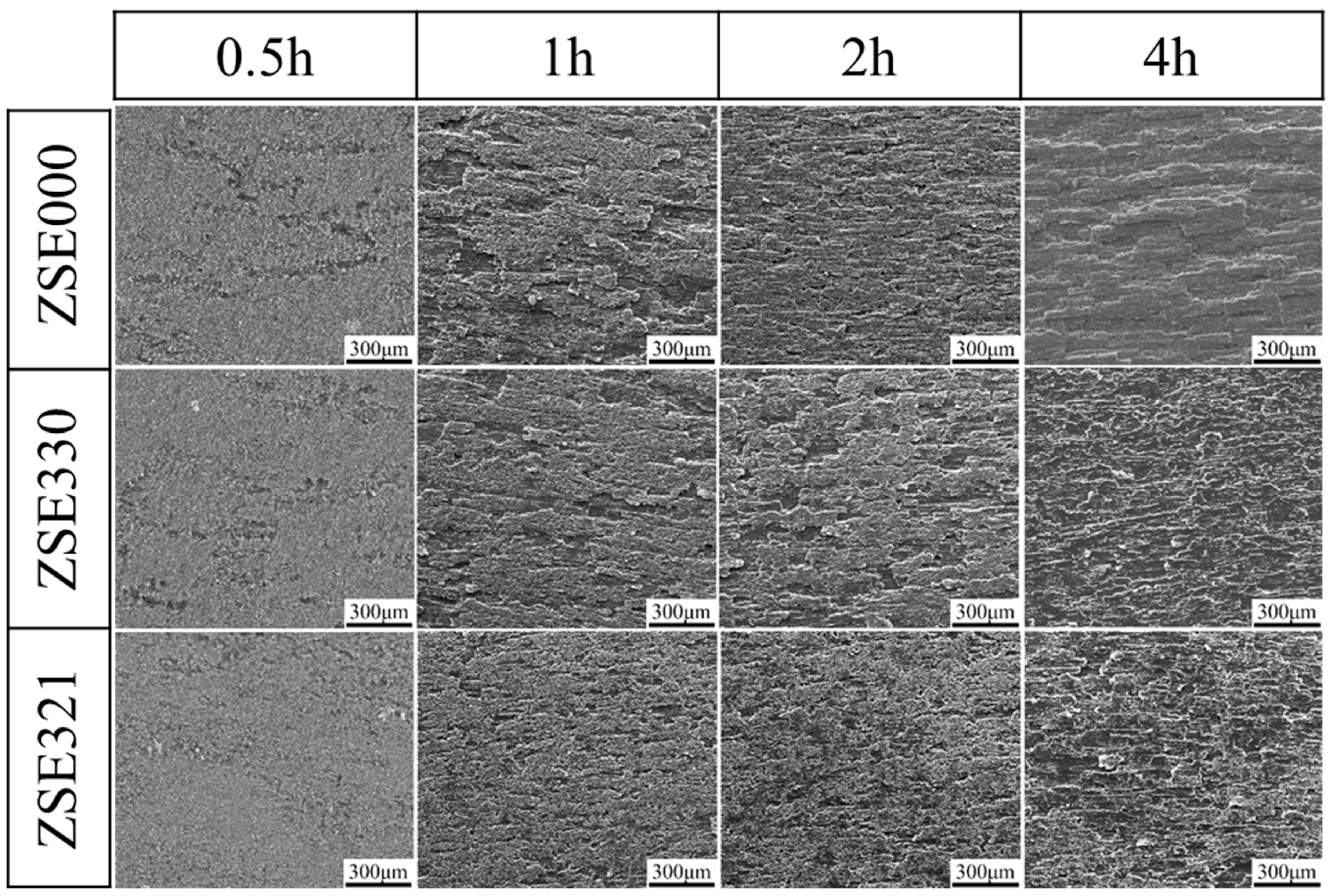
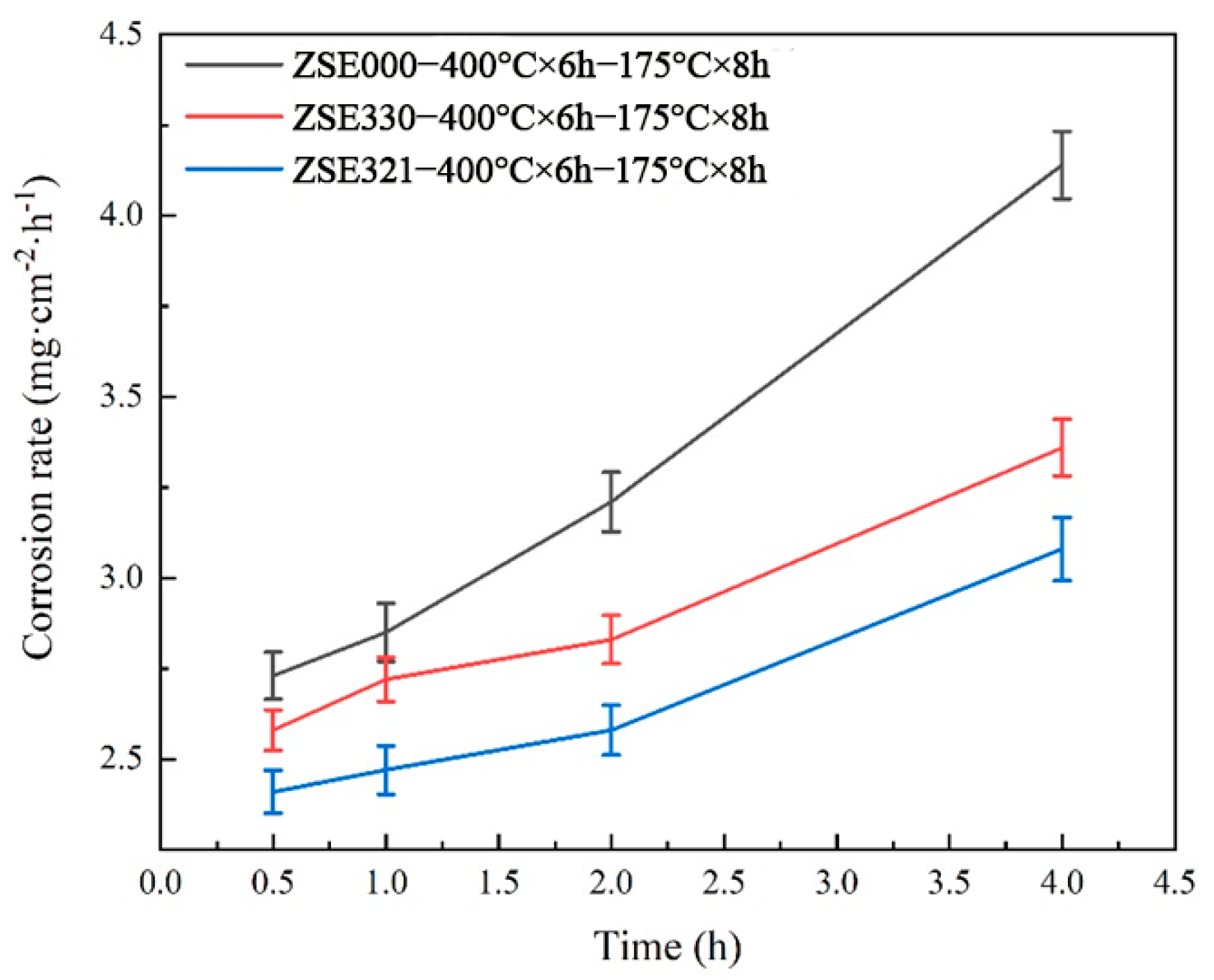
3.5. Electrochemical Corrosion Behavior
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| TBCC | Twin-belt continuous casting |
| TRC | Twin-roll casting |
| DC | Direct chill |
| TBCCRS | A simulated TBCC, subsequent direct rolling, intermediate annealing, cold rolling and stress-relief annealing |
| SCE | Saturated calomel electrode |
| EIS | Electrochemical impedance spectroscopies |
| OCP | Open circuit potential |
| HAMLT | Hydrochloric acid mass loss test |
| HAGBs | High-angle grain boundaries |
| LAGBs | Low-angle grain boundaries |
| UTS | Ultimate tensile strength |
| 0.2% PS | 0.2% proof stress |
| EF | Elongation to failure |
| ZSE000 | Standard (Unmodified) AA5083 |
| ZSE330 | AA5083 with 0.3% Zr and 0.3% Sc addition |
| ZSE321 | AA5083 with 0.3% Zr, 0.2% Sc and 0.1% Er addition |
References
- Liu, Y.; Luo, L.; Han, C.; Ou, L.; Wang, J.; Liu, C. Effect of Fe, Si and Cooling Rate on the Formation of Fe- and Mn-rich Intermetallics in Al–5Mg–0.8Mn Alloy. J. Mater. Sci. Technol. 2016, 32, 305–312. [Google Scholar] [CrossRef]
- Algendy, A.Y.; Liu, K.; Rometsch, P.; Parson, N.; Chen, X.G. Evolution of discontinuous/continuous Al3(Sc,Zr) precipitation in Al-Mg-Mn 5083 alloy during thermomechanical process and its impact on tensile properties. Mater. Charact. 2022, 192, 112241. [Google Scholar] [CrossRef]
- Wu, M.; Tang, Z.; Ye, P.; Long, M.; Wu, F.; Jiang, F. Effect of annealing treatment on microstructure of micro arc oxidation ceramic film on Al-Mg-Sc alloy. Mater. Charact. 2023, 202, 112991. [Google Scholar] [CrossRef]
- Zhao, S.B.; Yan, Y.; Li, X.W.; Xue, P.; Ni, D.R.; Ma, Z.Y.; Tian, Y.Z. Achieving high tensile ductility in a fully nanostructured Al–Mg alloy by low-temperature annealing. Mater. Sci. Eng. A 2022, 853, 143778. [Google Scholar] [CrossRef]
- Shen, J.; Chen, B.; Wan, J.; Shen, J.; Li, J. Effect of annealing on microstructure and mechanical properties of an Al–Mg-Sc-Zr alloy. Mater. Sci. Eng. A 2022, 838, 142821. [Google Scholar] [CrossRef]
- Stemper, L.; Tunes, M.A.; Dumitraschkewitz, P.; Mendez-Martin, F.; Tosone, R.; Marchand, D.; Curtin, W.A.; Uggowitzer, P.J.; Pogatscher, S. Giant hardening response in AlMgZn(Cu) alloys. Acta Mater. 2021, 206, 116617. [Google Scholar] [CrossRef]
- Haghayeghi, R.; Zoqui, E.J.; Eskin, D.G.; Bahai, H. Grain refinement of an Al–10% Mg alloy by intensive shearing in the liquid state. J. Alloys Compd. 2009, 485, 807–811. [Google Scholar] [CrossRef]
- Portnoy, V.K.; Rylov, D.S.; Levchenko, V.S.; Mikhaylovskaya, A.V. The influence of chromium on the structure and superplasticity of Al–Mg–Mn alloys. J. Alloys Compd. 2013, 581, 313–317. [Google Scholar] [CrossRef]
- Jung, J.-G.; Farkoosh, A.R.; Seidman, D.N. Microstructural and mechanical properties of precipitation-strengthened Al-Mg-Zr-Sc-Er-Y-Si alloys. Acta Mater. 2023, 257, 119167. [Google Scholar] [CrossRef]
- Du, P.; Sun, H.; Kong, L.; Wang, Z.; Zhang, J.; Liu, W.; Xue, X.; He, Y. A study on recrystallization behavior and recrystallization texture of high pressure heat-treated Al–Mg alloy. J. Mater. Sci. 2023, 58, 2876–2892. [Google Scholar] [CrossRef]
- Chen, Y.D.; Dan, C.Y.; Shi, Q.W.; Jin, L.; Liu, J.; Chen, C.; Li, C.; Wang, H.W.; Chen, Z. Influence of heating rate on the recrystallization temperature of Al–Mg alloy. J. Mater. Res. Technol. 2023, 22, 2206–2211. [Google Scholar] [CrossRef]
- Vetrano, J.S.; Bruemmer, S.M.; Pawlowski, L.M.; Robertson, I.M. Influence of the particle size on recrystallization and grain growth in Al-Mg-X alloys. Mater. Sci. Eng. A 1997, 238, 101–107. [Google Scholar] [CrossRef]
- Tong, M.; Jiang, F.; Wang, H.; Jiang, J.; Wu, M.; Tang, Z. The evolutions of flow stress and microstructure of Al-Mg-Mn-Sc-Zr alloy at elevated temperatures. Mater. Charact. 2021, 182, 111560. [Google Scholar] [CrossRef]
- Yin, Z.; Pan, Q.; Zhang, Y.; Jiang, F. Effect of minor Sc and Zr on the microstructure and mechanical properties of Al–Mg based alloys. Mater. Sci. Eng. A 2000, 280, 151–155. [Google Scholar] [CrossRef]
- Pozdniakov, A.V.; Yarasu, V.; Barkov, R.Y.; Yakovtseva, O.A.; Makhov, S.V.; Napalkov, V.I. Microstructure and mechanical properties of novel Al-Mg-Mn-Zr-Sc-Er alloy. Mater. Lett. 2017, 202, 116–119. [Google Scholar] [CrossRef]
- Zhang, H.-M.; Jiang, P.; Pan, H.-J.; Peng, J.; Wang, Z.-Z.; Yan, K.-T.; Zha, M. Dynamic recrystallization-dependent high-temperature tensile properties and deformation mechanisms in Al-Mg-Sc-Zr alloys. Mater. Sci. Eng. A 2023, 880, 145304. [Google Scholar] [CrossRef]
- Fuller, C.B.; Krause, A.R.; Dunand, D.C.; Seidman, D.N. Microstructure and mechanical properties of a 5754 aluminum alloy modified by Sc and Zr additions. Mater. Sci. Eng. A 2002, 338, 8–16. [Google Scholar] [CrossRef]
- Ocenasek, V.; Slamova, M. Resistance to recrystallization due to Sc and Zr addition to Al–Mg alloys. Mater. Charact. 2001, 47, 157–162. [Google Scholar] [CrossRef]
- Miyake, Y.; Sato, Y.; Teranishi, R.; Kaneko, K. Effect of heat treatments on the microstructure and formability of Al–Mg–Mn–Sc–Zr alloy. Micron 2017, 101, 151–155. [Google Scholar] [CrossRef]
- Xue, D.; Wei, W.; Shi, W.; Guo, Y.W.; Wen, S.P.; Wu, X.L.; Huang, H.; Nie, Z.R. Effect of cold rolling on mechanical and corrosion properties of stabilized Al–Mg–Mn–Er–Zr alloy. J. Mater. Res. Technol. 2021, 15, 6329–6339. [Google Scholar] [CrossRef]
- Gerber, A.G.; Sousa, A.C.M. A parametric study of the Hazelett thin-slab casting process. J. Mater. Process. Technol. 1995, 49, 41–56. [Google Scholar] [CrossRef]
- Penumakala, P.K.; Nallathambi, A.K.; Specht, E.; Urlau, U.; Hamilton, D.; Dykes, C. Feasibility Study of Continuous Casting of Steel Billets in Twin-Belt Caster. Met. Mater. Trans. B 2019, 50, 42–51. [Google Scholar] [CrossRef]
- Li, J.; Liu, W.C.; Zhai, T.; Kenik, E.A. Comparison of recrystallization texture in cold-rolled continuous cast AA5083 and 5182 aluminum alloys. Scr. Mater. 2005, 52, 163–168. [Google Scholar] [CrossRef]
- Gras, C.; Meredith, M.; Hunt, J.D. Microdefects formation during the twin-roll casting of Al–Mg–Mn aluminium alloys. J. Mater. Process. Technol. 2005, 167, 62–72. [Google Scholar] [CrossRef]
- Gras, C.; Meredith, M.; Hunt, J.D. Microstructure and texture evolution after twin roll casting and subsequent cold rolling of Al–Mg–Mn aluminium alloys. J. Mater. Process. Technol. 2005, 169, 156–163. [Google Scholar] [CrossRef]
- Das, S.K.; Lim, N.S.; Seol, J.B.; Kim, H.W.; Park, C.G. Effect of the rolling speed on microstructural and mechanical properties of aluminum―magnesium alloys prepared by twin roll casting. Mater. Des. 2010, 31, 1633–1638. [Google Scholar] [CrossRef]
- Sohi, M.; Poole, W.J.; Sinclair, C.W.; Gallerneault, M. Yield strength of twin-belt cast Al–Mg–Sc–Zr alloy after annealing. Mater. Sci. Technol. 2015, 31, 460–467. [Google Scholar] [CrossRef]
- Penumakala, P.K.; Nallathambi, A.K.; Specht, E.; Urlau, U.; Hamilton, D.; Dykes, C. Influence of process parameters on solidification length of twin-belt continuous casting. Appl. Therm. Eng. 2018, 134, 275–286. [Google Scholar] [CrossRef]
- Cieslar, M.; Bajer, J.; Zimina, M.; Šlapáková, M.; Grydin, O. Properties and microstructure of twin-roll cast Al-Mg alloy containing Sc and Zr. IOP Conf. Ser. Mater. Sci. Eng. 2017, 179, 012012. [Google Scholar] [CrossRef]
- Abrams, H. Grain size measurement by the intercept method. Metallography 1971, 4, 59–78. [Google Scholar] [CrossRef]
- ASTM E8/E8M-21; Standard Test Methods for Tension Testing of Metallic Materials. ASTM International: West Conshohocken, PA, USA, 2004.
- ASTM G67-99; Standard Test Method for Determining the Susceptibility to Intergranular Corrosion of 5XXX Series Aluminum Alloys by Mass Loss after Exposure to Nitric Acid (NAMLT Test). ASTM International: West Conshohocken, PA, USA, 1999.
- Mei, D.; Wang, C.; Nienaber, M.; Pacheco, M.; Barros, A.; Neves, S.; Reis, R.L.; Zhu, S.; Bohlen, J.; Letzig, D.; et al. Corrosion behavior of Mg wires for ureteral stent in artificial urine solution. Corros. Sci. 2021, 189, 109567. [Google Scholar] [CrossRef]
- Deng, M.; Wang, L.; Höche, D.; Lamaka, S.V.; Snihirova, D.; Jiang, P.; Zheludkevich, M.L. Corrosion and discharge properties of Ca/Ge micro-alloyed Mg anodes for primary aqueous Mg batteries. Corros. Sci. 2020, 177, 108958. [Google Scholar] [CrossRef]
- Wang, Y.; Freiberg, D.; Huo, Y.; Zhu, W.; Williams, R.; Li, M.; Wang, Y. Shapes of nano Al6Mn precipitates in Mn-containing Al-alloys. Acta Mater. 2023, 249, 118819. [Google Scholar] [CrossRef]
- Ekaputra, C.N.; Rakhmonov, J.U.; Weiss, D.; Mogonye, J.-E.; Dunand, D.C. Microstructure and mechanical properties of cast Al-Ce-Sc-Zr-(Er) alloys strengthened by Al11Ce3 micro-platelets and L12 Al3(Sc,Zr,Er) nano-precipitates. Acta Mater. 2022, 240, 118354. [Google Scholar] [CrossRef]
- Yasin, M.R.M.; Razak, S.N.A.A. Effect of high temperature solution heat treatment time on quality index and morphology of A356 DC alloy. Mater. Today Proc. 2022, 48, 1924–1928. [Google Scholar] [CrossRef]
- Xu, C.; Xiao, W.; Hanada, S.; Yamagata, H.; Ma, C. The effect of scandium addition on microstructure and mechanical properties of Al–Si–Mg alloy: A multi-refinement modifier. Mater. Charact. 2015, 110, 160–169. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, D.; Yan, X.; Chen, Y.; Jia, Z.; Zhang, D.; Han, M.; Wang, X.; Liu, G.; Liu, X.; et al. Insights into the dual effects of Ti on the grain refinement and mechanical properties of hypoeutectic Al–Si alloys. J. Mater. Sci. Technol. 2024, 189, 44–59. [Google Scholar] [CrossRef]
- Yang, X.; Xu, C.; Lu, G.; Guan, S. Towards strength-ductility synergy through an optimized two-stage solution treatment in Al–7Si–3Cu-0.5Mg alloys. Mater. Sci. Eng. A 2022, 849, 143504. [Google Scholar] [CrossRef]
- Tiryakioğlu, M.; Staley, J.T.; Campbell, J. Evaluating structural integrity of cast Al–7%Si–Mg alloys via work hardening characteristics: II. A new quality index. Mater. Sci. Eng. A 2004, 368, 231–238. [Google Scholar] [CrossRef]
- Cáceres, C.H.; Sokolowski, J.H.; Gallo, P. Effect of ageing and Mg content on the quality index of two model Al Cu Si Mg alloys. Mater. Sci. Eng. A 1999, 271, 53–61. [Google Scholar] [CrossRef]
- Clouet, E.; Nastar, M.; Sigli, C. Nucleation of Al3Zr and Al3Sc in aluminum alloys: From kinetic Monte Carlo simulations to classical theory. Phys. Rev. B 2004, 69, 064109. [Google Scholar] [CrossRef]
- Zhang, R.; Qiu, Y.; Qi, Y.; Birbilis, N. A closer inspection of a grain boundary immune to intergranular corrosion in a sensitised Al-Mg alloy. Corros. Sci. 2018, 133, 1–5. [Google Scholar] [CrossRef]
- Deng, P.; Mo, W.; Ouyang, Z.; Tang, C.; Luo, B.; Bai, Z. Mechanical properties and corrosion behaviors of (Sc, Zr) modified Al-Cu-Mg alloy. Mater. Charact. 2023, 196, 112619. [Google Scholar] [CrossRef]
- Qiu, Y.; Yang, X.; Li, J.; Xiang, S.; Shi, J.; Xu, J.; Sanders, R.E. The influence of Sc and Zr additions on microstructure and corrosion behavior of AA5182 alloy sheet. Corros. Sci. 2022, 199, 110181. [Google Scholar] [CrossRef]








| Alloys | Mg | Mn | Cr | Ti | Zr | Sc | Er | Al |
|---|---|---|---|---|---|---|---|---|
| ZSE000 (AA5083) | 4.73 | 0.60 | 0.16 | 0.14 | - | - | - | Bal. |
| ZSE330 | 4.71 | 0.61 | 0.16 | 0.14 | 0.29 | 0.30 | - | Bal. |
| ZSE321 | 4.71 | 0.60 | 0.16 | 0.14 | 0.29 | 0.20 | 0.10 | Bal. |
| Samples | Ecorr (V) | Icorr (A·cm−2) |
|---|---|---|
| ZSE000-175 °C × 8 h | −0.874 | 3.12 × 10−6 |
| ZSE330-175 °C × 8 h | −0.852 | 2.39 × 10−6 |
| ZSE321-175 °C × 8 h | −0.833 | 1.51 × 10−6 |
| ZSE000-400 °C × 6 h–175 °C × 8 h | −0.867 | 2.94 × 10−6 |
| ZSE330-400 °C × 6 h–175 °C × 8 h | −0.838 | 1.85 × 10−6 |
| ZSE321-400 °C × 6 h–175 °C × 8 h | −0.816 | 1.07 × 10−6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, G.; Liang, Y.; Xu, C.; Rao, W.; Xue, Y.; Li, L.; Zhang, L.; Guan, S. Influence of Partial Er Substitution for Sc on the Microstructure, Mechanical Properties and Corrosion Resistance of Short-Processed Al-4.7Mg-0.6Mn-0.3Zr-0.3Sc Sheets. Metals 2024, 14, 1013. https://doi.org/10.3390/met14091013
Lu G, Liang Y, Xu C, Rao W, Xue Y, Li L, Zhang L, Guan S. Influence of Partial Er Substitution for Sc on the Microstructure, Mechanical Properties and Corrosion Resistance of Short-Processed Al-4.7Mg-0.6Mn-0.3Zr-0.3Sc Sheets. Metals. 2024; 14(9):1013. https://doi.org/10.3390/met14091013
Chicago/Turabian StyleLu, Guangxi, Yabo Liang, Cong Xu, Wenfei Rao, Yaodong Xue, Longfei Li, Li Zhang, and Shaokang Guan. 2024. "Influence of Partial Er Substitution for Sc on the Microstructure, Mechanical Properties and Corrosion Resistance of Short-Processed Al-4.7Mg-0.6Mn-0.3Zr-0.3Sc Sheets" Metals 14, no. 9: 1013. https://doi.org/10.3390/met14091013






