Study of Intergranular Corrosion Behaviors of Mn-Increased 5083 Al Alloy with Controlled Precipitation States of Al6Mn Formed during Homogenization Annealing
Abstract
:1. Introduction
2. Materials and Methods
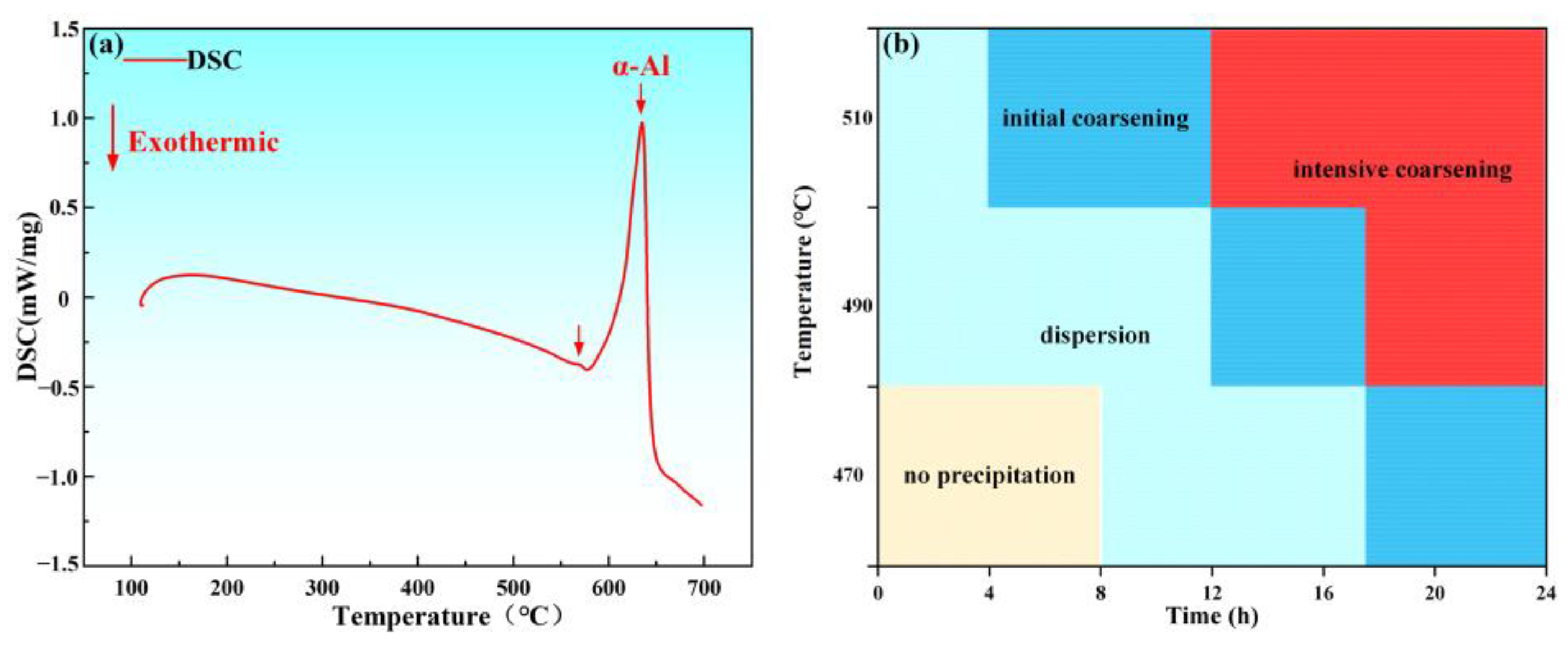
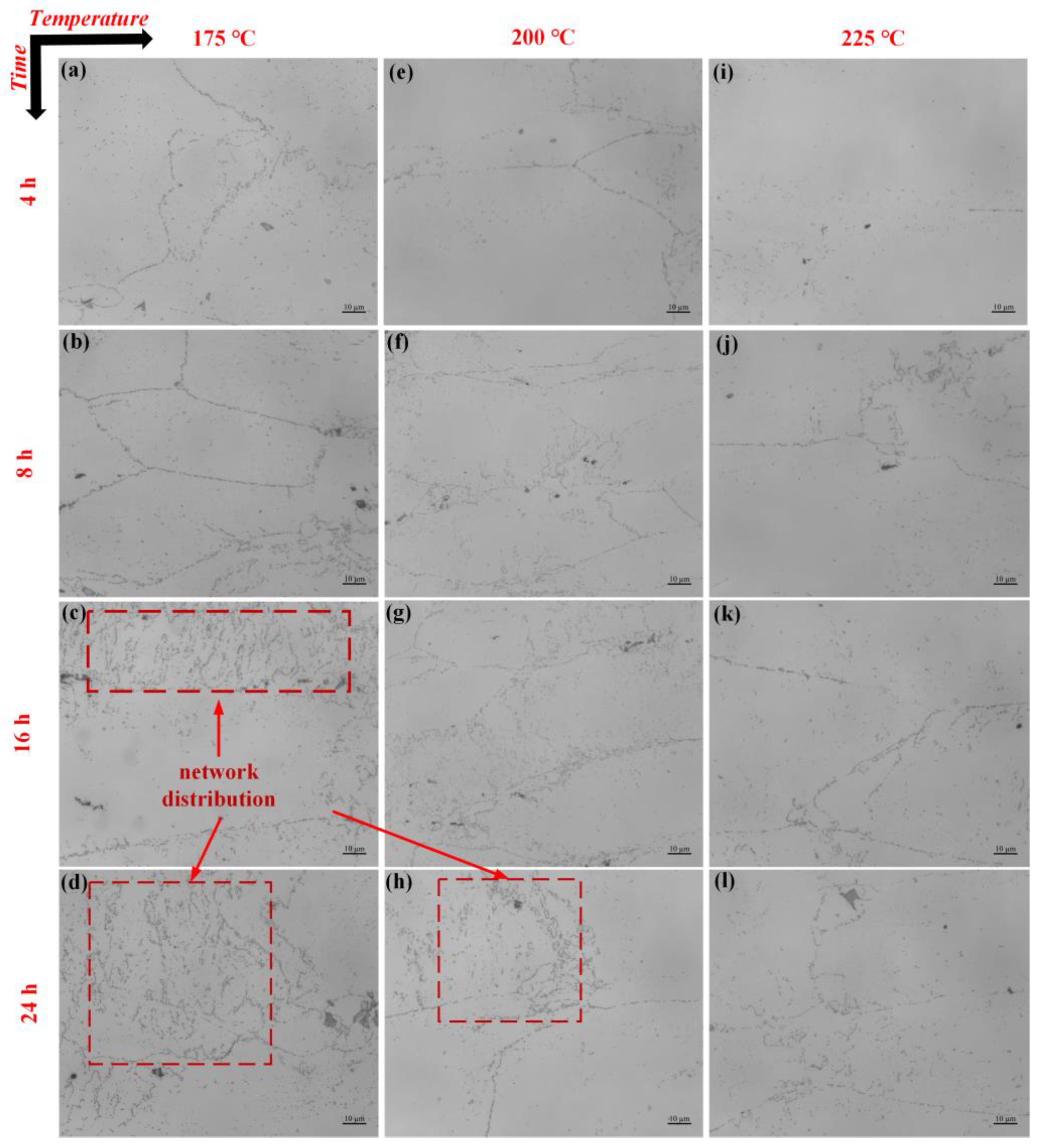
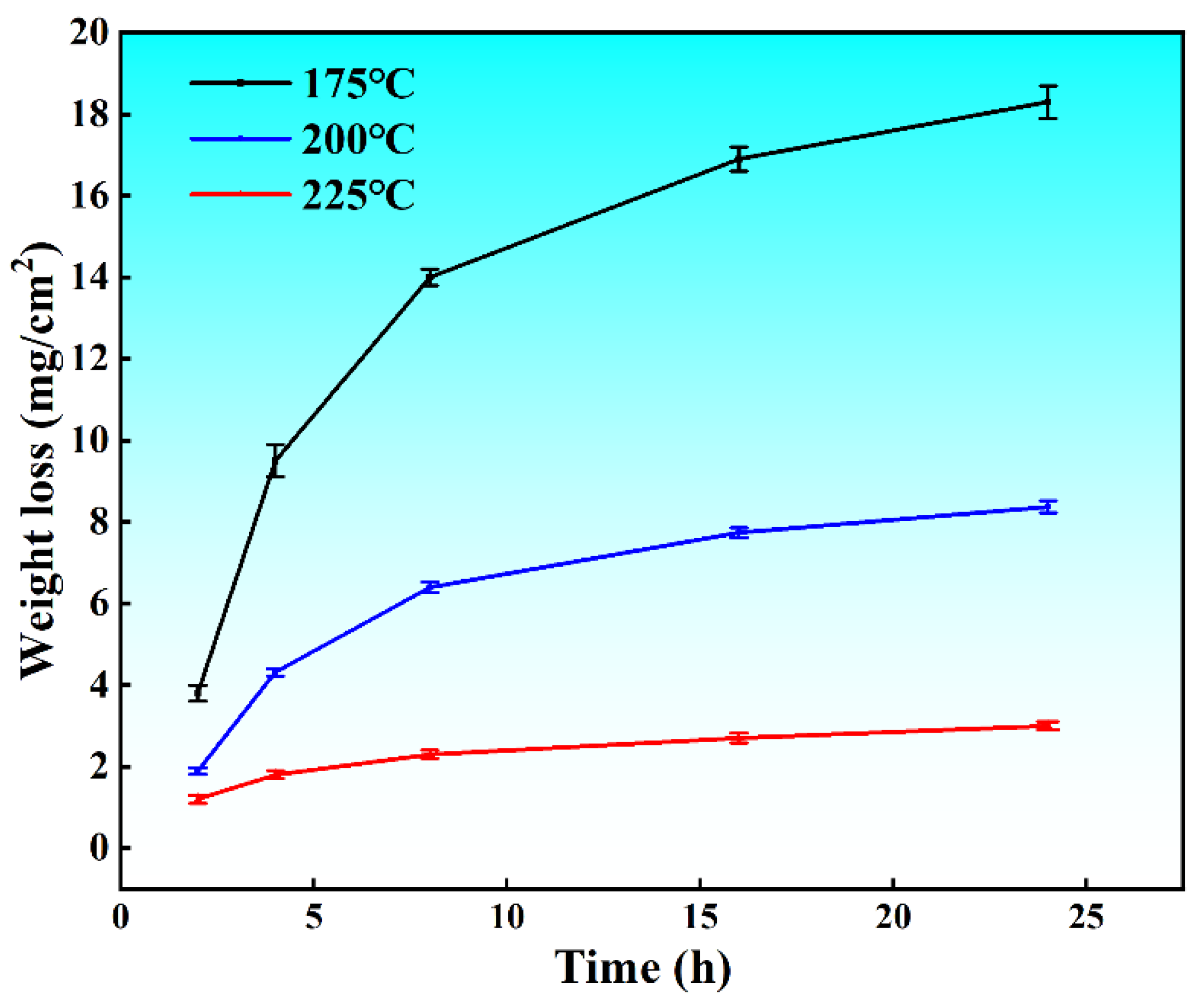
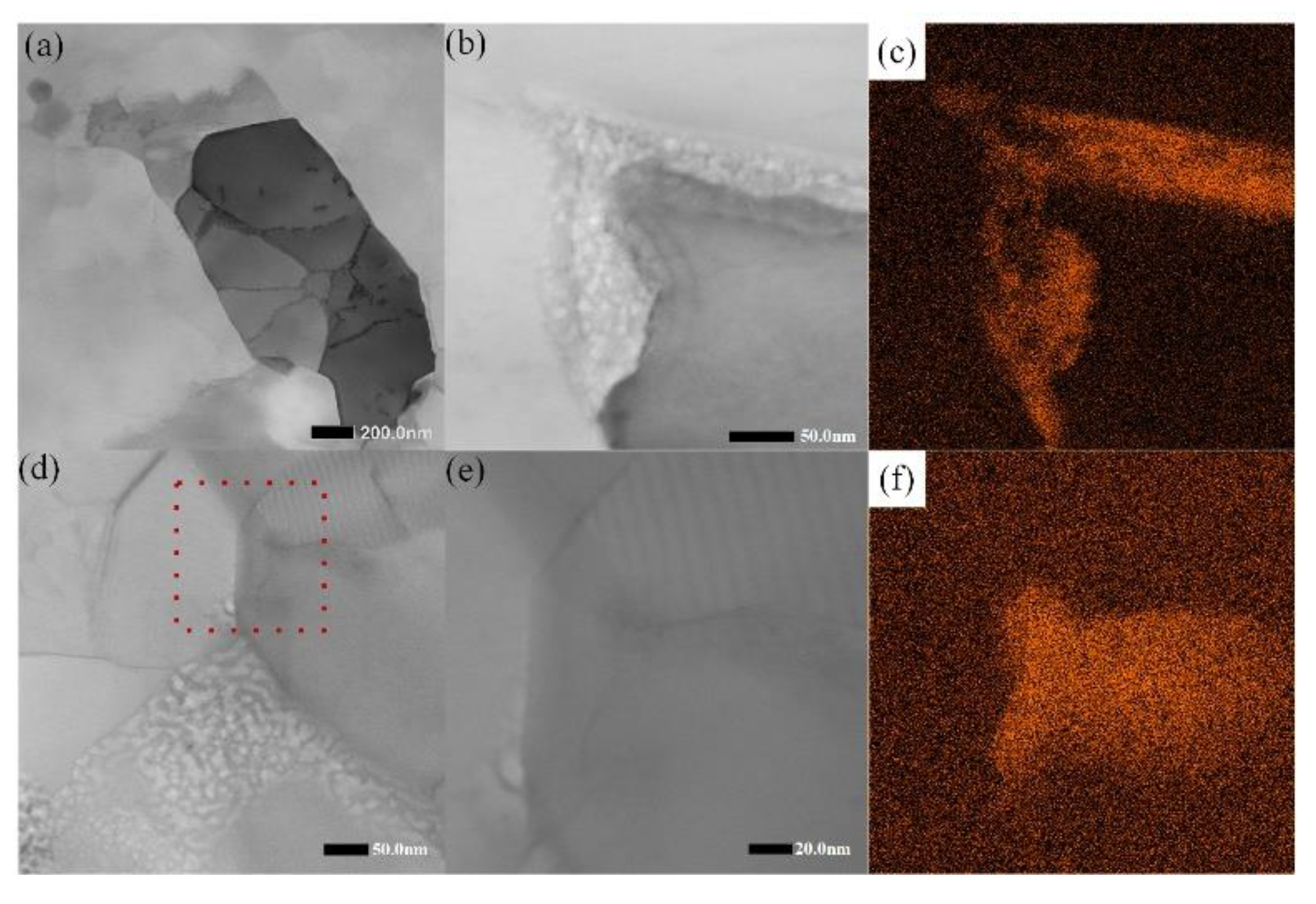
3. Results and Discussion
4. Conclusions
- (1)
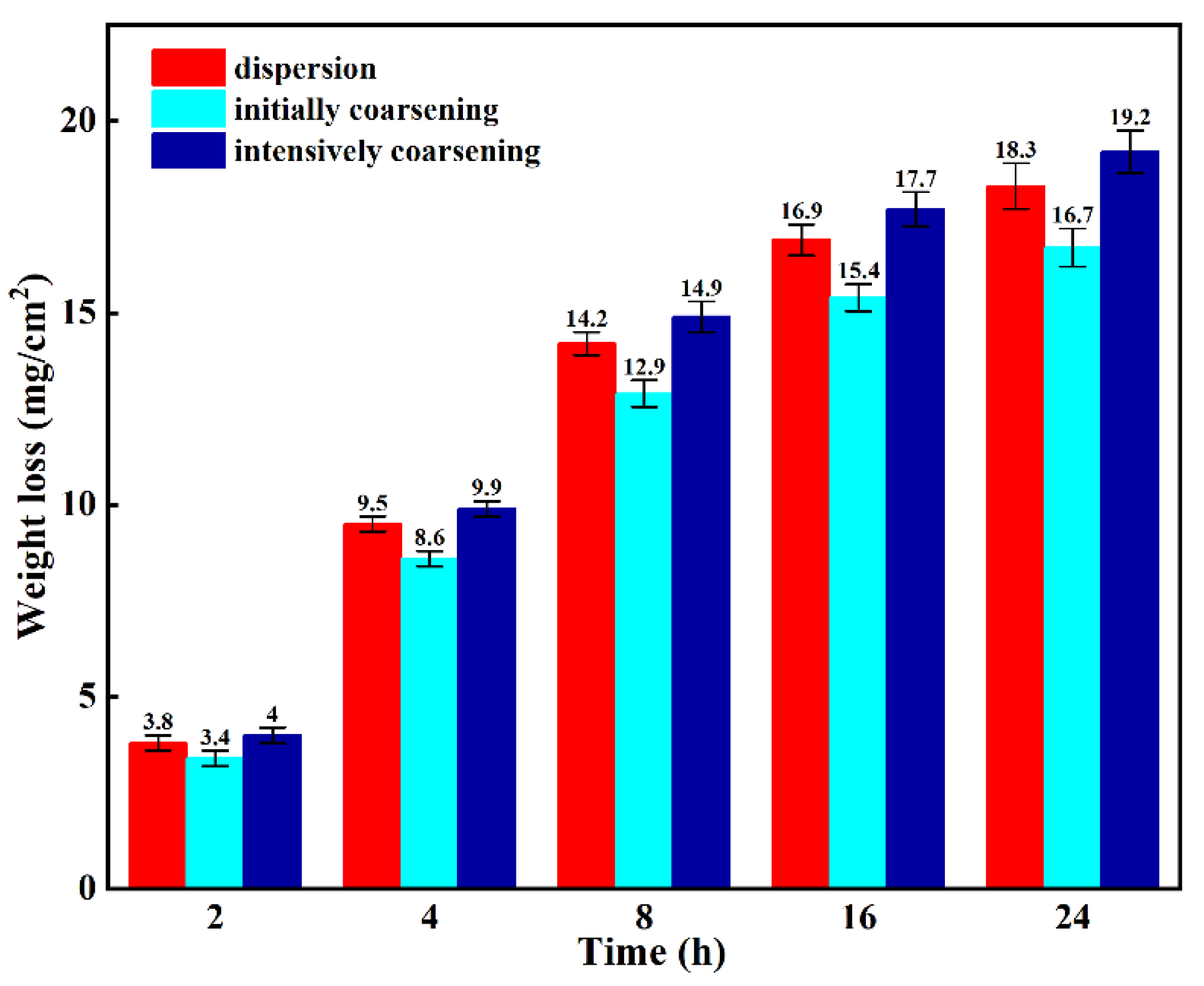
- Sensitization temperature determination: Through metallographic etching, TEM observation, and NAMLT testing, it was determined that 175 °C is the sensitization temperature for the material, 200 °C is the transition temperature, and 225 °C is the stabilization temperature.
- (2)
- The changes in β phase precipitation behavior: As the temperature increases from sensitization to transition to stabilization, the precipitation behavior of the β phase changes accordingly. At the sensitization temperature, the β phase is continuously distributed along the grain boundaries; at the transition temperature, this continuous distribution weakens, with some β phase forming at the triple grain junctions; at the stabilization temperature, the β phase is almost absent at the grain boundaries.
- (3)
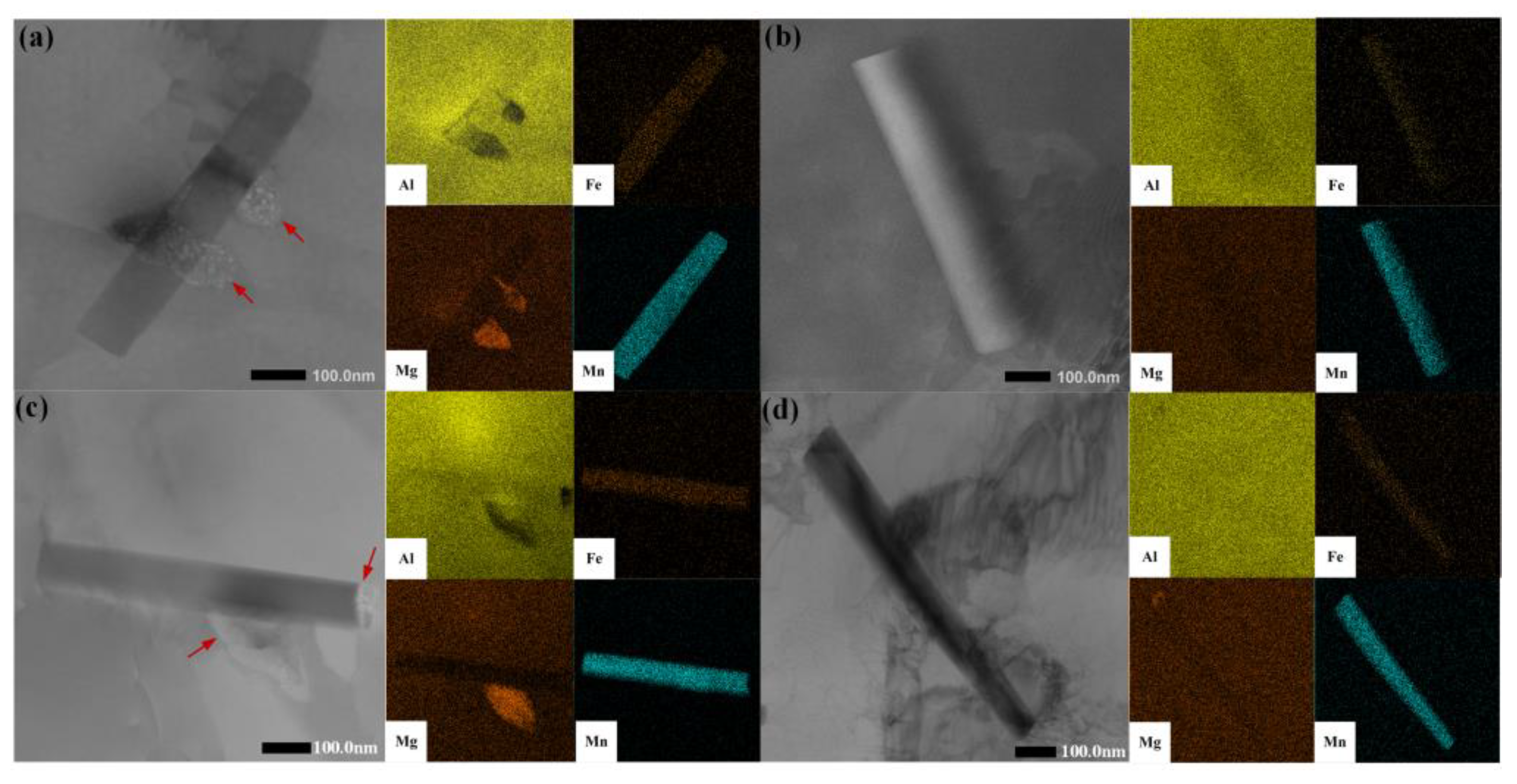
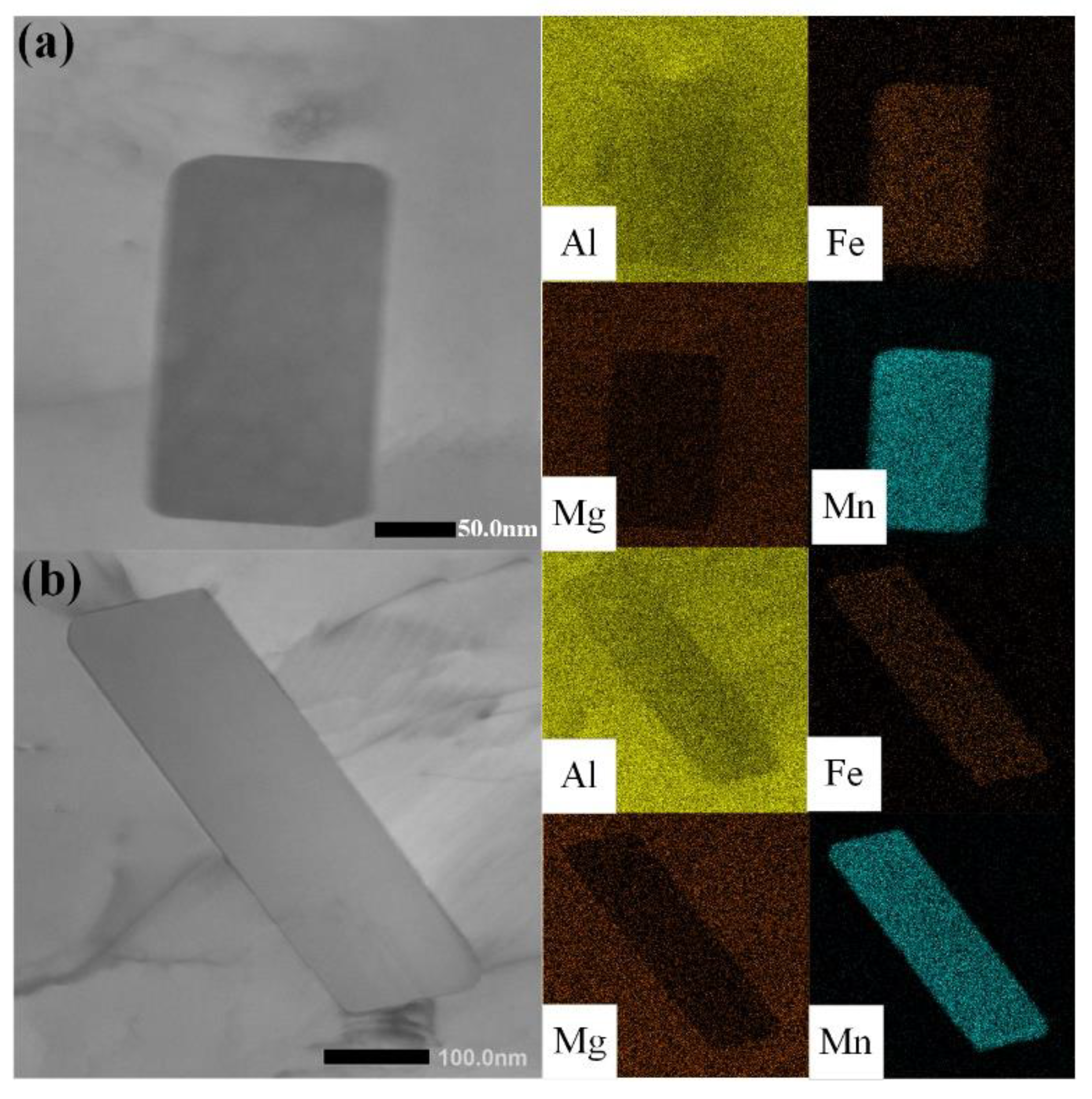
- The effect of Al6Mn on the β phase: the TEM observations of sensitized samples containing different Al6Mn phases showed that the β phase preferentially precipitates on plate-like Al6Mn, leading to intragranular precipitation, which improves the material’s resistance to intergranular corrosion. Further, the NAMLT testing indicated that plate-like Al6Mn can reduce the degree of sensitization by 15%.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, Y.; Li, Y.; Xiao, Z.; Liu, Y.; Huang, Y.; Ren, X. Effect of Homogenization on the Corrosion Behavior of 5083-H321 Aluminum Alloy. J. Alloys Compd. 2016, 673, 73–79. [Google Scholar] [CrossRef]
- Ding, Y.S.; Gao, K.Y.; Li, C.J.; Wen, S.P.; Huang, H.; Wu, X.L.; Nie, Z.R.; Zhou, D.J. The Effect of Mg Content on Intergranular Corrosion of Al-Mg-Mn Alloys after Annealing. Mater. Sci. Forum 2017, 877, 514–521. [Google Scholar] [CrossRef]
- Wen, W.; Zhao, Y.; Morris, J.G. The Effect of Mg Precipitation on the Mechanical Properties of 5xxx Aluminum Alloys. Mater. Sci. Eng. A 2005, 392, 136–144. [Google Scholar] [CrossRef]
- Li, Y.; Yang, B.; Zhang, M.; Wang, H.; Gong, W.; Lai, R.; Li, Y.; Teng, J. The Corrosion Behavior and Mechanical Properties of 5083 Al-Mg Alloy Manufactured by Additive Friction Stir Deposition. Corros. Sci. 2023, 213, 110972. [Google Scholar] [CrossRef]
- Crane, C.B.; Gangloff, R.P. Stress Corrosion Cracking of Al-Mg Alloy 5083 Sensitized at Low Temperature. Corrosion 2016, 72, 221–241. [Google Scholar] [CrossRef]
- Goswami, R.; Spanos, G.; Pao, P.S.; Holtz, R.L. Precipitation Behavior of the β Phase in Al-5083. Mater. Sci. Eng. A 2010, 527, 1089–1095. [Google Scholar] [CrossRef]
- Seong, J.; Frankel, G.S.; Sridhar, N. Inhibition of Stress Corrosion Cracking of Sensitized AA5083. Corrosion 2016, 72, 284–296. [Google Scholar] [CrossRef]
- Choi, D.H.; Ahn, B.W.; Quesnel, D.J.; Jung, S.B. Behavior of β Phase (Al3Mg2) in AA 5083 during Friction Stir Welding. Intermetallics 2013, 35, 120–127. [Google Scholar] [CrossRef]
- Birbilis, N.; Knight, S.; Zhang, R.; Holtz, R.; Goswami, R.; Davies, C. A Survey of Sensitisation in 5xxx Series Aluminium Alloys. Corrosion 2015, 72, 144–159. [Google Scholar]
- Goswami, R.; Holtz, R.L. Transmission Electron Microscopic Investigations of Grain Boundary Beta Phase Precipitation in Al 5083 Aged at 373 °K (100 °C). Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2013, 44, 1279–1289. [Google Scholar] [CrossRef]
- Carroll, M.C.; Gouma, P.I.; Mills, M.J.; Daehn, G.S.; Dunbar, B.R. Effects of Zn Additions on the Grain Boundary Precipitation and Corrosion of Al-5083. Scr. Mater. 2000, 42, 335–340. [Google Scholar] [CrossRef]
- Meng, C.; Zhang, D.; Cui, H.; Zhuang, L.; Zhang, J. Mechanical Properties, Intergranular Corrosion Behavior and Microstructure of Zn Modified Al-Mg Alloys. J. Alloys Compd. 2014, 617, 925–932. [Google Scholar] [CrossRef]
- Qin, J.; Li, Z.; Ma, M.-Y.; Yi, D.-Q.; Wang, B. Diversity of intergranular corrosion and stress corrosion cracking for 5083 Al alloy with different grain sizes. Trans. Nonferrous Met. Soc. China 2022, 32, 765–777. [Google Scholar] [CrossRef]
- Li, Z.; Yi, D.; Tan, C.; Wang, B. Investigation of the Stress Corrosion Cracking Behavior in Annealed 5083 Aluminum Alloy Sheets with Different Texture Types. J. Alloys Compd. 2020, 817, 152690. [Google Scholar] [CrossRef]
- Scotto D’Antuono, D.; Gaies, J.; Golumbfskie, W.; Taheri, M.L. Direct Measurement of the Effect of Cold Rolling on β Phase Precipitation Kinetics in 5xxx Series Aluminum Alloys. Acta Mater. 2017, 123, 264–271. [Google Scholar] [CrossRef]
- Mofarrehi, M.; Javidani, M.; Chen, X.G. Effect of Mn Content on the Hot Deformation Behavior and Microstructure Evolution of Al–Mg–Mn 5xxx Alloys. Mater. Sci. Eng. A 2022, 845, 143217. [Google Scholar] [CrossRef]
- G67-04; Standard Test Method for Determining the Susceptibility to Intergranular Corrosion of 5XXX Series Aluminum Alloys by Mass Loss After Exposure to Nitric Acid (NAMLT Test). ASTM: West Conshohocken, PA, USA, 2004.
- Algendy, A.Y.; Liu, K.; Rometsch, P.; Parson, N.; Chen, X.G. Effects of AlMn Dispersoids and Al3(Sc,Zr) Precipitates on the Microstructure and Ambient/Elevated-Temperature Mechanical Properties of Hot-Rolled AA5083 Alloys. Mater. Sci. Eng. A 2022, 855, 143950. [Google Scholar] [CrossRef]
- Algendy, A.Y.; Liu, K.; Chen, X.G. Evolution of Dispersoids during Multistep Heat Treatments and Their Effect on Rolling Performance in an Al-5% Mg–0.8% Mn Alloy. Mater. Charact. 2021, 181, 111487. [Google Scholar] [CrossRef]
- Mills, R.J.; Lattimer, B.Y.; Case, S.W.; Mouritz, A.P. The Influence of Sensitization and Corrosion on Creep of 5083-H116. Corros. Sci. 2018, 143, 1–9. [Google Scholar] [CrossRef]
- Krishnamurthy, S.C.; Arseenko, M.; Kashiwar, A.; Dufour, P.; Marchal, Y.; Delahaye, J.; Idrissi, H.; Pardoen, T.; Mertens, A.; Simar, A. Controlled Precipitation in a New Al-Mg-Sc Alloy for Enhanced Corrosion Behavior While Maintaining the Mechanical Performance. Mater. Charact. 2023, 200, 112886. [Google Scholar] [CrossRef]
- Zhang, R.; Li, J.; Li, Q.; Qi, Y.; Zeng, Z.; Qiu, Y.; Chen, X.; Kairy, S.K.; Thomas, S.; Birbilis, N. Analysing the Degree of Sensitisation in 5xxx Series Aluminium Alloys Using Artificial Neural Networks: A Tool for Alloy Design. Corros. Sci. 2019, 150, 268–278. [Google Scholar] [CrossRef]
- Harris, Z.D.; Burns, J.T. On the Loading Rate Dependence of Environment-Assisted Cracking in Sensitized AA5456-H116 Exposed to Marine Environments. Corros. Sci. 2022, 201, 110267. [Google Scholar] [CrossRef]
- Ning, J.; Gao, W.; Gu, X.; Zhang, H.; Guan, W.; Li, W.; Liang, H.; Wang, D.; Lewandowski, J.J. Precipitation Behavior and Corrosion Properties of Friction Stir Welded AA5083 Al–Mg Alloy after Sensitization. Mater. Charact. 2023, 199, 112782. [Google Scholar] [CrossRef]
- Jones, R.H.; Baer, D.R.; Danielson, M.J.; Vetrano, J.S. Role of Mg in the Stress Corrosion Cracking of an Al-Mg Alloy. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2001, 32, 1699–1711. [Google Scholar] [CrossRef]
- Yan, J.; Heckman, N.M.; Velasco, L.; Hodge, A.M. Improve Sensitization and Corrosion Resistance of an Al-Mg Alloy by Optimization of Grain Boundaries. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, L.; Han, C.; Ou, L.; Wang, J.; Liu, C. Effect of Fe, Si and Cooling Rate on the Formation of Fe- and Mn-Rich Intermetallics in Al-5Mg–0.8Mn Alloy. J. Mater. Sci. Technol. 2016, 32, 305–312. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, D.; Wang, H.; Jia, Y.; Lin, B.; Tang, Y.; Tang, Y.; Shu, D.; Sun, Z.; Fu, Y.; et al. Revealing the Influence of Fe on Fe-Rich Phases Formation and Mechanical Properties of Cast Al-Mg-Mn-Fe Alloys. J. Alloys Compd. 2022, 901, 163666. [Google Scholar] [CrossRef]

| Number | Material and State | Mg Content (wt.%) | Sensitization Conditions |
|---|---|---|---|
| 1 [20] | 5083 H116 | 4.4 | 150 °C–1250 h |
| 2 [21] | 5028 H116 | 3.2–4.8 | 120 °C–7 day |
| 3 [22] | 5083 H | 4.2 | 150 °C–7 day |
| 4 [23] | 5456 H116 | 5.0 | 100 °C–14 day |
| 5 [24] | 5083 H112 | 4.0 | 175 °C–100 h |
| 6 [25] | 5083 H321 | 4.8 | 175 °C–100 h |
| 7 [26] | 5182 T6 | 4.5 | 150 °C–100 h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Wang, Y.; Zhao, P.; Jiang, Z.; Tian, Y.; Yang, Y.; Han, J. Study of Intergranular Corrosion Behaviors of Mn-Increased 5083 Al Alloy with Controlled Precipitation States of Al6Mn Formed during Homogenization Annealing. Metals 2024, 14, 1053. https://doi.org/10.3390/met14091053
Zhang P, Wang Y, Zhao P, Jiang Z, Tian Y, Yang Y, Han J. Study of Intergranular Corrosion Behaviors of Mn-Increased 5083 Al Alloy with Controlled Precipitation States of Al6Mn Formed during Homogenization Annealing. Metals. 2024; 14(9):1053. https://doi.org/10.3390/met14091053
Chicago/Turabian StyleZhang, Peng, Yue Wang, Pizhi Zhao, Zhengyi Jiang, Yinbao Tian, Yang Yang, and Jian Han. 2024. "Study of Intergranular Corrosion Behaviors of Mn-Increased 5083 Al Alloy with Controlled Precipitation States of Al6Mn Formed during Homogenization Annealing" Metals 14, no. 9: 1053. https://doi.org/10.3390/met14091053








