Sintering Mechanism and Leaching Kinetics of Low-Grade Mixed Lithium Ore and Limestone
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Methods
2.2.1. Limestone Sintering Procedure
2.2.2. Leaching Procedure
2.3. Characterization
3. Results and Discussion
3.1. Mineralogical Analysis
3.1.1. Phase Analysis of Lithium Ore
3.1.2. Microstructure of Lithium Ore
3.1.3. Mineral Liberation Analysis of Lithium Ore
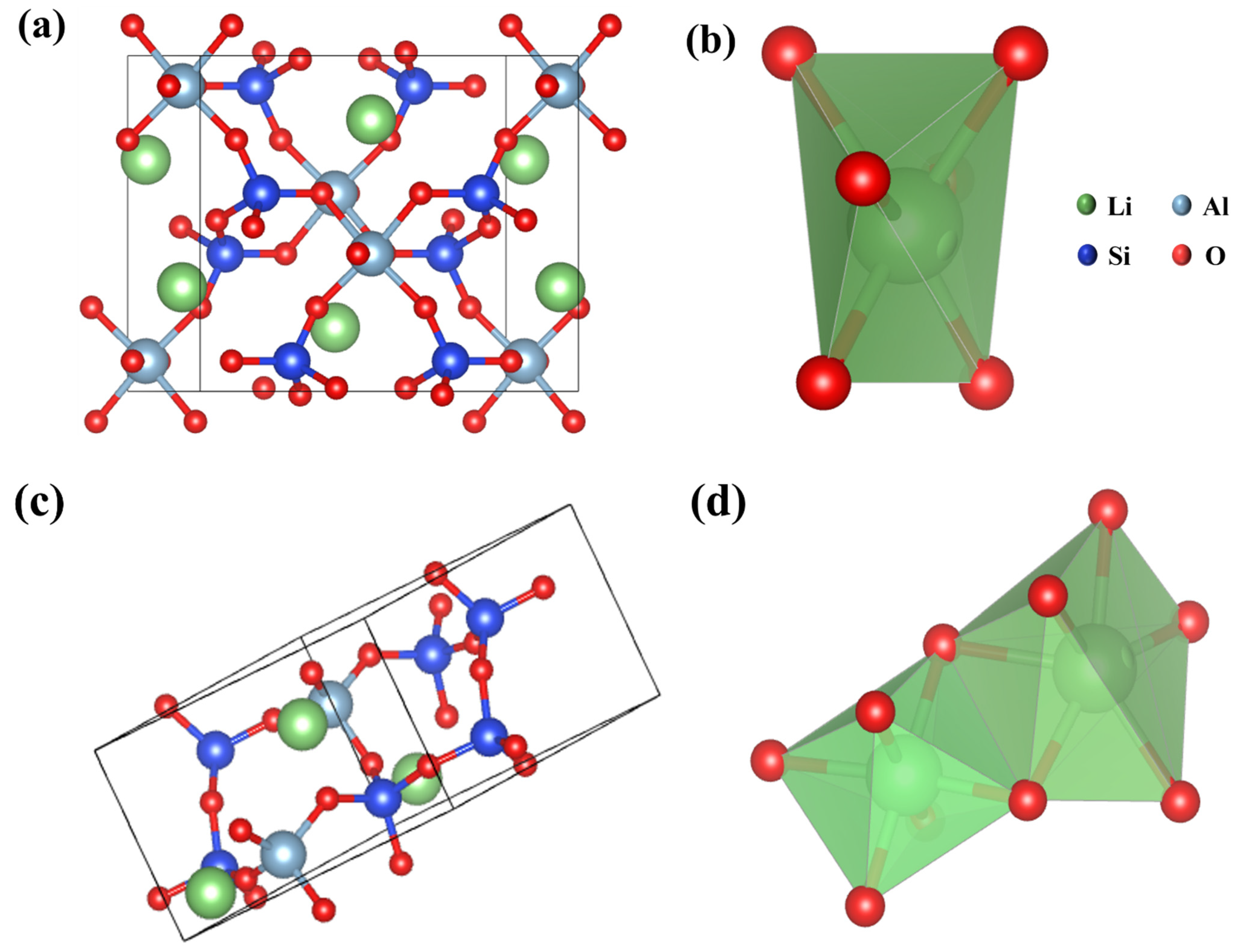
3.1.4. The Crystal Structure of Lithium Ore
3.2. Limestone Sintering Process
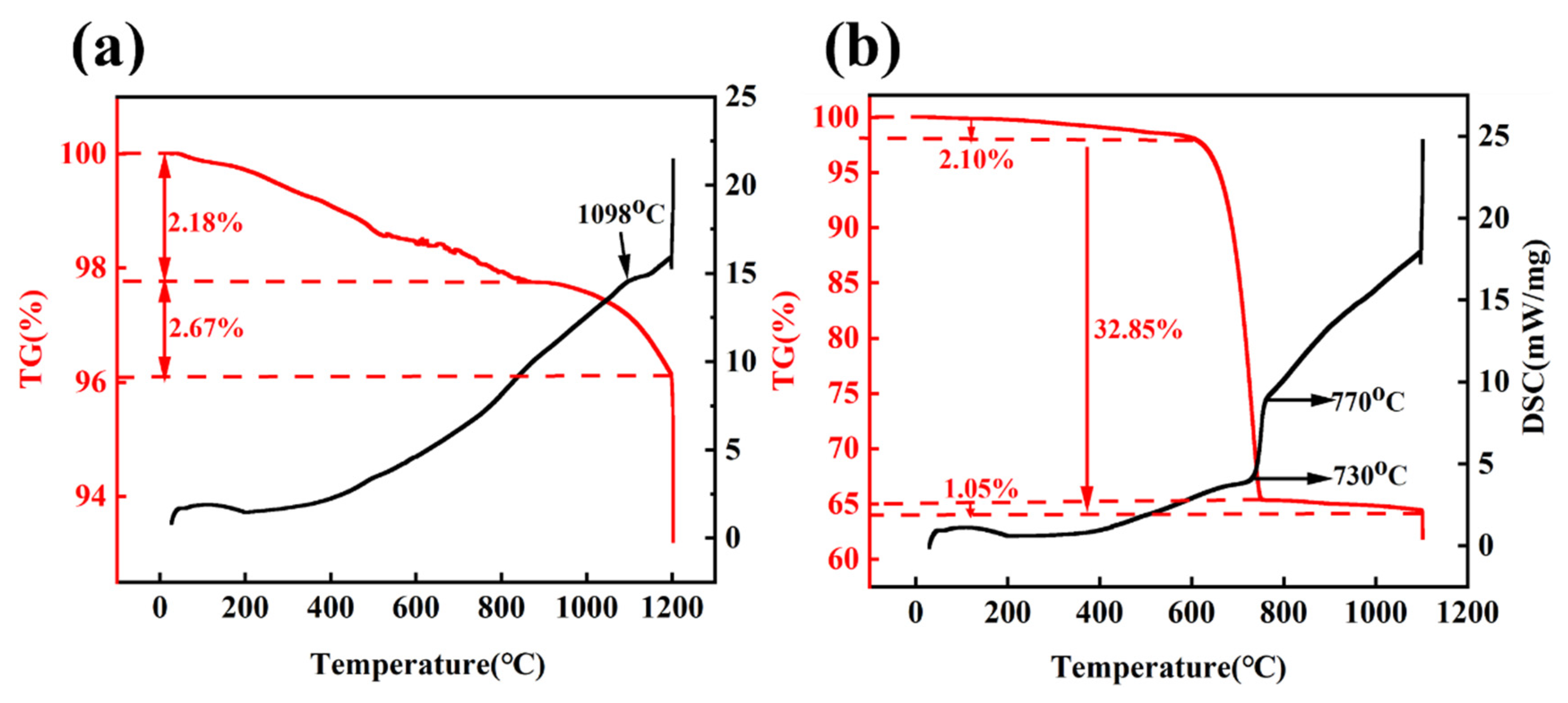
3.2.1. TG–DSC Analysis
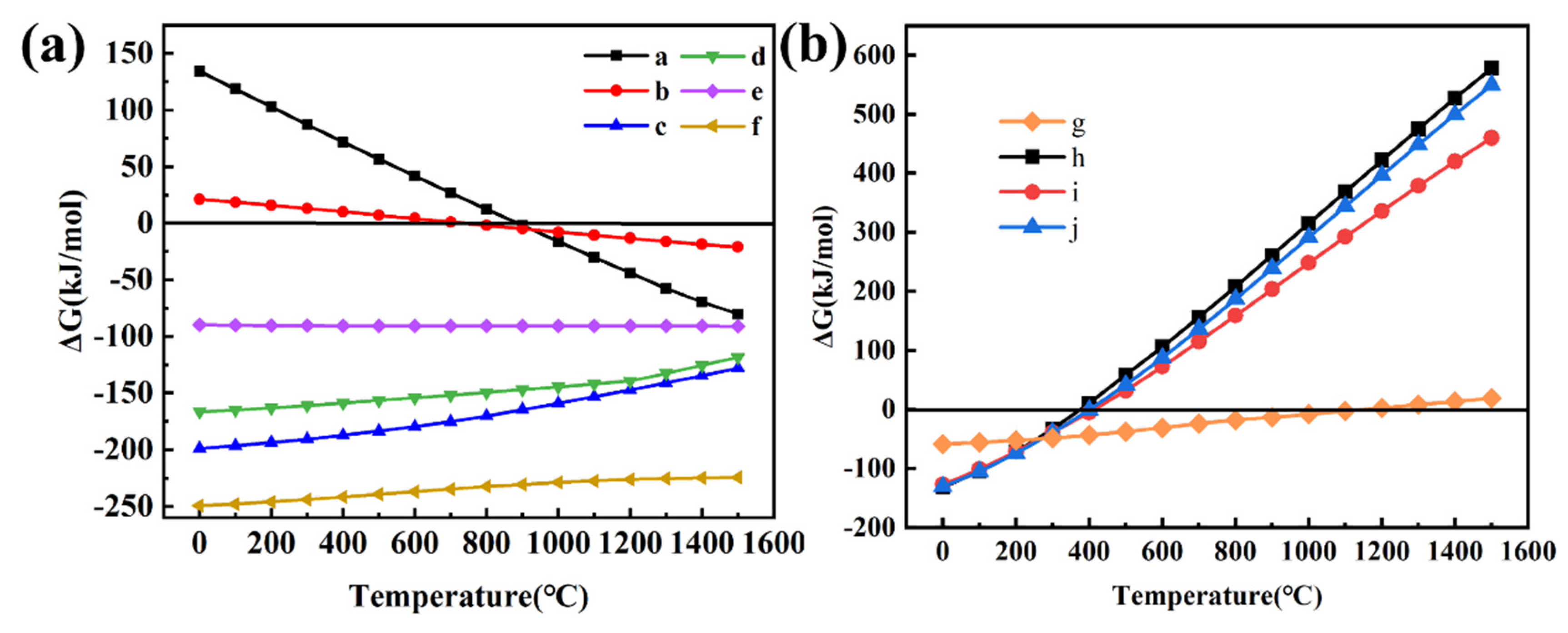
3.2.2. Thermodynamic Calculation
3.2.3. Effects of Sintering Conditions on the Leaching Rate of Li
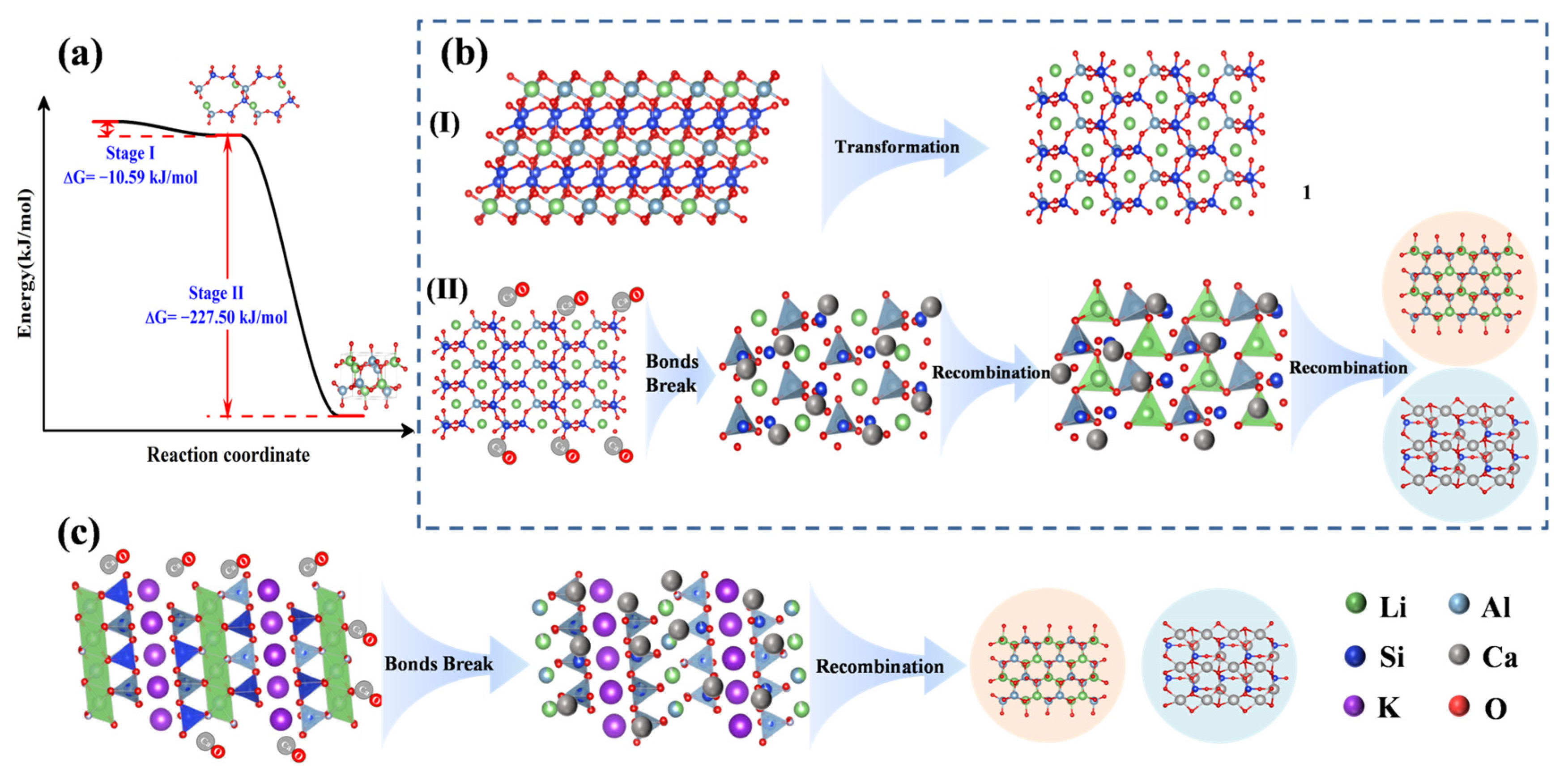
3.3. Mechanism of Sintering Process
3.4. Leaching Process
3.4.1. Effects of Leaching Conditions on the Leaching Rate of Li
3.4.2. Leaching Kinetics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Christmann, P.; Gloaguen, E.; Labbé, J.-F.; Melleton, J.; Piantone, P. Global Lithium Resources and Sustainability Issues. In Lithium Process Chemistry; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1–40. ISBN 978-0-12-801417-2. [Google Scholar]
- Dessemond, C.; Lajoie-Leroux, F.; Soucy, G.; Laroche, N.; Magnan, J.-F. Spodumene: The Lithium Market, Resources and Processes. Minerals 2019, 9, 334. [Google Scholar] [CrossRef]
- Martin, G.; Rentsch, L.; Höck, M.; Bertau, M. Lithium Market Research–Global Supply, Future Demand and Price Development. Energy Storage Mater. 2017, 6, 171–179. [Google Scholar] [CrossRef]
- Han, S.; Zhenghao, M.; Meilin, L.; Xiaohui, Y.; Xiaoxue, W. Global Supply Sustainability Assessment of Critical Metals for Clean Energy Technology. Resour. Policy 2023, 85, 103994. [Google Scholar] [CrossRef]
- Xu, C.; Dai, Q.; Gaines, L.; Hu, M.; Tukker, A.; Steubing, B. Future Material Demand for Automotive Lithium-Based Batteries. Commun. Mater. 2020, 1, 99. [Google Scholar] [CrossRef]
- Li, X.; Mo, Y.; Qing, W.; Shao, S.; Tang, C.Y.; Li, J. Membrane-Based Technologies for Lithium Recovery from Water Lithium Resources: A Review. J. Membr. Sci. 2019, 591, 117317. [Google Scholar] [CrossRef]
- Choubey, P.K.; Kim, M.; Srivastava, R.R.; Lee, J.; Lee, J.-Y. Advance Review on the Exploitation of the Prominent Energy-Storage Element: Lithium. Part I: From Mineral and Brine Resources. Miner. Eng. 2016, 89, 119–137. [Google Scholar] [CrossRef]
- Averill, W.A.; Olson, D.L. A review of extractive processes for lithium from ores and brines. In Lithium Needs and Resources; Elsevier: Amsterdam, The Netherlands, 1978; pp. 305–313. ISBN 978-0-08-022733-7. [Google Scholar]
- Hu, Y.; Su, H.; Zhu, Z.; Qi, T.; Pang, Q. Environmentally Benign Techniques of Lithium Extraction from Salt Lakes: A Review. Environ. Chem. Lett. 2024, 22, 105–120. [Google Scholar] [CrossRef]
- Xiang, W.; Liang, S.; Zhou, Z.; Qin, W.; Fei, W. Lithium Recovery from Salt Lake Brine by Counter-Current Extraction Using Tributyl Phosphate/FeCl3 in Methyl Isobutyl Ketone. Hydrometallurgy 2017, 171, 27–32. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, B.; Lü, Y.; Wang, C.; Chen, Y. A Review of Lithium Extraction from Natural Resources. Int. J. Miner. Metall. Mater. 2023, 30, 209–224. [Google Scholar] [CrossRef]
- Sun, X.; Luo, H.; Dai, S. Ionic Liquids-Based Extraction: A Promising Strategy for the Advanced Nuclear Fuel Cycle. Chem. Rev. 2012, 112, 2100–2128. [Google Scholar] [CrossRef]
- Mudring, A.; Tang, S. Ionic Liquids for Lanthanide and Actinide Chemistry. Eur. J. Inorg. Chem. 2010, 2010, 2569–2581. [Google Scholar] [CrossRef]
- Shi, C.; Jia, Y.; Zhang, C.; Liu, H.; Jing, Y. Extraction of Lithium from Salt Lake Brine Using Room Temperature Ionic Liquid in Tributyl Phosphate. Fusion Eng. Des. 2015, 90, 1–6. [Google Scholar] [CrossRef]
- Su, H.; Li, Z.; Zhang, J.; Zhu, Z.; Wang, L.; Qi, T. Recovery of Lithium from Salt Lake Brine Using a Mixed Ternary Solvent Extraction System Consisting of TBP, FeCl3 and P507. Hydrometallurgy 2020, 197, 105487. [Google Scholar] [CrossRef]
- Xie, R.; Zhao, Z.; Tong, X.; Xie, X.; Song, Q.; Fan, P. Review of the Research on the Development and Utilization of Clay-Type Lithium Resources. Particuology 2024, 87, 46–53. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, B.; Lv, Y.; Wang, C.; Chen, Y. An Effective Method for Directly Extracting Lithium from α-Spodumene by Activated Roasting and Sulfuric Acid Leaching. J. Ind. Eng. Chem. 2023, 122, 540–550. [Google Scholar] [CrossRef]
- Rioyo, J.; Tuset, S.; Grau, R. Lithium Extraction from Spodumene by the Traditional Sulfuric Acid Process: A Review. Miner. Process. Extr. Metall. Rev. 2022, 43, 97–106. [Google Scholar] [CrossRef]
- Han, S.; Sagzhanov, D.; Pan, J.; Vaziri Hassas, B.; Rezaee, M.; Akbari, H.; Mensah-Biney, R. Direct Extraction of Lithium from α-Spodumene by Salt Roasting–Leaching Process. ACS Sustain. Chem. Eng. 2022, 10, 13495–13504. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, T.; He, L.; Zhao, Z.; Liu, X. A Promising Approach for Directly Extracting Lithium from α-Spodumene by Alkaline Digestion and Precipitation as Phosphate. Hydrometallurgy 2019, 189, 105141. [Google Scholar] [CrossRef]
- Wu, X.; Zeng, L.; Wu, S.; Qing, J.; Li, Q.; Cao, Z.; Wang, M.; Zhang, G.; Guan, W. Eco-Friendly Extraction of Arsenic and Tungsten from Hazardous Tungsten Residue Waste by Pressure Oxidation Leaching in Alkaline Solutions: Mechanism and Kinetic Model. J. Environ. Manag. 2023, 325, 116586. [Google Scholar] [CrossRef]
- Xiang, J.; Huang, Q.; Lv, X.; Bai, C. Extraction of Vanadium from Converter Slag by Two-Step Sulfuric Acid Leaching Process. J. Clean. Prod. 2018, 170, 1089–1101. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, D.; Qiu, S.; Liu, C.; Yu, J. Lithium Recovery from Pretreated α -spodumene Residue through Acid Leaching at Ambient Temperature. Can. J. Chem. Eng. 2023, 101, 4360–4373. [Google Scholar] [CrossRef]
- Gao, L.; Wang, H.; Li, J.; Wang, M. Recovery of Lithium from Lepidolite by Sulfuric Acid and Separation of Al/Li by Nanofiltration. Minerals 2020, 10, 981. [Google Scholar] [CrossRef]
- Zhang, X.; Tan, X.; Li, C.; Yi, Y.; Liu, W.; Zhang, L. Energy-Efficient and Simultaneous Extraction of Lithium, Rubidium and Cesium from Lepidolite Concentrate via Sulfuric Acid Baking and Water Leaching. Hydrometallurgy 2019, 185, 244–249. [Google Scholar] [CrossRef]
- Su, H.; Ju, J.; Zhang, J.; Yi, A.; Lei, Z.; Wang, L.; Zhu, Z.; Qi, T. Lithium Recovery from Lepidolite Roasted with Potassium Compounds. Miner. Eng. 2020, 145, 106087. [Google Scholar] [CrossRef]
- Mulwanda, J.; Senanayake, G.; Oskierski, H.; Altarawneh, M.; Dlugogorski, B.Z. Leaching of Lepidolite and Recovery of Lithium Hydroxide from Purified Alkaline Pressure Leach Liquor by Phosphate Precipitation and Lime Addition. Hydrometallurgy 2021, 201, 105538. [Google Scholar] [CrossRef]
- Nazir, M.K.; Dyer, L.; Tadesse, B.; Albijanic, B.; Kashif, N. Lithium Deportment by Size of a Calcined Spodumene Ore. Sci. Rep. 2022, 12, 18335. [Google Scholar] [CrossRef]
- Rosales, G.D.; Resentera, A.C.J.; Wuilloud, R.G.; Rodriguez, M.H.; Esquivel, M.R. Optimization of Combined Mechanical Activation-Leaching Parameters of Low-Grade α-Spodumene/NaF Mixture Using Response Surface Methodology. Miner. Eng. 2022, 184, 107633. [Google Scholar] [CrossRef]
- Wang, J.-M.; Hou, K.-S.; Yang, L.; Liu, X.-C.; Wang, R.-C.; Li, G.-M.; Fu, J.-G.; Hu, F.-Y.; Tian, Y.-L.; Wu, F.-Y. Mineralogy, Petrology and P-T Conditions of the Spodumene Pegmatites and Surrounding Meta-Sediments in Lhozhag, Eastern Himalaya. Lithos 2023, 456–457, 107295. [Google Scholar] [CrossRef]
- Roy, T.; Plante, B.; Benzaazoua, M.; Demers, I. Geochemistry and Mineralogy of a Spodumene-Pegmatite Lithium Ore at Various Mineral Beneficiation Stages. Miner. Eng. 2023, 202, 108312. [Google Scholar] [CrossRef]
- Han, P.; Zhao, F.; Liu, D.; Zhang, Q.; Zhang, Q.; Ullah, S. Occurrence and Favorable Enrichment Environment of Lithium in Gaoping Coal Measures: Evidence from Mineralogy and Geochemistry. Appl. Sci. 2024, 14, 7298. [Google Scholar] [CrossRef]
- Aylmore, M.G.; Merigot, K.; Rickard, W.D.A.; Evans, N.J.; McDonald, B.J.; Catovic, E.; Spitalny, P. Assessment of a Spodumene Ore by Advanced Analytical and Mass Spectrometry Techniques to Determine Its Amenability to Processing for the Extraction of Lithium. Miner. Eng. 2018, 119, 137–148. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, L.; Pan, J.; He, X.; Shi, S.; Long, X.; Yang, Y.; Zhao, X.; Zhou, C. Study on Modes of Occurrence and Selective Leaching of Lithium in Coal Gangue via Grinding-Thermal Activation. Chem. Eng. J. 2024, 482, 148941. [Google Scholar] [CrossRef]
- Lajoie-Leroux, F.; Dessemond, C.; Soucy, G.; Laroche, N.; Magnan, J.-F. Impact of the Impurities on Lithium Extraction from β-Spodumene in the Sulfuric Acid Process. Miner. Eng. 2018, 129, 1–8. [Google Scholar] [CrossRef]
- Vuković, F.; Garcia, N.A.; Perera, S.; Turchi, M.; Andersson, M.P.; Solvang, M.; Raiteri, P.; Walsh, T.R. Atomistic Simulations of Calcium Aluminosilicate Interfaced with Liquid Water. J. Chem. Phys. 2023, 159, 104704. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Duan, Y.; Li, M.; Hou, D.; Wang, P.; Chen, J.; Li, S. Reaction Molecular Dynamics Study of Calcium Alumino-Silicate Hydrate Gel in the Hydration Deposition Process at the Calcium Silicate Hydrate Interface: The Influence of Al/Si. J. Build. Eng. 2024, 86, 108823. [Google Scholar] [CrossRef]
- Cao, R.; Jia, Z.; Zhang, Z.; Zhang, Y.; Banthia, N. Leaching Kinetics and Reactivity Evaluation of Ferronickel Slag in Alkaline Conditions. Cem. Concr. Res. 2020, 137, 106202. [Google Scholar] [CrossRef]
- Sethurajan, M.; Huguenot, D.; Jain, R.; Lens, P.N.L.; Horn, H.A.; Figueiredo, L.H.A.; Van Hullebusch, E.D. Leaching and Selective Zinc Recovery from Acidic Leachates of Zinc Metallurgical Leach Residues. J. Hazard. Mater. 2017, 324, 71–82. [Google Scholar] [CrossRef]
- Ning, Y.; Zhang, Y.; Zhu, B.; Wei, G.; Wang, Q.; Qu, J. Reducing the Environmental Impact of Lithium-Ion Battery Recycling through Co-Processing of NCM and LFP. Process Saf. Environ. Prot. 2024, 187, 810–819. [Google Scholar] [CrossRef]
- Sharp, J.H.; Brindley, G.W.; Achar, B.N.N. Numerical Data for Some Commonly Used Solid State Reaction Equations. J. Am. Ceram. Soc. 1966, 49, 379–382. [Google Scholar] [CrossRef]
- Wang, X. Effect of Mechanical Activation on the Leaching Kinetics of Niobium-Bearing Mineralisation in KOH Hydrothermal System. Hydrometallurgy 2018, 181, 123–129. [Google Scholar] [CrossRef]

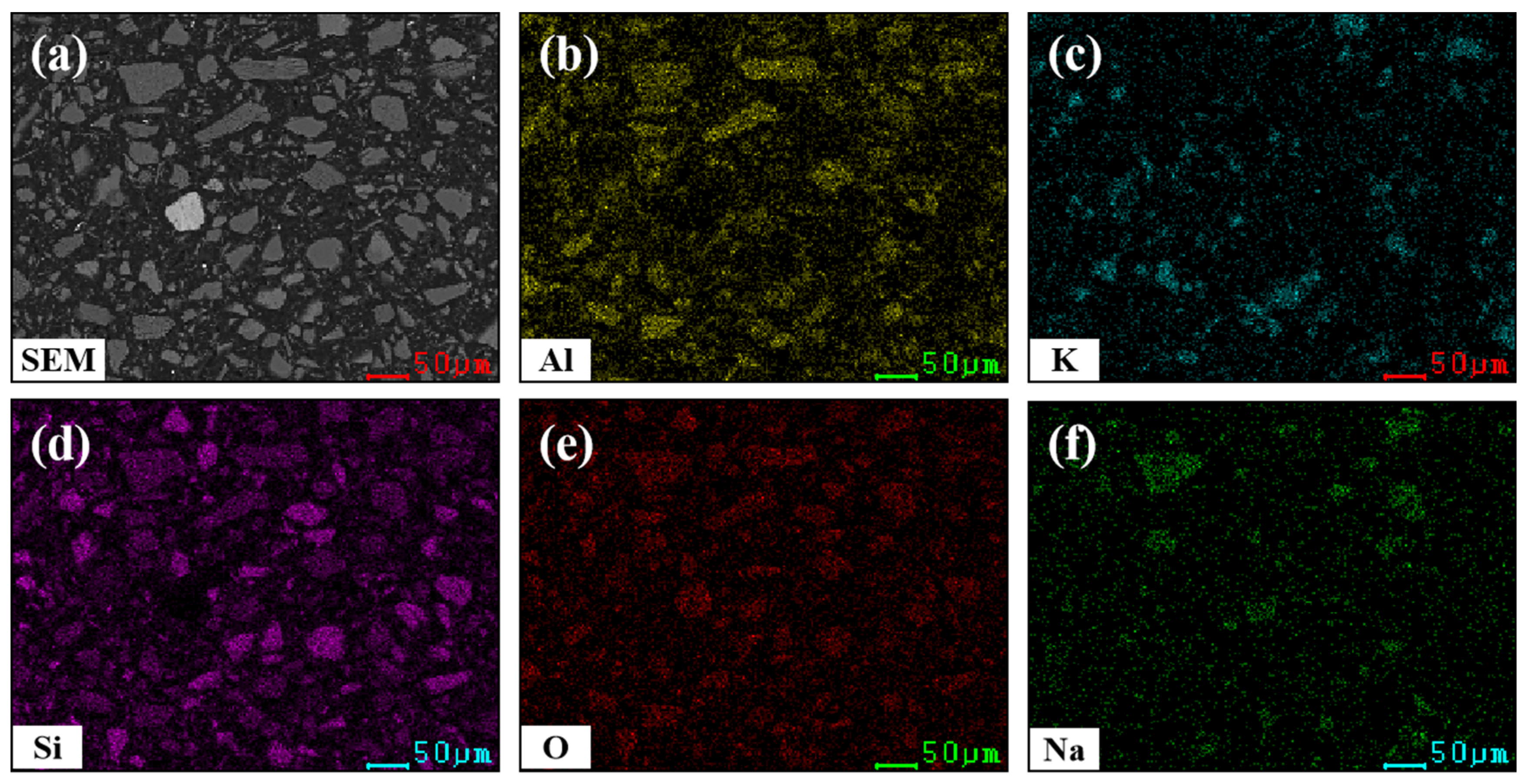
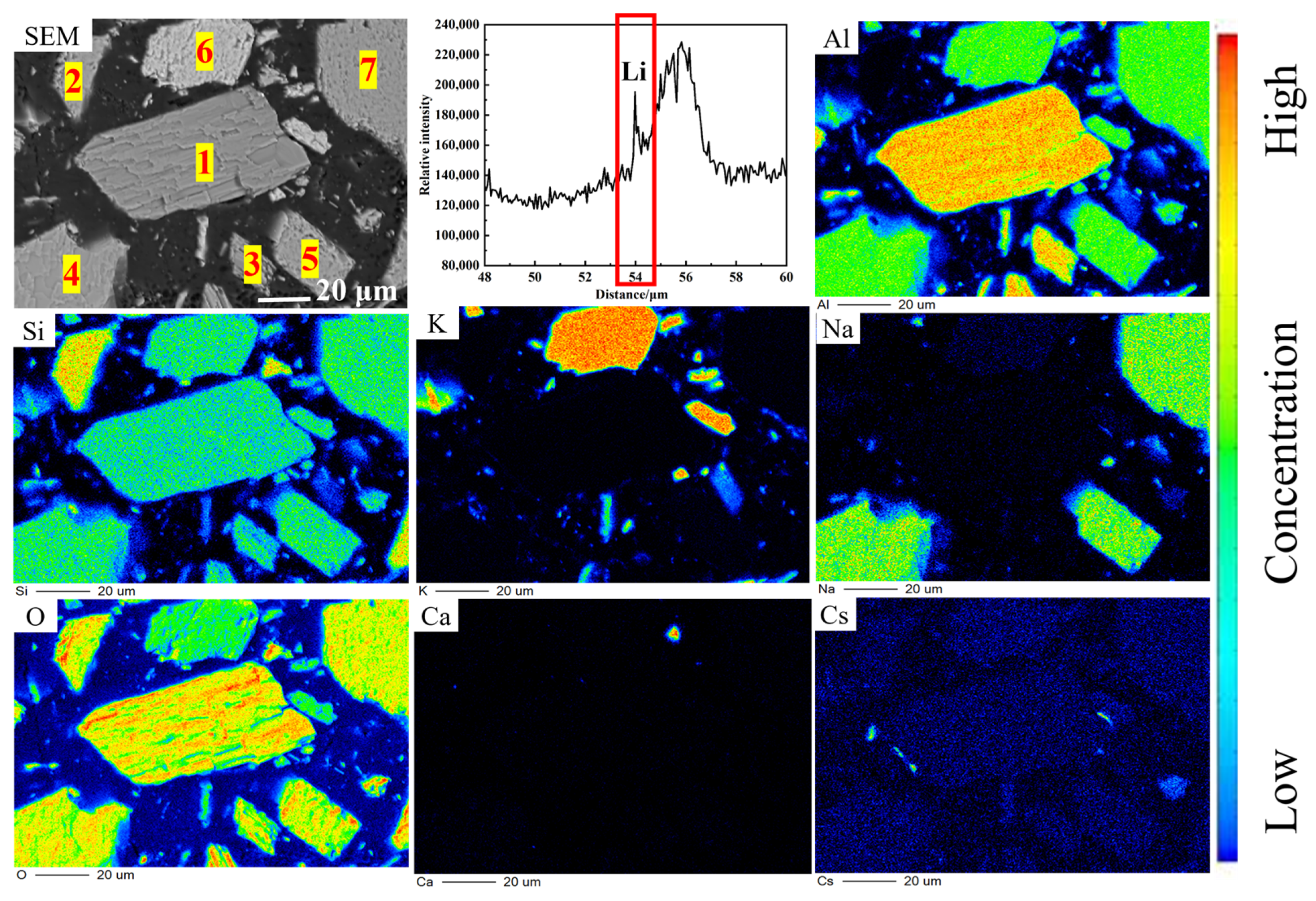
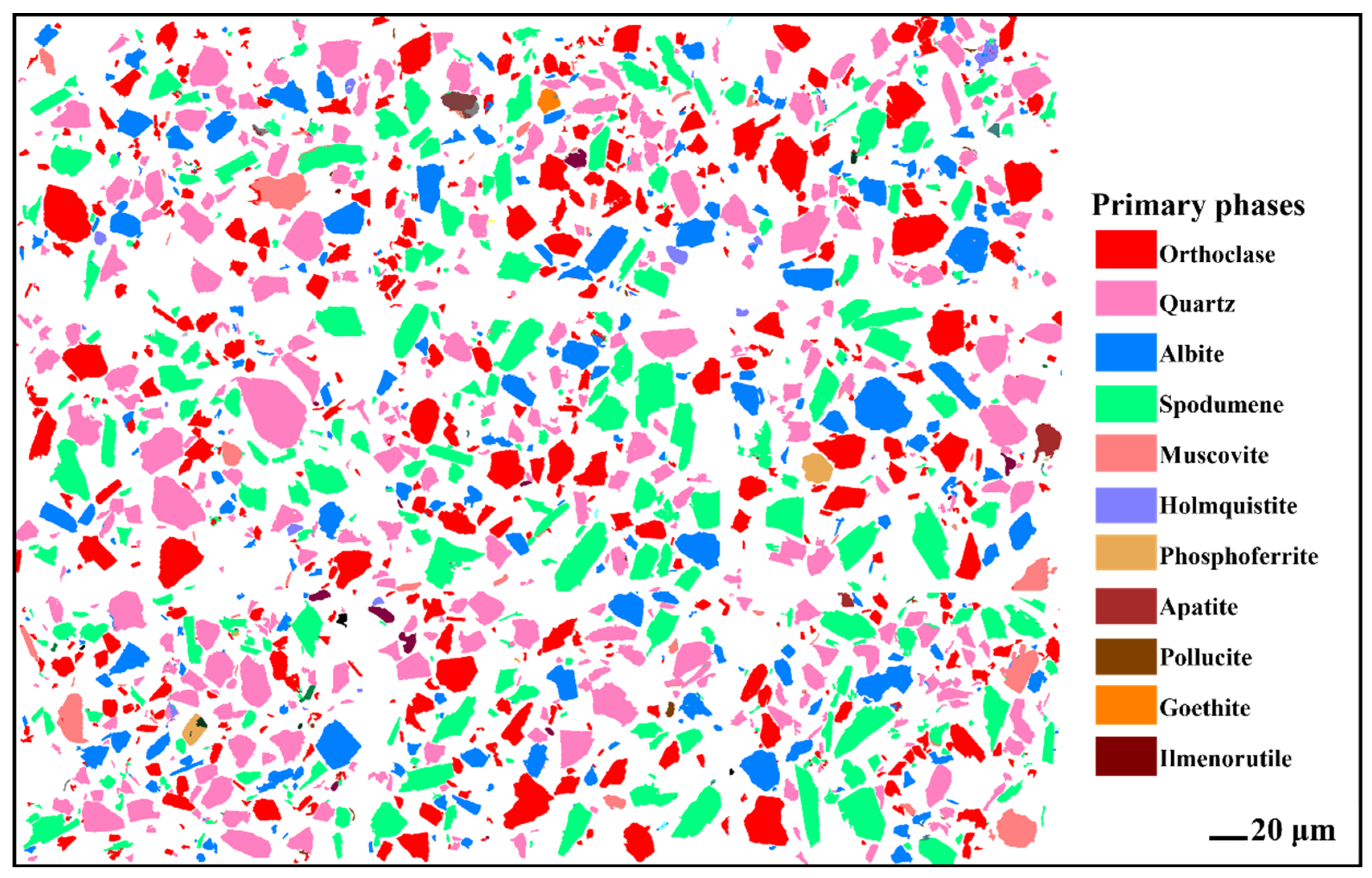
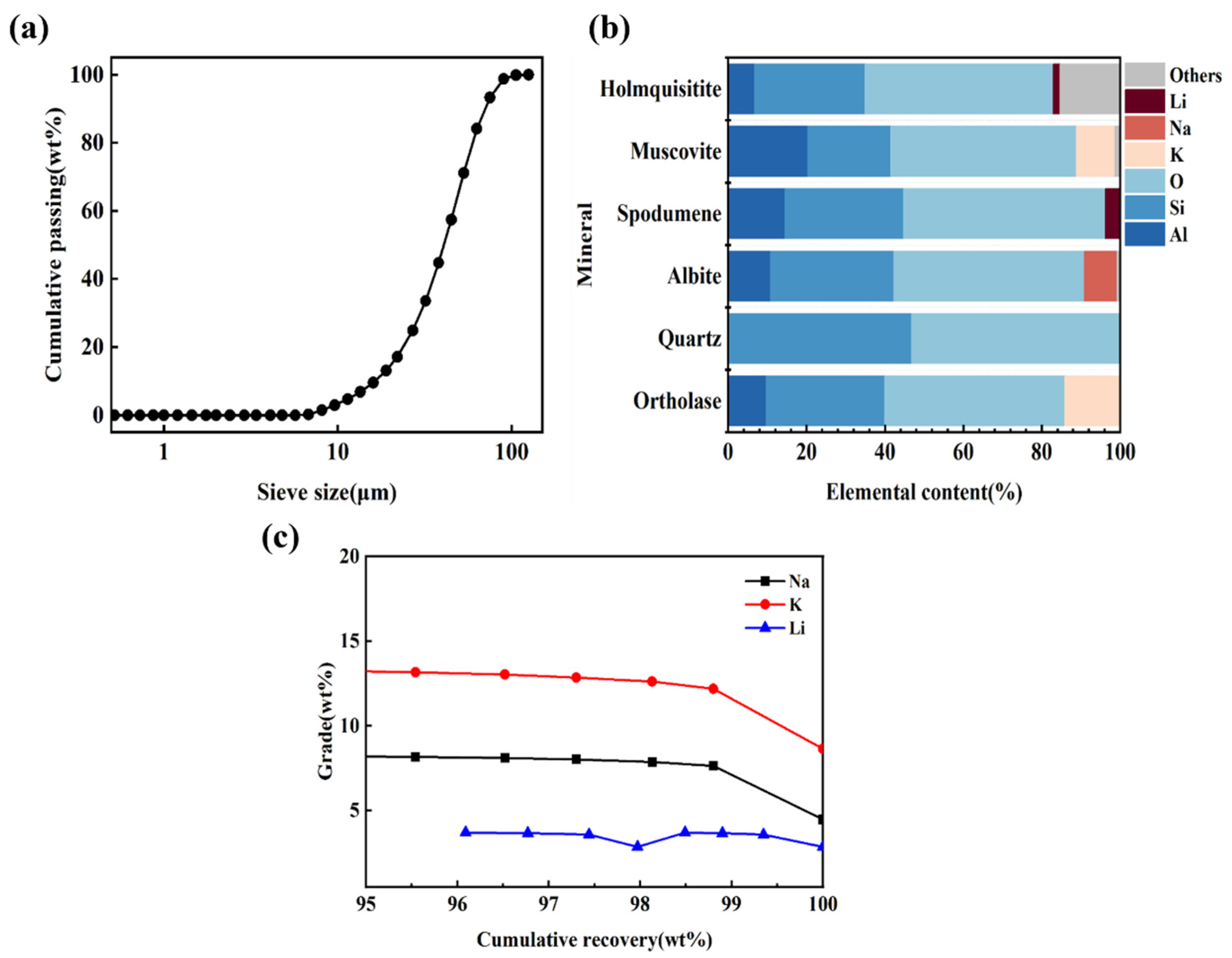






| Oxide | Li2O | SiO2 | Al2O3 | K2O | Na2O | Rb2O | Cs2O | Fe2O3 | Cr2O3 | CaO | MgO |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Content | 2.72 | 74.40 | 17.18 | 4.80 | 1.73 | 0.24 | 0.02 | 0.95 | 0.12 | 0.15 | 0.06 |
| Point | SiO2 | Al2O3 | CaO | Na2O | K2O | Fe2O3 | Cs2O |
|---|---|---|---|---|---|---|---|
| 1 | 64.597 | 35.313 | 0.013 | 0.032 | 0.160 | 0.028 | 0.001 |
| 2 | 99.880 | 0.044 | 0.006 | 0.018 | 0.024 | 0.026 | 0.000 |
| 3 | 61.468 | 32.288 | 0.033 | 0.102 | 0.016 | 0.086 | 0.006 |
| 4 | 67.991 | 26.306 | 0.138 | 5.537 | 0.027 | 0.000 | 0.000 |
| 5 | 68.748 | 26.306 | 0.138 | 5.537 | 0.027 | 0.000 | 0.000 |
| 6 | 59.165 | 23.093 | 0.022 | 0.255 | 17.413 | 0.021 | 0.031 |
| 7 | 71.278 | 25.956 | 0.039 | 2.517 | 0.055 | 0.145 | 0.010 |
| Mineral | Orthoclase | Quartz | Albite | Spodumene | Muscovite | Holmquisite | Others |
|---|---|---|---|---|---|---|---|
| Content | 22.66 | 28.97 | 14.35 | 29.85 | 1.87 | 0.59 | 2.30 |
| a | b | c | α | β | γ | Vol/(nm3) | |
|---|---|---|---|---|---|---|---|
| α–LiAlSi2O6 | 9.466 | 8.394 | 5.221 | 90.00 | 69.67 | 90.00 | 389.01 |
| β–LiAlSi2O6 | 7.548 | 7.548 | 9.177 | 90.00 | 90.00 | 90.00 | 520.36 |
| Bonds Type | Li–O | Si–O | Al–O |
|---|---|---|---|
| Bonds length/(nm) | 2.211 | 1.598 | 2.028 |
| Number | Process | Chemical Reaction |
|---|---|---|
| a | Sintering | CaCO3 →CaO + CO2↑ |
| b | α–Li2O·Al2O3·4SiO2 → β–Li2O·Al2O3·4SiO2 | |
| c | 3CaO + NaAlSi3O8→NaAlO2 + 3CaSiO3 | |
| d | 3CaO + KAlSi3O8→KAlO2 + 3CaSiO3 | |
| e | CaO + SiO2→CaSiO3 | |
| f | 4CaO + LiAlSi2O6→LiAlO2 + 2Ca2SiO4 | |
| g | Leaching | CaO + 2H2O→Ca(OH)2 |
| h i j | 2LiAlO2 + 3Ca(OH)2 + 4H2O→2LiOH + 3CaO·Al2O3·6H2O 2NaAlO2 + 3Ca(OH)2 + 4H2O→2NaOH + 3CaO·Al2O3·6H2O 2KAlO2 + 3Ca(OH)2 + 4H2O→2KOH + 3CaO·Al2O3·6H2O |
| Functional Group | Si–O–Si | Si–O–Al | Al–O | Si–O |
|---|---|---|---|---|
| Wavenumber (cm−1) | 772, 726 and 691 | 535 and 466 | 648 and 585 | 1011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, W.; Meng, L.; Qu, J. Sintering Mechanism and Leaching Kinetics of Low-Grade Mixed Lithium Ore and Limestone. Metals 2024, 14, 1075. https://doi.org/10.3390/met14091075
Fu W, Meng L, Qu J. Sintering Mechanism and Leaching Kinetics of Low-Grade Mixed Lithium Ore and Limestone. Metals. 2024; 14(9):1075. https://doi.org/10.3390/met14091075
Chicago/Turabian StyleFu, Wanying, Long Meng, and Jingkui Qu. 2024. "Sintering Mechanism and Leaching Kinetics of Low-Grade Mixed Lithium Ore and Limestone" Metals 14, no. 9: 1075. https://doi.org/10.3390/met14091075
APA StyleFu, W., Meng, L., & Qu, J. (2024). Sintering Mechanism and Leaching Kinetics of Low-Grade Mixed Lithium Ore and Limestone. Metals, 14(9), 1075. https://doi.org/10.3390/met14091075






