Mechanical, Corrosion and Wear Characteristics of Cu-Based Composites Reinforced with Zirconium Diboride Consolidated by SPS
Abstract
1. Introduction
2. Materials and Methods
3. Results
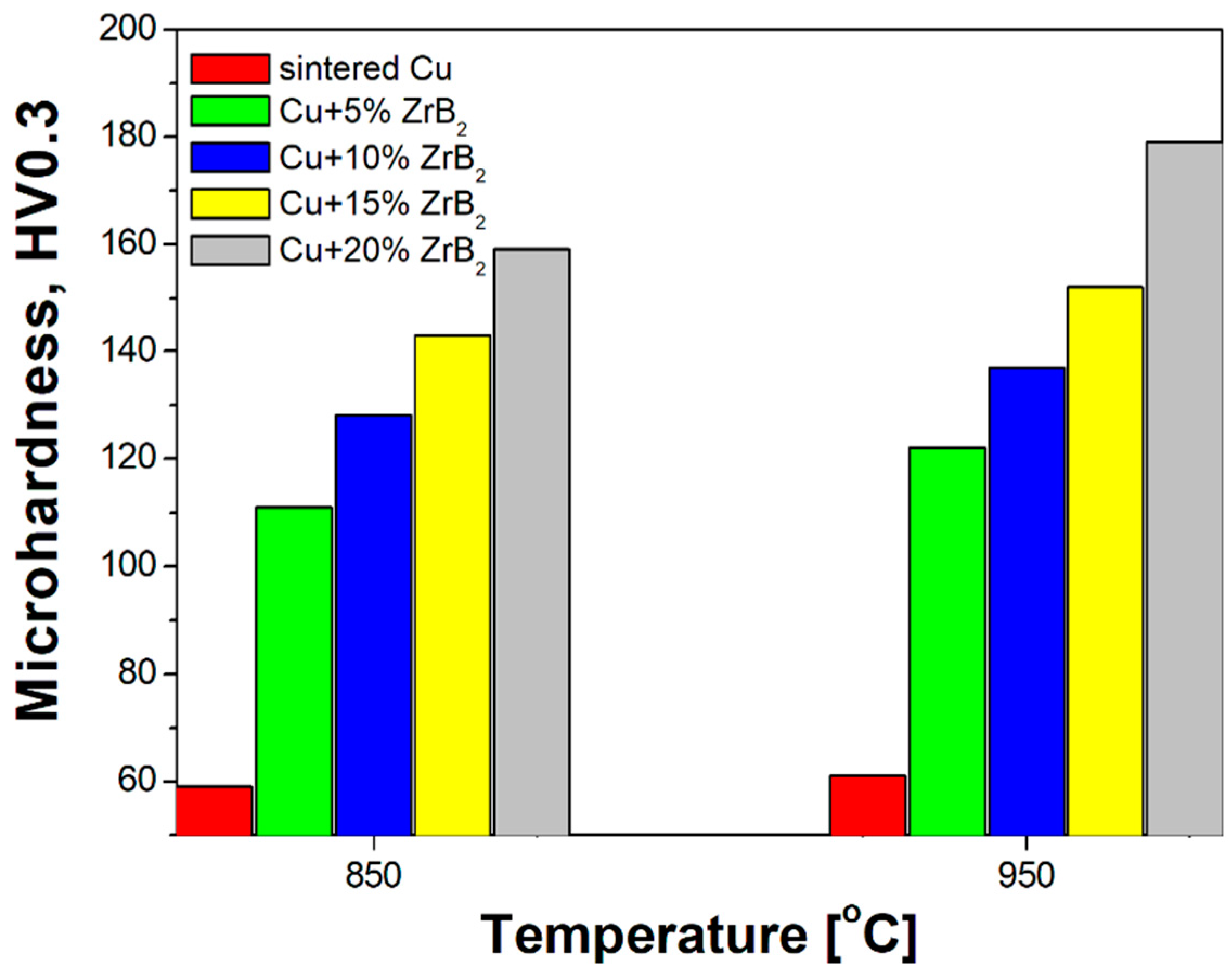
3.1. Physical and Mechanical Properties
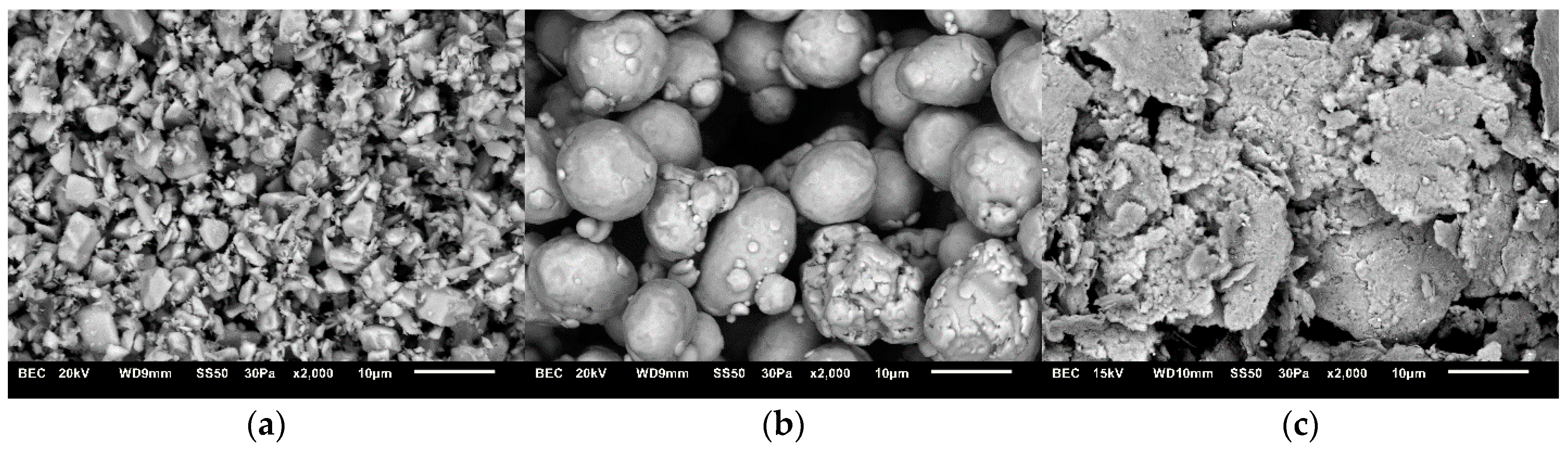
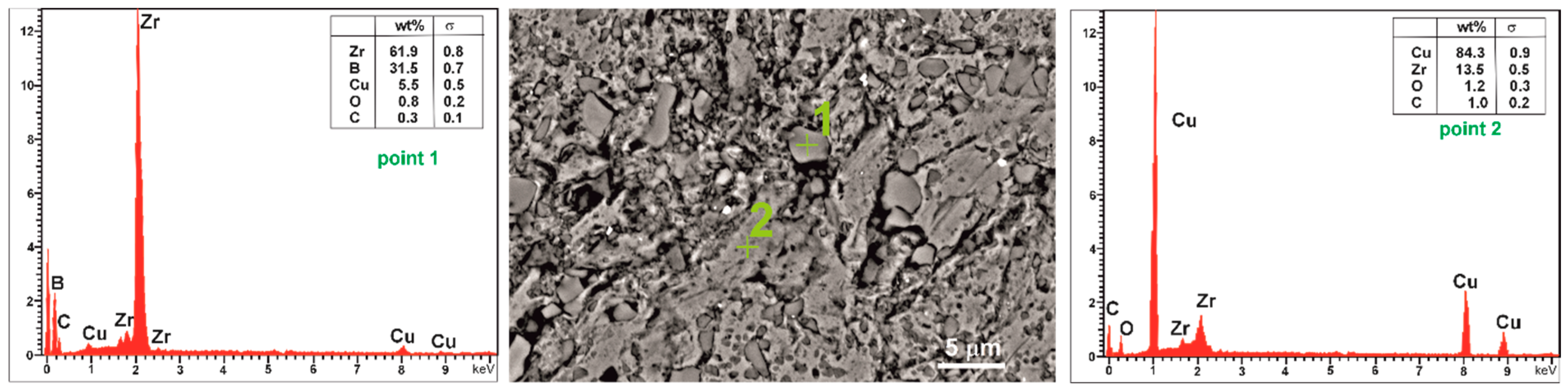
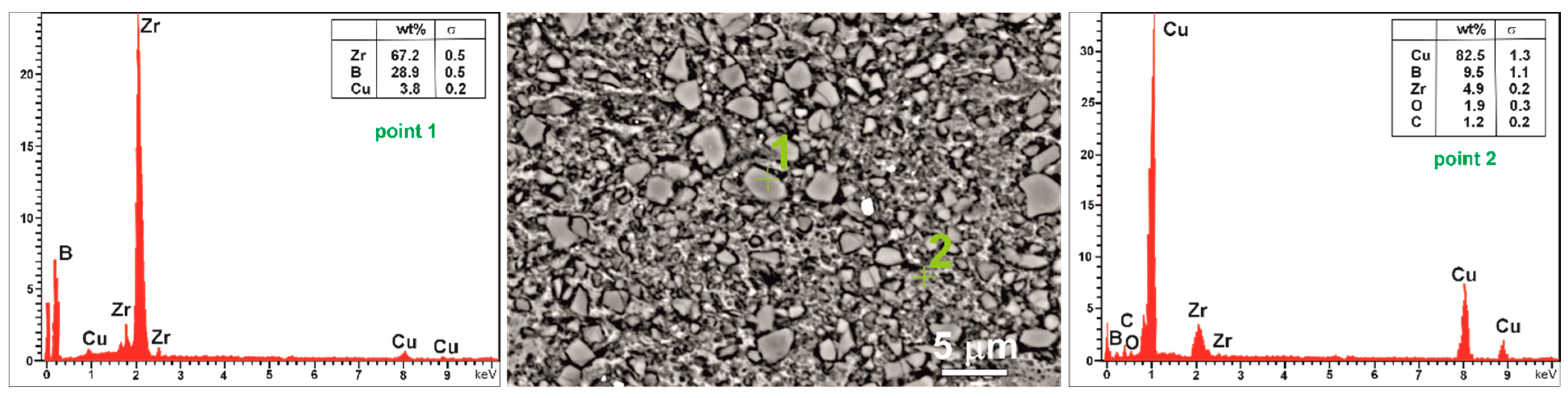
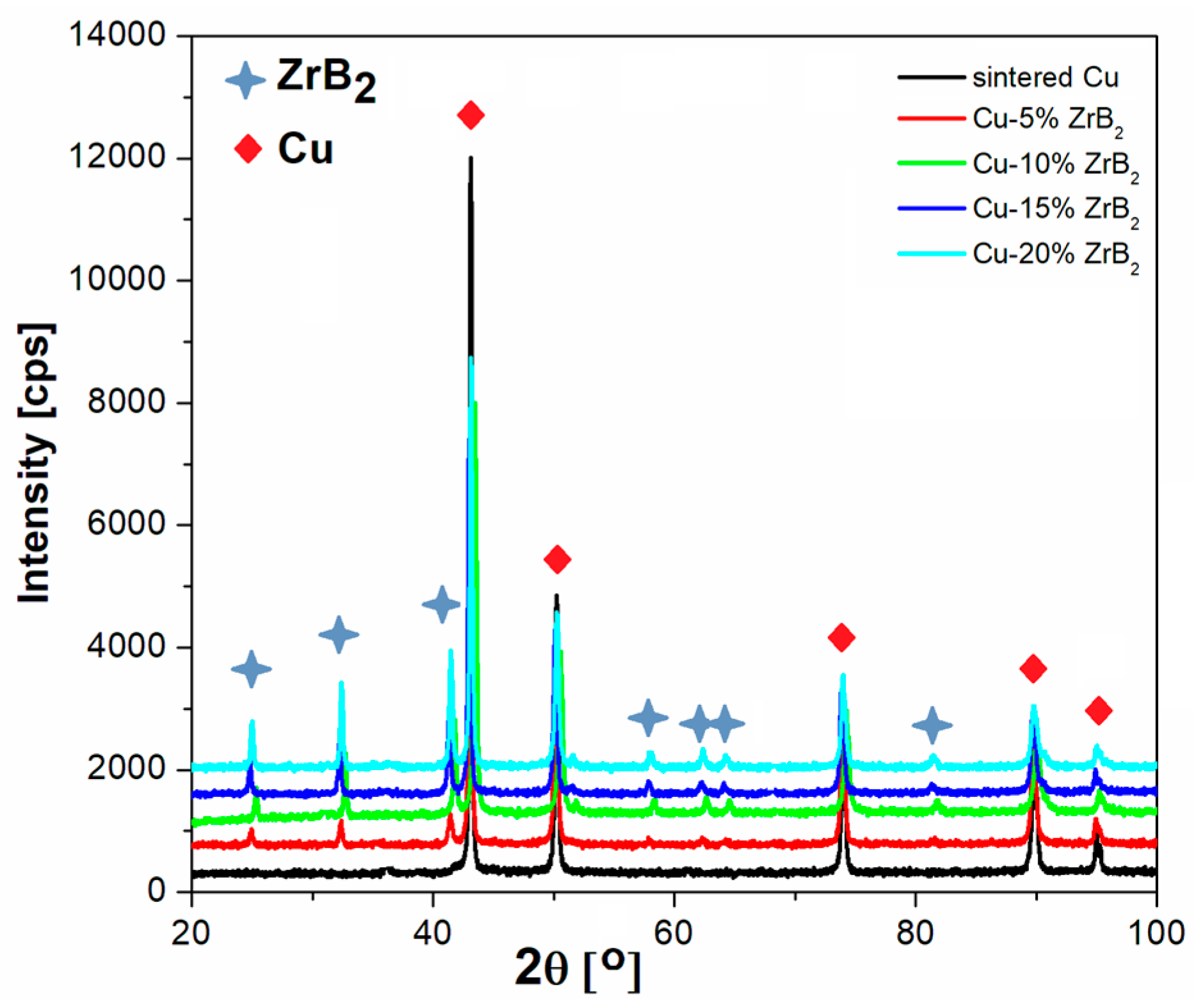
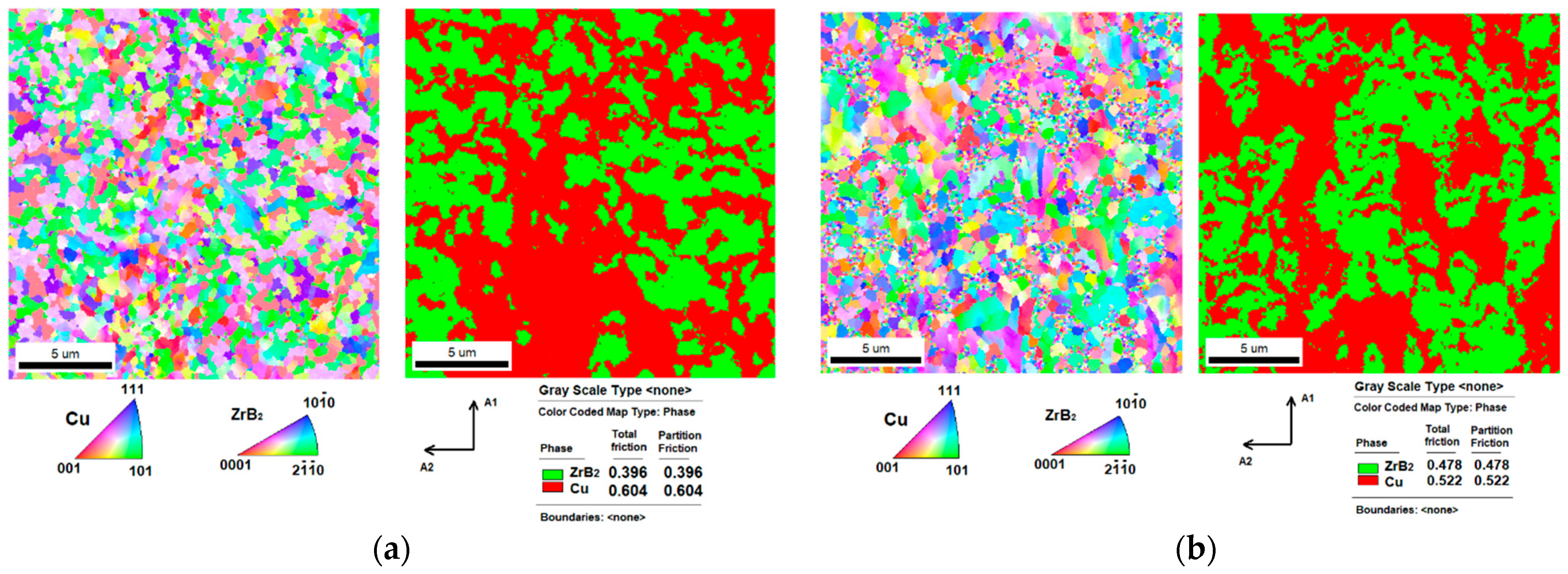
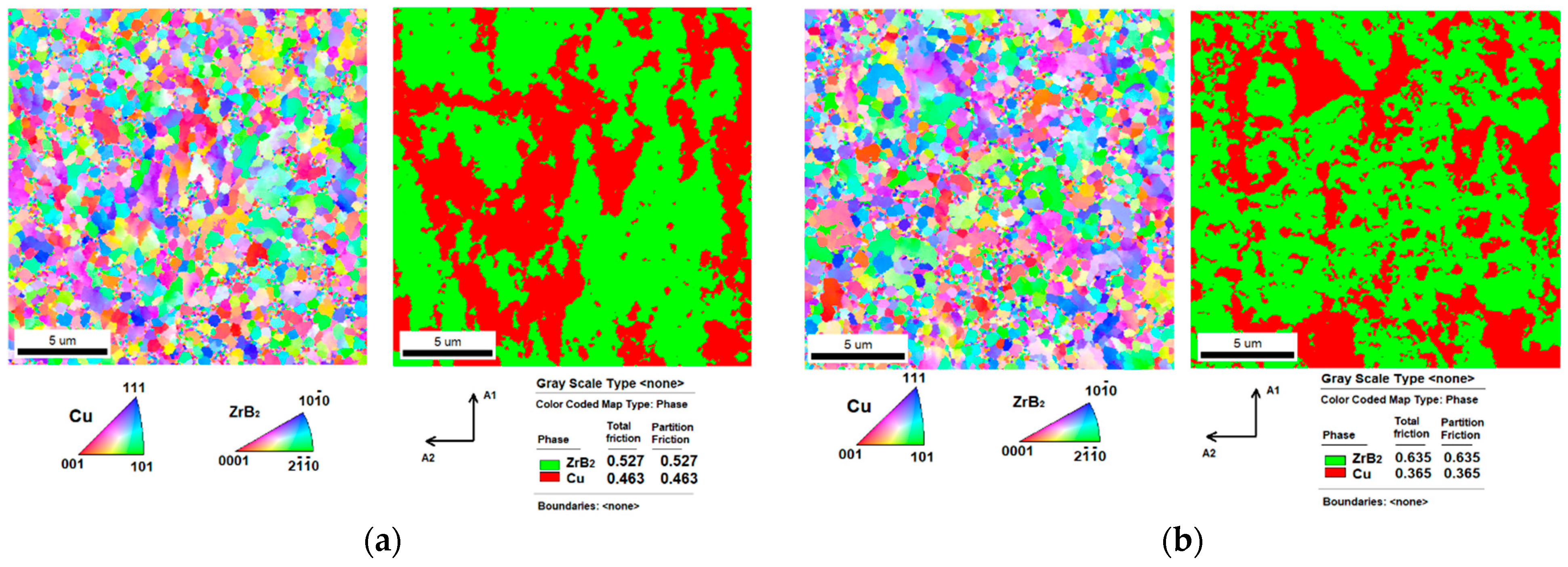
3.2. Microstructures
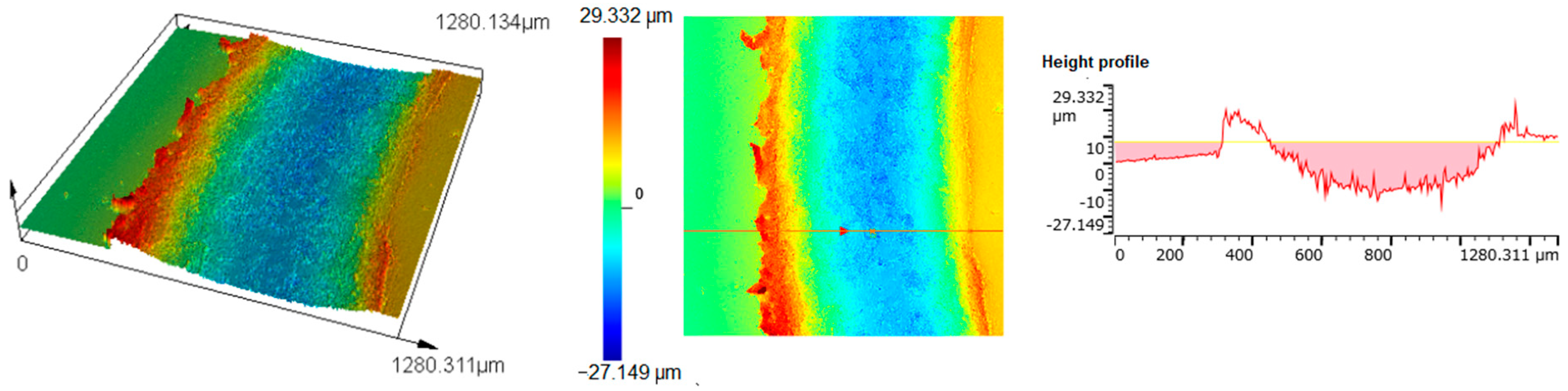
3.3. Wear Properties
3.4. Corrosion Properties
4. Conclusions
- Using a temperature of 950 °C in the SPS process is beneficial for obtaining a high degree of densification of Cu + ZrB2 composites, for which the apparent density is 93–97% of the theoretical density. The densification of the composites was observed to decrease with increasing ZrB2 phase.
- The microhardness increased significantly with the change in the content of ZrB2. Among all the composites, Cu + 20% ZrB2 showed the maximum hardness (179 HV0.3).
- The wear resistance of the Cu + ZrB2 composites increases with the increasing content of ZrB2.
- Adding ZrB2 above 5% significantly reduces the resistance of the composite to corrosion in chloride solutions. Regardless of sintering temperature, composites with a ZrB2 content of up to 5% do not show a significant difference in corrosion resistance compared to pure copper.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Renwick, S. Copper Alloys: Processes, Applications and Developments; New York Research Press: New York, NY, USA, 2015. [Google Scholar]
- Davis, J.R. Copper and Copper Alloys. In ASM Specialty Handbook; ASM International: Materials Park, OH, USA, 2001. [Google Scholar]
- Pang, Y.; Xia, C.; Wang, M.; Li, Z.; Xiao, Z.; Wei, H.; Sheng, X.; Jia, Y.; Chen, C. Effects of Zr and (Ni, Si) additions on properties and microstructure of Cu–Cr alloy. J. Alloys Compd. 2014, 582, 786–792. [Google Scholar] [CrossRef]
- Wang, M.; Yang, Q.R.; Jiang, Y.B.; Li, Z.; Xiao, Z.; Gong, S.; Wang, Y.R.; Guo, C.L.; Wei, H.G. Effects of Fe content on microstructure and properties of Cu–Fe alloy. Trans. Nonferrous Met. Soc. China 2021, 31, 3039–3049. [Google Scholar] [CrossRef]
- Krüger, C.; Mortensen, A. In situ copper-alumina composites. Mater. Sci. Eng. A-Struct. 2013, 585, 396–407. [Google Scholar] [CrossRef]
- Shaik, M.A.; Golla, B.R. Two body abrasion wear behaviour of Cu–ZrB2 composites against SiC emery paper. Wear 2020, 450–451, 203260. [Google Scholar] [CrossRef]
- Taha, M.A.; Zawrah, M.F. Effect of nano ZrO2 on strengthening and electrical properties of Cu-matrix nanocomposites prepared by mechanical alloying. Ceram. Int. 2017, 43, 12698–12704. [Google Scholar] [CrossRef]
- Yan, Y.F.; Kou, S.Q.; Yang, H.Y.; Shu, S.L.; Qiu, F.; Jiang, Q.C.; Zhang, L.C. Ceramic particles reinforced copper matrix composites manufactured by advanced powder metallurgy: Preparation, performance, and mechanisms. Int. J. Extrem. Manuf. 2023, 5, 032006. [Google Scholar] [CrossRef]
- Mittal, P.; Mehta, J.; Mahto, S.; Mehta, S. Copper Matrix Composites—Synthesis and Applications, Metal Matrix Composites; Chapter 3; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Qu, X.; Zhang, L.; Wu, M.; Ren, S. Review of metal matrix composites with high thermal conductivity for thermal management applications. Prog. Nat. Sci. Mater. Int. 2011, 21, 189–197. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, D.L.; Kong, C.; Munroe, P. Factors controlling the tensile properties of ultrafine structured Cu–5vol%Al2O3 nanocomposite prepared by high energy mechanical milling and powder compact extrusion. Mater. Sci. Eng. A 2013, 584, 67–72. [Google Scholar] [CrossRef]
- Huang, B.; Hishinuma, Y.; Noto, H.; Muroga, T. Mechanochemical processing of Cu-Y2O3 alloy by MA-HIP for heat sink materials application. Fusion Eng. Des. 2019, 140, 33–40. [Google Scholar] [CrossRef]
- Fuentes, S.; Tapia, A.; Pozo, P. Synthesis, characterization, and antibacterial activity evaluation of Cu-TiO2 nanocomposites. Mater. Lett. 2021, 296, 129885. [Google Scholar] [CrossRef]
- Prajapati, P.K.; Chaira, D. Fabrication and characterization of Cu–B4C metal matrix composite by powder metallurgy: Effect of B4C on microstructure, mechanical properties and electrical conductivity. Transit. Indian Inst. Met. 2018, 72, 673–684. [Google Scholar] [CrossRef]
- Wang, F.; Li, Y.; Wang, X.; Koizumi, Y.; Kenta, Y.; Chiba, A. In-situ fabrication and characterization of ultrafine structured Cu–TiC composites with high strength and high conductivity by mechanical milling. J. Alloys Compd. 2016, 657, 122–132. [Google Scholar] [CrossRef]
- Zeng, W.; Xie, J.; Zhou, D.; Fu, Z.; Zhang, D.; Lavernia, E.J. Bulk Cu-NbC nanocomposites with high strength and high electrical conductivity. J. Alloys Compd. 2018, 745, 55–62. [Google Scholar] [CrossRef]
- Liu, Q.; Miao, W.; Ding, H.; Glandut, N.; Jia, H.; Li, C. The introduction of SiC into Cu melts based on Ti–SiC system and its transformation. J. Mater. Res. Technol. 2020, 9, 2881–2891. [Google Scholar] [CrossRef]
- Wang, F.; Jiang, C.; Li, J.; Huang, J. Metal-Nitride nanocomposite thin film of nanomaze-like Cu embedded in TiN. Mater. Lett. 2021, 294, 129780. [Google Scholar] [CrossRef]
- Ding, H.; Zhang, X.; Liu, Q.; Miao, W.; Zhou, J.; Wang, J. The microstructures of the TiN–Ti5Si3 hybrid reinforced Cu matrix composites fabricated by the Ti–Si3N4 reaction and its preparation mechanism. J. Mater. Res. Technol. 2021, 14, 1709–1723. [Google Scholar] [CrossRef]
- Ramírez-Vinasco, D.; Le, C.A.; Nanko, M.; Aguilar-Reyes, E.A. Consolidation behaviour of Cu/AlN composites by pulse electric current sintering of copper-coated aluminium nitride precursors. Powder Technol. 2021, 377, 723–732. [Google Scholar] [CrossRef]
- Soloviova, T.O.; Solodkyi, I.V.; Loboda, P.I. Spark Plasma Sintering of Cu-(LaB6-TiB2) Metal-Ceramic Composite and Its Physical-Mechanical Properties. J. Superhard Mater. 2019, 41, 213–220. [Google Scholar] [CrossRef]
- Rino, J.J.; Prabu, S.B.; Paskaramoorthy, R. Comparison of thermal and mechanical properties of Al-5wt.%TiB2 and Al-5wt.%ZrB2 composites processed through salt-melt reaction route. Mater. Today Proc. 2017, 4, 8739–8750. [Google Scholar] [CrossRef]
- Ružić, J.; Stašić, J.; Marković, S.; Raić, K.; Božić, D. Synthesis and Characterization of Cu-ZrB2 Alloy Produced byPM Techniques. Sci. Sinter. 2014, 46, 217–224. [Google Scholar] [CrossRef]
- Guo, X.; Jia, L.; Lu, Z.; Xing, Z.; Xie, H.; Kondoh, K. Preparation of Cu/CrB2 composites with well-balanced mechanical properties and electrical conductivity by ex-situ powder metallurgy. J. Mater. Res. Technol. 2022, 17, 1605–1615. [Google Scholar] [CrossRef]
- López, M.; Jiménez, J.A.; Corredor, D. Precipitation strengthened high strength-conductivity copper alloys containing ZrC ceramics. Compos. Part A Appl. Sci. Manuf. 2007, 38, 272–279. [Google Scholar] [CrossRef]
- Stępień, M.; Sulima, I.; Hyjek, P.; Kowalik, R. Evaluation of the corrosion resistance of spark plasma sintered stainless steel 316L matrix composites with zirconium diboride in sulfuric acid. Arch. Civ. Mech. Eng. 2022, 22, 127. [Google Scholar] [CrossRef]
- Kear, G.; Barker, B.D.; Walsh, F.C. Electrochemical corrosion of unalloyed copper in chloride media—A critical review. Corros. Sci. 2004, 46, 109–135. [Google Scholar] [CrossRef]
- Hutchings, I.; Shipway, P. Tribology: Friction and Wear of Engineering Materials; Butterworth-Heinemann: Oxford, UK, 2017. [Google Scholar]
- Yang, Y.; Guo, X.; Song, K.; Long, F.; Wang, X.; Li, S.; Lsi, Z. Electrical wear performance of copper matrix composites reinforced with hybrid CNTs and TiB2 particles. Ind. Lubr. Tribol. 2022, 74, 609–618. [Google Scholar] [CrossRef]
- Zhou, J.; Ma, C.; Kang, X.; Zhang, L.; Liu, X. Effect of WS2 particle size on mechanical properties and tribological behaviors of Cu–WS2 composites sintered by SPS. Trans. Nonferrous Met. Soc. China 2018, 28, 1176–1185. [Google Scholar] [CrossRef]
- Su, Y.; Jiang, F.; Long, M.; Wu, F.; Xiao, Z.; Wu, M. Microstructure and frictional properties of copper-tin composites containing graphite and MoS2 by rapid hot-press sintering. Tribol. Int. 2023, 183, 108392. [Google Scholar] [CrossRef]
- Bagheri, G.H.A. The effect of reinforcement percentages on properties of copper matrix composites reinforced with TiC particles. J. Alloys Compd. 2016, 676, 120–126. [Google Scholar] [CrossRef]
- Aleksandar, V.; Viseslava, R.; Fatima, Z. Friction and wear properties of copper based composites reinforced with micro and nano-sized Al2O3 particles. In Proceedings of the 8th International Conference on Tribology, Sinaia, Romania, 30 October–1 November 2014; pp. 357–358. [Google Scholar]
- Raja, R.; Jannet, S.; Ruban, S.R.; George, L. Mechanical, wear, and microstructural examination of copper surface composites reinforced with SiC nanoparticles done by FSP. Mater. Today Proc. 2023, 92, 376–381. [Google Scholar] [CrossRef]
- Fan, X.; Liu, Q.; Ding, H.; Wang, H.; Hao, C. The microstructures and properties of in-situ ZrB2 reinforced Cu matrix composites. Results Phys. 2019, 14, 102494. [Google Scholar] [CrossRef]
- Taylor, R.P.; McClain, S.T.; Berry, J.T. Uncertainty analysis of metal-casting porosity measurements using Archimedes’ principle. Int. J. Cast Met. Res. 1999, 11, 247–257. [Google Scholar] [CrossRef]
- ISO 20808:2016(E); International Standard, Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)- Determination of friction and wear characteristics of monolithic ceramics by ball-on-disc method, Edition 2. American National Standards Institute: New York, NY, USA, 2016.
- Meozzi, M. Special use of the ball on disc standard test. Tribol. Int. 2006, 39, 496–505. [Google Scholar] [CrossRef]
- Wang, C.; Lin, H.; Zhang, Z.; Li, W. Fabrication, Interfacial characteristics and strengthening mechanisms of ZrB2 microparticles reinforced Cu composites prepared by hot-pressed sintering. J. Alloys Compd. 2018, 748, 546–552. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, C.; Zhou, S.; Guo, B.; Zhang, Z.; Yu, Z.; Li, W. Effect of Sintering Temperature on the Microstructure and Properties of High-Strength and Highly Conductive 5 wt.% ZrB2/Cu Composite. Powder Metall. Met. Ceram. 2023, 61, 560–573. [Google Scholar]
- Fahrenholtz, W.G.; Hilmas, G.E.; Talmy, I.G.; Zaykoski, J.A. Refractory Diborides of Zirconium and Hafnium. J. Am. Ceram. Soc. 2007, 90, 1347–1364. [Google Scholar] [CrossRef]
- Pouyani, M.R.; Rajabi, M. Microwave-assisted synthesis of Cu–ZrB2 MM Nanocomposite using double pressing double sintering method. J. Mater. Sci. Mater. Electron. 2019, 30, 266–276. [Google Scholar] [CrossRef]
- Bengough, G.D.; Jones, R.M.; Pirret, R. Diagnosis of brass condenser tube corrosion. J. Inst. Met. 1920, 23, 65–158. [Google Scholar]
- Ahmad, Z. Principles of Corrosion Engineering and Corrosion Control; Butterworth-Heinemann: Oxford, UK, 2006. [Google Scholar] [CrossRef]



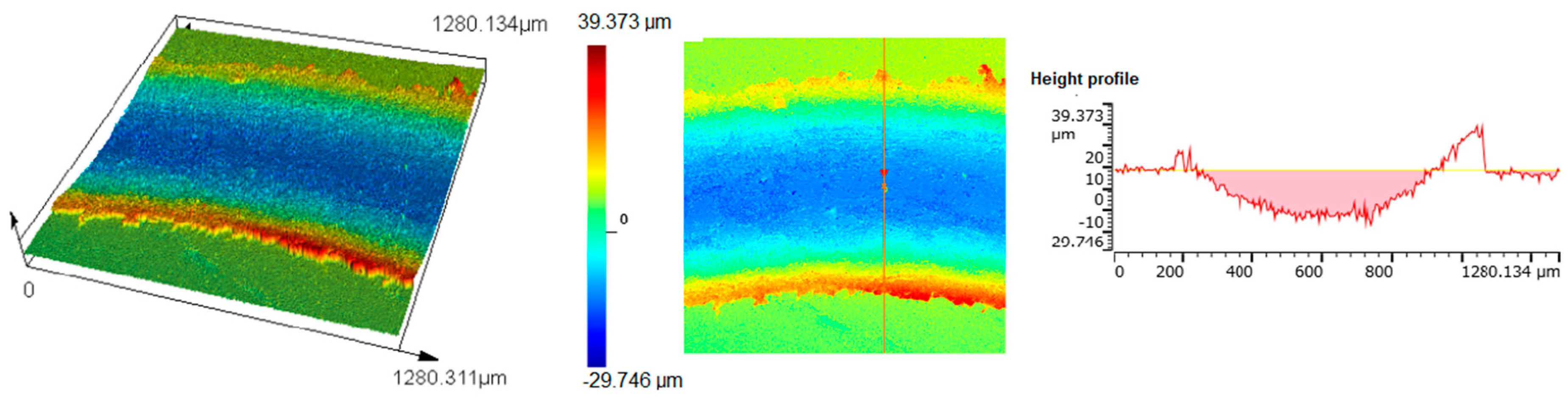


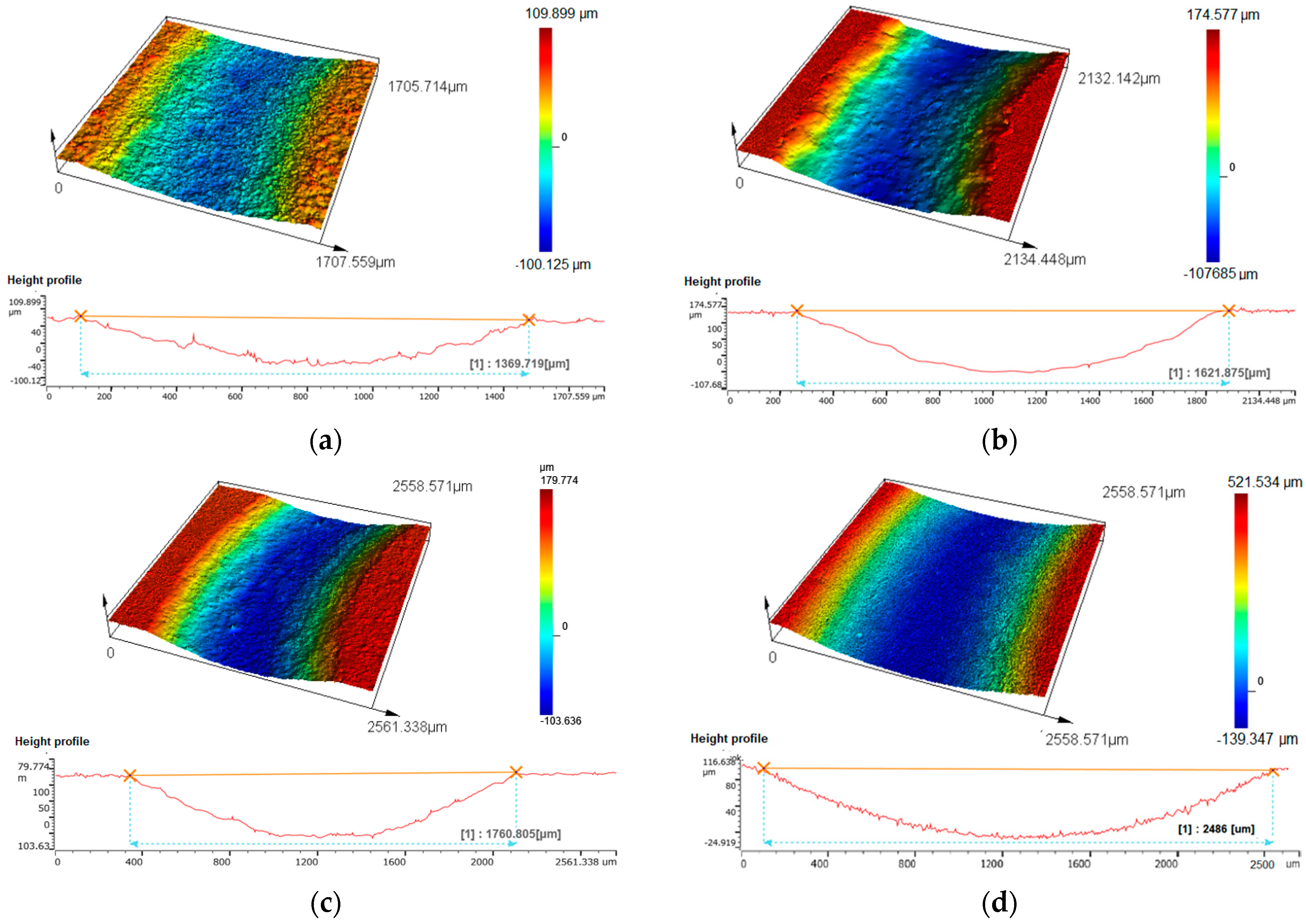
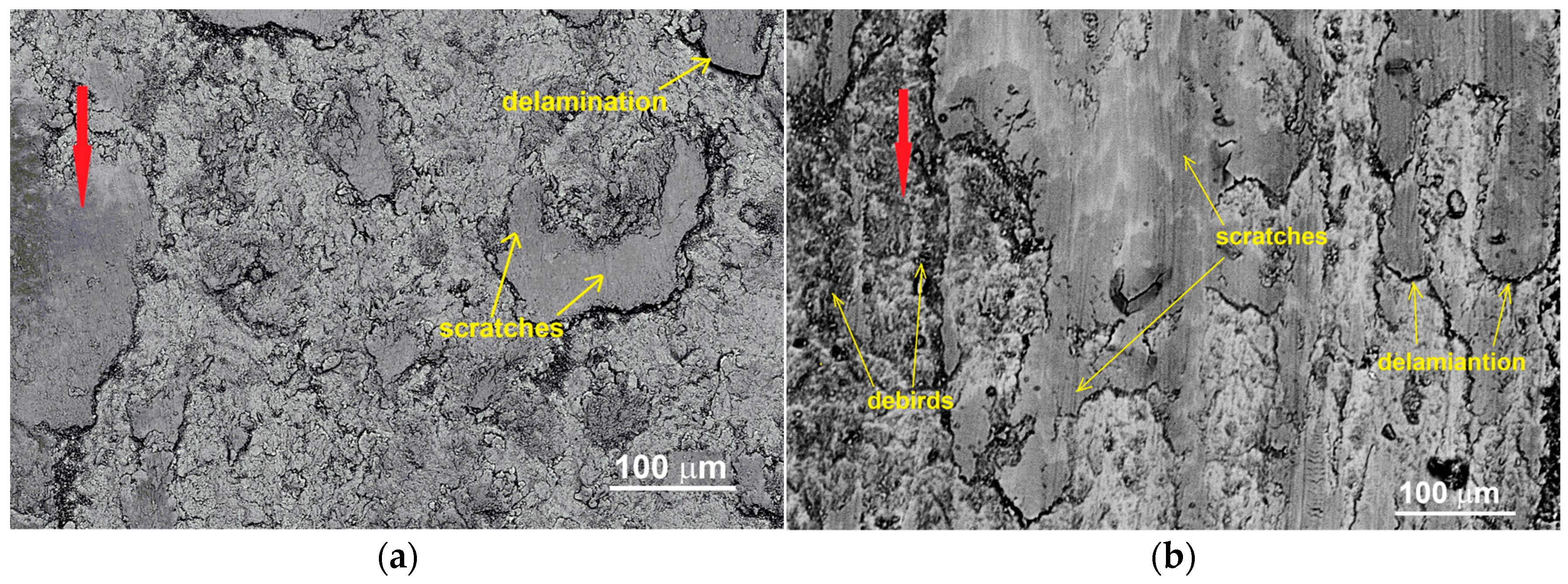

| Sintered Materials | Sintering Temperature (°C) | Friction Coefficient μ (-) | Specific Wear Rate Wv⋅10−4 (mm3/Nm) | Loss of Weight Δm (%) |
|---|---|---|---|---|
| Cu | 850 | 0.61 | 8.16 | 0.36 |
| 950 | 0.60 | 8.08 | 0.29 | |
| Cu + 5% ZrB2 | 850 | 0.64 | 6.56 | 0.27 |
| 950 | 0.62 | 6.26 | 0.24 | |
| Cu + 10% ZrB2 | 850 | 0.62 | 4.42 | 0.18 |
| 950 | 0.58 | 4.28 | 0.17 | |
| Cu + 15% ZrB2 | 850 | 0.56 | 3.98 | 0.12 |
| 950 | 0.54 | 3.61 | 0.11 | |
| Cu + 20% ZrB2 | 850 | 0.49 | 2.59 | 0.09 |
| 950 | 0.48 | 2.37 | 0.09 |
| Samples | Sintering Temperature | Erest (mV) | Ecorr (mV) | Icorr (µA/cm2) | βa (mV/dec) | βc (mV/dec) |
|---|---|---|---|---|---|---|
| Cu solid | ----- | −0.183 | −0.171 | 3.33 | 26 | 201 |
| Cu (sintered) | 850 °C | −0.190 | −0.194 | 4.62 | 67 | 222 |
| Cu + 5% ZrB2 | −0.187 | −0.245 | 6.33 | 86 | 133 | |
| Cu + 10% ZrB2 | −0.136 | −0.168 | 16.68 | 71 | 160 | |
| Cu + 15% ZrB2 | −0.136 | −0.184 | 34.51 | 101 | 199 | |
| Cu + 20% ZrB2 | −0.133 | −0.172 | 27.67 | 121 | 161 | |
| Cu (sintered) | 950 °C | −0.183 | −0.253 | 3.30 | 179 | 163 |
| Cu + 5% ZrB2 | −0.201 | −0.244 | 4.48 | 74 | 189 | |
| Cu + 10% ZrB2 | −0.149 | −0.208 | 22.70 | 106 | 166 | |
| Cu + 15% ZrB2 | −0.118 | −0.208 | 52.60 | 145 | 136 | |
| Cu + 20% ZrB2 | −0.094 | −0.177 | 124.45 | 110 | 123 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sulima, I.; Stępień, M.; Hyjek, P.; Boczkal, S.; Kowalik, R. Mechanical, Corrosion and Wear Characteristics of Cu-Based Composites Reinforced with Zirconium Diboride Consolidated by SPS. Metals 2024, 14, 974. https://doi.org/10.3390/met14090974
Sulima I, Stępień M, Hyjek P, Boczkal S, Kowalik R. Mechanical, Corrosion and Wear Characteristics of Cu-Based Composites Reinforced with Zirconium Diboride Consolidated by SPS. Metals. 2024; 14(9):974. https://doi.org/10.3390/met14090974
Chicago/Turabian StyleSulima, Iwona, Michał Stępień, Paweł Hyjek, Sonia Boczkal, and Remigiusz Kowalik. 2024. "Mechanical, Corrosion and Wear Characteristics of Cu-Based Composites Reinforced with Zirconium Diboride Consolidated by SPS" Metals 14, no. 9: 974. https://doi.org/10.3390/met14090974
APA StyleSulima, I., Stępień, M., Hyjek, P., Boczkal, S., & Kowalik, R. (2024). Mechanical, Corrosion and Wear Characteristics of Cu-Based Composites Reinforced with Zirconium Diboride Consolidated by SPS. Metals, 14(9), 974. https://doi.org/10.3390/met14090974







