Mechanical, Corrosion and Wear Characteristics of Cu-Based Composites Reinforced with Zirconium Diboride Consolidated by SPS
Abstract
:1. Introduction
2. Materials and Methods
3. Results
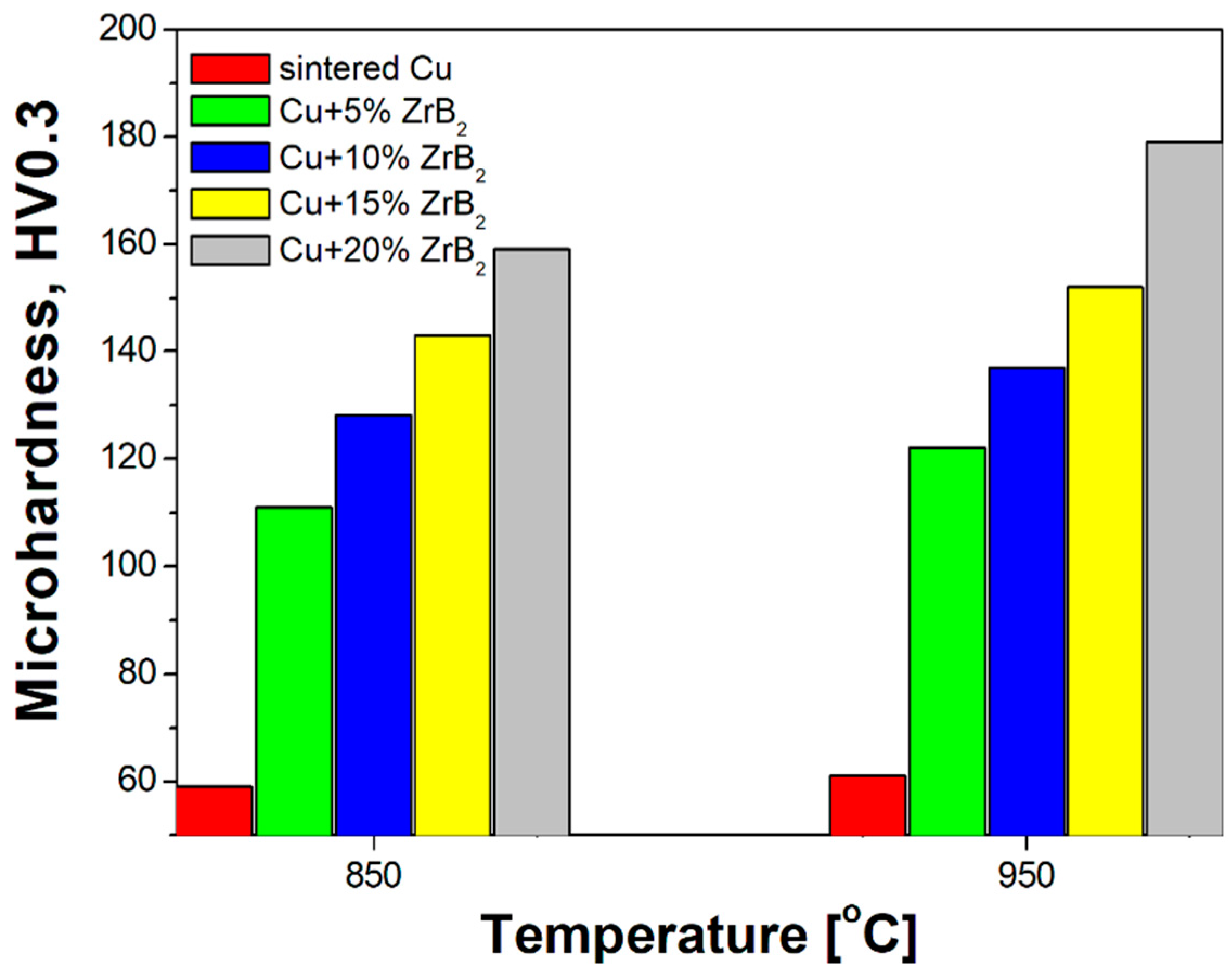
3.1. Physical and Mechanical Properties
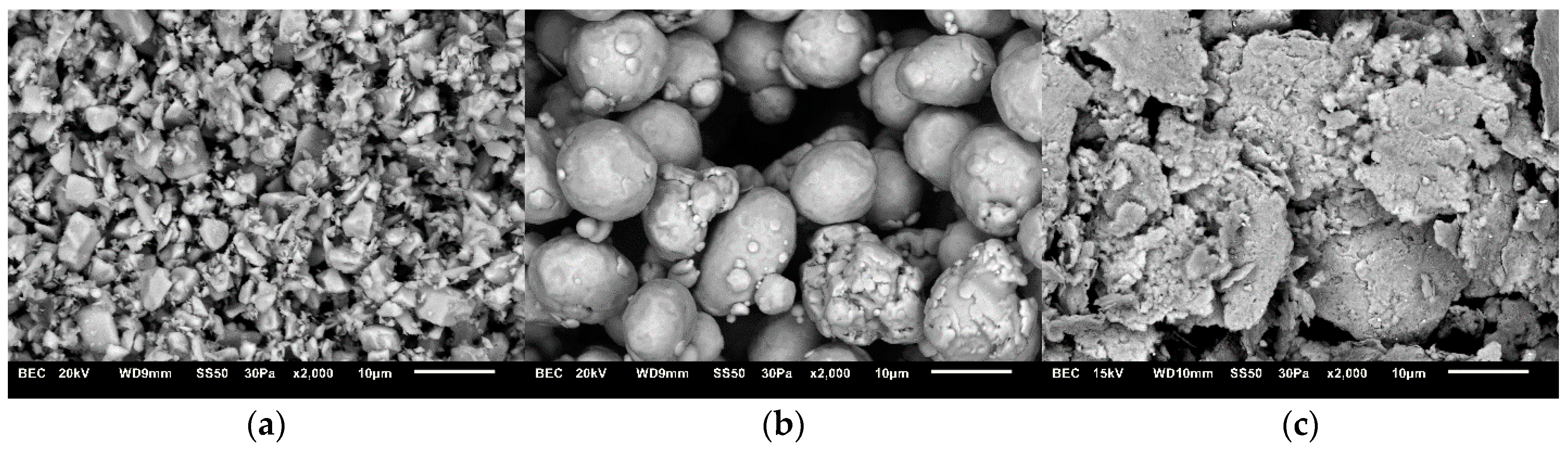
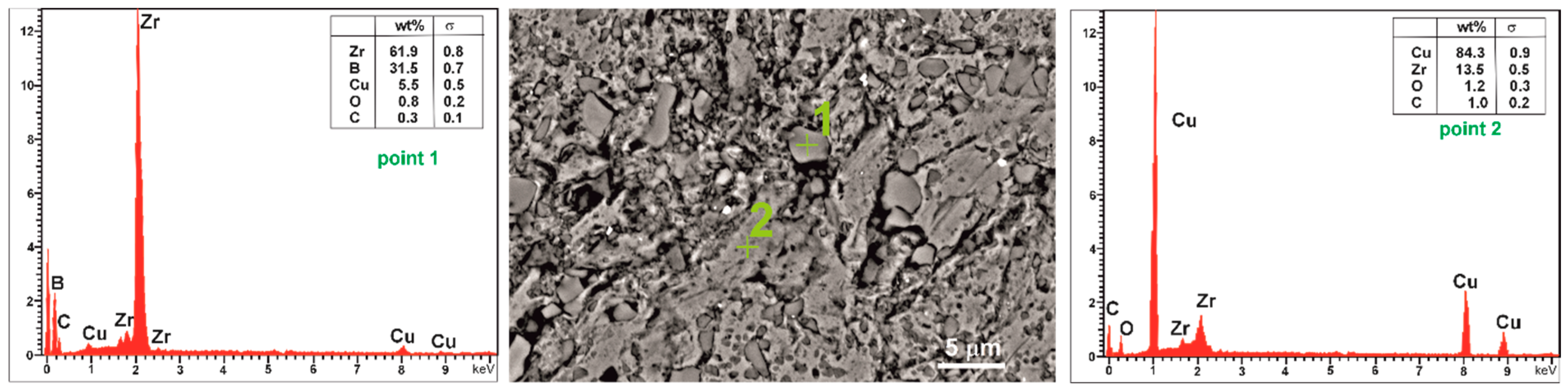
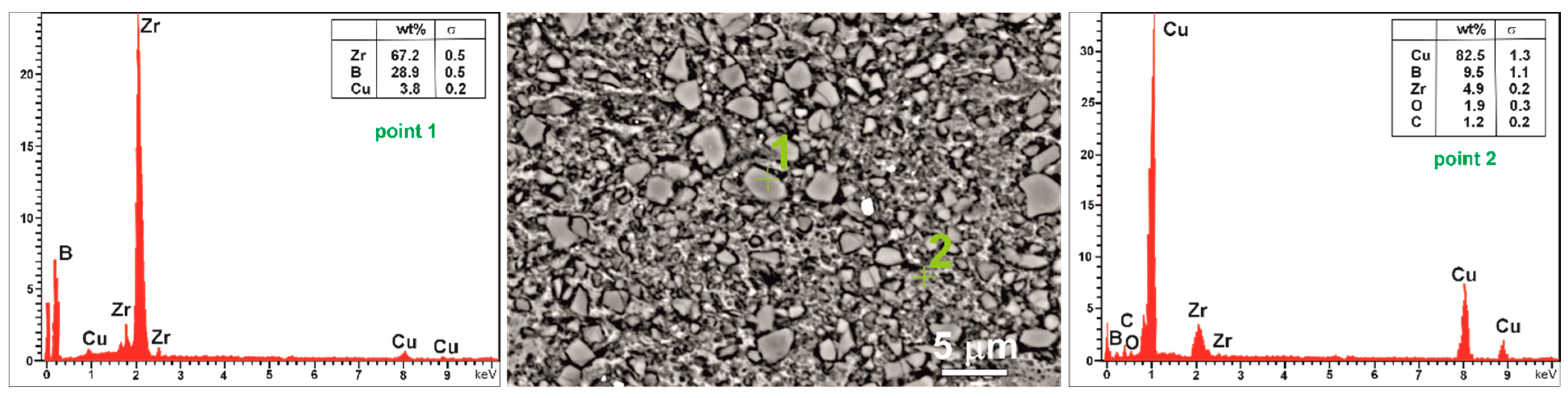
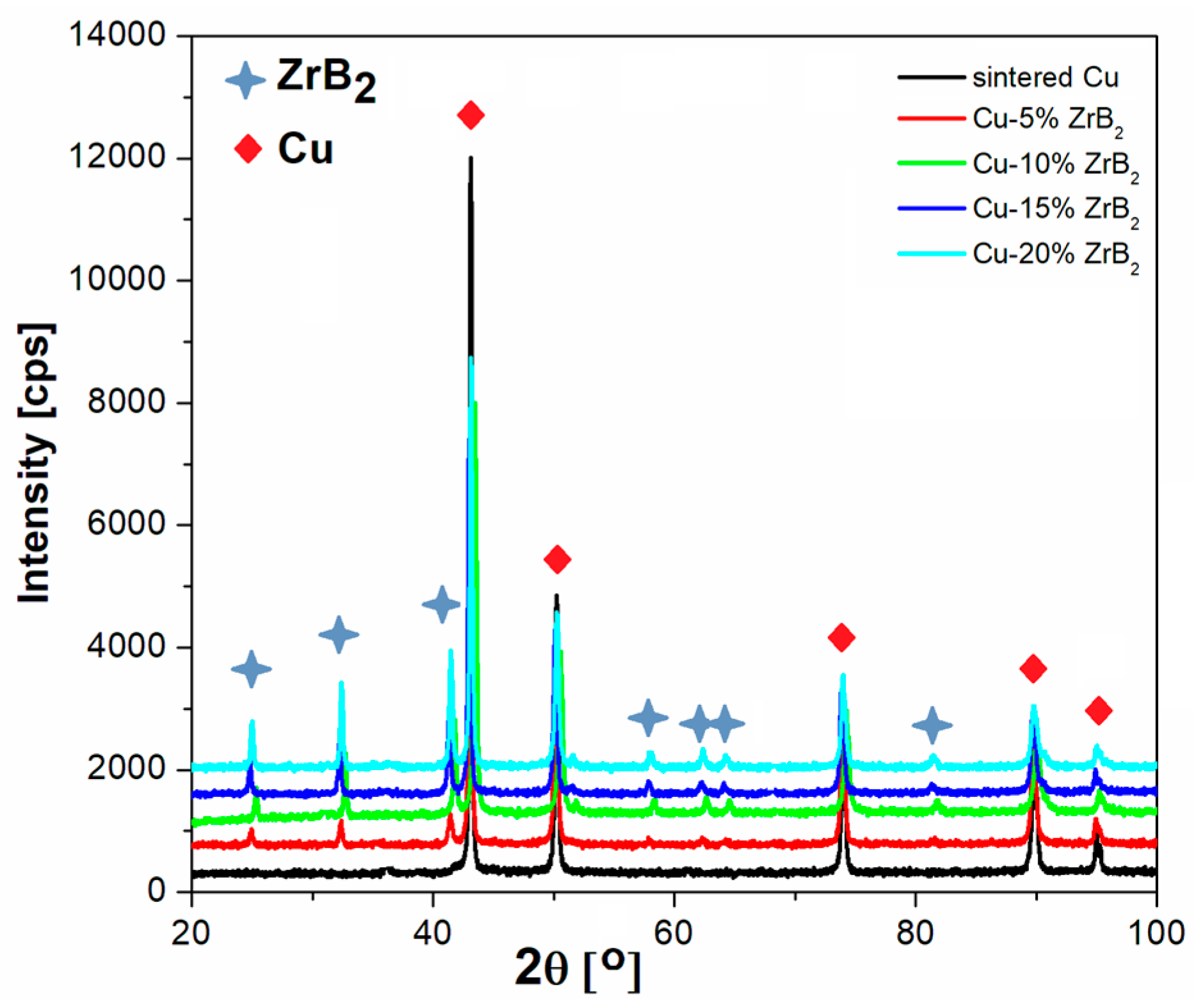
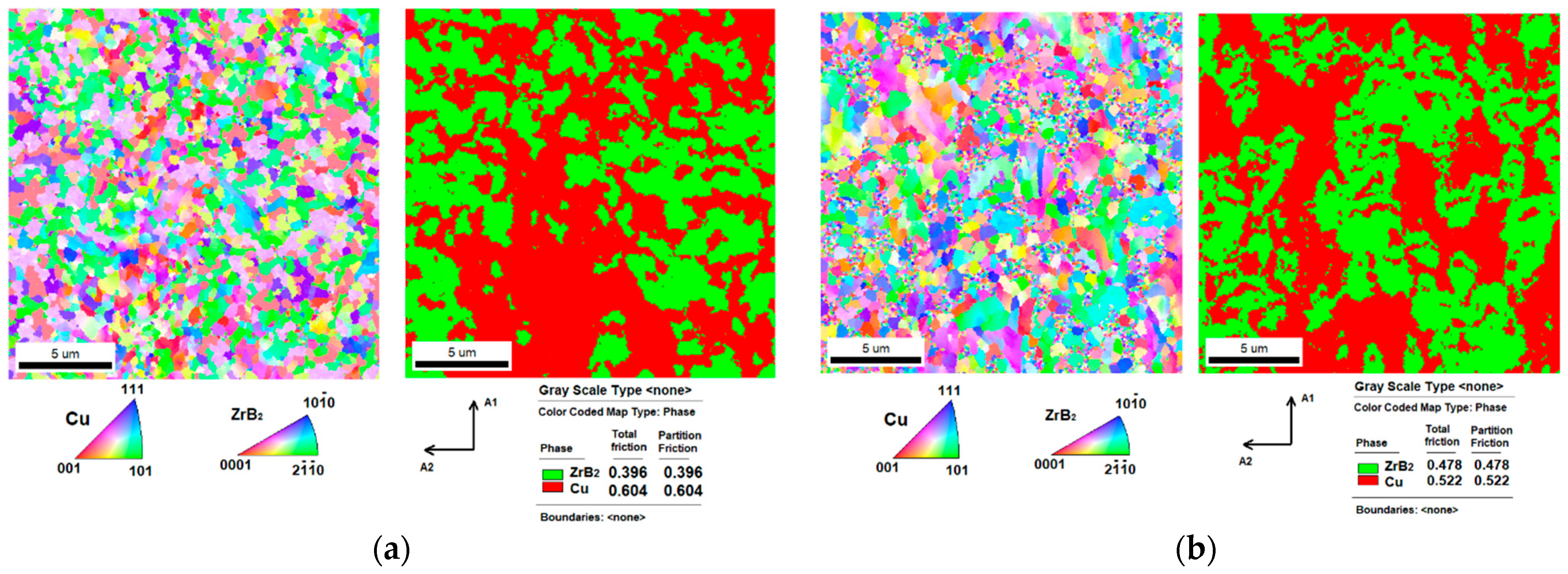
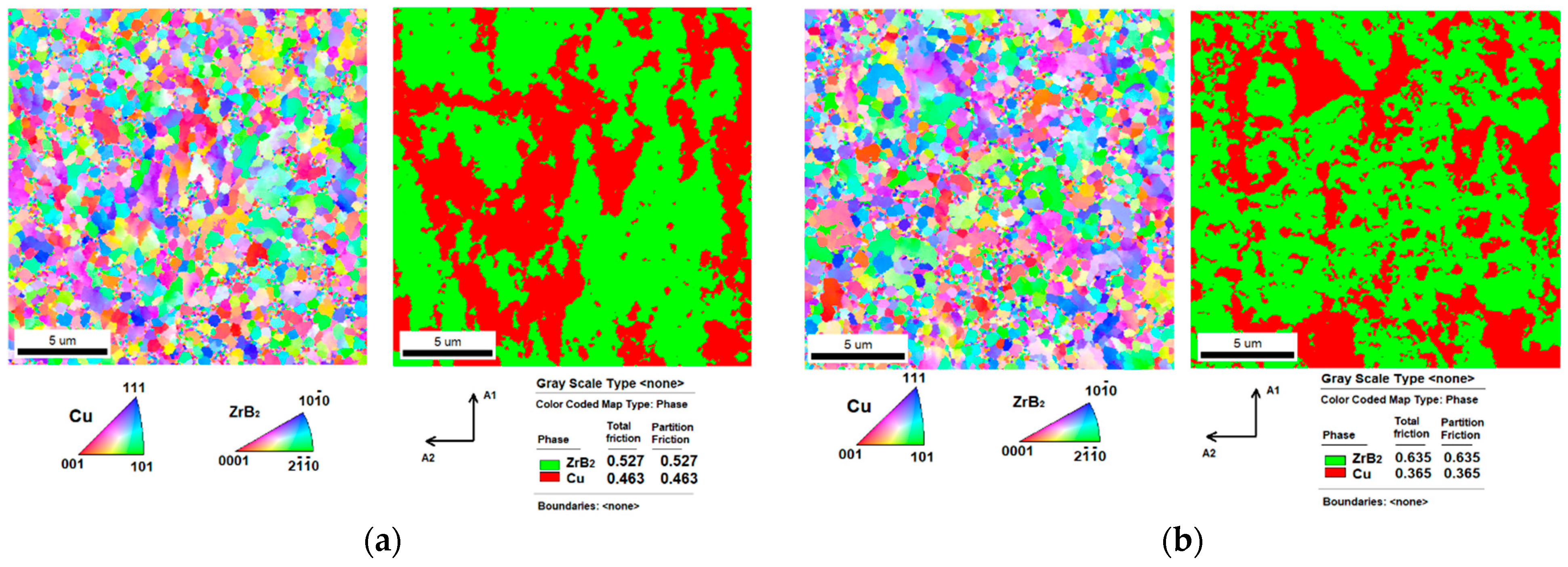
3.2. Microstructures
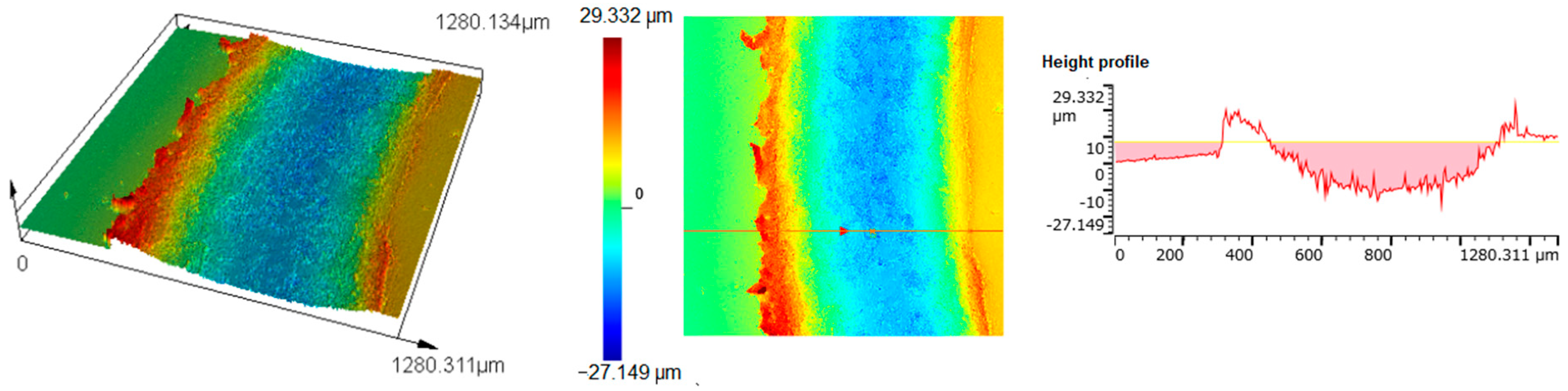
3.3. Wear Properties
3.4. Corrosion Properties
4. Conclusions
- Using a temperature of 950 °C in the SPS process is beneficial for obtaining a high degree of densification of Cu + ZrB2 composites, for which the apparent density is 93–97% of the theoretical density. The densification of the composites was observed to decrease with increasing ZrB2 phase.
- The microhardness increased significantly with the change in the content of ZrB2. Among all the composites, Cu + 20% ZrB2 showed the maximum hardness (179 HV0.3).
- The wear resistance of the Cu + ZrB2 composites increases with the increasing content of ZrB2.
- Adding ZrB2 above 5% significantly reduces the resistance of the composite to corrosion in chloride solutions. Regardless of sintering temperature, composites with a ZrB2 content of up to 5% do not show a significant difference in corrosion resistance compared to pure copper.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Renwick, S. Copper Alloys: Processes, Applications and Developments; New York Research Press: New York, NY, USA, 2015. [Google Scholar]
- Davis, J.R. Copper and Copper Alloys. In ASM Specialty Handbook; ASM International: Materials Park, OH, USA, 2001. [Google Scholar]
- Pang, Y.; Xia, C.; Wang, M.; Li, Z.; Xiao, Z.; Wei, H.; Sheng, X.; Jia, Y.; Chen, C. Effects of Zr and (Ni, Si) additions on properties and microstructure of Cu–Cr alloy. J. Alloys Compd. 2014, 582, 786–792. [Google Scholar] [CrossRef]
- Wang, M.; Yang, Q.R.; Jiang, Y.B.; Li, Z.; Xiao, Z.; Gong, S.; Wang, Y.R.; Guo, C.L.; Wei, H.G. Effects of Fe content on microstructure and properties of Cu–Fe alloy. Trans. Nonferrous Met. Soc. China 2021, 31, 3039–3049. [Google Scholar] [CrossRef]
- Krüger, C.; Mortensen, A. In situ copper-alumina composites. Mater. Sci. Eng. A-Struct. 2013, 585, 396–407. [Google Scholar] [CrossRef]
- Shaik, M.A.; Golla, B.R. Two body abrasion wear behaviour of Cu–ZrB2 composites against SiC emery paper. Wear 2020, 450–451, 203260. [Google Scholar] [CrossRef]
- Taha, M.A.; Zawrah, M.F. Effect of nano ZrO2 on strengthening and electrical properties of Cu-matrix nanocomposites prepared by mechanical alloying. Ceram. Int. 2017, 43, 12698–12704. [Google Scholar] [CrossRef]
- Yan, Y.F.; Kou, S.Q.; Yang, H.Y.; Shu, S.L.; Qiu, F.; Jiang, Q.C.; Zhang, L.C. Ceramic particles reinforced copper matrix composites manufactured by advanced powder metallurgy: Preparation, performance, and mechanisms. Int. J. Extrem. Manuf. 2023, 5, 032006. [Google Scholar] [CrossRef]
- Mittal, P.; Mehta, J.; Mahto, S.; Mehta, S. Copper Matrix Composites—Synthesis and Applications, Metal Matrix Composites; Chapter 3; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Qu, X.; Zhang, L.; Wu, M.; Ren, S. Review of metal matrix composites with high thermal conductivity for thermal management applications. Prog. Nat. Sci. Mater. Int. 2011, 21, 189–197. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, D.L.; Kong, C.; Munroe, P. Factors controlling the tensile properties of ultrafine structured Cu–5vol%Al2O3 nanocomposite prepared by high energy mechanical milling and powder compact extrusion. Mater. Sci. Eng. A 2013, 584, 67–72. [Google Scholar] [CrossRef]
- Huang, B.; Hishinuma, Y.; Noto, H.; Muroga, T. Mechanochemical processing of Cu-Y2O3 alloy by MA-HIP for heat sink materials application. Fusion Eng. Des. 2019, 140, 33–40. [Google Scholar] [CrossRef]
- Fuentes, S.; Tapia, A.; Pozo, P. Synthesis, characterization, and antibacterial activity evaluation of Cu-TiO2 nanocomposites. Mater. Lett. 2021, 296, 129885. [Google Scholar] [CrossRef]
- Prajapati, P.K.; Chaira, D. Fabrication and characterization of Cu–B4C metal matrix composite by powder metallurgy: Effect of B4C on microstructure, mechanical properties and electrical conductivity. Transit. Indian Inst. Met. 2018, 72, 673–684. [Google Scholar] [CrossRef]
- Wang, F.; Li, Y.; Wang, X.; Koizumi, Y.; Kenta, Y.; Chiba, A. In-situ fabrication and characterization of ultrafine structured Cu–TiC composites with high strength and high conductivity by mechanical milling. J. Alloys Compd. 2016, 657, 122–132. [Google Scholar] [CrossRef]
- Zeng, W.; Xie, J.; Zhou, D.; Fu, Z.; Zhang, D.; Lavernia, E.J. Bulk Cu-NbC nanocomposites with high strength and high electrical conductivity. J. Alloys Compd. 2018, 745, 55–62. [Google Scholar] [CrossRef]
- Liu, Q.; Miao, W.; Ding, H.; Glandut, N.; Jia, H.; Li, C. The introduction of SiC into Cu melts based on Ti–SiC system and its transformation. J. Mater. Res. Technol. 2020, 9, 2881–2891. [Google Scholar] [CrossRef]
- Wang, F.; Jiang, C.; Li, J.; Huang, J. Metal-Nitride nanocomposite thin film of nanomaze-like Cu embedded in TiN. Mater. Lett. 2021, 294, 129780. [Google Scholar] [CrossRef]
- Ding, H.; Zhang, X.; Liu, Q.; Miao, W.; Zhou, J.; Wang, J. The microstructures of the TiN–Ti5Si3 hybrid reinforced Cu matrix composites fabricated by the Ti–Si3N4 reaction and its preparation mechanism. J. Mater. Res. Technol. 2021, 14, 1709–1723. [Google Scholar] [CrossRef]
- Ramírez-Vinasco, D.; Le, C.A.; Nanko, M.; Aguilar-Reyes, E.A. Consolidation behaviour of Cu/AlN composites by pulse electric current sintering of copper-coated aluminium nitride precursors. Powder Technol. 2021, 377, 723–732. [Google Scholar] [CrossRef]
- Soloviova, T.O.; Solodkyi, I.V.; Loboda, P.I. Spark Plasma Sintering of Cu-(LaB6-TiB2) Metal-Ceramic Composite and Its Physical-Mechanical Properties. J. Superhard Mater. 2019, 41, 213–220. [Google Scholar] [CrossRef]
- Rino, J.J.; Prabu, S.B.; Paskaramoorthy, R. Comparison of thermal and mechanical properties of Al-5wt.%TiB2 and Al-5wt.%ZrB2 composites processed through salt-melt reaction route. Mater. Today Proc. 2017, 4, 8739–8750. [Google Scholar] [CrossRef]
- Ružić, J.; Stašić, J.; Marković, S.; Raić, K.; Božić, D. Synthesis and Characterization of Cu-ZrB2 Alloy Produced byPM Techniques. Sci. Sinter. 2014, 46, 217–224. [Google Scholar] [CrossRef]
- Guo, X.; Jia, L.; Lu, Z.; Xing, Z.; Xie, H.; Kondoh, K. Preparation of Cu/CrB2 composites with well-balanced mechanical properties and electrical conductivity by ex-situ powder metallurgy. J. Mater. Res. Technol. 2022, 17, 1605–1615. [Google Scholar] [CrossRef]
- López, M.; Jiménez, J.A.; Corredor, D. Precipitation strengthened high strength-conductivity copper alloys containing ZrC ceramics. Compos. Part A Appl. Sci. Manuf. 2007, 38, 272–279. [Google Scholar] [CrossRef]
- Stępień, M.; Sulima, I.; Hyjek, P.; Kowalik, R. Evaluation of the corrosion resistance of spark plasma sintered stainless steel 316L matrix composites with zirconium diboride in sulfuric acid. Arch. Civ. Mech. Eng. 2022, 22, 127. [Google Scholar] [CrossRef]
- Kear, G.; Barker, B.D.; Walsh, F.C. Electrochemical corrosion of unalloyed copper in chloride media—A critical review. Corros. Sci. 2004, 46, 109–135. [Google Scholar] [CrossRef]
- Hutchings, I.; Shipway, P. Tribology: Friction and Wear of Engineering Materials; Butterworth-Heinemann: Oxford, UK, 2017. [Google Scholar]
- Yang, Y.; Guo, X.; Song, K.; Long, F.; Wang, X.; Li, S.; Lsi, Z. Electrical wear performance of copper matrix composites reinforced with hybrid CNTs and TiB2 particles. Ind. Lubr. Tribol. 2022, 74, 609–618. [Google Scholar] [CrossRef]
- Zhou, J.; Ma, C.; Kang, X.; Zhang, L.; Liu, X. Effect of WS2 particle size on mechanical properties and tribological behaviors of Cu–WS2 composites sintered by SPS. Trans. Nonferrous Met. Soc. China 2018, 28, 1176–1185. [Google Scholar] [CrossRef]
- Su, Y.; Jiang, F.; Long, M.; Wu, F.; Xiao, Z.; Wu, M. Microstructure and frictional properties of copper-tin composites containing graphite and MoS2 by rapid hot-press sintering. Tribol. Int. 2023, 183, 108392. [Google Scholar] [CrossRef]
- Bagheri, G.H.A. The effect of reinforcement percentages on properties of copper matrix composites reinforced with TiC particles. J. Alloys Compd. 2016, 676, 120–126. [Google Scholar] [CrossRef]
- Aleksandar, V.; Viseslava, R.; Fatima, Z. Friction and wear properties of copper based composites reinforced with micro and nano-sized Al2O3 particles. In Proceedings of the 8th International Conference on Tribology, Sinaia, Romania, 30 October–1 November 2014; pp. 357–358. [Google Scholar]
- Raja, R.; Jannet, S.; Ruban, S.R.; George, L. Mechanical, wear, and microstructural examination of copper surface composites reinforced with SiC nanoparticles done by FSP. Mater. Today Proc. 2023, 92, 376–381. [Google Scholar] [CrossRef]
- Fan, X.; Liu, Q.; Ding, H.; Wang, H.; Hao, C. The microstructures and properties of in-situ ZrB2 reinforced Cu matrix composites. Results Phys. 2019, 14, 102494. [Google Scholar] [CrossRef]
- Taylor, R.P.; McClain, S.T.; Berry, J.T. Uncertainty analysis of metal-casting porosity measurements using Archimedes’ principle. Int. J. Cast Met. Res. 1999, 11, 247–257. [Google Scholar] [CrossRef]
- ISO 20808:2016(E); International Standard, Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)- Determination of friction and wear characteristics of monolithic ceramics by ball-on-disc method, Edition 2. American National Standards Institute: New York, NY, USA, 2016.
- Meozzi, M. Special use of the ball on disc standard test. Tribol. Int. 2006, 39, 496–505. [Google Scholar] [CrossRef]
- Wang, C.; Lin, H.; Zhang, Z.; Li, W. Fabrication, Interfacial characteristics and strengthening mechanisms of ZrB2 microparticles reinforced Cu composites prepared by hot-pressed sintering. J. Alloys Compd. 2018, 748, 546–552. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, C.; Zhou, S.; Guo, B.; Zhang, Z.; Yu, Z.; Li, W. Effect of Sintering Temperature on the Microstructure and Properties of High-Strength and Highly Conductive 5 wt.% ZrB2/Cu Composite. Powder Metall. Met. Ceram. 2023, 61, 560–573. [Google Scholar]
- Fahrenholtz, W.G.; Hilmas, G.E.; Talmy, I.G.; Zaykoski, J.A. Refractory Diborides of Zirconium and Hafnium. J. Am. Ceram. Soc. 2007, 90, 1347–1364. [Google Scholar] [CrossRef]
- Pouyani, M.R.; Rajabi, M. Microwave-assisted synthesis of Cu–ZrB2 MM Nanocomposite using double pressing double sintering method. J. Mater. Sci. Mater. Electron. 2019, 30, 266–276. [Google Scholar] [CrossRef]
- Bengough, G.D.; Jones, R.M.; Pirret, R. Diagnosis of brass condenser tube corrosion. J. Inst. Met. 1920, 23, 65–158. [Google Scholar]
- Ahmad, Z. Principles of Corrosion Engineering and Corrosion Control; Butterworth-Heinemann: Oxford, UK, 2006. [Google Scholar] [CrossRef]



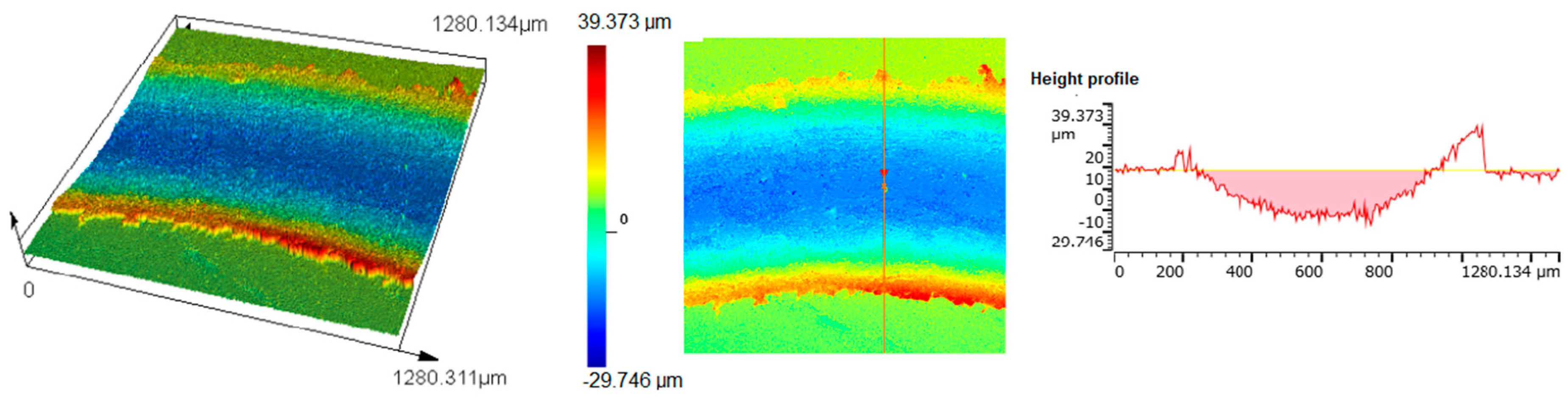


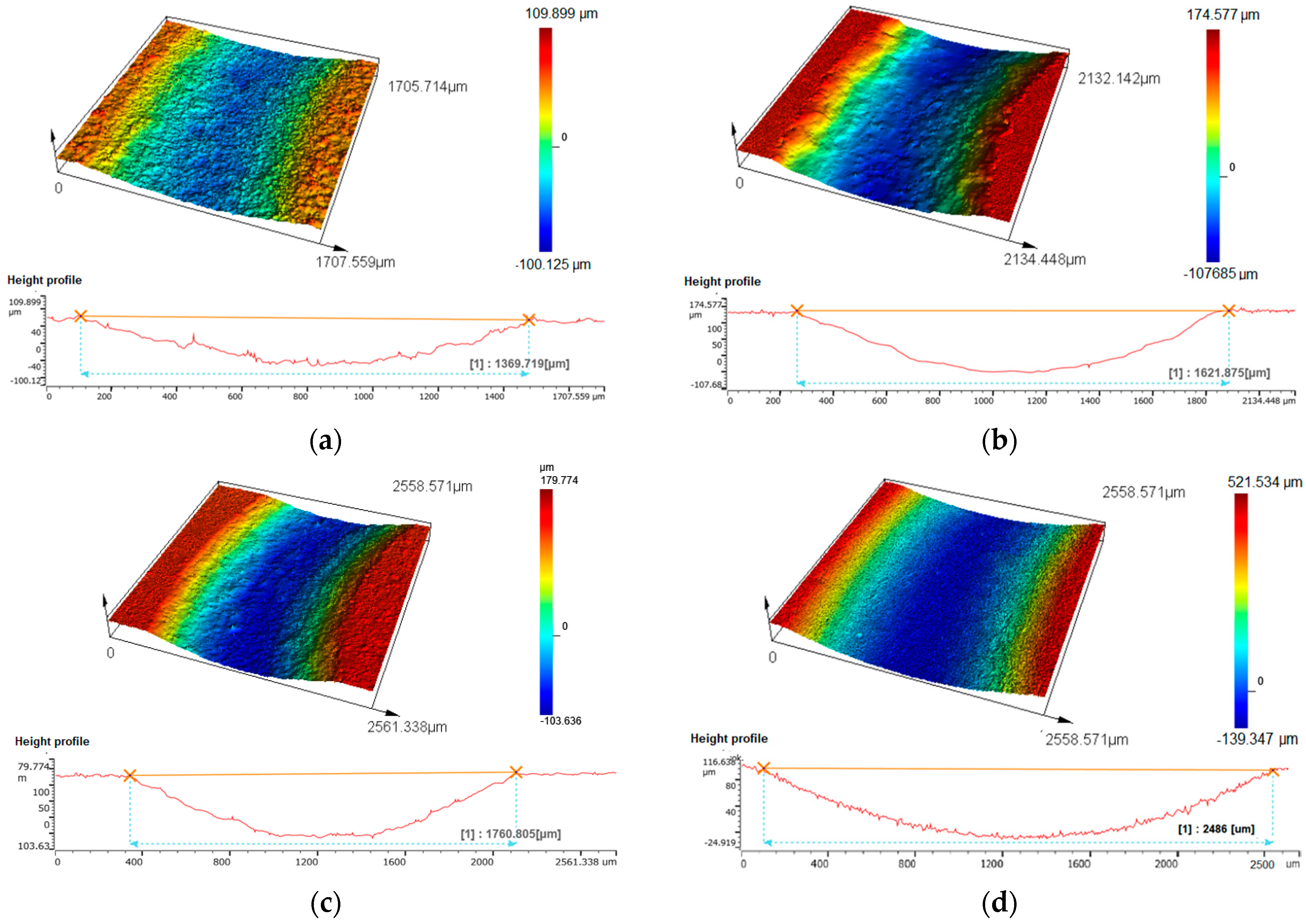
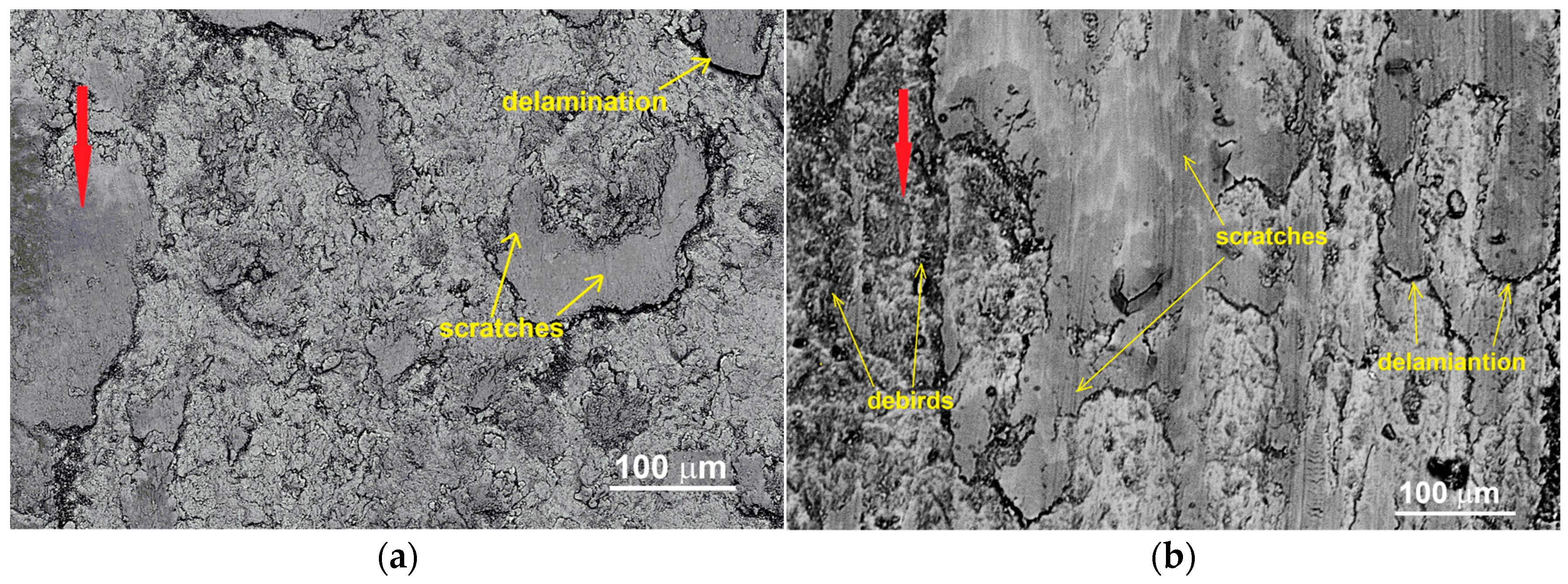

| Sintered Materials | Sintering Temperature (°C) | Friction Coefficient μ (-) | Specific Wear Rate Wv⋅10−4 (mm3/Nm) | Loss of Weight Δm (%) |
|---|---|---|---|---|
| Cu | 850 | 0.61 | 8.16 | 0.36 |
| 950 | 0.60 | 8.08 | 0.29 | |
| Cu + 5% ZrB2 | 850 | 0.64 | 6.56 | 0.27 |
| 950 | 0.62 | 6.26 | 0.24 | |
| Cu + 10% ZrB2 | 850 | 0.62 | 4.42 | 0.18 |
| 950 | 0.58 | 4.28 | 0.17 | |
| Cu + 15% ZrB2 | 850 | 0.56 | 3.98 | 0.12 |
| 950 | 0.54 | 3.61 | 0.11 | |
| Cu + 20% ZrB2 | 850 | 0.49 | 2.59 | 0.09 |
| 950 | 0.48 | 2.37 | 0.09 |
| Samples | Sintering Temperature | Erest (mV) | Ecorr (mV) | Icorr (µA/cm2) | βa (mV/dec) | βc (mV/dec) |
|---|---|---|---|---|---|---|
| Cu solid | ----- | −0.183 | −0.171 | 3.33 | 26 | 201 |
| Cu (sintered) | 850 °C | −0.190 | −0.194 | 4.62 | 67 | 222 |
| Cu + 5% ZrB2 | −0.187 | −0.245 | 6.33 | 86 | 133 | |
| Cu + 10% ZrB2 | −0.136 | −0.168 | 16.68 | 71 | 160 | |
| Cu + 15% ZrB2 | −0.136 | −0.184 | 34.51 | 101 | 199 | |
| Cu + 20% ZrB2 | −0.133 | −0.172 | 27.67 | 121 | 161 | |
| Cu (sintered) | 950 °C | −0.183 | −0.253 | 3.30 | 179 | 163 |
| Cu + 5% ZrB2 | −0.201 | −0.244 | 4.48 | 74 | 189 | |
| Cu + 10% ZrB2 | −0.149 | −0.208 | 22.70 | 106 | 166 | |
| Cu + 15% ZrB2 | −0.118 | −0.208 | 52.60 | 145 | 136 | |
| Cu + 20% ZrB2 | −0.094 | −0.177 | 124.45 | 110 | 123 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sulima, I.; Stępień, M.; Hyjek, P.; Boczkal, S.; Kowalik, R. Mechanical, Corrosion and Wear Characteristics of Cu-Based Composites Reinforced with Zirconium Diboride Consolidated by SPS. Metals 2024, 14, 974. https://doi.org/10.3390/met14090974
Sulima I, Stępień M, Hyjek P, Boczkal S, Kowalik R. Mechanical, Corrosion and Wear Characteristics of Cu-Based Composites Reinforced with Zirconium Diboride Consolidated by SPS. Metals. 2024; 14(9):974. https://doi.org/10.3390/met14090974
Chicago/Turabian StyleSulima, Iwona, Michał Stępień, Paweł Hyjek, Sonia Boczkal, and Remigiusz Kowalik. 2024. "Mechanical, Corrosion and Wear Characteristics of Cu-Based Composites Reinforced with Zirconium Diboride Consolidated by SPS" Metals 14, no. 9: 974. https://doi.org/10.3390/met14090974
APA StyleSulima, I., Stępień, M., Hyjek, P., Boczkal, S., & Kowalik, R. (2024). Mechanical, Corrosion and Wear Characteristics of Cu-Based Composites Reinforced with Zirconium Diboride Consolidated by SPS. Metals, 14(9), 974. https://doi.org/10.3390/met14090974







