Wettability of Polar and Apolar Liquids on Metal Surfaces
Abstract
:1. Introduction
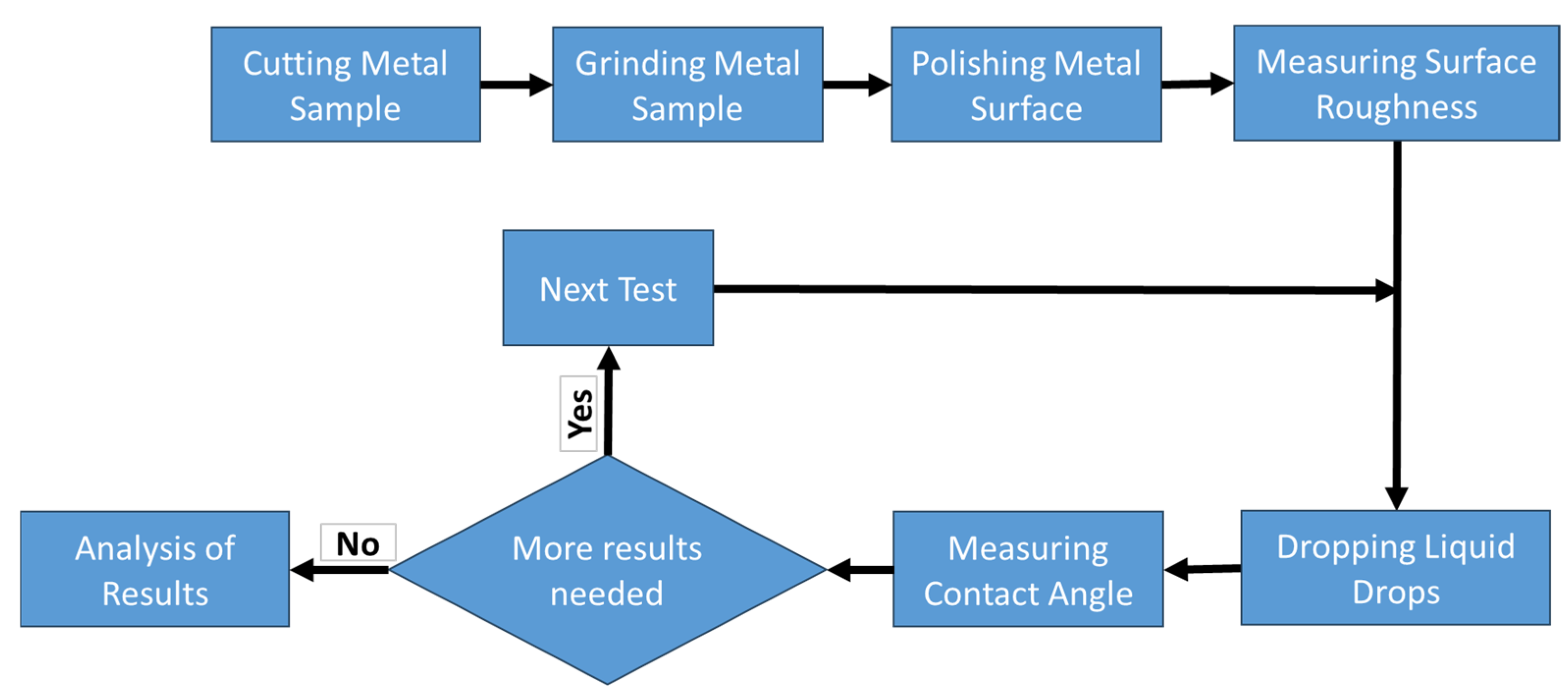
2. Materials and Methods
2.1. Preparation of the Samples
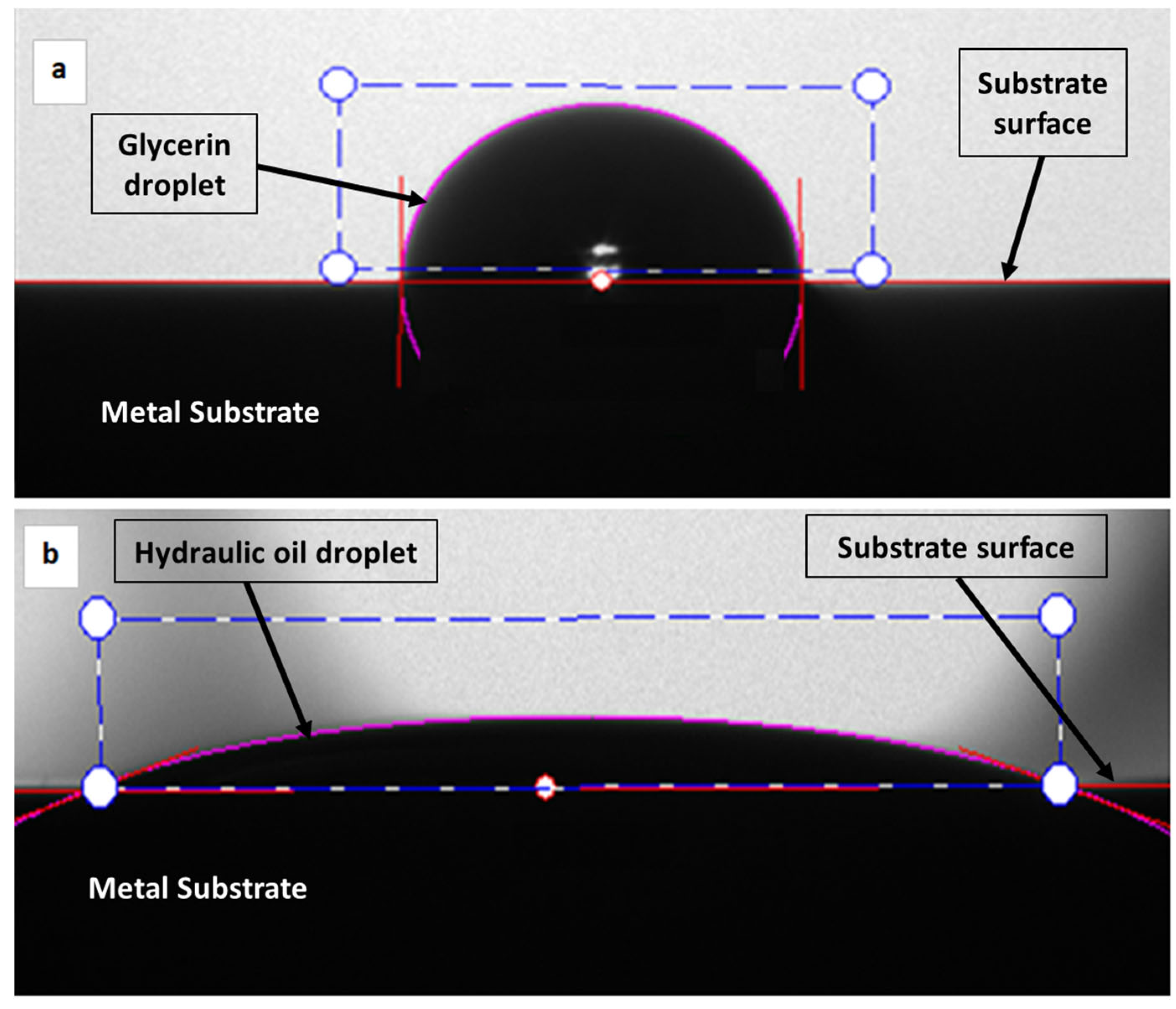
2.2. The Measurement of the Contact Angle (CA)
3. Results and Discussion
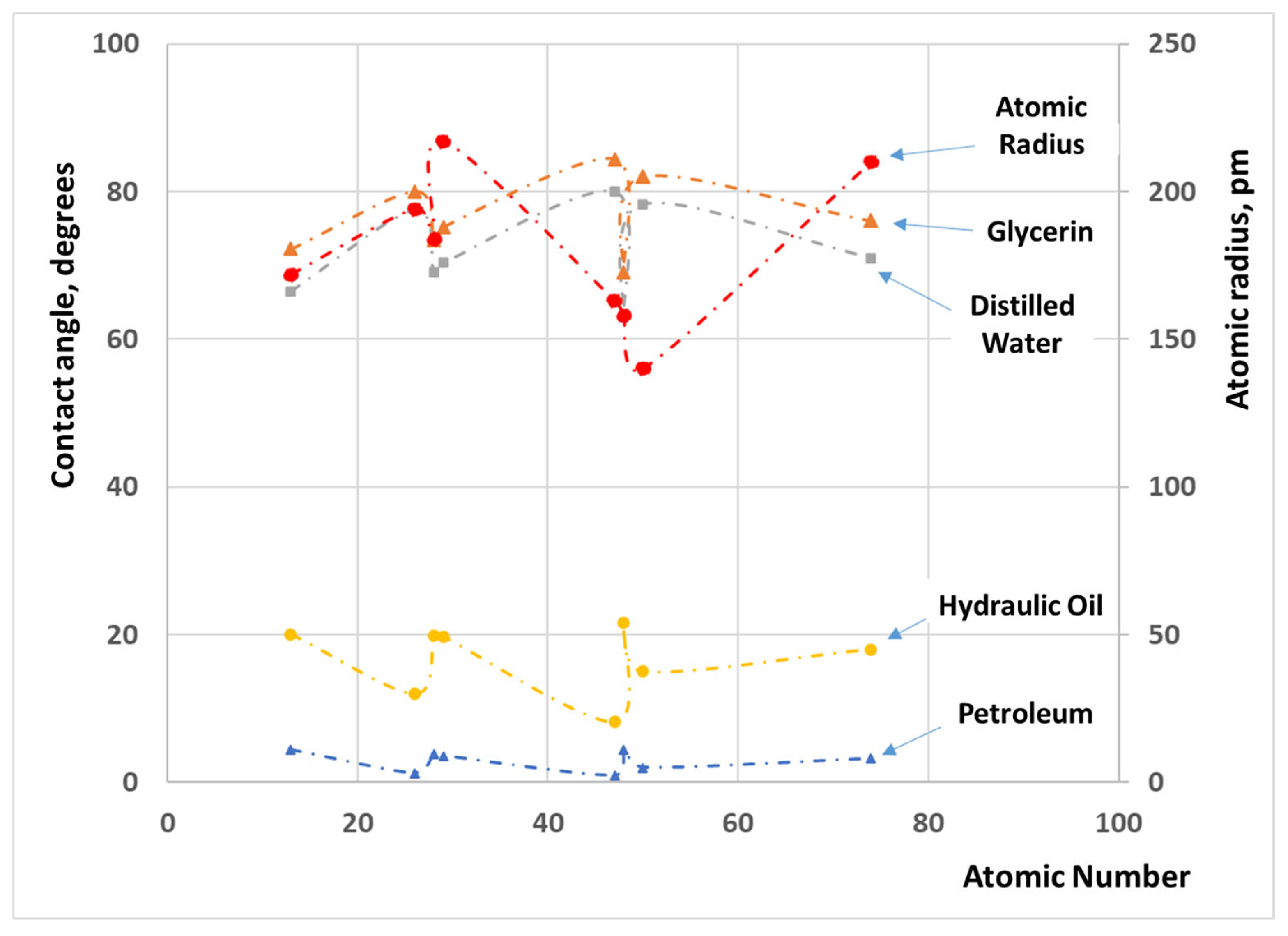
3.1. Experimental Results
- Atomic Interactions: The strength and type of interactions between the liquid molecules and the solid surface could be affected by the atomic radius of the substrate. A larger atomic radius often corresponds to a larger surface area and more available interaction sites. The concept depends on the idea that as the atomic radius increases, the overall size of atoms or molecules increases, potentially offering more surface area for interactions, especially in chemical reactions, adsorption processes, or catalytic activity [39,40]. Substrates with larger atomic radii might have more surface sites capable of forming hydrogen bonds, dipole–dipole interactions, or van der Waals forces with the liquid molecules.
- Pore size and Roughness: In porous substrates or surfaces with nanoscale roughness, the atomic radius of atoms making up a porous material can influence the overall structure of the pores, and larger atomic radii might lead to a more open structure that allows easy infiltration of the liquid, resulting in a lower contact angle. However, pore size is more directly related to the material structure (like the crystal lattice, the atom arrangement, or even the way the particles are packed) rather than only atomic radius. Pore size and surface roughness directly impact liquid infiltration and the contact angle, while a larger atomic radius can indirectly contribute to a more open structure [41,42].
- Surface Energy and Wetting: The surface energy of a solid substrate plays a significant role in wetting behavior. If the liquid’s cohesive forces are stronger than the adhesive forces between the liquid and the solid, the liquid tends to bead up and form a higher contact angle. On the other hand, if the adhesive forces are stronger, the liquid spreads out and forms a lower contact angle [43].
- Capillary Action: The ability of a liquid to flow in a narrow area against the force of gravity is called capillary action, which can also be affected by the atomic radius. This is usually a structural effect of the material rather than a direct result of atomic size, where capillary action is influenced by the dimensions of the capillaries, which in turn can be affected by the material’s atomic structure. It is determined by how atoms or molecules are arranged within the material rather than just their atomic radii, which might lead to capillaries with different dimensions. A material composed of atoms with a larger atomic radius might exhibit different pore or capillary sizes, indirectly influencing capillary action and potentially altering the contact angle [43,44,45].
- Chemical Interactions: The atomic radius is often linked to the chemical properties of the substrate. Substrates with larger atomic radii might have more polarizable electrons, affecting the strength of van der Waals forces or inducing stronger dipole interactions with the liquid [46].
- High and Low Atomic Density: Surfaces with high atomic density (Tightly Packed Atoms) are smoother and have fewer irregularities. Oil spreads more easily on such surfaces because there are fewer energy barriers, creating a more uniform interaction between the surface and the oil. This enhances the wettability of the surface by the oil. Surfaces with low atomic density (Loosely Packed Atoms) are rougher or have more gaps, which can reduce the wettability, as the oil may form patches instead of spreading uniformly [43].
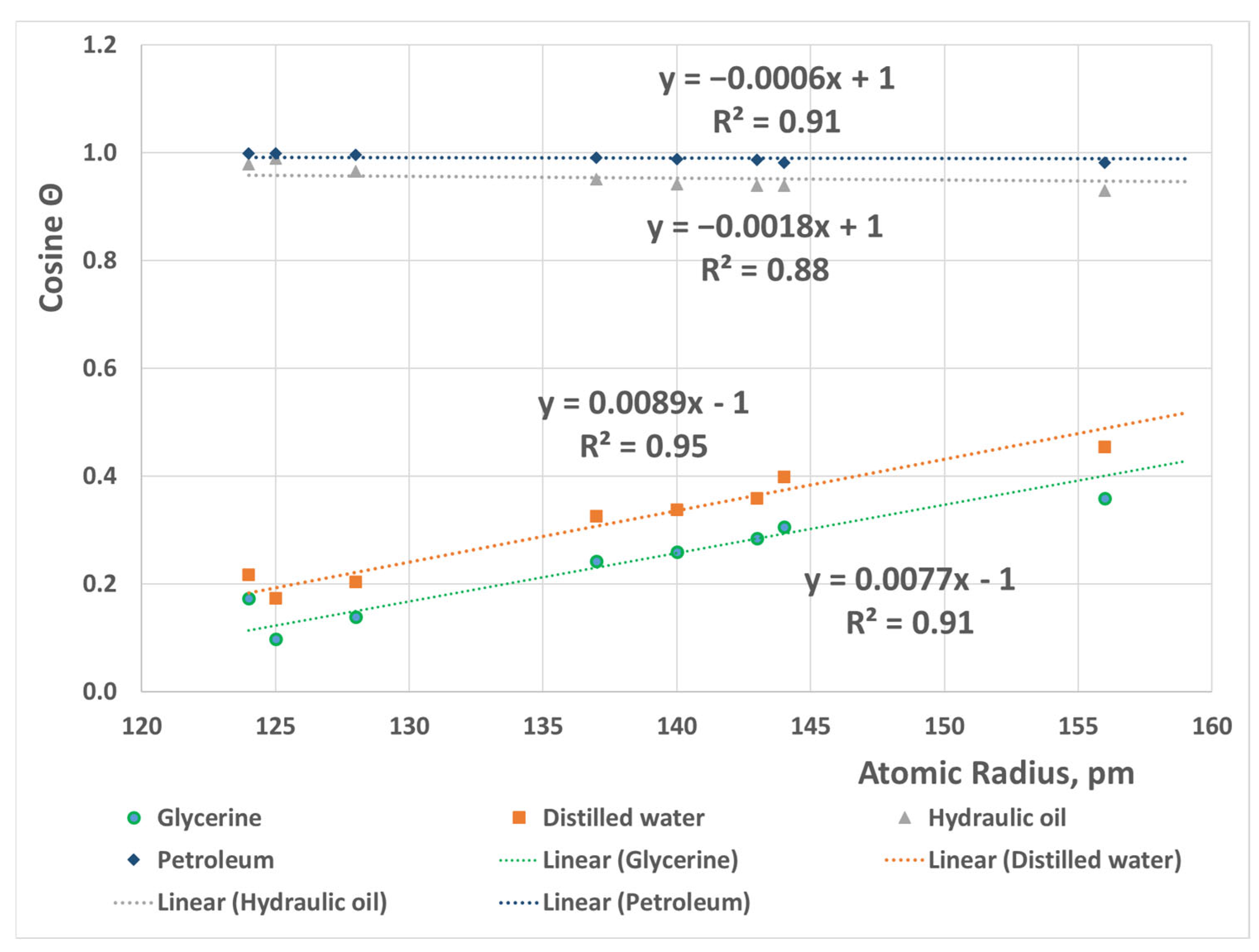
3.2. Broken Bond Model
4. Conclusions
- The atomic radius of the substrate significantly impacts the contact angle values, and therefore the overall wetting behavior will be impacted.
- Nickel exhibits superior oil wetting, displaying consistently lower contact angles throughout the testing. This distinction can be attributed to oxide formation on the surface of the other metals, resulting in their decreased surface energy and decreased wettability.
- Glycerin and distilled water share a similar wettability pattern on identical metal surfaces, owing to their relatively high surface tensions.
- Petroleum demonstrates remarkably rapid wetting compared to glycerin and distilled water. These novel observations could find applications in oil–water separation processes in the oil industry.
- The linear relation between atomic radius and wettability was proved by Becker’s broken bond model.
- An equation has been developed aiming to predict the wettability behavior of metals.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jung, Y.C.; Bhushan, B. Wetting Behavior of Water and Oil Droplets in Three-Phase Interfaces for Hydrophobicity/Philicity and Oleophobicity/Philicity. Langmuir 2009, 25, 14165–14173. [Google Scholar] [CrossRef]
- Liu, K.; Jiang, L. Metallic Surfaces with Special Wettability. Nanoscale 2011, 3, 825–838. [Google Scholar] [CrossRef] [PubMed]
- Ashokkumar, S.; Adler-Nissen, J.; Moller, P. Factors Affecting the Wettability of Different Surface Materials with Vegetable Oil at High Temperatures and Its Relation to Cleanability. Appl. Surf. Sci. 2012, 263, 86–94. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, H.; Wang, Z.; Liu, Y. Superhydrophobic Aluminum Alloy Surface: Fabrication, Structure, and Corrosion Resistance. Colloids Surf. A Physicochem. Eng. Asp. 2014, 441, 319–325. [Google Scholar] [CrossRef]
- Gupta, R.K.; Dunderdale, G.J.; England, M.W.; Hozumi, A. Oil/Water Separation Techniques: A Review of Recent Progresses and Future Directions. J. Mater. Chem. A 2017, 5, 16025–16058. [Google Scholar] [CrossRef]
- Kalin, M.; Polajnar, M. The Correlation between the Surface Energy, the Contact Angle and the Spreading Parameter, and Their Relevance for the Wetting Behaviour of DLC with Lubricating Oils. Tribol. Int. 2013, 66, 225–233. [Google Scholar] [CrossRef]
- Aspenes, G.; Høiland, S.; Barth, T.; Askvik, K.M. The Influence of Petroleum Acids and Solid Surface Energy on Pipeline Wettability in Relation to Hydrate Deposition. J. Colloid Interface Sci. 2009, 333, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, C.; Fu, J.; Gu, G. Wetting Behavior and Mechanism of Wetting Agents on Low-Energy Surface. Colloids Surf. A Physicochem. Eng. Asp. 2013, 424, 10–17. [Google Scholar] [CrossRef]
- Quéré, D. Wetting and Roughness. Annu. Rev. Mater. Res. 2008, 38, 71–99. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Y.; Cao, Y.; Li, G.; Liao, Y. Influence of Surface Roughness on Contact Angle Hysteresis and Spreading Work. Colloid Polym. Sci. 2020, 298, 1107–1112. [Google Scholar] [CrossRef]
- Zhu, H.; Guo, Z.; Liu, W. Adhesion Behaviors on Superhydrophobic Surfaces. Chem. Commun. 2014, 50, 3900–3913. [Google Scholar] [CrossRef]
- Samanta, A.; Huang, W.; Chaudhry, H.; Wang, Q.; Shaw, S.K.; Ding, H. Design of Chemical Surface Treatment for Laser-Textured Metal Alloys to Achieve Extreme Wetting Behavior. ACS Appl. Mater. Interfaces 2020, 12, 18032–18045. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Liu, J.; Xia, H.; Li, X.; Zhang, X.; Yang, H. Effects of Surface Treatment and Adhesive Thickness on the Shear Strength of Precision Bonded Joints. Polym. Test. 2021, 94, 107063. [Google Scholar] [CrossRef]
- Huhtamäki, T.; Tian, X.; Korhonen, J.T.; Ras, R.H.A. Surface-Wetting Characterization Using Contact-Angle Measurements. Nat. Protoc. 2018, 13, 1521–1538. [Google Scholar] [CrossRef] [PubMed]
- Drelich, J.W.; Boinovich, L.; Chibowski, E.; Della Volpe, C.; Hołysz, L.; Marmur, A.; Siboni, S. Contact Angles: History of over 200 Years of Open Questions. Surf. Innov. 2020, 8, 3–27. [Google Scholar] [CrossRef]
- Yin, Q.; Li, C.; Dong, L.; Bai, X.; Zhang, Y.; Yang, M.; Jia, D.; Li, R.; Liu, Z. Effects of Physicochemical Properties of Different Base Oils on Friction Coefficient and Surface Roughness in MQL Milling AISI 1045. Int. J. Precis. Eng. Manuf. Technol. 2021, 8, 1629–1647. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, Q.; Sari, A.; Brady, P.V.; Saeedi, A. Oil/Water/Rock Wettability: Influencing Factors and Implications for Low Salinity Water Flooding in Carbonate Reservoirs. Fuel 2018, 215, 171–177. [Google Scholar] [CrossRef]
- Ding, F.; Gao, M. Pore Wettability for Enhanced Oil Recovery, Contaminant Adsorption and Oil/Water Separation: A Review. Adv. Colloid Interface Sci. 2021, 289, 102377. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Cao, S.; Li, J.; Du, Z.; Cheng, F. Anti-Fouling TiO2 Nanowires Membrane for Oil/Water Separation: Synergetic Effects of Wettability and Pore Size. J. Membr. Sci. 2019, 572, 596–606. [Google Scholar] [CrossRef]
- Xu, J.; Che, P.; Zhang, H.; Zhang, Y.; Wu, J.; Li, W.; He, J.; Ma, Z.; Li, T.; Dong, Y.; et al. Superhydrophobic Modification of Biomass Cuttlebone Applied to Oil Spill Remediation. Materials 2022, 15, 4401. [Google Scholar] [CrossRef]
- Sun, Y.; Ke, Z.; Shen, C.; Wei, Q.; Sun, R.; Yang, W.; Yin, Z. Facile Construction and Fabrication of a Superhydrophobic and Super Oleophilic Stainless Steel Mesh for Separation of Water and Oil. Nanomaterials 2022, 12, 1661. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lee, B.; Wang, C.; Bajpayee, A.; Douglas, L.D.; Phillips, B.K.; Yu, G.; Rivera-Gonzalez, N.; Peng, B.J.; Jiang, Z.; et al. Photopolymerized Superhydrophobic Hybrid Coating Enabled by Dual-Purpose Tetrapodal ZnO for Liquid/Liquid Separation. Mater. Horizons 2022, 9, 452–461. [Google Scholar]
- Ren, W.; Lian, Z.; Wang, J.; Xu, J.; Yu, H. Fabrication of Durable Underoil Superhydrophobic Surfaces with Self-Cleaning and Oil-Water Separation Properties. RSC Adv. 2022, 12, 3838–3846. [Google Scholar] [CrossRef]
- Hou, B.; Jia, R.; Fu, M.; Wang, Y.; Jiang, C.; Yang, B.; Huang, Y. Wettability Alteration of Oil-Wet Carbonate Surface Induced by Self-Dispersing Silica Nanoparticles: Mechanism and Monovalent Metal Ion’s Effect. J. Mol. Liq. 2019, 294, 111601. [Google Scholar] [CrossRef]
- Gateman, S.M.; Gharbi, O.; Turmine, M.; Vivier, V. Measuring Changes in Wettability and Surface Area during Micro Droplet Corrosion Measurements. Electrochim. Acta 2021, 399, 139402. [Google Scholar] [CrossRef]
- Qu, W.; Li, S.; Chen, Z.; Li, C.; Pei, Y.; Gong, S. Hot Corrosion Behavior and Wettability of Calcium–Magnesium–Alumina–Silicate (CMAS) on LaTi2Al9O19 Ceramic. Corros. Sci. 2020, 162, 108199. [Google Scholar] [CrossRef]
- Lokhande, C.D.; Barkschat, A.; Tributsch, H. Contact angle measurements: An empirical diagnostic method for evaluation of thin film solar cell absorbers (CuInS2). Sol. Energy Mater Sol. Cells 2003, 79, 293–304. [Google Scholar] [CrossRef]
- Petkovšek, M.; Hočevar, M.; Gregorčič, P. Surface Functionalization by Nanosecond-Laser Texturing for Controlling Hydrodynamic Cavitation Dynamics. Ultrason. Sonochem. 2020, 67, 105126. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wang, S.; Zhou, R.; He, L.; Luo, X. The Migration and Adherence of Oil Droplets on Surfaces with Different Wettability in a Laminar Flow Field. Langmuir 2020, 36, 109–118. [Google Scholar] [CrossRef]
- Esmaeili, S.; Sarma, H.; Harding, T.; Maini, B. Review of the Effect of Temperature on Oil-Water Relative Permeability in Porous Rocks of Oil Reservoirs. Fuel 2019, 237, 91–116. [Google Scholar] [CrossRef]
- Lokanathan, M.; Wikramanayake, E.; Bahadur, V. Scalably Manufactured Textured Surfaces for Controlling Wettability in Oil-Water Systems. Mater. Res. Express. 2019, 6, 046507. [Google Scholar] [CrossRef]
- Kang, L.; Wang, B.; Zeng, J.; Cheng, Z.; Li, J.; Xu, J.; Gao, W.; Chen, K. Degradable Dual Superlyophobic Lignocellulosic Fibers for High-Efficiency Oil/Water Separation. Green Chem. 2020, 22, 504–512. [Google Scholar] [CrossRef]
- Pan, B.; Yin, X.; Iglauer, S. A Review on Clay Wettability: From Experimental Investigations to Molecular Dynamics Simulations. Adv. Colloid Interface Sci. 2020, 285, 102266. [Google Scholar] [CrossRef] [PubMed]
- Drelich, J.W. Contact Angles: From Past Mistakes to New Developments through Liquid-Solid Adhesion Measurements. Adv. Colloid Interface Sci. 2019, 267, 1–14. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, Y.; Drelich, J.W.; Choi, C.H. Spontaneous Spreading of a Droplet: The Role of Solid Continuity and Advancing Contact Angle. Langmuir 2018, 34, 4945–4951. [Google Scholar] [CrossRef] [PubMed]
- White, M.L. The Wetting of Gold Surfaces by Water. J. Phys. Chem. 1964, 68, 3083–3085. [Google Scholar] [CrossRef]
- Somlyai-Sipos, L.; Baumli, P. Wettability of Metals by Water. Metals 2022, 12, 1274. [Google Scholar] [CrossRef]
- Buckley, J.S.; Morrow, N.R. Characterization of Crude Oil Wetting Behavior by Adhesion Tests. In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 22–25 April 1990. [Google Scholar]
- Zhang, Y.; Zhao, R.; Sanchez-Sanchez, M.; Haller, G.L.; Hu, J.; Bermejo-Deval, R.; Liu, Y.; Lercher, J.A. Promotion of protolytic pentane conversion on H-MFI zeolite by proximity of extra-framework aluminum oxide and Brønsted acid sites. J. Catal. 2019, 370, 424–433. [Google Scholar] [CrossRef]
- Zumdahl, S.; Zumdahl, S.; DeCoste, D.J. Chemistry: An Atoms First Approach; Cengage Learning: Boston, MA, USA, 2020. [Google Scholar]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Adamson, A.W.; Gast, A.P. Physical Chemistry of Surfaces, 6th ed.; Wiley-Interscience: Toronto, Canada, 1997. [Google Scholar]
- Gennes, P.G.; Brochard-Wyart, F.; Quéré, D. Capillarity and Wetting Phenomena: Drops, Bubbles, Pearls, Waves, 1st ed.; Springer: New York, NY, USA, 2004. [Google Scholar]
- Dąbrowski, A. Adsorption—From theory to practice. Adv. Colloid Interface Sci. 2001, 93, 135–224. [Google Scholar] [CrossRef]
- Parsegian, V.A. Van der Waals Forces: A Handbook for Biologists, Chemists, Engineers, and Physicists; Cambridge University Press: New York, NY, USA, 2006. [Google Scholar]
- Becker, R. Die Keimbildung bei der Ausscheidung in metallischen Mischkristallen. Ann. Phys. 1938, 424, 128–140. [Google Scholar] [CrossRef]
- Nishizawa, T.; Ohnuma, I.; Ishida, K. Correlation between interfacial energy and phase diagram in ceramic-metal systems. J. Phase Equilibria 2001, 22, 269–275. [Google Scholar] [CrossRef]
| Liquid | Al | Fe | Ni | Cu | Sn | Ag | W | Cd |
|---|---|---|---|---|---|---|---|---|
| Hydraulic oil | 20 | 12 | 8 | 15 | 19 | 20 | 18 | 22 |
| Petroleum | 9 | 3 | 2 | 5 | 9 | 11 | 8 | 11 |
| Distilled water | 69 | 77 | 80 | 78 | 70 | 67 | 71 | 63 |
| Glycerin | 73 | 80 | 84 | 82 | 75 | 72 | 76 | 69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khairi, M.; Erdélyi, Z.; Baumli, P. Wettability of Polar and Apolar Liquids on Metal Surfaces. Metals 2025, 15, 23. https://doi.org/10.3390/met15010023
Khairi M, Erdélyi Z, Baumli P. Wettability of Polar and Apolar Liquids on Metal Surfaces. Metals. 2025; 15(1):23. https://doi.org/10.3390/met15010023
Chicago/Turabian StyleKhairi, Mohanad, Zoltán Erdélyi, and Peter Baumli. 2025. "Wettability of Polar and Apolar Liquids on Metal Surfaces" Metals 15, no. 1: 23. https://doi.org/10.3390/met15010023
APA StyleKhairi, M., Erdélyi, Z., & Baumli, P. (2025). Wettability of Polar and Apolar Liquids on Metal Surfaces. Metals, 15(1), 23. https://doi.org/10.3390/met15010023










