Experimental Study on Bismuth Removal from Lead with Auxiliary Calcium Magnesium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Equipment
2.2. Preparation of a Synthetic Sample
3. Results and Discussion
3.1. Theoretical Calculation Results
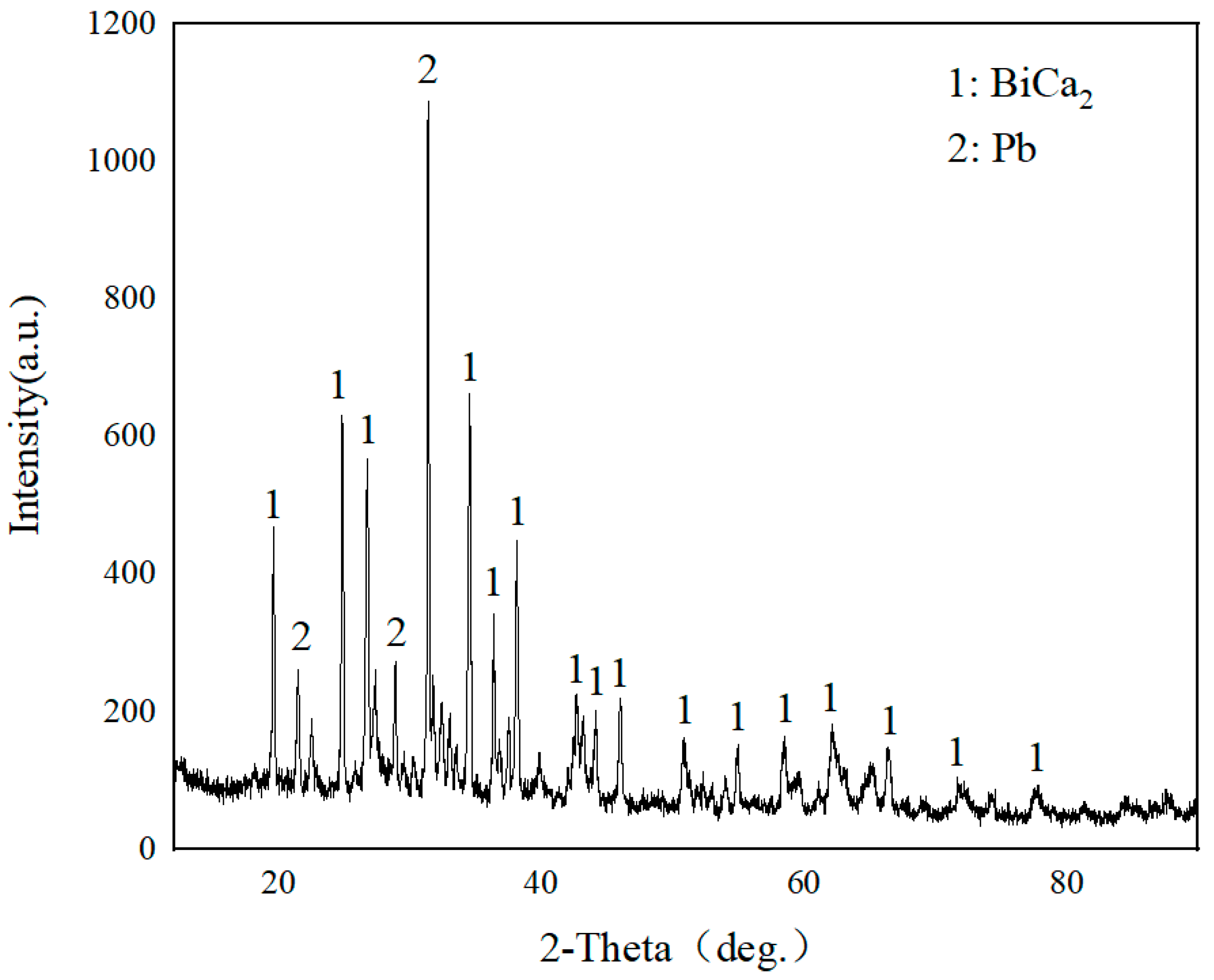
3.2. Experimental Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carrère, T.; Khalid, U.; Baumann, M.; Bouzidi, M.; Allard, B. Carbon footprint assessment of manufacturing of synthetic graphite battery anode material for electric mobility applications. J. Energy Storage 2024, 94, 112356. [Google Scholar] [CrossRef]
- Romero, A.; Urra, O.; Blecua, M.; Ocón, P.; Valenciano, J.; Trinidad, F. Effect on water consumption by metallic impurities into electrolyte of lead-acid batteries. J. Energy Storage 2021, 42, 103025. [Google Scholar] [CrossRef]
- Qi, L.; Wang, Y.; Kong, L.; Yi, M.; Song, J.; Hao, D.; Zhou, X.; Zhang, Z.; Yan, J. Manufacturing processes and recycling technology of automotive lithium-ion battery: A review. J. Energy Storage 2023, 67, 107533. [Google Scholar] [CrossRef]
- Yolshina, L.A.; Yolshina, V.A.; Yolshin, A.N.; Plaksin, S.V. Novel lead-graphene and lead-graphite metallic composite materials for possible applications as positive electrode grid in lead-acid battery. J. Power Sources 2015, 278, 87–97. [Google Scholar] [CrossRef]
- Feng, B.; Long, T.; Li, R.; Ding, Y.L. Rationally constructing metallic Sn-ZnO heterostructure via in-situ Mn doping for high-rate Na-ion batteries. Chin. Chem. Lett. 2025, 36, 110273. [Google Scholar] [CrossRef]
- Zhou, W.; Li, J.; Bian, Y.; Han, X.; Guan, R. Exploration of deep purification of aluminum alloy in vacuum based on adsorption of oxides by hydrogen bubbles. Vacuum 2024, 229, 113594. [Google Scholar] [CrossRef]
- Wang, S.; Chen, L.; Xu, B.; Jiang, W.; Kong, L.; Yang, B.; Xiong, H.; Qu, C.; Zhang, T.; Zhang, S.; et al. Theoretical calculation and experimental investigation on vacuum gasification separation of Ag-Cu-Au ternary alloy. J. Alloys Compd. 2023, 948, 169685. [Google Scholar] [CrossRef]
- Ghodsi, A.; Fashandi, H. Influence of photothermal nanomaterials localization within the electrospun membrane structure on purification of saline oily wastewater based on photothermal vacuum membrane distillation. J. Environ. Manage 2024, 366, 121866. [Google Scholar] [CrossRef]
- Wan, H.; Xu, B.; Yang, B.; Zhao, J.; Dai, Y. The impurities distribution and purification efficiency of high-purity aluminum preparation by zone melting in vacuum. Vacuum 2020, 171, 108839. [Google Scholar] [CrossRef]
- Qiu, S.; Wen, S.; Fang, M.; Zhang, L.; Gan, C.; Jiang, D.; Tan, Y.; Li, J.; Luo, X. Process parameters influence on the growth rate during silicon purification by vacuum directional solidification. Vacuum 2016, 125, 40–47. [Google Scholar] [CrossRef]
- Ngulezhu, T.; Abdulkarim, A.S.; Rawat, S.; Singh, R.C.; Singh, P.; Singh, D.; Strzałkowski, K.; Srivastava, M. Stable lead free perovskite solar cells based on bismuth doped perovskite materials. Chem. Phys. Impact 2024, 9, 100689. [Google Scholar] [CrossRef]
- Hu, W.; He, X.; Fang, Z.; Lian, W.; Shang, Y.; Li, X.; Zhou, W.; Zhang, M.; Chen, T.; Lu, Y.; et al. Bulk heterojunction gifts bismuth-based lead-free perovskite solar cells with record efficiency. Nano Energy 2019, 68, 104362. [Google Scholar] [CrossRef]
- Jain, S.M.; Phuyal, D.; Davies, M.L.; Li, M.; Philippe, B.; De Castro, C.; Qiu, Z.; Kim, J.; Watson, T.; Tsoi, W.C.; et al. An effective approach of vapour assisted morphological tailoring for reducing metal defect sites in lead-free, (CH3NH3)3Bi2I9 bismuth-based perovskite solar cells for improved performance and long-term stability. Nano Energy 2018, 49, 614–624. [Google Scholar] [CrossRef]
- Lan, C.; Luo, J.; Zhao, S.; Zhang, C.; Liu, W.; Hayase, S.; Ma, T. Effect of lead-free (CH3NH3)3Bi2I9 perovskite addition on spectrum absorption and enhanced photovoltaic performance of bismuth triiodide solar cells. J. Alloys Compd. 2017, 701, 834–840. [Google Scholar] [CrossRef]
- Waykar, R.; Bhorde, A.; Nair, S.; Pandharkar, S.; Gabhale, B.; Aher, R.; Rondiya, S.; Waghmare, A.; Doiphode, V.; Punde, A.; et al. Environmentally stable lead-free cesium bismuth iodide (Cs3Bi2I9) perovskite: Synthesis to solar cell application. J. Phys. Chem. Solids 2020, 146, 109608. [Google Scholar] [CrossRef]
- Wang, C.; Wang, G.; Zhu, W.; Xiang, H.; Zheng, L.; Zhang, J.; Chen, J.; Fu, G.; Pan, J.; Wang, R. Advancements in the separation, purification, and smelting technology of bismuth: A review. Sep. Purif. Technol. 2024, 355, 129684. [Google Scholar] [CrossRef]
- Lu, D.; Jin, Z.; Jiang, K. Fine debismuthizing with calcium, magnesium and antimony. Trans. Nonferrous Met. Soc. China 2011, 21, 2311–2316. [Google Scholar] [CrossRef]
- Majhi, J.; Ganguly, S.; Basu, A.; Mondal, A. Improved corrosion response of squeeze-cast AZ91 magnesium alloy with calcium and bismuth additions. J. Alloys Compd. 2021, 873, 159600. [Google Scholar] [CrossRef]
- Perumal, S.; Lee, W.; Atchudan, R. A review on bismuth-based materials for the removal of organic and inorganic pollutants. Chemosphere 2022, 306, 135521. [Google Scholar] [CrossRef] [PubMed]
- Emeje, K.; Aghemenloh, E.; Onate, C. Analytical determination of enthalpy, heat capacity and Gibbs free energy for nitrogen and iodine. Chem. Phys. Lett. 2024, 844, 141271. [Google Scholar] [CrossRef]
- Safaeipour, S.; Kalantarian, M.M. Insight into the origin of electrochemical potential: Fermi vs. Gibbs free energies. Nano-Structures Nano-Objects 2024, 39, 101213. [Google Scholar] [CrossRef]
- Park, J.; Nicholas, J.D.; Qi, Y. Surface Gibbs free energy analyses of Sr segregation in lanthanum strontium iron oxide. Surf. Sci. 2023, 732, 122268. [Google Scholar] [CrossRef]
- Zhou, Y. Free energy of formation of Mg3Bi2, Ca3Bi2, CaMg2Bi2 and K9Mg6Bi7. Acta Metall. Sin. 1964, 2, 217–219. [Google Scholar]
- Liang, Y.; Che, Y. Manual of Inorganic Thermodynamics; Northeast Institute of Technology Press: Shenyang, China, 1994. [Google Scholar]




| Metal | Source | Purity |
|---|---|---|
| Lead | Henan Yuguang Gold&Lead Co., Ltd., Jiyuan, China | 99.992% |
| Bismuth | Henan Yuguang Gold&Lead Co., Ltd., Jiyuan, China | 99.999% |
| Calcium | Yifeng Metal Materials Co., Ltd., Dongguan, China | 99.995% |
| Magnesium | Yifeng Metal Materials Co., Ltd., Dongguan, China | 99.996% |
| Instrument | Manufacturer | Equipment Type |
|---|---|---|
| Electric blast drying oven | Yuyao Star Instrument Factory, Yuyao, China | XGQ-2000 |
| Planetary ball mill | Nanjing University Instrument Factory, Nanjing, China | QM-BP |
| Powder block press | Tianjin Keqi High-tech Company, Tianjin, China | 769YP-40C |
| Electronic balance | Ruian Yingheng Electric Appliance Co., Ltd., Ruian, China | JCS-31002C |
| Pit furnace | National Engineering Research Center of Vacuum Metallurgy, Kunming, China | Self-control |
| Argon | Kunming Stone Headman gas products Co., Ltd., Kunming, China | - |
| High-purity graphite crucible | National Engineering Research Center of Vacuum Metallurgy, Kunming, China | Self-control |
| Substance | Standard Free Energy of Formation kJ/mol (600 K) |
|---|---|
| Ca (s) | –27.94 |
| Mg (s) | –22.66 |
| Bi (s) | –38.34 |
| Bi (l) | –39.49 |
| Bi2Mg3 (s) | –22.56 |
| Bi2Ca3 (s) | –154.48 |
| CaMg2Bi2 (s) | –118.16 |
| Impurity | As | Fe | Cu | Sn | Ag | Bi |
|---|---|---|---|---|---|---|
| content | 2 | 1 | 4 | 1 | 3 | 6 |
| Element (%) | Bi | Ca | Mg |
|---|---|---|---|
| Initial content | 4.85 | 0.5 | 0.66 |
| Product content (at 1223 K) | 2.74 | 0.012 | 0.047 |
| Product content (at 1323 K) | 1.81 | 0.0045 | 0.0077 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, L.; Qin, H.; Yang, W.; Kong, X. Experimental Study on Bismuth Removal from Lead with Auxiliary Calcium Magnesium. Metals 2025, 15, 156. https://doi.org/10.3390/met15020156
Deng L, Qin H, Yang W, Kong X. Experimental Study on Bismuth Removal from Lead with Auxiliary Calcium Magnesium. Metals. 2025; 15(2):156. https://doi.org/10.3390/met15020156
Chicago/Turabian StyleDeng, Linxing, Haocheng Qin, Weichen Yang, and Xiangfeng Kong. 2025. "Experimental Study on Bismuth Removal from Lead with Auxiliary Calcium Magnesium" Metals 15, no. 2: 156. https://doi.org/10.3390/met15020156
APA StyleDeng, L., Qin, H., Yang, W., & Kong, X. (2025). Experimental Study on Bismuth Removal from Lead with Auxiliary Calcium Magnesium. Metals, 15(2), 156. https://doi.org/10.3390/met15020156






