Gold Leaching from an Auriferous Ore by Alkaline Thiosulfate–Glycine–Copper Solution
Abstract
:1. Introduction
1.1. Traditional Methods for Gold Recovery
1.2. Thiosulfate–Glycine System: An Alternative for Gold Extraction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Ore Characterization
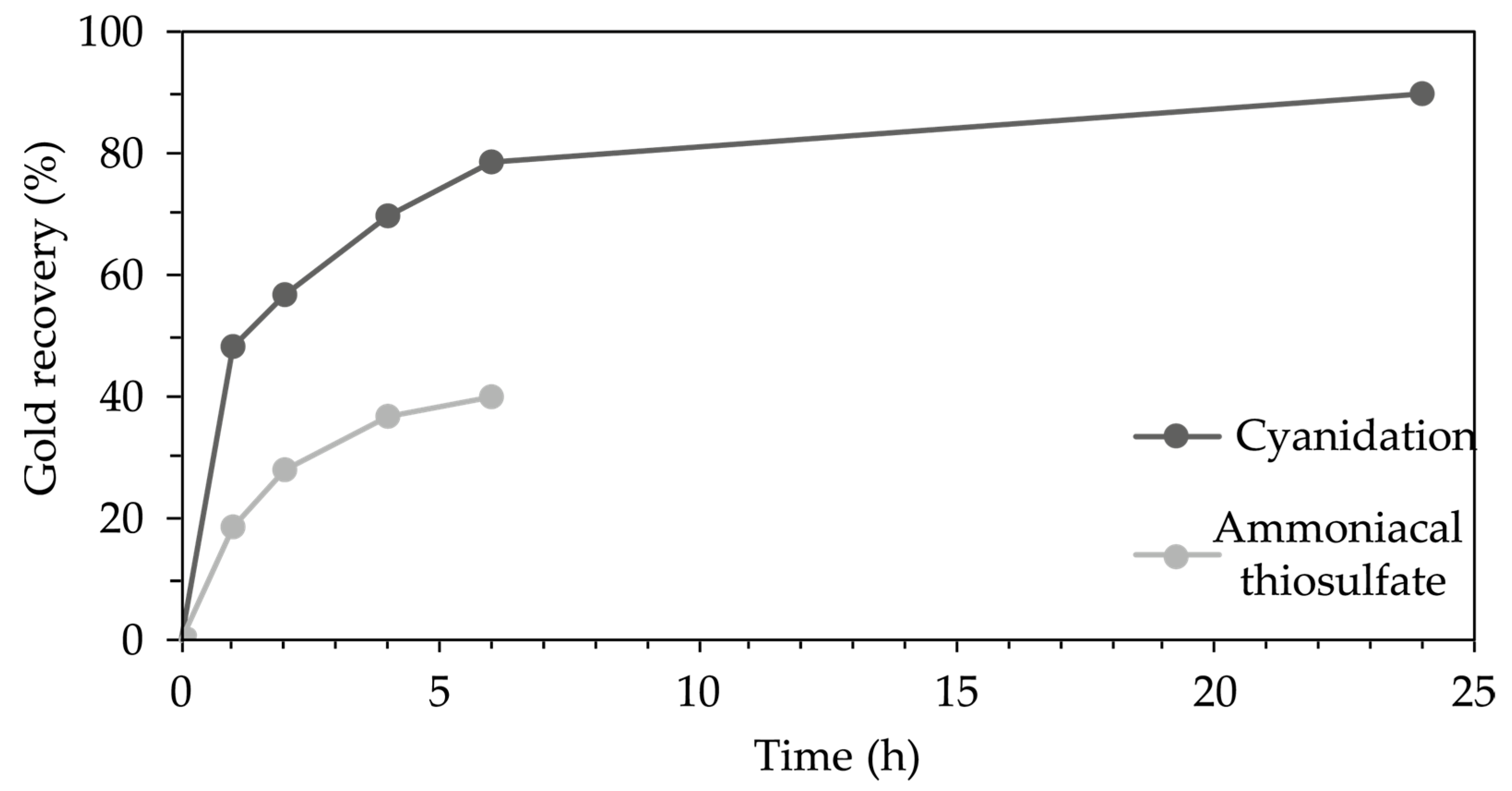
2.2.2. Sodium Cyanide Leaching
2.2.3. Ammonium Thiosulfate Leaching
2.2.4. Thiosulfate–Glycine Leaching
2.2.5. Glycine Leaching
3. Results
3.1. Characterization of the Auriferous Ore
3.2. Sodium Cyanide Leaching
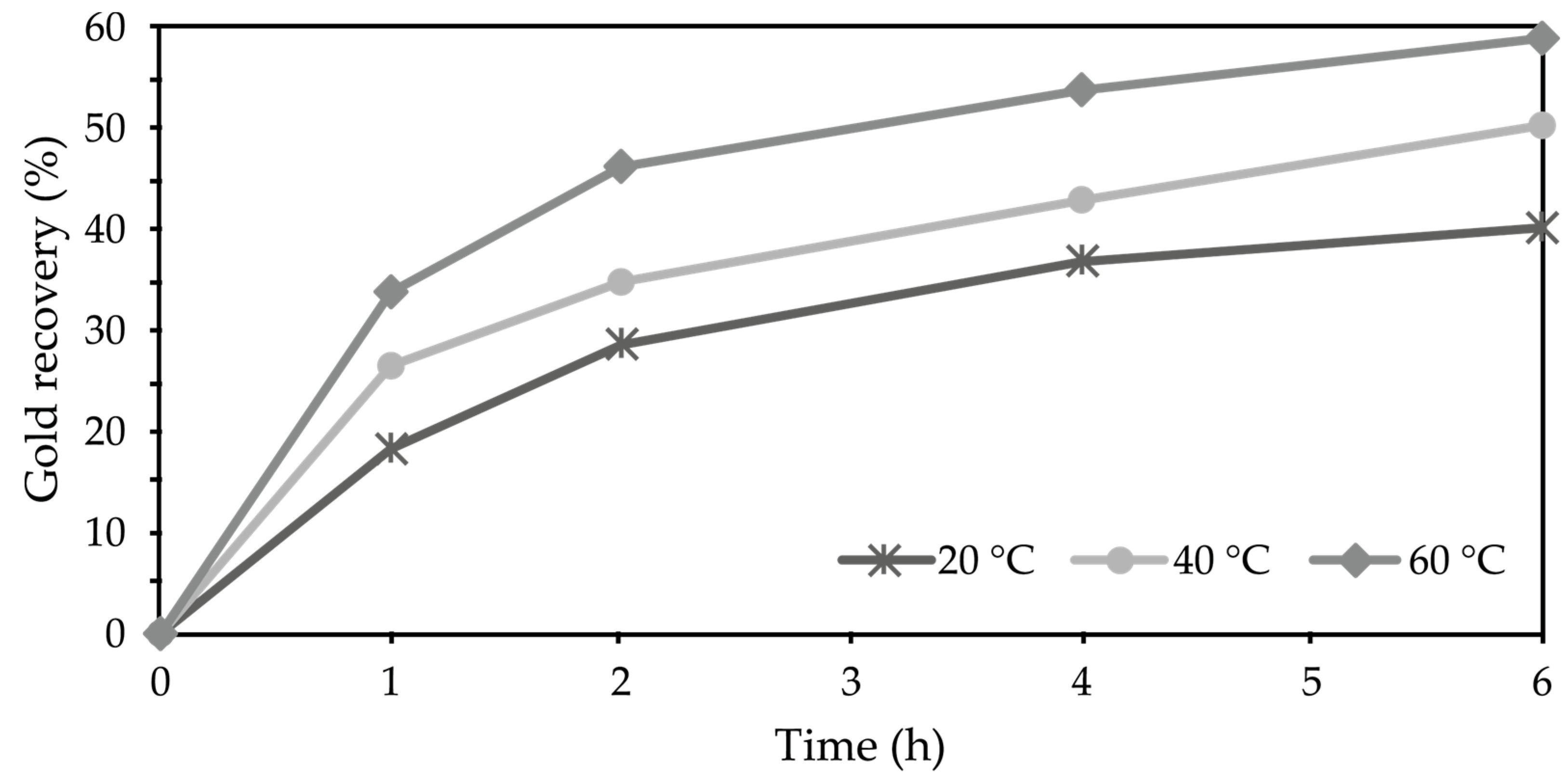
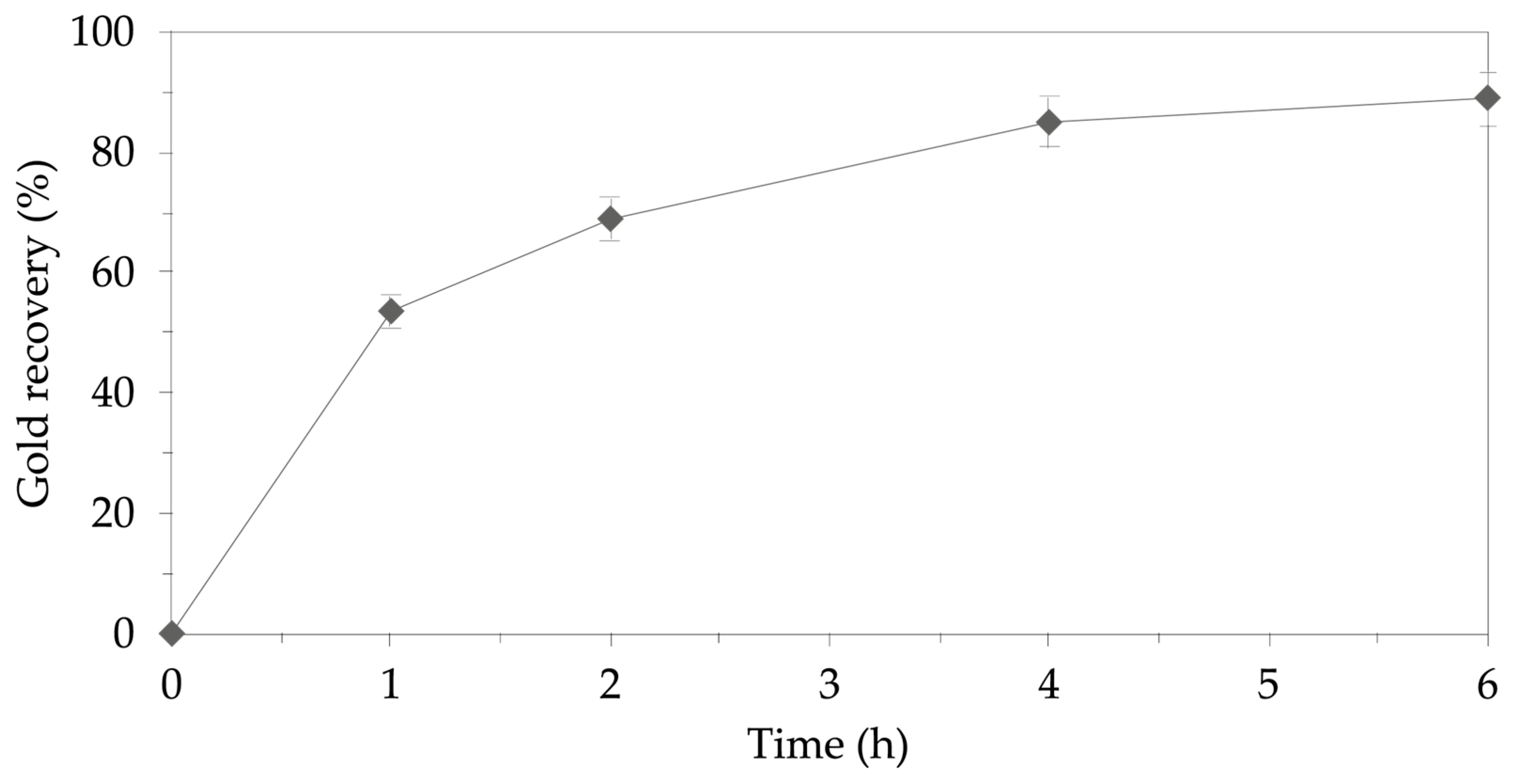
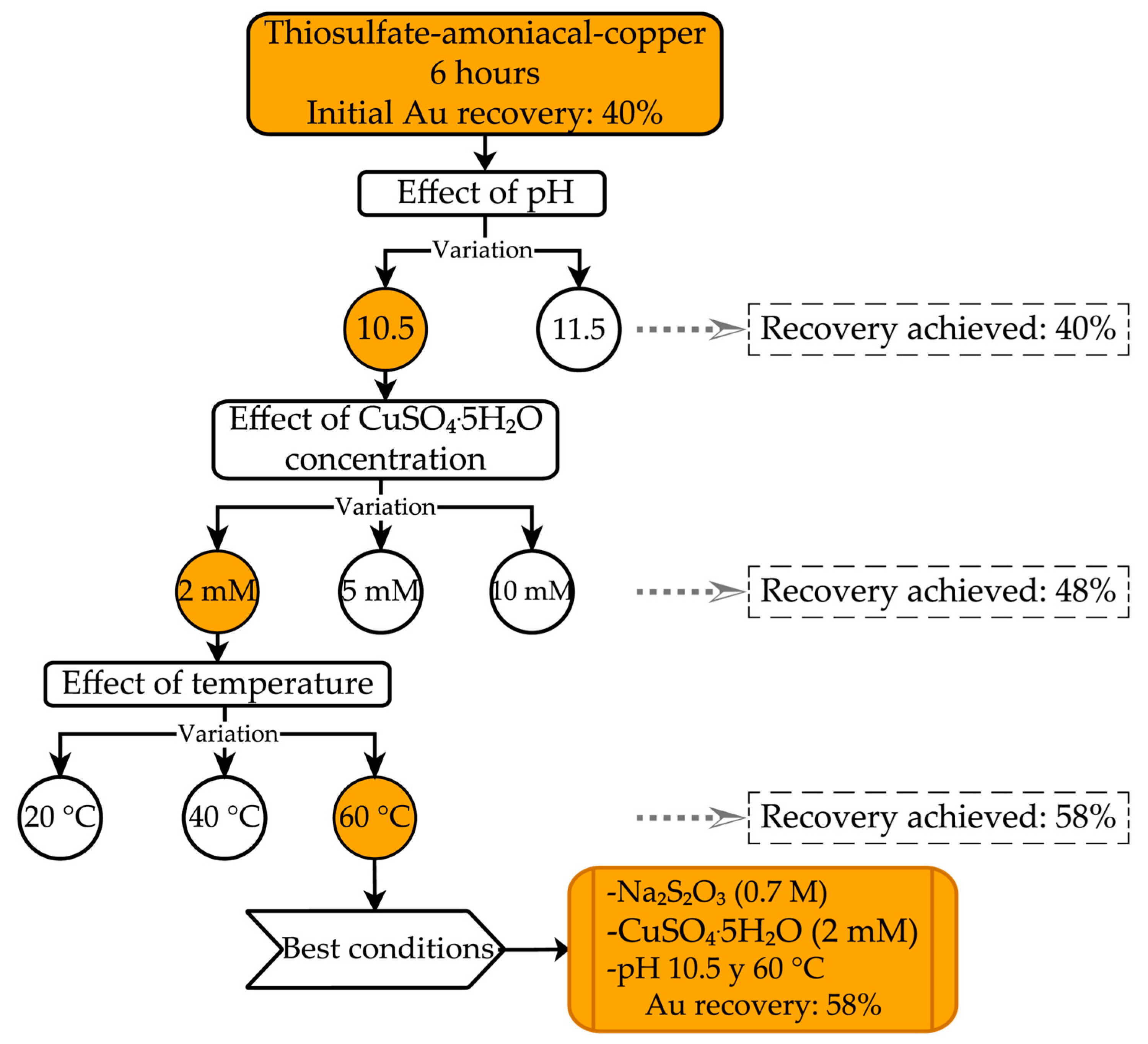
3.3. Ammoniacal Thiosulfate–Copper Leaching
Evaluation of Leaching Conditions for Gold Recovery Using the Ammoniacal Thiosulfate System
3.4. Thiosulfate–Glycine–Copper Leaching
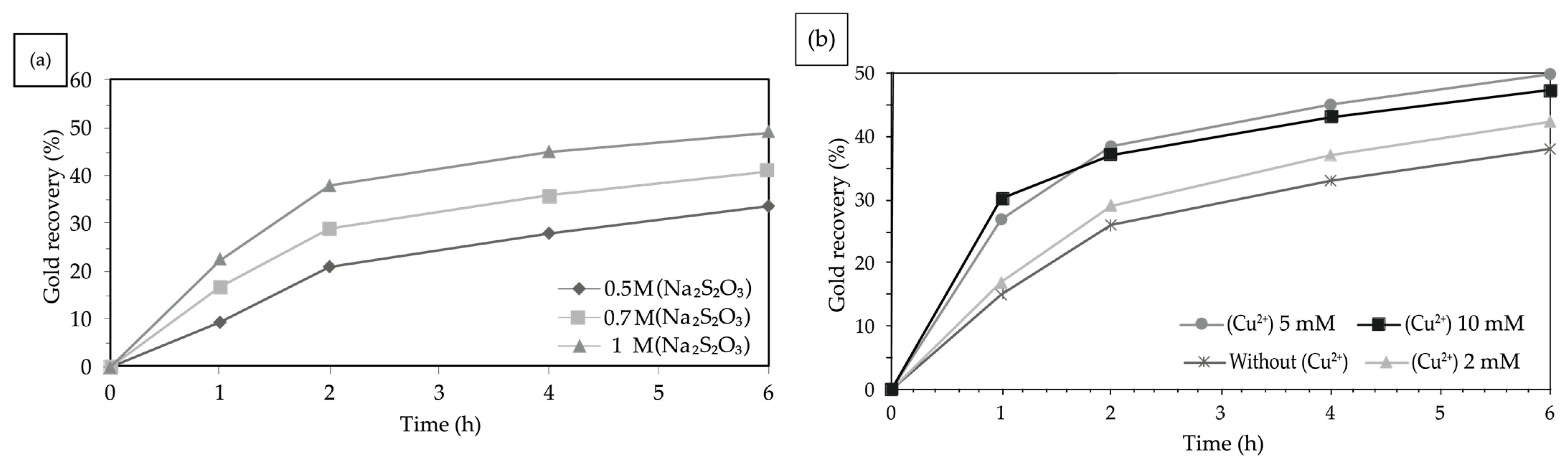
3.4.1. Effect of Sodium Thiosulfate Concentration
3.4.2. Effect of Copper Ion Concentration
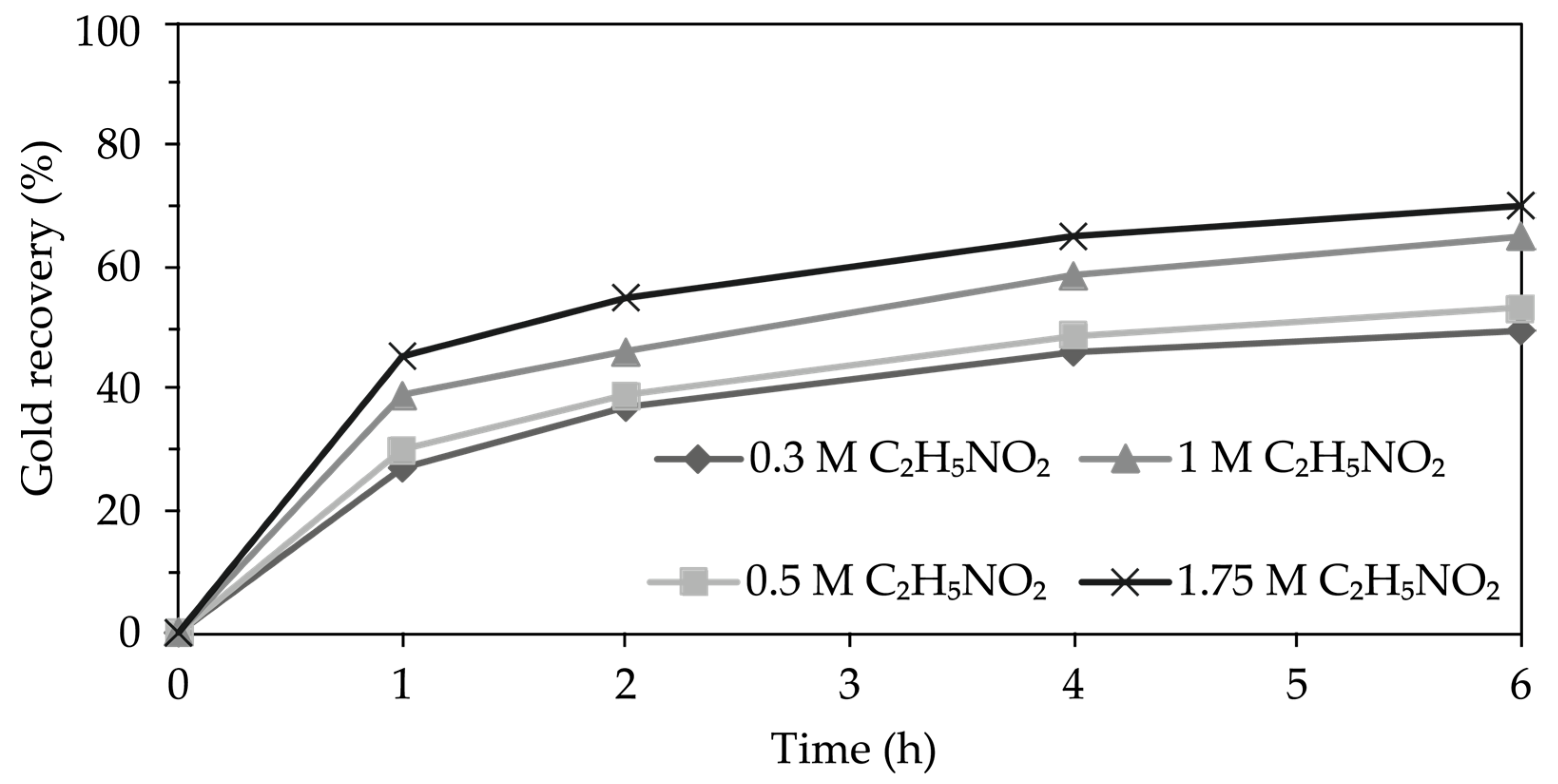
3.4.3. Effect of Glycine Concentration
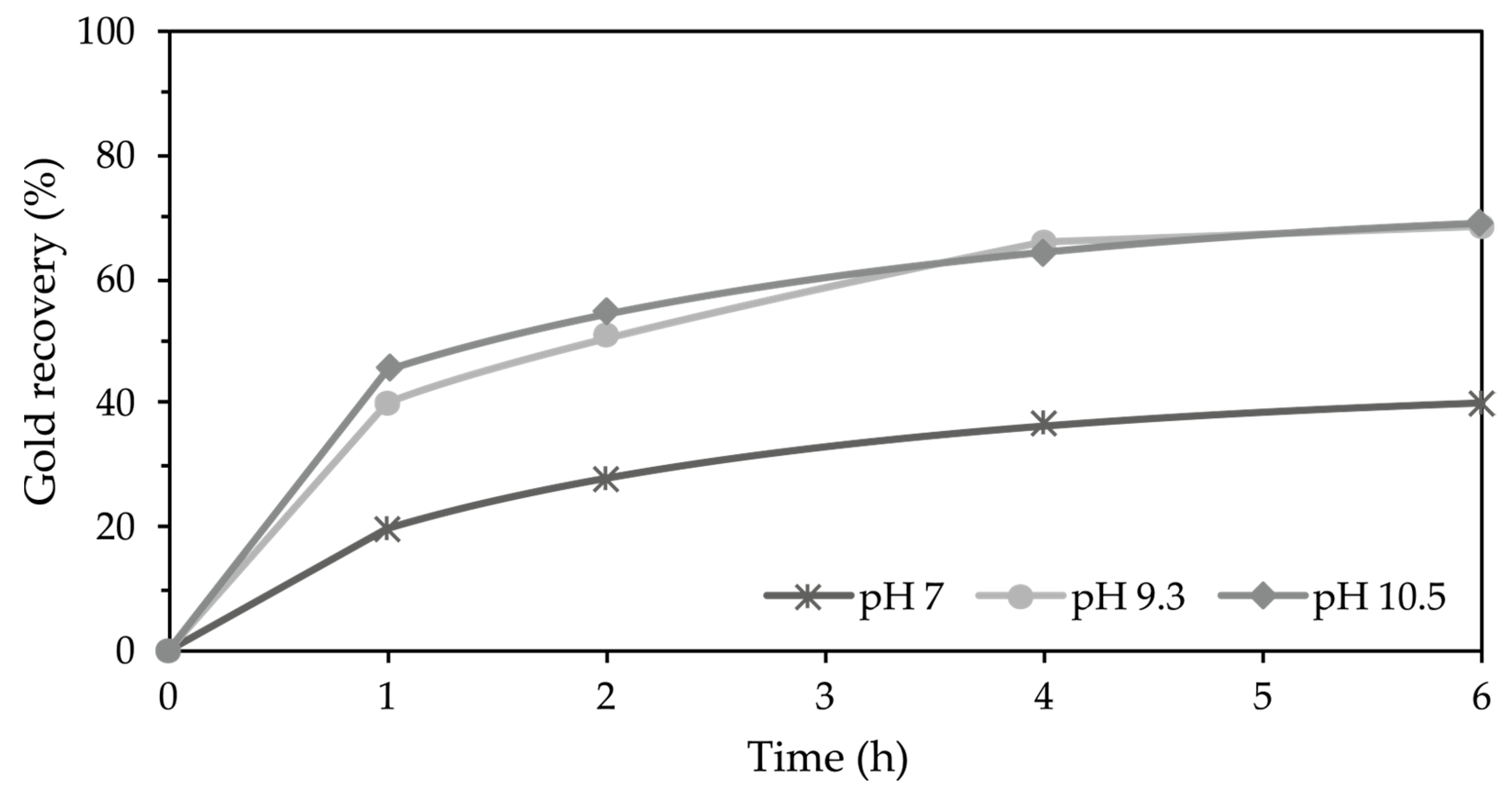
3.4.4. Effect of pH
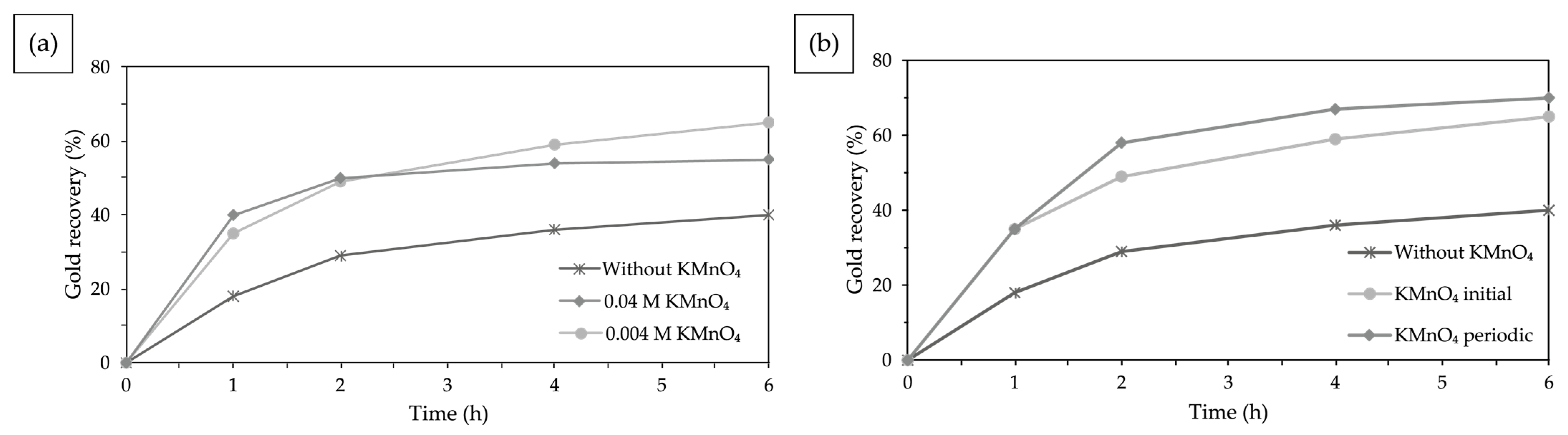
3.4.5. Effect of Oxidizing Agents: Potassium Permanganate
Effect of Potassium Permanganate Concentration
Effect of Periodic Addition of Potassium Permanganate
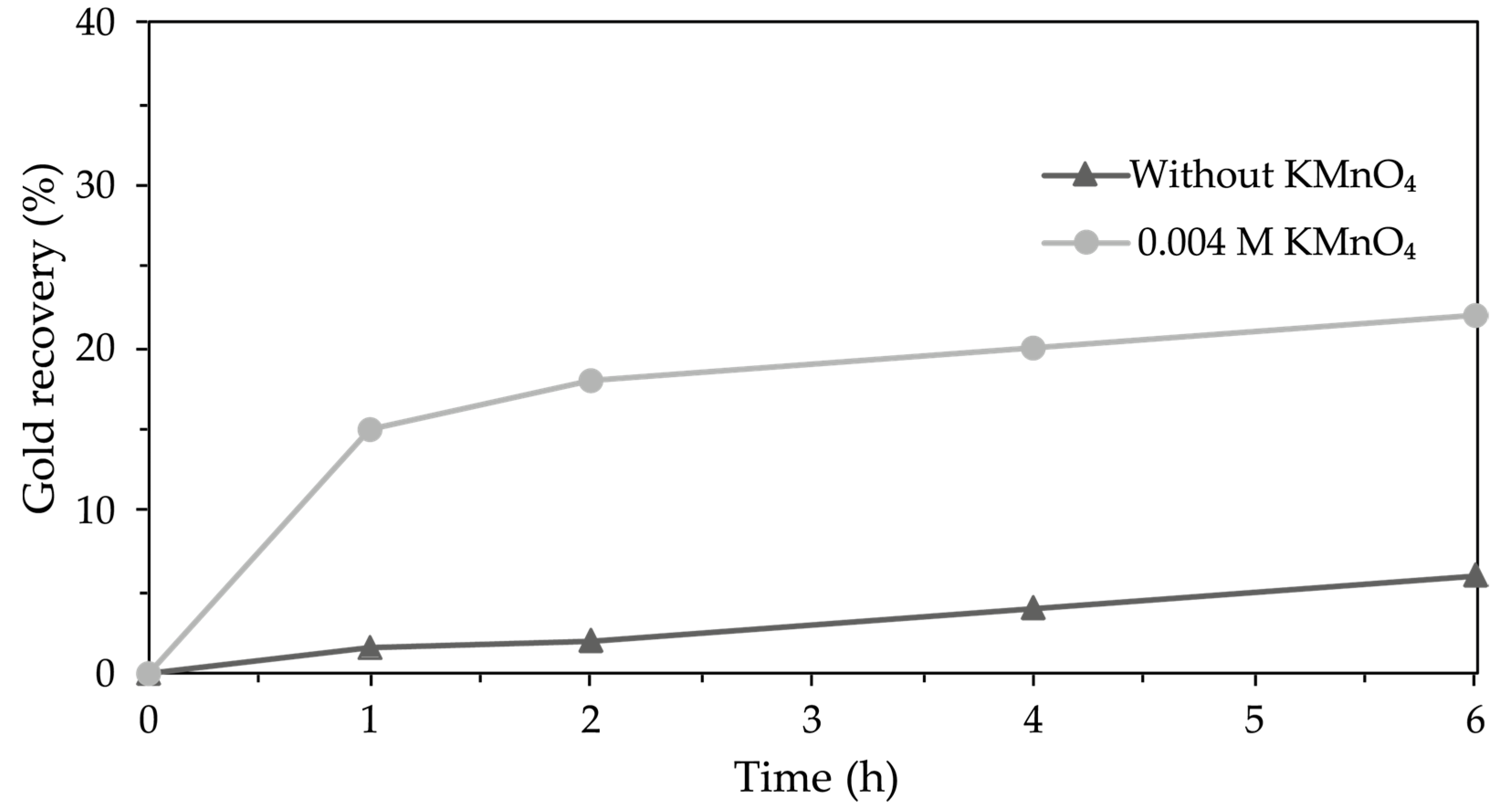
3.4.6. Synergy of Conditions for Gold Recovery with a Thiosulfate–Glycine–Copper-Permanganate System
4. Discussion
4.1. Characterization of Auriferous Ore
4.2. Gold Recovery by Cyanidation Leaching
4.3. Ammoniacal Thiosulfate-Copper Leaching System
Evaluation of Leaching Conditions for Gold Recovery Using the Ammoniacal Thiosulfate System
4.4. Thiosulfate–Glycine–Copper-Leaching System
4.4.1. Effect of Sodium Thiosulfate Concentration
4.4.2. Effect of Copper Ion Concentration
4.4.3. Effect of Glycine Concentration
4.4.4. Effect of pH on Thiosulfate–Glycine System
4.4.5. Effect of Oxidizing Agents: Potassium Permanganate
Effect of Potassium Permanganate Concentration
Effect of Periodic Addition of Potassium Permanganate
4.4.6. Synergy of Conditions for Gold Recovery with a Thiosulfate–Glycine–Copper–Permanganate System
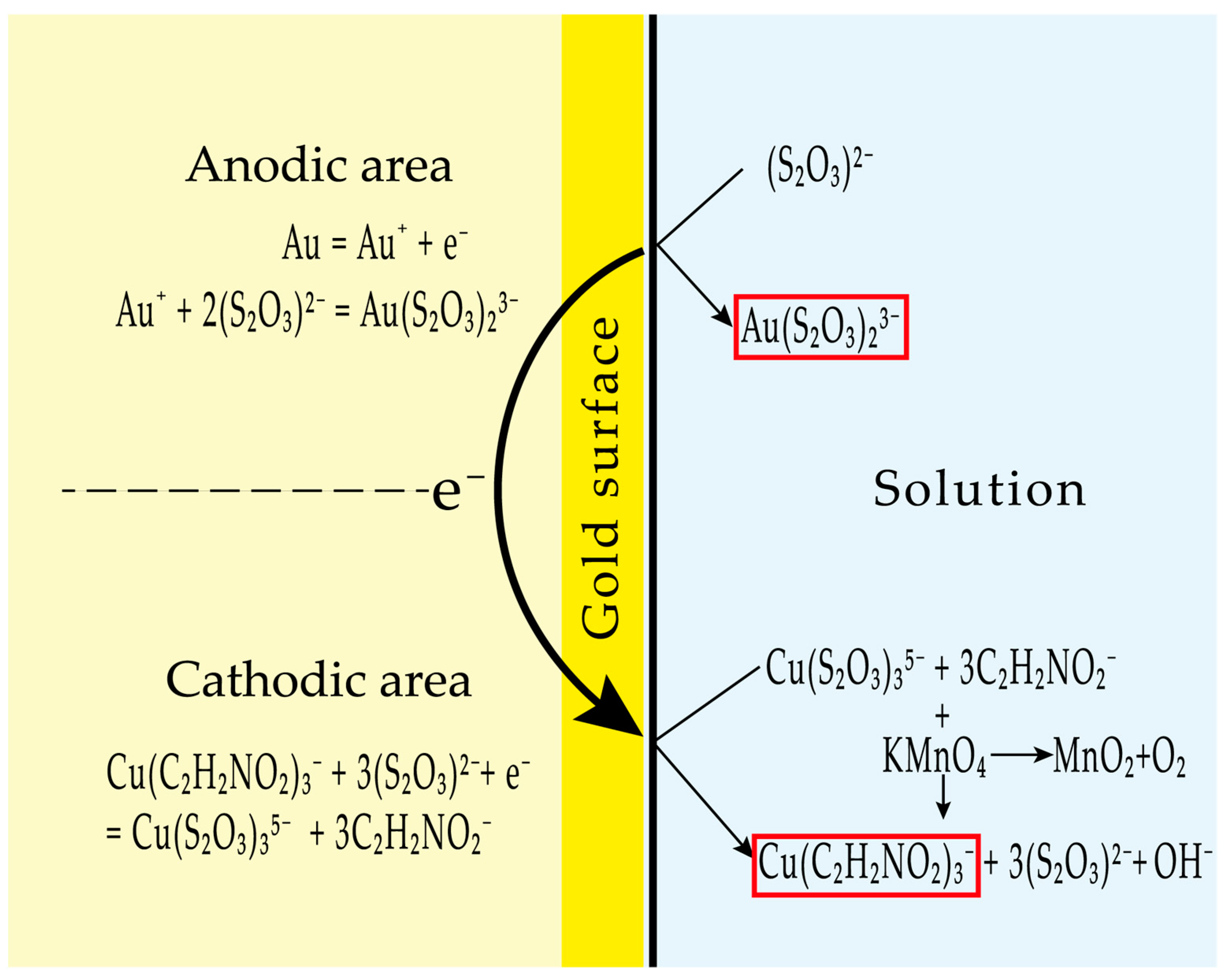
4.4.7. Mechanism of Gold Dissolution in the Thiosulfate–Glycine–Copper–Permanganate System
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Altinkaya, P.; Wang, Z.; Korolev, I.; Hamuyuni, J.; Haapalainen, M.; Kolehmainen, E.; Yliniemi, K.; Lundström, M. Leaching and recovery of gold from ore in cyanide-free glycine media. Miner. Eng. 2020, 158, 106610. [Google Scholar] [CrossRef]
- Barani, K.; Dehghani, M.; Azadi, M.R.; Karrech, A. Leaching of a polymetal gold ore and reducing cyanide consumption using cyanide-glycine solutions. Miner. Eng. 2021, 163, 106802. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, X.; Zi, F.; Yang, P.; Chen, Y.; Chen, S. Environmentally friendly extraction of gold from refractory concentrate using a copper—Ethylenediamine—Thiosulfate solution. J. Clean. Prod. 2019, 214, 860–872. [Google Scholar] [CrossRef]
- Wu, H.; Feng, Y.; Huang, W.; Li, H.; Liao, S. The role of glycine in the ammonium thiocyanate leaching of gold. Hydrometallurgy 2019, 185, 111–116. [Google Scholar] [CrossRef]
- Xie, F.; Chen, J.; Wang, J.; Wang, W. Review of gold leaching in thiosulfate-based solutions. Trans. Nonferr. Met. Soc. China 2021, 31, 3506–3529. [Google Scholar] [CrossRef]
- Ospina-Correa, J.D.; Mejía-Restrepo, E.; Serna-Zuluaga, C.M.; Posada-Montoya, A.; Osorio-Cachaya, J.G.; Ta-mayo-Sepúlveda, J.A.; Calderón-Gutiérrez, J.A. Process mineralogy of refractory gold ore in thiosulfate solutions. Hydrometallurgy 2018, 182, 104–113. [Google Scholar] [CrossRef]
- Gámez, S.; Garcés, K.; de la Torre, E.; Guevara, A. Precious metals recovery from waste printed circuit boards using thiosulfate leaching and ion exchange resin. Hydrometallurgy 2019, 186, 1–11. [Google Scholar] [CrossRef]
- Wu, J.; Ahn, J.; Lee, J. Characterization of gold deportment and thiosulfate extraction for a copper gold concentrate treated by pressure oxidation. Hydrometallurgy 2022, 207, 105771. [Google Scholar] [CrossRef]
- Celep, O.; Altinkaya, P.; Yazici, E.Y.; Deveci, H. Thiosulphate leaching of silver from an arsenical refractory ore. Miner. Eng. 2018, 122, 285–295. [Google Scholar] [CrossRef]
- Sitando, O.; Dai, X.; Senanayake, G.; Nikoloski, A.N.; Breuer, P. A fundamental study of gold leaching in a thiosulfate oxygen copper system in the presence of activated carbon. Hydrometallurgy 2020, 192, 105232. [Google Scholar] [CrossRef]
- Barani, K.; Kogani, Y.; Nazarian, F. Leaching of complex gold ore using a cyanide-glycine solution. Miner. Eng. 2022, 180, 107475. [Google Scholar] [CrossRef]
- Wang, J.; Xie, F.; Pan, Y.; Wang, W. Leaching of Gold with Copper-citrate-thiosulfate Solutions. Miner. Process. Extr. Metall. Rev. 2022, 43, 916–925. [Google Scholar] [CrossRef]
- Gámez, S.A.; De la Torre, E. Recuperación de oro a partir de minerales polisulfurados con soluciones amoniacales de tiosulfato de sodio. Rev. Politéc. 2015, 36, 42. [Google Scholar]
- Escobar-Ledesma, F.R.; Aragón-Tobar, C.F.; Espinoza-Montero, P.J.; De La Torre-Chauvin, E. In-creased recovery of gold thiosulfate alkaline solutions by adding thiol groups in the porous structure of activated carbon. Molecules 2020, 25, 2902. [Google Scholar] [CrossRef]
- Olvera, O.G.; Domanski, D.F.R. Effect of activated carbon on the thiosulfate leaching of gold. Hydrometallurgy 2019, 188, 47–53. [Google Scholar] [CrossRef]
- Sitando, O.; Senanayake, G.; Dai, X.; Breuer, P. The adsorption of gold(I) on minerals and activated carbon (preg-robbing) in non-ammoniacal thiosulfate solutions—Effect of calcium thiosulfate, silver(I), copper(I) and polythionate ions. Hydrometallurgy 2019, 184, 206–217. [Google Scholar] [CrossRef]
- Chen, Y.; Zi, F.; Hu, X.; Yang, P.; Ma, Y.; Cheng, H.; Wang, Q.; Qin, X.; Liu, Y.; Chen, S.; et al. The use of new modified activated carbon in thiosulfate solution: A green gold recovery technology. Sep. Purif. Technol. 2020, 230, 115834. [Google Scholar] [CrossRef]
- He, P.; Hu, X.; Zi, F.; Wang, C. A Method of Recovery Au(I) from Thiosulfate Solution by Novel Functionalised Activated Carbon. Russ. J. Non-Ferr. Met. 2022, 63, 46–56. [Google Scholar] [CrossRef]
- Yu, H.; Zi, F.; Hu, X.; Nie, Y.; Chen, Y.; Cheng, H. Adsorption of gold from thiosulfate solutions with chemically modified activated carbon. Adsorpt. Sci. Technol. 2017, 36, 408–428. [Google Scholar] [CrossRef]
- Chen, Y.; Zi, F.; Hu, X.; Yu, H.; Nie, Y.; Yang, P.; Cheng, H.; Wang, Q.; Qin, X.; Chen, S.; et al. Grafting of organic sulfur-containing functional groups on activated carbon for gold(I) adsorption from thiosulfate solution. Hydrometallurgy 2019, 185, 102–110. [Google Scholar] [CrossRef]
- Muir, D.M.; Aylmore, M.G. Thiosulphate as an Alternative to Cyanide for Gold Processing—Issues and Impediments. In Proceedings of the Green Processing 2002—Proceedings: International Conference on the Sustainable Proceesing of Minerals, Cairns, QLD, Australia, 29–31 May 2002; pp. 125–133. Available online: https://www.scopus.com/record/display.uri?eid=2-s2.0-2342642835&origin=resultslist&sort=plf-f&src=s&sot=b&sdt=b&s=TITLE-ABS-KEY%28Thiosulphate+as+an+Alternative+to+Cyanide+for+Gold+Processing+-+Issues+and+Impediments%29&sessionSearchId=ec33dcdea3835817912a4605780cdc08&relpos=1 (accessed on 1 October 2024).
- Oraby, E.A.; Eksteen, J.J.; Tanda, B.C. Gold and copper leaching from gold-copper ores and concentrates using a synergistic lixiviant mixture of glycine and cyanide. Hydrometallurgy 2017, 169, 339–345. [Google Scholar] [CrossRef]
- Tauetsile, P.J.; Oraby, E.A.; Eksteen, J.J. Activated carbon adsorption of gold from cyanide-starved glycine solutions containing copper. Part 1: Isotherms. Sep. Purif. Technol. 2019, 211, 594–601. [Google Scholar] [CrossRef]
- Eksteen, J.J.; Oraby, E.A. The leaching and adsorption of gold using low concentration amino acids and hydrogen peroxide: Effect of catalytic ions, sulphide minerals and amino acid type. Miner. Eng. 2015, 70, 36–42. [Google Scholar] [CrossRef]
- Li, H.; Deng, Z.; Oraby, E.; Eksteen, J. Amino acids as lixiviants for metals extraction from natural and secondary resources with emphasis on glycine: A literature review. Hydrometallurgy 2023, 216, 106008. [Google Scholar] [CrossRef]
- Feng, D.; van Deventer, J.S.J. The role of amino acids in the thiosulphate leaching of gold. Miner. Eng. 2011, 24, 1022–1024. [Google Scholar] [CrossRef]
- Wang, J.; Wang, R.; Pan, Y.; Liu, F.; Xu, Z. Thermodynamic analysis of gold leaching by copper-glycine-thiosulfate solutions using Eh-pH and species distribution diagrams. Miner. Eng. 2022, 179, 107438. [Google Scholar] [CrossRef]
- Li, J.; Kou, J.; Sun, C.; Zhang, N.; Zhang, H. A review of environmentally friendly gold lixiviants: Fundamentals, applications, and commonalities. Miner. Eng. 2023, 197, 108074. [Google Scholar] [CrossRef]
- Oraby, E.A.; Eksteen, J.J. The leaching of gold, silver and their alloys in alkaline glycine–peroxide solutions and their adsorption on carbon. Hydrometallurgy 2015, 152, 199–203. [Google Scholar] [CrossRef]
- Feng, D.; van Deventer, J.S.J. Thiosulphate leaching of gold in the presence of carboxymethyl cellulose (CMC). Miner. Eng. 2011, 24, 115–121. [Google Scholar] [CrossRef]
- Senanayake, G. Gold leaching by thiosulphate solutions: A critical review on copper(II)-thiosulphate-oxygen interactions. Miner. Eng. 2005, 18, 995–1009. [Google Scholar] [CrossRef]
- Zhang, X.M.; Senanayake, G. A Review of Ammoniacal Thiosulfate Leaching of Gold: An Update Useful for Further Research in Non-cyanide Gold Lixiviants. Miner. Process. Extr. Metall. Rev. 2016, 37, 385–411. [Google Scholar] [CrossRef]
- Oraby, E.A.; Eksteen, J.J.; O’Connor, G.M. Gold leaching from oxide ores in alkaline glycine solutions in the presence of permanganate. Hydrometallurgy 2020, 198, 105527. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Q.; Liu, X.; Jiang, T. A thermodynamic analysis on thiosulfate leaching of gold under the catalysis of Fe3+/Fe2+ complexes. Miner. Eng. 2022, 180, 107511. [Google Scholar] [CrossRef]
- Oraby, E.A.; Eksteen, J.J. The selective leaching of copper from a gold–copper concentrate in glycine solutions. Hydrometallurgy 2014, 150, 14–19. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, M.; Wang, J.; Liu, X.; Lyu, X. A review of gold extraction using alternatives to cyanide: Focus on current status and future prospects of the novel eco-friendly synthetic gold lixiviants. Miner. Eng. 2022, 176, 107336. [Google Scholar] [CrossRef]
- Liedberg, B.; Lundström, I.; Wu, C.R.; Salaneck, W.R. Adsorption of glycine on hydrophilic gold. J. Colloid Interface Sci. 1985, 108, 123–132. [Google Scholar] [CrossRef]
- Aylmore, M.G.; Muir, D.M. Thiosulfate leaching of gold—A review. Miner. Eng. 2001, 14, 135–174. [Google Scholar] [CrossRef]
- Grosse, A.; Dicinoski, G.; Shaw, M.; Haddad, P. Leaching and recovery of gold using ammoniacal thiosulfate leach liquors (a review). Hydrometallurgy 2003, 69, 1–21. [Google Scholar] [CrossRef]
- Senanayake, G. Review of rate constants for thiosulphate leaching of gold from ores, concentrates and flat surfaces: Effect of host minerals and pH. Miner. Eng. 2007, 20, 1–15. [Google Scholar] [CrossRef]
- Xu, B.; Kong, W.; Li, Q.; Yang, Y.; Jiang, T.; Liu, X. A review of thiosulfate leaching of gold: Focus on thiosulfate consumption and gold recovery from pregnant solution. Metals 2017, 7, 222. [Google Scholar] [CrossRef]
- Xing, H.; Peng, M.; Li, Z.; Chen, J.; Zhang, H.; Teng, X. Ammonia inhalation-mediated mir-202-5p leads to cardiac autophagy through PTEN/AKT/mTOR pathway. Chemosphere 2019, 235, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Khezri, M.; Rezai, B.; Akbar Abdollahzadeh, A.; Molaeinasab, M.; Wilson, B.P.; Lundström, M. Glycine leaching of Sarcheshmeh chalcopyrite concentrate at high pulp densities in a stirred tank reactor. Miner. Eng. 2020, 157, 106555. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, X.; Tian, Q.; Zhang, L. A systematic review of gold extraction: Fundamentals, advancements, and challenges toward alternative lixiviants. J. Hazard. Mater. 2022, 440, 129778. [Google Scholar] [CrossRef]
- Senanayake, G. Gold leaching by copper(II) in ammoniacal thiosulphate solutions in the presence of additives. Part I: A review of the effect of hard-soft and Lewis acid-base properties and interactions of ions. Hydrometallurgy 2012, 115–116, 1–20. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, X.; Kou, J.; Xing, Y. A review of gold extraction using noncyanide lixiviants: Fundamentals, advancements, and challenges toward alkaline sulfur-containing leaching agents. Int. J. Miner. Metall. Mater. 2020, 27, 417–431. [Google Scholar] [CrossRef]
- Ferreira, C.M.H.; Pinto, I.S.S.; Soares, E.V.; Soares, H.M.V.M. (Un) suitability of the use of pH buffers in biological, biochemical and environmental studies and their interaction with metal ions—A review. RSC Adv. 2015, 5, 30989–31003. [Google Scholar] [CrossRef]
- Knight, J.; Assimos, D.G.; Callahan, M.F.; Holmes, R.P. Metabolism of primed, constant infusions of [1,2-13C2] glycine and [1-13C1] phenylalanine to urinary oxalate. Metabolism 2011, 60, 950–956. [Google Scholar] [CrossRef]
- Yoo, K.; Kim, H. Development of Ammoniacal leaching processes; a review. Resour. Recycl. 2012, 21, 3–17. [Google Scholar] [CrossRef]
- Sitando, O.; Senanayake, G.; Dai, X.; Nikoloski, A.N.; Breuer, P. A review of factors affecting gold leaching in non-ammoniacal thiosulfate solutions including degradation and in-situ generation of thiosulfate. Hydrometallurgy 2018, 178, 151–175. [Google Scholar] [CrossRef]

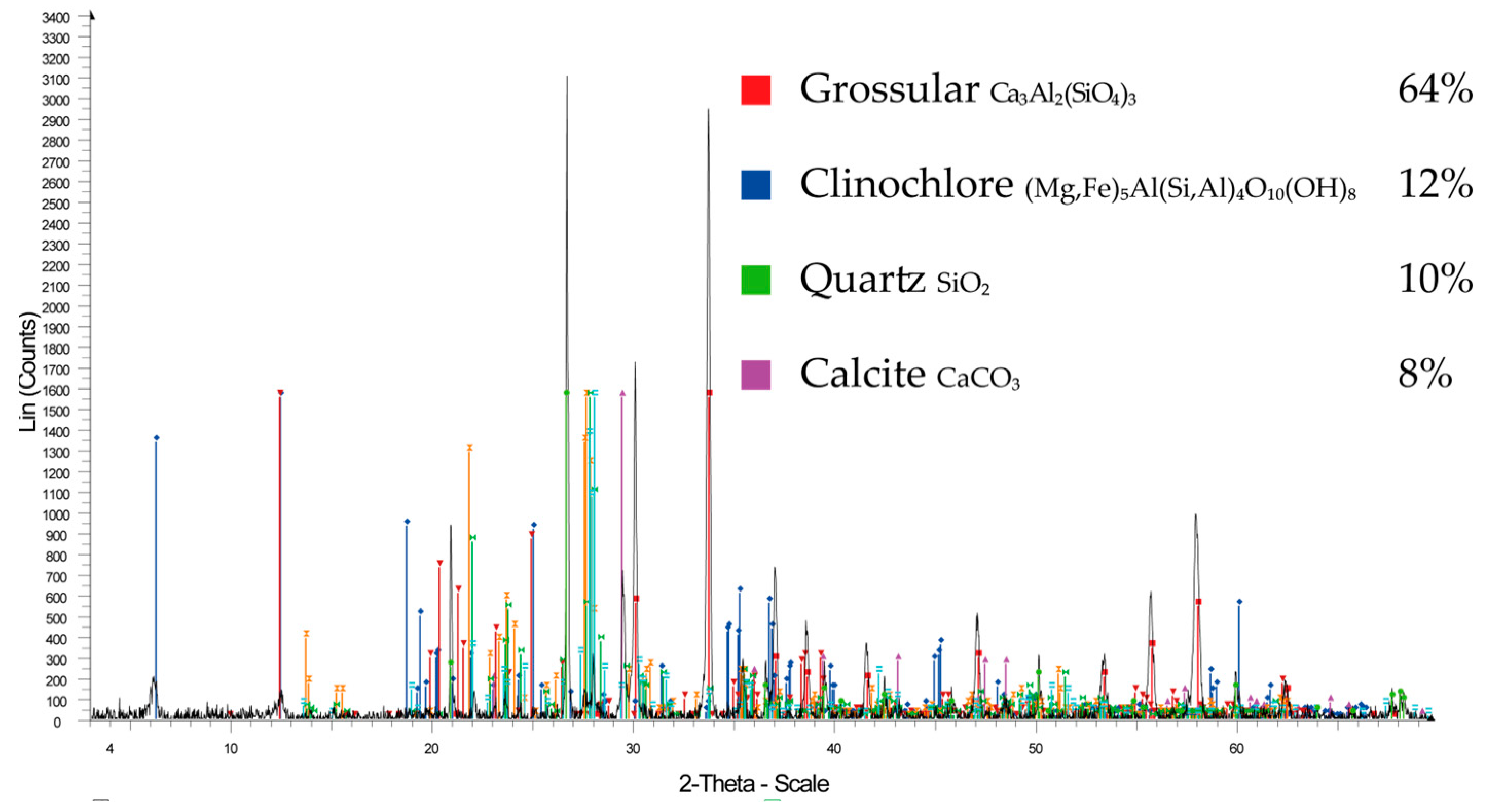


| Mineral | Formula | Content (%) |
|---|---|---|
| Grossular | Ca3Al2(SiO4)3 | 64 |
| Clinochlore | (Mg, Fe)5Al (Si, Al)4O10 (OH)8 | 12 |
| Quartz | SiO2 | 10 |
| Calcite | CaCO3 | 8 |
| Kaolinite | Al2(Si2O5) (OH)4 | 4 |
| Plagioclases | (Na, Ca) Al (Si, Al) Si2O8 | 2 |
| Element | Concentration |
|---|---|
| Au | 10 g t−1 |
| Cu | 0.008% |
| Si | 18.8% |
| Ca | 15.1% |
| Fe | 5% |
| Al | 4.5% |
| Mg | 1.6% |
| Mn | 0.9% |
| K | 0.8% |
| Na | 0.4% |
| S | 0.1% |
| Sodium Thiosulfate Concentration | Gold Recovery (%) |
|---|---|
| 0.5 M | 33.7 |
| 0.7 M | 40.8 |
| 1 M | 49.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Redrovan, A.S.; Torre, E.d.l.; Aragón-Tobar, C.F. Gold Leaching from an Auriferous Ore by Alkaline Thiosulfate–Glycine–Copper Solution. Metals 2025, 15, 204. https://doi.org/10.3390/met15020204
Redrovan AS, Torre Edl, Aragón-Tobar CF. Gold Leaching from an Auriferous Ore by Alkaline Thiosulfate–Glycine–Copper Solution. Metals. 2025; 15(2):204. https://doi.org/10.3390/met15020204
Chicago/Turabian StyleRedrovan, Alex S., Ernesto de la Torre, and Carlos F. Aragón-Tobar. 2025. "Gold Leaching from an Auriferous Ore by Alkaline Thiosulfate–Glycine–Copper Solution" Metals 15, no. 2: 204. https://doi.org/10.3390/met15020204
APA StyleRedrovan, A. S., Torre, E. d. l., & Aragón-Tobar, C. F. (2025). Gold Leaching from an Auriferous Ore by Alkaline Thiosulfate–Glycine–Copper Solution. Metals, 15(2), 204. https://doi.org/10.3390/met15020204







