Feasibility Exploration and Research Examples of On-Site Metallographic Inspection Methods in the Analysis of Bronze Artifacts—A Case Study of Ming Jiajing Bronze Lions and the Shang Bronze Tripod Vessel with Cicada Designs
Abstract
:1. Introduction
2. Background
2.1. Fundamentals of Classical Metallography Theory
2.2. The Advantages and Disadvantages of Laboratory Metallographic Inspection Methods
2.3. Theoretical Basis of On-Site Metallographic Inspection Technique
- (1)
- Metallographic etching reaction.
- (2)
- Hydrogen peroxide oxidation sealing method.
3. Materials and Methods
3.1. Materials
3.2. Experimental Instruments and Methods
3.2.1. On-Site Metallographic Inspection
3.2.2. X-Ray Fluorescence Spectrometer (XRF)
3.2.3. Scanning Electron Microscopy and Energy Dispersive Spectroscopy Analysis
3.3. Verify Simulation Experiment
3.4. The Specific Operation of On-Site Metallographic Inspection Technique
3.5. Examples of On-Site Metallographic Inspection
3.5.1. The Ming Jiajing Bronze Lions Inlaid with Coins
Summary
3.5.2. The Shang Bronze Tripod Vessel with Cicada Designs
Summary
4. Conclusions
- (1)
- The coins inlaid on the Ming Jiajing Bronze Lions are the actual currency of the time, “Jiajing Tongbao (嘉靖通宝)”, using the casting-inlay method on the surface of the lions.
- (2)
- The Shang Bronze Tripod Vessel with Cicada Designs has two legs that were restored and supplemented historically. After the partial restoration of the feet, the Bronze Tripod Vessel was put back into use. After the full restoration of the feet, the Bronze Tripod Vessel was never heated and used again.
- (3)
- During the study of partial craftsmanship of large bronzeware, the on-site metallographic inspection method can observe the local metallographic structure of the object by only damaging a very small area of the surface oxide layer and accurately indicate the direction of heating and other directional structural details at the observation location.
- (4)
- When facing bronzeware that has been processed multiple times overall, the on-site metallographic inspection method can flexibly explore the craftsmanship differences of each part, and the damage to the object is minimal, making it applicable to complete objects that are not suitable for sampling.
5. Patents
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xie, C.; Fu, C.; Li, S.; Liao, L.; Chen, G.; Pan, C. A Special Ancient Bronze Sword and Its Possible Manufacturing Technique from Materials Science Analysis. Materials 2022, 15, 2491. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, X.; Chen, Z.; Zhang, S.; Niu, Q.; Zhao, X. Study on the Bronze Weapons Excavated from Xichuan, China. Metals 2024, 14, 395. [Google Scholar] [CrossRef]
- Aiestaran, M.; Velaza, J.; Gorrochategui, J.; Usúa, C.; Pujol, P.; Alonso, E.; Iriarte, E.; Narbarte, J.; Ruiz-González, D.; Mendizabal-Sandonís, O.; et al. A Vasconic inscription on a bronze hand: Writing and rituality in the Iron Age Irulegi settlement in the Ebro Valley. Antiquity 2024, 98, 66–84. [Google Scholar] [CrossRef]
- Chen, J.; Chen, L.; Zhang, L.; Cui, X.; Liu, X. Analysis of Mineralization and Disease Characteristics of Bronzes Unearthed in Archaeology. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2024. [Google Scholar] [CrossRef]
- Liu, X.; Xu, D.; Lin, J.; Zhang, L.; Zeng, Y.; Chen, Q. Failure analysis and factors influencing spline wear in hydraulic motors of charging pumps in nuclear power plants. Eng. Fail. Anal. 2023, 156, 107780. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, J.Q.; Ma, P.Z.; Zhang, X.H. Effect of welding thermal cycle on microstructural evolution of Al–Zn–Mg–Cu alloy. Mater. Sci. Eng. A 2018, 717, 85–94. [Google Scholar] [CrossRef]
- Beisenov, A.Z.; Panichkin, A.V.; Shashenov, D.T. Iron Axe Head from the Tasmola Culture Barrow in the Qyzylzhartas Burial Ground: Results of Metallographic and Chemical Analysis. MAIASP 2023, 15, 102–120. (In Russian) [Google Scholar] [CrossRef]
- Callister, W.D., Jr.; Rethwisch, D.G. Materials Science and Engineering: An Introduction; Wiley: Hoboken, NJ, USA, 2018; pp. 110–120. [Google Scholar]
- Greene, N.D.; Rudaw, P.S.; Lee, L. Principles of metallographic etching. Corros. Sci. 1966, 6, 371–379. [Google Scholar] [CrossRef]
- William, F.; Smith, J.H. Foundations of Materials Science and Engineering; McGraw Hill LLC: New York, NY, USA, 2023; pp. 191–206. [Google Scholar]
- Samberger, S.; Kremmer, T.; Stemper, L.; Tourey, S.; Uggowitzer, P.J.; Pogatscher, S. Metallographic Etching of Al–Mg–Zn–(Cu) Crossover Alloys. Adv. Eng. Mater. 2024, 26, 2400576. [Google Scholar] [CrossRef]
- Lu, Z.; Qian, L.; Tianliang, Z. Corrosion Behavior and Sealing Technologies of Bronze. J. Chin. Soc. Corros. Prot. 2023, 43, 1165–1177. (In Chinese) [Google Scholar]
- Robbiola, L.; Blengino, J.M.; Fiaud, C. Morphology and mechanisms of formation of natural patinas on archaeological Cu–Sn alloys. Corros. Sci. 1998, 40, 2083–2111. [Google Scholar] [CrossRef]
- Kwon, H. Corrosion Behaviors of Artificial Chloride Patina for Studying Bronze Sculpture Corrosion in Marine Environments. Coatings 2023, 13, 1630. [Google Scholar] [CrossRef]
- Song, Z.; Tegus, O. Microstructure and Chlorine Ion Corrosion Performance in Bronze Earring Relics. Materials 2024, 17, 1734. [Google Scholar] [CrossRef]
- Maurya, R.C. Inorganic Chemistry; De Gruyter: Berlin, Germany, 2021; pp. 124–132. [Google Scholar]
- Colomban, P.; Tournié, A.; Maucuer, M.; Meynard, P. On-site Raman and XRF analysis of Japanese/Chinese bronze/brass patina—The search for specific Raman signatures. J. Raman Spectrosc. 2012, 43, 799–808. [Google Scholar] [CrossRef]
- Porcu, D.; Innocenti, S.; Galeotti, M.; Striova, J.; Dei, L.; Carretti, E.; Fontana, R. Spectroscopic and Morphologic Investigation of Bronze Disease: Performance Evaluation of Portable Devices. Heritage 2022, 5, 3548–3561. [Google Scholar] [CrossRef]
- Radivojević, M.; Pendić, J.; Srejić, A.; Korać, M.; Davey, C.; Benzonelli, A.; Martinón-Torres, M.; Jovanović, N.; Kamberović, Ž. Experimental design of the Cu-As-Sn ternary colour diagram. J. Archaeol. Sci. 2017, 90, 106–119. [Google Scholar] [CrossRef]
- Letardi, P.; Salvadori, B.; Galeotti, M.; Cagnini, A.; Porcinai, S.; Santagostino Barbone, A.; Sansonetti, A. An in situ multi-analytical approach in the restoration of bronze artefacts. Microchem. J. 2016, 125, 151–158. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, C.; Guo, Y. Corrosion and Protection of Chinese Bronze Relics: A Review. Coatings 2024, 14, 1196. [Google Scholar] [CrossRef]
- Li, Z. The Imperial Qing-court Copperware Manufacture in The Yongzheng and Qianlong Periods. Palace Mus. J. 2013, 94–133+163. (In Chinese) [Google Scholar] [CrossRef]
- Juan, H. An Analysis of the Cask Pattern of the Han Bronze Mirror. J. Natl. Mus. China 2019, 5, 40–51. (In Chinese) [Google Scholar]
- Xinnan, H.; Guojun, S. Seigniorage Reform in Ming Dynasty as Seen from Alloy Composition. J. Chifeng Univ. Philos. Soc. Sci. Chin. Ed. 2024, 45, 24–30. (In Chinese) [Google Scholar] [CrossRef]
- Filippov, A.; Shamarin, N.; Moskvichev, E.; Savchenko, N.; Kolubaev, E.; Khoroshko, E.; Tarasov, S. The Effect of Heat Input, Annealing, and Deformation Treatment on Structure and Mechanical Properties of Electron Beam Additive Manufactured (EBAM) Silicon Bronze. Materials 2022, 15, 3209. [Google Scholar] [CrossRef] [PubMed]
- Baige, L.; Jing, T. Analysis of Bronze Wares with Copper-inlay Decoration in Mianzhu Museum. Sichuan Cult. Relics 2023, 1, 107–114. (In Chinese) [Google Scholar]
- Cong, W.; Zhenlong, G.; Qingzhu, W.; Jun, G.; Quanyu, W. The diversity of bronze production technologies during the Eastern Zhou dynasty revealed by analysis of slags from the Baidian and Xincun sites in Central China. Herit. Sci. 2024, 12, 333. [Google Scholar] [CrossRef]
- Guoshuo, Z.; Jian, Z. A Preliminary Exploration into the Using System of Ding-Tripod in Shang Dynasty. Huaxia Archaeol. 2018, 45–53+105. (In Chinese) [Google Scholar] [CrossRef]
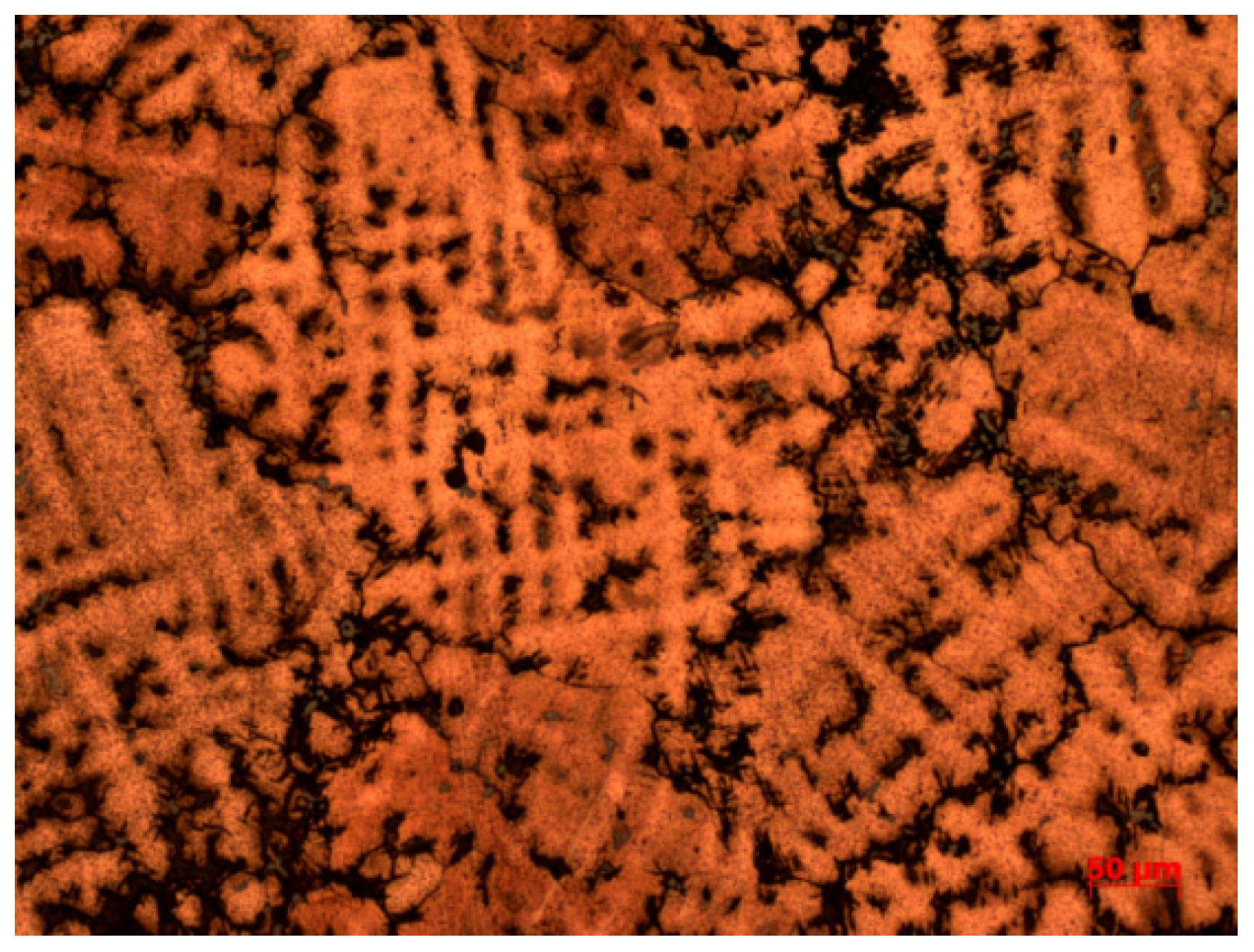


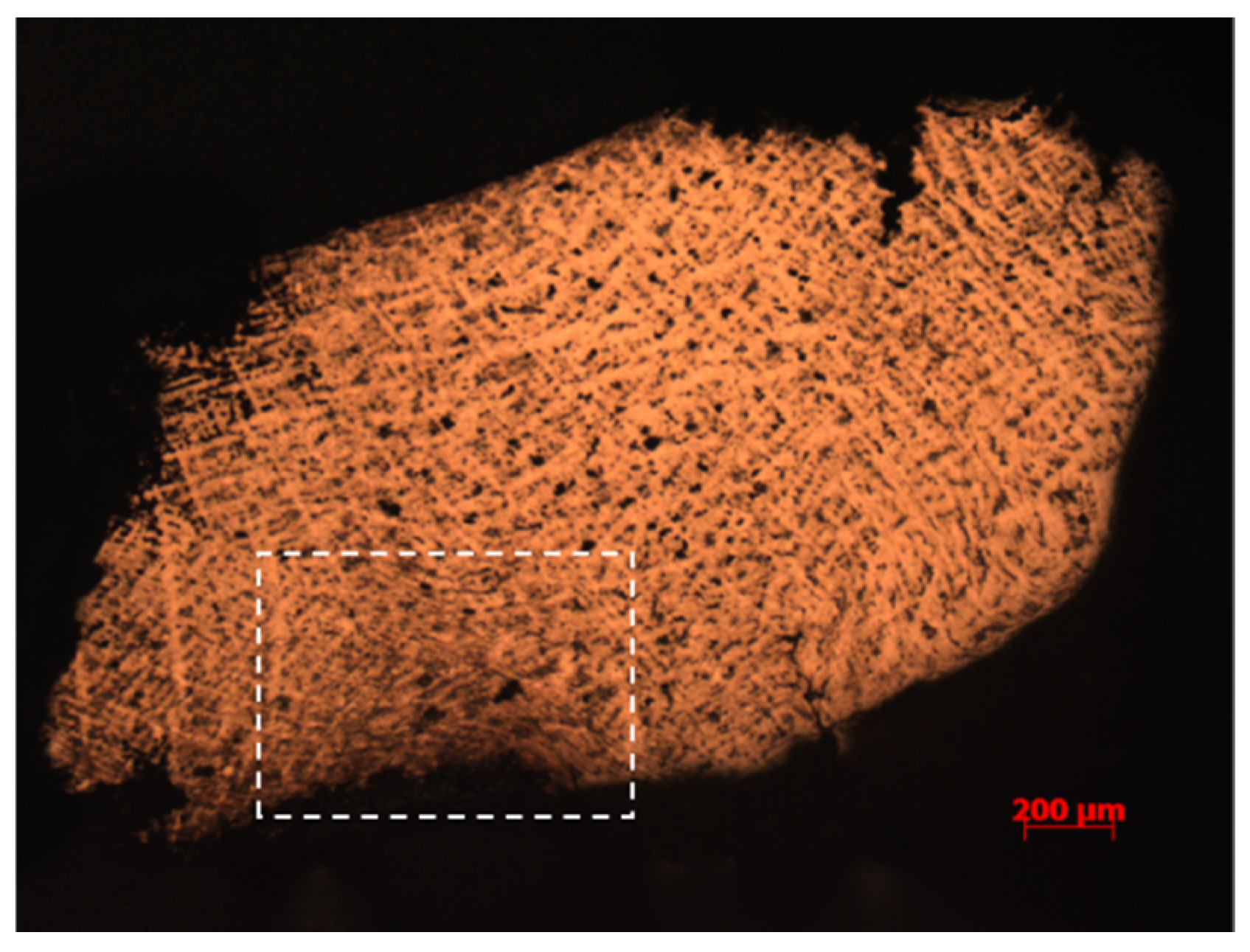


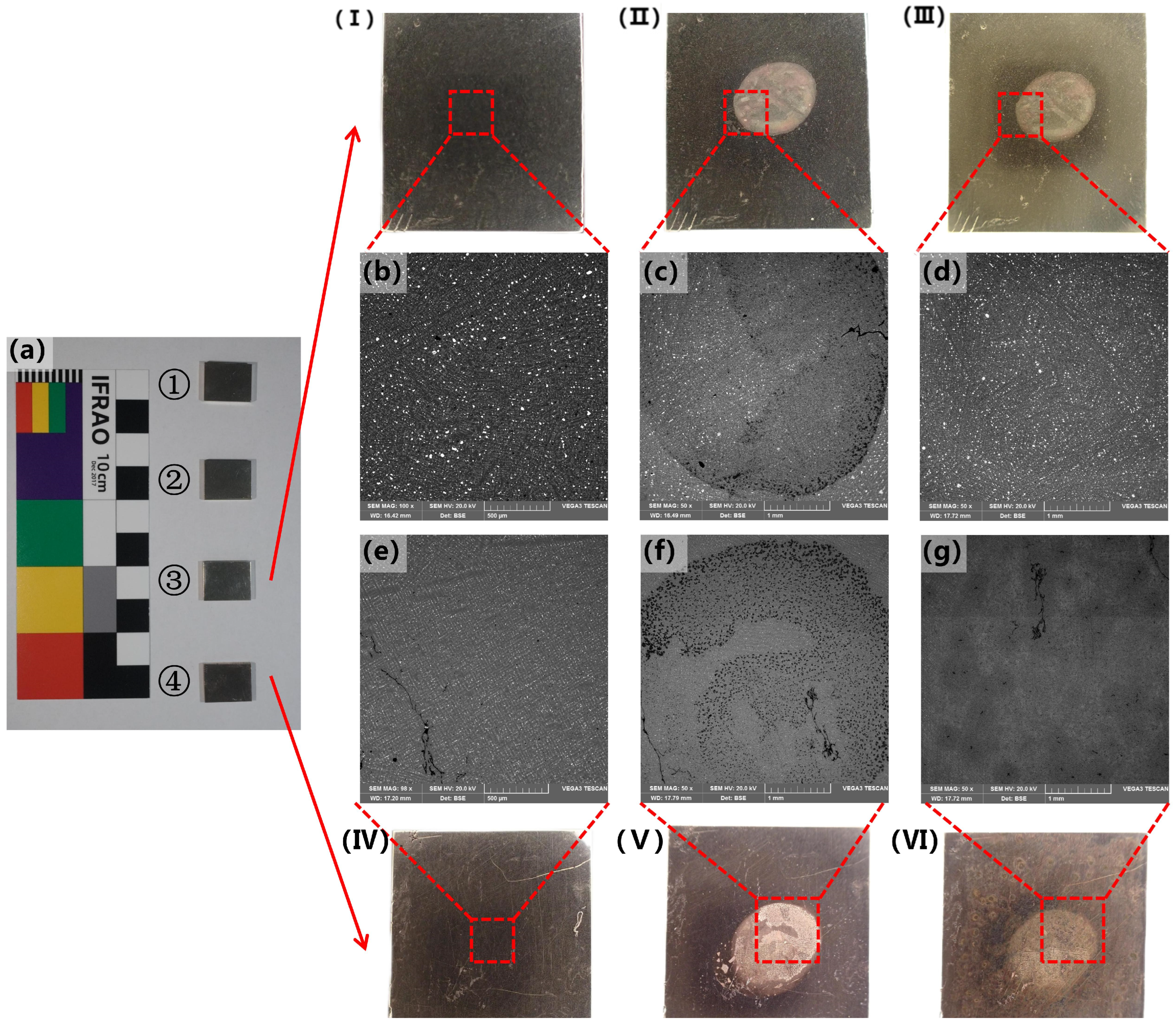




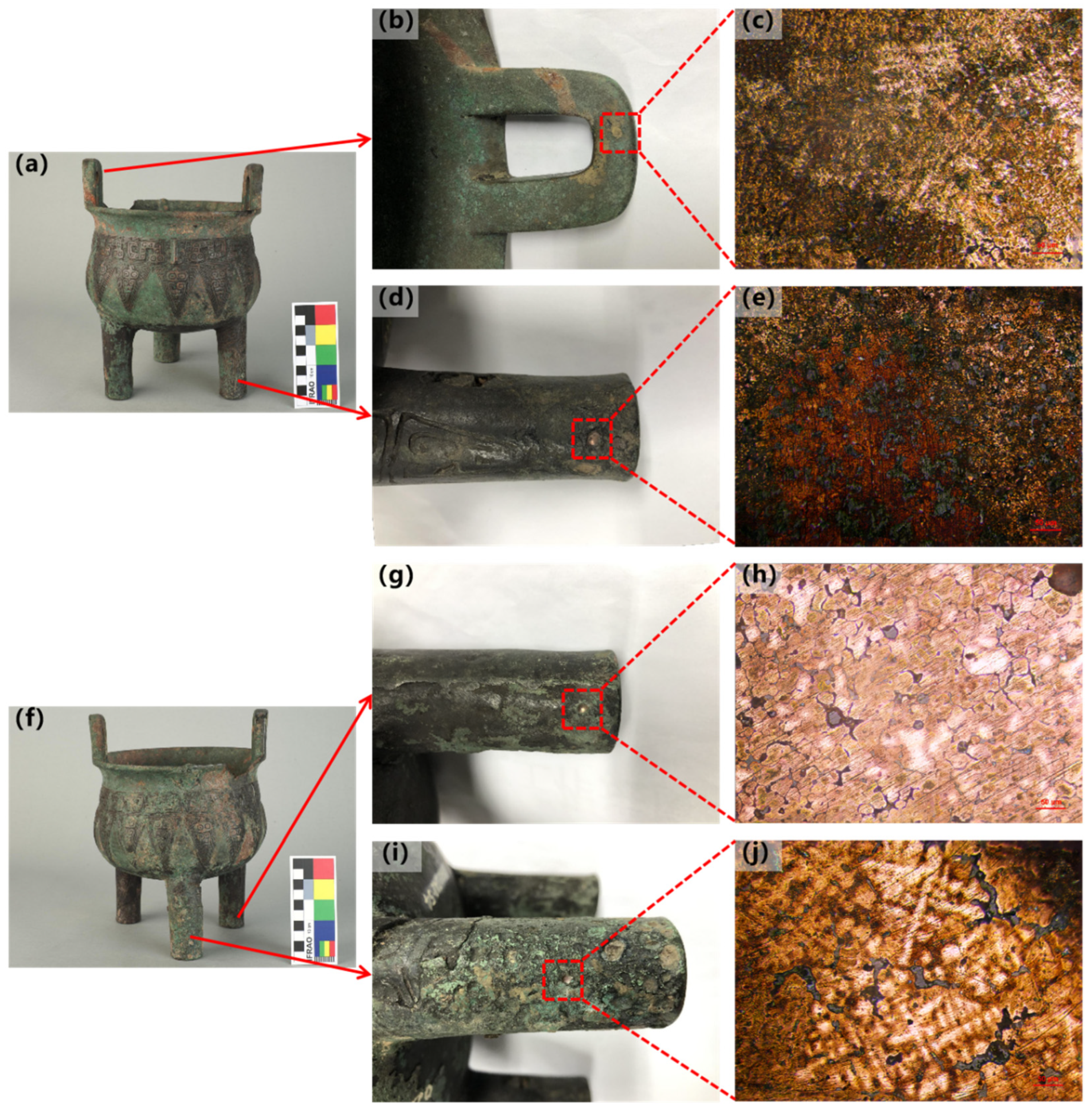
| No. | Sample No. | Method of Processing | Main Element Content (Wt%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cl | Fe | Cu | Sn | Pb | O | Other | Summation | |||
| 1.1 | ① | - | - | 0.05 ± 0.08 | 77.45 ± 0.40 | 14.10 ± 0.23 | 7.41 ± 0.33 | 0.75 ± 0.19 | 0.23 | 100.00 |
| 1.2 | ① | etching | 3.57 ± 0.08 | 1.45 ± 0.10 | 72.21 ± 0.41 | 13.19 ± 0.22 | 5.01 ± 0.31 | 4.37 ± 0.26 | 0.20 | 100.00 |
| 1.3 | ① | C2H6O + H2O2 | - | - | 70.37 ± 0.42 | 12.26 ± 0.22 | 10.82 ± 0.32 | 6.16 ± 0.22 | 0.39 | 100.00 |
| 2.1 | ② | - | - | 0.06 ± 0.09 | 71.19 ± 0.39 | 19.65 ± 0.25 | 7.91 ± 0.32 | 1.05 ± 0.21 | 0.14 | 100.00 |
| 2.2 | ② | etching | 3.70 ± 0.08 | 1.54 ± 0.10 | 67.43 ± 0.39 | 18.89 ± 0.25 | 5.69 ± 0.32 | 2.03 ± 0.22 | 0.72 | 100.00 |
| 2.3 | ② | C2H6O | 1.23 ± 0.07 | 0.03 ± 0.09 | 71.35 ± 0.39 | 19.40 ± 0.26 | 6.13 ± 0.33 | 1.51 ± 0.21 | 0.36 | 100.00 |
| 3.1 | ③ | - | - | - | 70.26 ± 0.38 | 20.40 ± 0.25 | 8.19 ± 0.32 | 0.96 ± 0.20 | 0.19 | 100.00 |
| 3.2 | ③ | etching | 2.93 ± 0.09 | 1.22 ± 0.1 | 68.37 ± 0.39 | 20.06 ± 0.26 | 5.10 ± 0.31 | 2.00 ± 0.22 | 0.32 | 100.00 |
| 3.3 | ③ | C2H6O | 1.06 ± 0.07 | 0.04 ± 0.09 | 71.11 ± 0.40 | 20.48 ± 0.26 | 5.66 ± 0.33 | 1.41 ± 0.22 | 0.23 | 100.00 |
| 4.1 | ④ | - | - | 0.07 ± 0.08 | 90.54 ± 0.41 | 6.27 ± 0.21 | 1.97 ± 0.29 | 0.87 ± 0.17 | 0.28 | 100.00 |
| 4.2 | ④ | etching | 6.18 ± 0.12 | 1.16 ± 0.09 | 83.53 ± 0.40 | 5.76 ± 0.20 | 1.80 ± 0.29 | 1.27 ± 0.17 | 0.30 | 100.00 |
| 4.3 | ④ | C2H6O + H2O2 | - | - | 86.55 ± 0.40 | 5.84 ± 0.18 | 3.55 ± 0.30 | 3.72 ± 0.19 | 0.34 | 100.00 |
| Name of the Artifact | Sampling Location | Metallographic Structure | Component Content/% | Alloy Material | Manufacturing Process | ||||
|---|---|---|---|---|---|---|---|---|---|
| Cu | Sn | Pb | Zn | Other | |||||
| Female lion | Surface coins | The middle part of the α solid solution still has a dendritic morphology, with intracrystalline segregation not being very obvious. A large number of polygonal island-like (α + δ) eutectoid and free-state lead particles are distributed between the crystals. Near the edge of the coin, the α solid solution exhibits the equiaxial structure. | 64.6 ± 0.16 | 8.8 ± 0.12 | 15.9 ± 0.23 | 9.70 ± 0.05 | 1.0 (Fe, Ag, Sb, et al.) | Cu–Zn–Sn–Pb | Post-casting heat-affected |
| Male lion | Surface coins | The middle part of the α solid solution still has a dendritic morphology, with intracrystalline segregation not being very obvious. A large number of polygonal island-like (α + δ) eutectoid and free-state lead particles are distributed between the crystals. Near the edge of the coin, there is a distinct area where equiaxed crystals have become significantly smaller. The α solid solution dendrites exhibit clear intragranular segregation, with a small amount of lead particles distributed between the grains, and no obvious eutectoid structure is observed. | 72.2 ± 0.17 | 10.1 ± 0.13 | 6.7 ± 0.14 | 9.70 ± 0.05 | 1.20 (Fe, As, Sb, et al.) | Cu–Zn–Sn–Pb | Post-casting heat-affected |
| Coin 1 | - | α solid solution dendrites with significant intragranular segregation, a small amount of lead particles distributed between grains, and no obvious eutectoid structure observed. | 70.62 ± 0.16 | 6.00 ± 0.11 | 6.09 ± 0.14 | 16.41 ± 0.06 | 0.89 (Fe, As, et al.) | Cu–Zn–Sn–Pb | Casting |
| Coin 2 | - | 68.05 ± 0.15 | 6.32 ± 0.10 | 7.63 ± 0.10 | 17.30 ± 0.07 | 0.69 (Fe, As, et al.) | Cu–Zn–Sn–Pb | Casting | |
| Coin 3 | - | 69.42 ± 0.15 | 6.73 ± 0.12 | 5.51 ± 0.09 | 17.67 ± 0.07 | 0.67 (Fe, As, et al.) | Cu–Zn–Sn–Pb | Casting | |
| Name of the Artifact | Sampling Location | Metallographic Structure | Component Content/% | Alloy Material | Manufacturing Process | ||||
|---|---|---|---|---|---|---|---|---|---|
| Cu | Sn | Pb | As | Other | |||||
| The Shang Bronze Tripod Vessel with Cicada Designs | Leg A | The α solid solution twins and equiaxed grains show no significant intragranular segregation, with a small amount of polygonal island-like (α + δ) eutectoid distributed between grains. A few lead particles are distributed between grains, typical of a post-casting heat-treated structure, and due to the longest preservation time, a large number of grains have already been mineralized. | 70.28 ± 0.15 | 18.24 ± 0.22 | 9.41 ± 0.23 | 0.63 ± 0.06 | 1.44 (Fe, et al.) | Cu–Sn–Pb | Post-casting heat-affected |
| Leg C | Equiaxed grains of α solid solution show no significant intragranular segregation, with a small amount of polygonal island-like (α + δ) eutectoid distributed between grains. Spherical lead particles are also distributed between grains. | 75.95 ± 0.20 | 8.37 ± 0.13 | 13.87 ± 0.21 | 0.81 ± 0.06 | 1.01 (Fe, et al.) | Cu–Sn–Pb | Post-casting heat-affected | |
| Leg B | The α solid solution dendrites show significant intragranular segregation, with large blocks of lead particles and a small amount of eutectoid structure distributed at the grain boundaries. | 87.73 ± 0.18 | 0.26 ± 0.12 | 10.47 ± 0.19 | 0.78 ± 0.08 | 0.75 (Fe, et al.) | Cu–Sn–Pb | Casting | |
| Ear | The α solid solution dendrites show significant intragranular segregation, with a small amount of lead particles and a large amount of (α + δ) eutectoid structures distributed at the grain boundaries. | 70.27 ± 0.16 | 17.84 ± 0.17 | 9.67 ± 0.20 | 0.40 ± 0.07 | 1.83 (Fe, et al.) | Cu–Sn–Pb | Casting | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Liu, C.; Zhang, S.; Cui, R.; Li, Y. Feasibility Exploration and Research Examples of On-Site Metallographic Inspection Methods in the Analysis of Bronze Artifacts—A Case Study of Ming Jiajing Bronze Lions and the Shang Bronze Tripod Vessel with Cicada Designs. Metals 2025, 15, 209. https://doi.org/10.3390/met15020209
Zhang K, Liu C, Zhang S, Cui R, Li Y. Feasibility Exploration and Research Examples of On-Site Metallographic Inspection Methods in the Analysis of Bronze Artifacts—A Case Study of Ming Jiajing Bronze Lions and the Shang Bronze Tripod Vessel with Cicada Designs. Metals. 2025; 15(2):209. https://doi.org/10.3390/met15020209
Chicago/Turabian StyleZhang, Kaige, Cheng Liu, Siyu Zhang, Ruihua Cui, and Yi Li. 2025. "Feasibility Exploration and Research Examples of On-Site Metallographic Inspection Methods in the Analysis of Bronze Artifacts—A Case Study of Ming Jiajing Bronze Lions and the Shang Bronze Tripod Vessel with Cicada Designs" Metals 15, no. 2: 209. https://doi.org/10.3390/met15020209
APA StyleZhang, K., Liu, C., Zhang, S., Cui, R., & Li, Y. (2025). Feasibility Exploration and Research Examples of On-Site Metallographic Inspection Methods in the Analysis of Bronze Artifacts—A Case Study of Ming Jiajing Bronze Lions and the Shang Bronze Tripod Vessel with Cicada Designs. Metals, 15(2), 209. https://doi.org/10.3390/met15020209






