Effect of Ag and Ti Addition on the Deformation and Tribological Behavior of Zr-Co-Al Bulk Metallic Glass
Abstract
1. Introduction
2. Materials and Methods
2.1. Alloy Synthesis and Microstructure Characterization
2.2. Mechanical Characterization
2.3. Wear Characterization
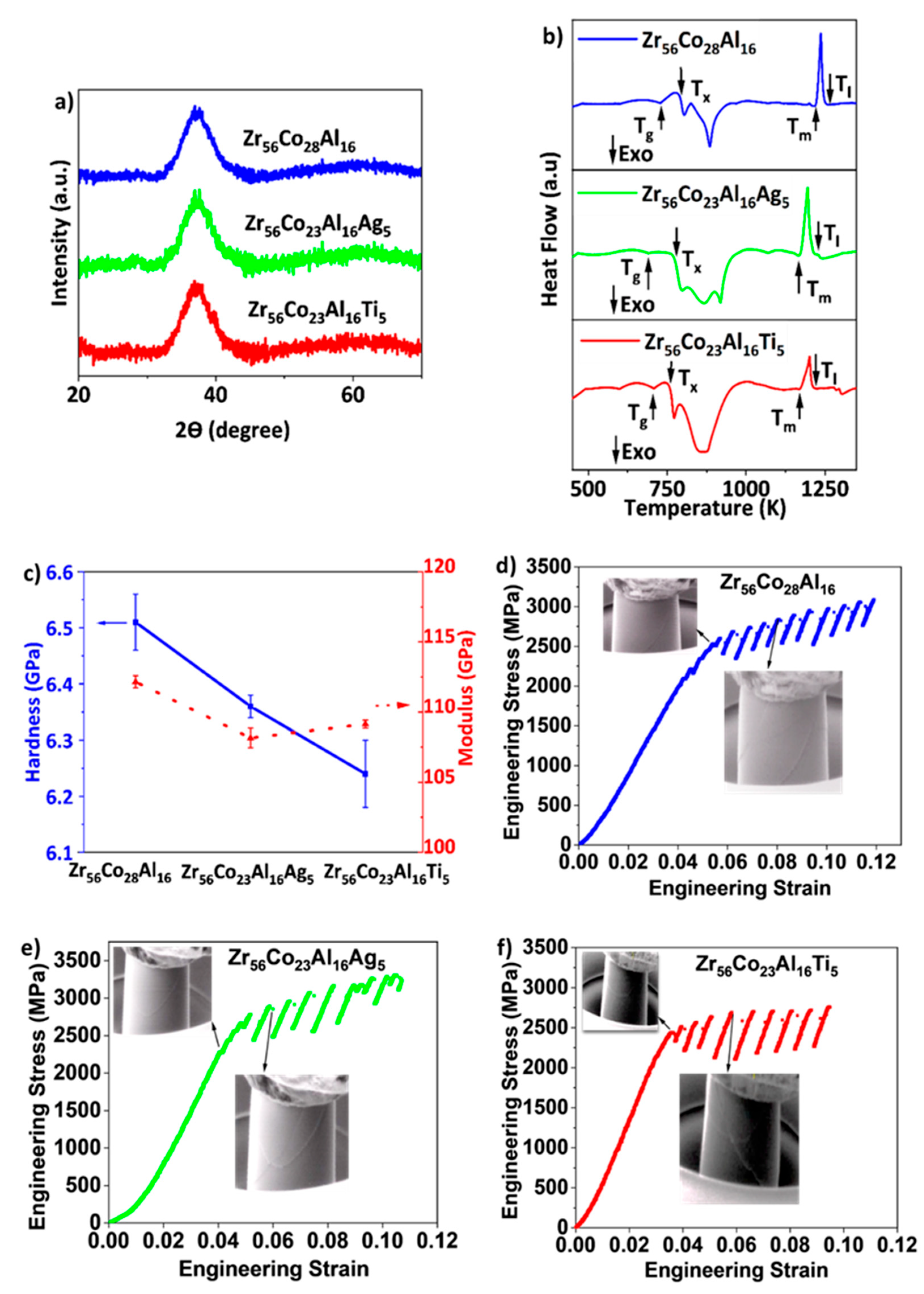
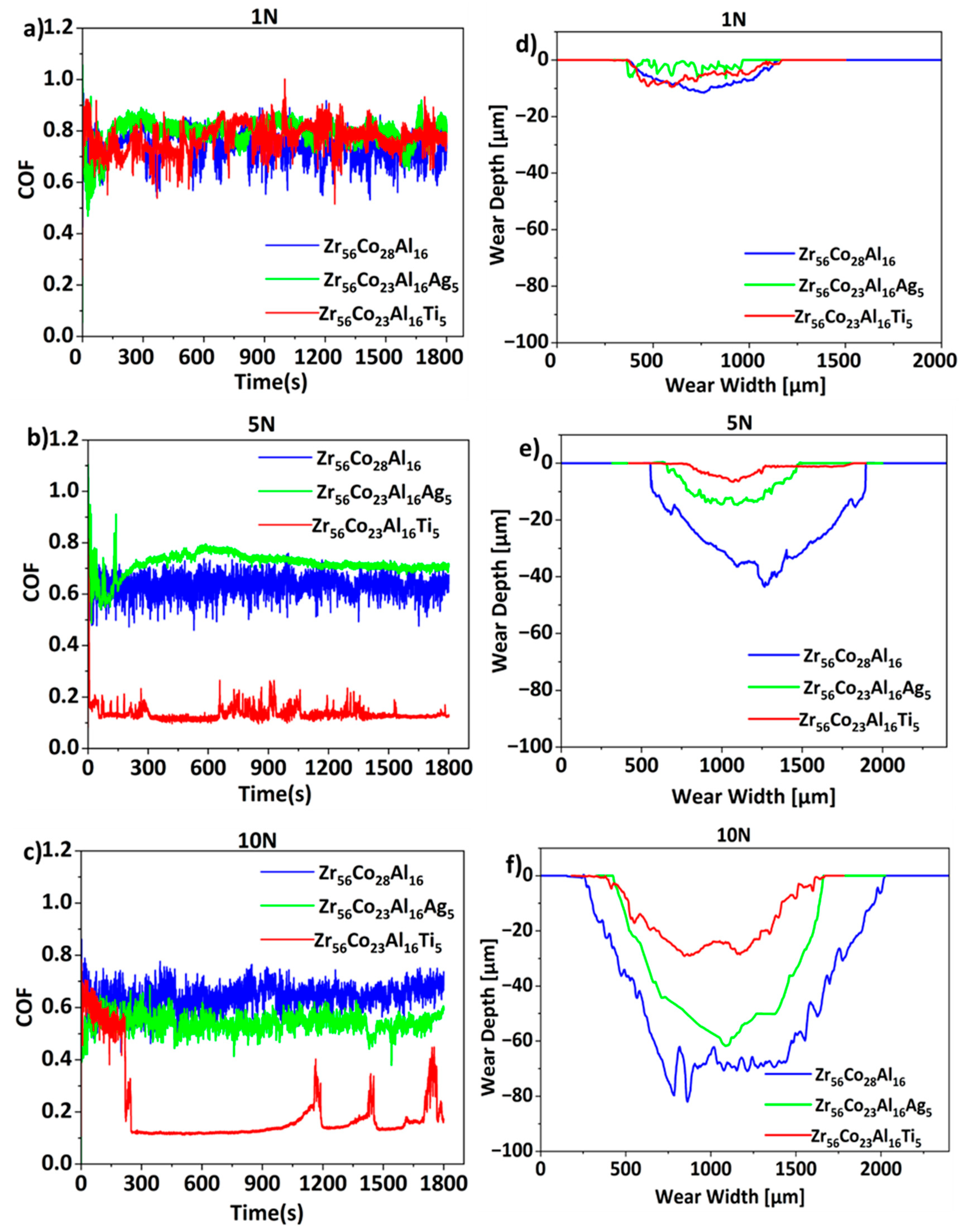
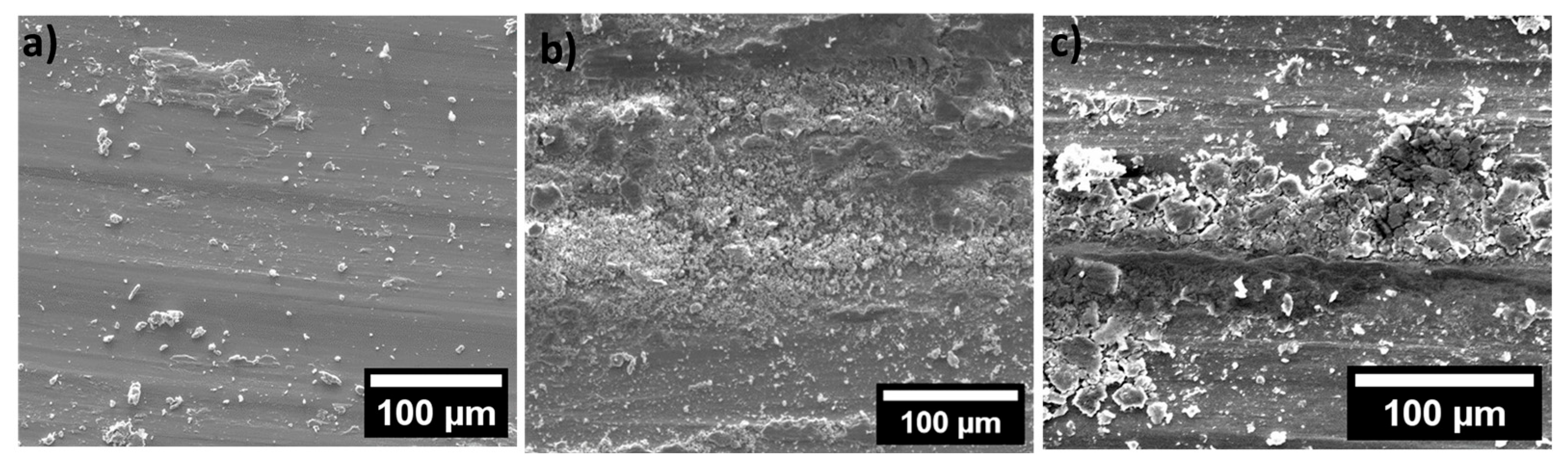
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hua, N.; Chen, W.; Wang, W.; Lu, H.; Ye, X.; Li, G.; Lin, C.; Huang, X. Tribological Behavior of a Ni-Free Zr-Based Bulk Metallic Glass with Potential for Biomedical Applications. Mater. Sci. Eng. C 2016, 66, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Aditya, A.; Felix Wu, H.; Arora, H.; Mukherjee, S. Amorphous Metallic Alloys: Pathways for Enhanced Wear and Corrosion Resistance. JOM 2017, 69, 2150–2155. [Google Scholar] [CrossRef]
- Ji, X.; Pole, M.; Ho, T.; Akhtar, M.A.; Pantawane, M.; Mukherjee, S.; Dahotre, N.B. Tribology of Rejuvenated CuZr-Based Amorphous Alloys. Wear 2021, 484–485, 204018. [Google Scholar] [CrossRef]
- Arora, H.S.; Grewal, H.S.; Singh, H.; Mukherjee, S. Zirconium Based Bulk Metallic Glass-Better Resistance to Slurry Erosion Compared to Hydroturbine Steel. Wear 2013, 307, 28–34. [Google Scholar] [CrossRef]
- Hua, N.; Huang, L.; Wang, J.; Cao, Y.; He, W.; Pang, S.; Zhang, T. Corrosion Behavior and in Vitro Biocompatibility of Zr-Al-Co-Ag Bulk Metallic Glasses: An Experimental Case Study. J. Non. Cryst. Solids 2012, 358, 1599–1604. [Google Scholar] [CrossRef]
- Tan, J.; Pan, F.S.; Zhang, Y.; Sun, B.A.; He, J.; Zheng, N.; Stoica, M.; Kühn, U.; Eckert, J. Formation of Zr-Co-Al Bulk Metallic Glasses with High Strength and Large Plasticity. Intermetallics 2012, 31, 282–286. [Google Scholar] [CrossRef]
- Tan, J.; Zhang, Y.; Stoica, M.; Kühn, U.; Mattern, N.; Pan, F.S.; Eckert, J. Study of Mechanical Property and Crystallization of a ZrCoAl Bulk Metallic Glass. Intermetallics 2011, 19, 567–571. [Google Scholar] [CrossRef]
- Chae, W.S.; Li, M.W.; Cao, Q.P.; Wang, X.D.; Ding, S.Q.; Zhang, D.X.; Jiang, J.Z.; Caron, A. Excellent Corrosion and Wear Resistance of Amorphous and Crystalline ZrCoAl Alloys. J. Alloys Compd. 2023, 968, 172055. [Google Scholar] [CrossRef]
- Dong, Q.; Pan, Y.J.; Tan, J.; Qin, X.M.; Li, C.J.; Gao, P.; Feng, Z.X.; Calin, M.; Eckert, J. A Comparative Study of Glass-Forming Ability, Crystallization Kinetics and Mechanical Properties of Zr55Co25Al20 and Zr52Co25Al23 Bulk Metallic Glasses. J. Alloys Compd. 2019, 785, 422–428. [Google Scholar] [CrossRef]
- Tan, J.; Pan, F.S.; Zhang, Y.; Wang, Z.; Stoica, M.; Sun, B.A.; Kühn, U.; Eckert, J. Effect of Fe Addition on Glass Forming Ability and Mechanical Properties in Zr-Co-Al-(Fe) Bulk Metallic Glasses. Mater. Sci. Eng. A 2012, 539, 124–127. [Google Scholar] [CrossRef]
- Mohammadi Rahvard, M.; Tamizifar, M.; Boutorabi, S.M.A. The Effect of Ag Addition on the Non-Isothermal Crystallization Kinetics and Fragility of Zr56Co28Al16 Bulk Metallic Glass. J. Non. Cryst. Solids 2018, 481, 74–84. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, C.; Han, J.; Yang, S.; Xie, G.; Jiang, H.; Chen, Y.; Liu, X. Formation of Zr-Based Bulk Metallic Glass with Large Amount of Yttrium Addition. Intermetallics 2018, 92, 55–61. [Google Scholar] [CrossRef]
- Rack, H.J.; Qazi, J.I. Titanium Alloys for Biomedical Applications. Mater. Sci. Eng. C 2006, 26, 1269–1277. [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, J.; Zhao, X.; Ma, L. Ti Substituted Ni-Free Zr65-XTixCu17.5Fe10Al7.5 Bulk Metallic Glasses with Significantly Enhanced Glass-Forming Ability and Mechanical Properties. J. Alloys Compd. 2019, 773, 713–718. [Google Scholar] [CrossRef]
- Zhou, W.; Hu, J.; Weng, W.; Gao, L.; Xu, G. Enhancement of Plasticity in Zr-Cu-Ni-Al-Ti Bulk Metallic Glass by Heterogeneous Microstructure. J. Non. Cryst. Solids 2018, 481, 530–536. [Google Scholar] [CrossRef]
- Shi, H.; Zhao, W.; Wei, X.; Ding, Y.; Shen, X.; Liu, W. Effect of Ti Addition on Mechanical Properties and Corrosion Resistance of Ni-Free Zr-Based Bulk Metallic Glasses for Potential Biomedical Applications. J. Alloys Compd. 2020, 815, 152636. [Google Scholar] [CrossRef]
- Peng, L.; Li, J.; Zhang, M.; Lin, H.; Li, Z.; Li, W. Effects of Normal Load and Reciprocating Frequency on the Tribological Behaviors of a Zr-Based Bulk Metallic Glass. Wear 2023, 520–521, 204732. [Google Scholar] [CrossRef]
- Greer, A.L.; Rutherford, K.L.; Hutchings, I.M. Wear Resistance of Amorphous Alloys and Related Materials. Int. Mater. Rev. 2002, 47, 87–112. [Google Scholar] [CrossRef]
- Fleury, E.; Lee, S.M.; Ahn, H.S.; Kim, W.T.; Kim, D.H. Tribological Properties of Bulk Metallic Glasses. Mater. Sci. Eng. A 2004, 375–377, 276–279. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Wang, T.; Hui, X.D.; Chen, W.; Feng, C.F. Effect of Sliding Velocity on the Transition of Wear Mechanism in (Zr,Cu)95Al5 Bulk Metallic Glass. Tribol. Int. 2016, 101, 141–151. [Google Scholar] [CrossRef]
- Kong, J.; Xiong, D.; Li, J.; Yuan, Q.; Tyagi, R. Effect of Flash Temperature on Tribological Properties of Bulk Metallic Glasses. Tribol. Lett. 2009, 35, 151–158. [Google Scholar] [CrossRef]
- Hopur, P.; Chen, W.; Zhou, Y.; Zhou, J.; Wang, T. The Correlation among the Atomic Structure, Electronic Valence Band and Properties of Zr-Cu-Al-Ag Bulk Metallic Glasses. Metals 2023, 13, 1181. [Google Scholar] [CrossRef]
- Ding, D.W.; Tan, J.; Cai, A.H.; Liu, Y.; Wu, H.; An, Q.; Li, P.W.; Zhang, Y.; Yang, Q. Effect of Ti Addition on Properties of Zr54Al10.2Ni9.4Cu26.4 Glass Forming Alloy. J. Alloys Compd. 2021, 864, 158911. [Google Scholar] [CrossRef]
- Pan, J.; Chan, K.C.; Chen, Q.; Li, N.; Guo, S.F.; Liu, L. The Effect of Microalloying on Mechanical Properties in CuZrAl Bulk Metallic Glass. J. Alloys Compd. 2010, 504, S74–S77. [Google Scholar] [CrossRef]
- Jiang, F.; Qu, J.; Fan, G.; Jiang, W.; Qiao, D.; Freels, M.W.; Liaw, P.K.; Choo, H. Tribological Studies of a Zr-Based Glass-Forming Alloy with Different States. Adv. Eng. Mater. 2009, 11, 925–931. [Google Scholar] [CrossRef]
- Tariq, N.H.; Hasan, B.A.; Akhter, J.I.; Ali, F. Mechanical and Tribological Properties of Zr-Al-Ni-Cu Bulk Metallic Glasses. J. Alloys Compd. 2009, 469, 179–185. [Google Scholar] [CrossRef]
- Du, Y.; Zhou, Q.; Pei, X.; Wang, H.; Wang, H.; Liu, W. Enhancing the Tribological Performance of the TiZrHfCuBe High Entropy Bulk Metallic Glass by Sn Addition. Tribol. Int. 2022, 171, 107529. [Google Scholar] [CrossRef]
- Bajpai, S.; Nisar, A.; Sharma, R.K.; Schwarz, U.D.; Balani, K.; Datye, A. Effect of Fictive Temperature on Tribological Properties of Zr44Ti11Cu10Ni10Be25 Bulk Metallic Glasses. Wear 2021, 486–487, 204075. [Google Scholar] [CrossRef]
- Fu, X.-Y.; Kasai, T.; Falk, M.L.; Rigney, D.A. Sliding Behavior of Metallic Glass Part I. Experimental Investigations. Wear 2001, 250, 409–419. [Google Scholar] [CrossRef]
- Aouadi, S.M.; Gao, H.; Martini, A.; Scharf, T.W.; Muratore, C. Lubricious Oxide Coatings for Extreme Temperature Applications: A Review. Surf. Coat. Technol. 2014, 257, 266–277. [Google Scholar] [CrossRef]
- Ayyagari, A.; Scharf, T.W.; Mukherjee, S. Dry Reciprocating Sliding Wear Behavior and Mechanisms of Bulk Metallic Glass Composites. Wear 2016, 350–351, 56–62. [Google Scholar] [CrossRef]
- Zou, Y.; Qiu, Z.; Zheng, Z.; Wang, G.; Yan, X.; Yin, S.; Liu, M.; Zeng, D. Ex-Situ Additively Manufactured FeCrMoCB/Cu Bulk Metallic Glass Composite with Well Wear Resistance. Tribol. Int. 2021, 162, 107112. [Google Scholar] [CrossRef]
- Liu, Y.; Pang, S.; Yang, W.; Hua, N.; Liaw, P.K.; Zhang, T. Tribological Behaviors of a Ni-Free Ti-Based Bulk Metallic Glass in Air and a Simulated Physiological Environment. J. Alloys Compd. 2018, 766, 1030–1036. [Google Scholar] [CrossRef]
- Villapún, V.M.; Medina, J.; Pérez, P.; Esat, F.; Inam, F.; González, S. Strategy for Preventing Excessive Wear Rate at High Loads in Bulk Metallic Glass Composites. Mater Des. 2017, 135, 300–308. [Google Scholar] [CrossRef]
- Jiang, X.; Song, J.; Fan, H.; Su, Y.; Zhang, Y.; Hu, L. Sliding Friction and Wear Mechanisms of Cu36Zr48Ag8Al8 Bulk Metallic Glass under Different Sliding Conditions: Dry Sliding, Deionized Water, and NaOH Corrosive Solutions. Tribol. Int. 2020, 146, 106211. [Google Scholar] [CrossRef]
- Żyła, M.; Smoła, G.; Knapik, A.; Rysz, J.; Sitarz, M.; Grzesik, Z. The Formation of the Co3O4 Cobalt Oxide within CoO Substrate. Corros. Sci. 2016, 112, 536–541. [Google Scholar] [CrossRef]
- Kim, B.; Hamaguchi, H. Mode Assignments of the Raman Spectrum of Monoclinic Zirconia by Isotopic Exchange Technique. Phys. Status Solidi (b) 1997, 203, 557–563. [Google Scholar] [CrossRef]
- Thomas, P.V.; Ramakrishnan, V.; Vaidyan, V.K. Oxidation Studies of Aluminum Thin Films by Raman Spectroscopy. Thin Solid Films 1989, 170, 35–40. [Google Scholar] [CrossRef]
- Shirani, A.; Nunn, N.; Shenderova, O.; Osawa, E.; Berman, D. Nanodiamonds for Improving Lubrication of Titanium Surfaces in Simulated Body Fluid. Carbon 2019, 143, 890–896. [Google Scholar] [CrossRef]
- Hadjiev, V.G.; Iliev, M.N.; Vergilov, I.V. The Raman Spectra of Co3O4. J. Phys. C Solid State Phys. 1988, 21, L199. [Google Scholar] [CrossRef]
- Mohrbacher, H.; Blanpain, B.; Celts, J.-P.; Roos, J.R. Raman Spectroscopy on Defective Wear Debris Generated by Contact Vibrations. J. Mater. Sci. Lett. 1995, 14, 279–281. [Google Scholar] [CrossRef]
- Porto, S.P.S.; Fleury, P.A.; Damen, T.C. Raman Spectra of TiO2, MgF2, ZnF2, FeF2, and MnF2. Phys. Rev. 1967, 154, 522. [Google Scholar] [CrossRef]
- Wu, X.; Yeow, E.K.L. Fluorescence Blinking Dynamics of Silver Nanoparticle and Silver Nanorod Films. Nanotechnology 2007, 19, 035706. [Google Scholar] [CrossRef] [PubMed]
- Ravi Chandra Raju, N.; Jagadeesh Kumar, K. Photodissociation Effects on Pulsed Laser Deposited Silver Oxide Thin Films: Surface-Enhanced Resonance Raman Scattering. J. Raman Spectrosc. 2011, 42, 1505–1509. [Google Scholar] [CrossRef]
- Al Sulaimi, R.; Eskandari, M.; Shirani, A.; Macknojia, A.Z.; Miller, W.; Berman, D. Effect of Cu and Ni Inclusion on Tribological Performance of Tribocatalytically Active Coatings in Hydrocarbon Environments. Coatings 2023, 14, 61. [Google Scholar] [CrossRef]
- Shirani, A.; Al Sulaimi, R.; Macknojia, A.Z.; Eskandari, M.; Berman, D. Tribocatalytically-Active Nickel/Cobalt Phosphorous Films for Universal Protection in a Hydrocarbon-Rich Environment. Sci. Rep. 2023, 13, 10914. [Google Scholar] [CrossRef] [PubMed]
- Tsang, J.C.; Demuth, J.E.; Sanda, P.N.; Kirtley, J.R. Enhanced Raman Scattering from Carbon Layers on Silver. Chem. Phys. Lett. 1980, 76, 54–57. [Google Scholar] [CrossRef]
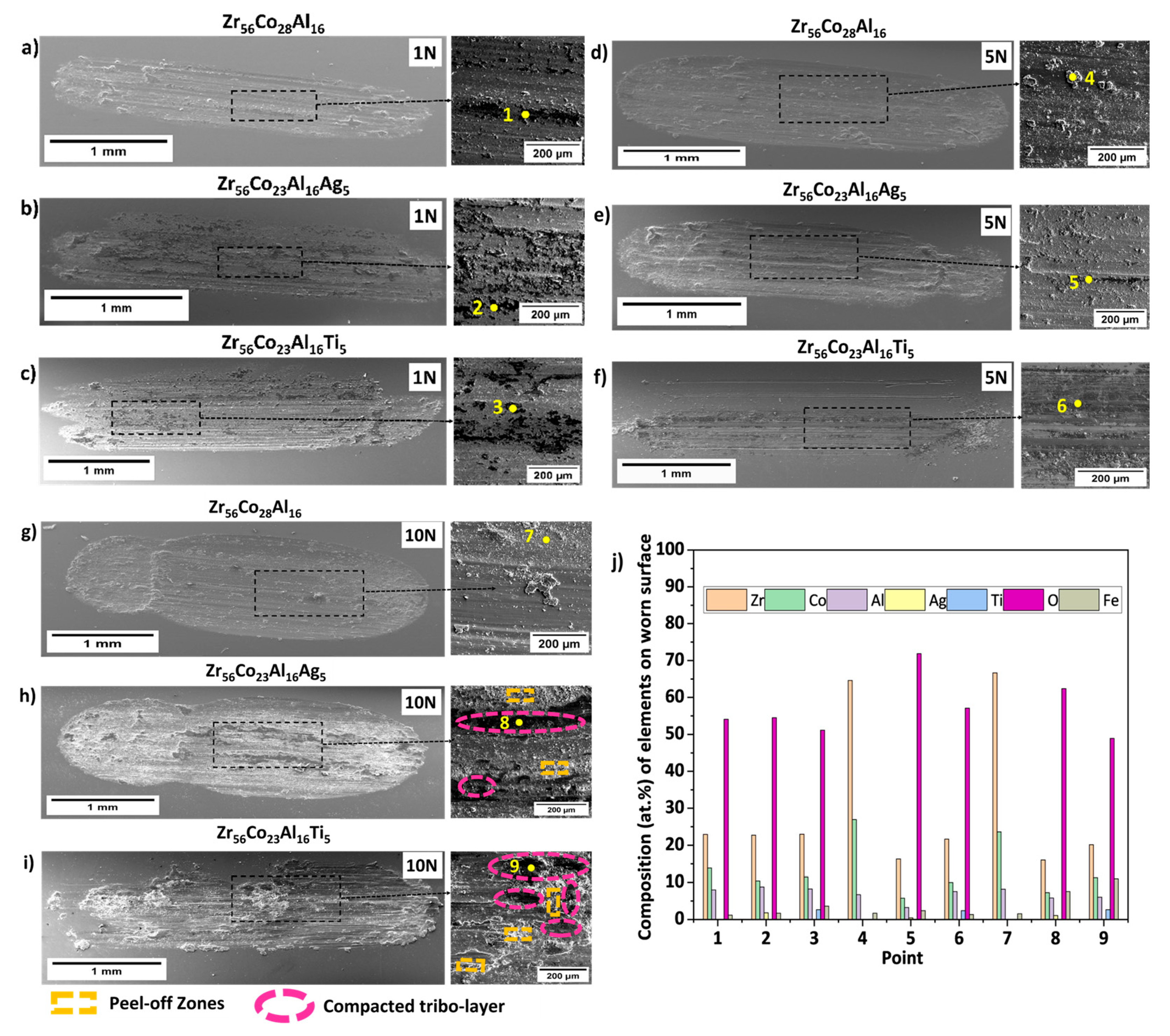
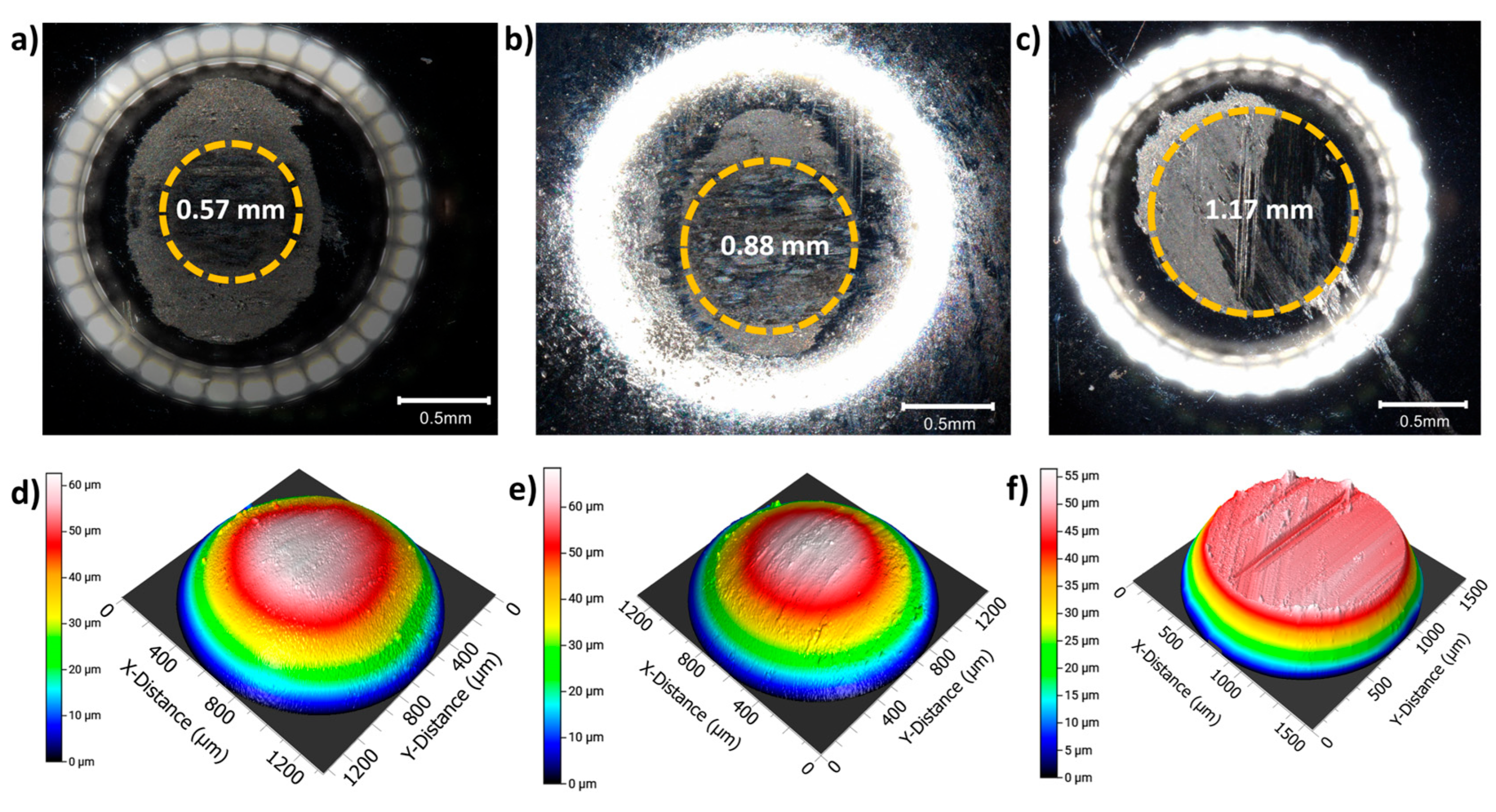
 indicates outside the wear track and
indicates outside the wear track and  indicates inside the wear track for the three BMGs studied.
indicates inside the wear track for the three BMGs studied.
 indicates outside the wear track and
indicates outside the wear track and  indicates inside the wear track for the three BMGs studied.
indicates inside the wear track for the three BMGs studied.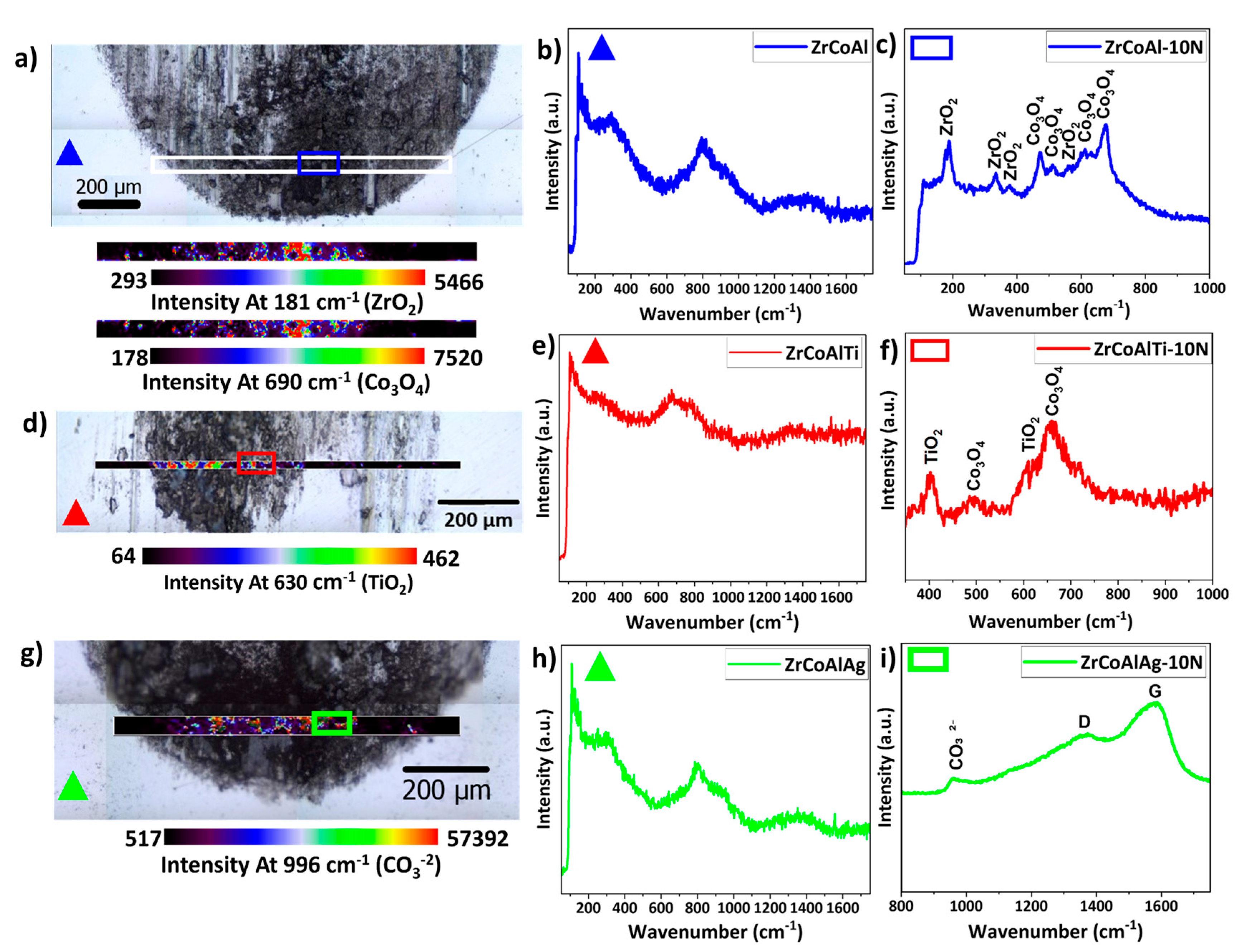
| BMG | Tg (K) | Tx (K) | ΔT = Tx − Tg (K) |
|---|---|---|---|
| Zr-Co-Al | 739 | 787 | 48 |
| Zr-Co-Al-Ag | 688 | 762 | 74 |
| Zr-Co-Al-Ti | 709 | 763 | 54 |
| Alloy | COF | Wear rate [10−5 mm3/N·m] | ||||
|---|---|---|---|---|---|---|
| 1 N | 5 N | 10 N | 1 N | 5 N | 10 N | |
| Zr-Co-Al | 0.73 ± 0.2 | 0.63 ± 0.2 | 0.64 ± 0.2 | 3 ± 0.1 | 25 ± 0.2 | 60 ± 0.1 |
| Zr-Co-Al-Ag | 0.73 ± 0.2 | 0.71 ± 0.1 | 0.53 ± 0.1 | 6 ± 0.1 | 7 ± 0.1 | 17 ± 0.5 |
| Zr-Co-Al-Ti | 0.77 ± 0.2 | 0.14 ± 0.1 | 0.2 ± 0.1 | 4 ± 0.5 | 6 ± 0.4 | 15 ± 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alla, S.S.; Eskandari, M.; Jha, S.; Pei, Z.; Vincent, S.; Ryu, W.H.; Park, E.S.; Mukherjee, S. Effect of Ag and Ti Addition on the Deformation and Tribological Behavior of Zr-Co-Al Bulk Metallic Glass. Metals 2025, 15, 213. https://doi.org/10.3390/met15020213
Alla SS, Eskandari M, Jha S, Pei Z, Vincent S, Ryu WH, Park ES, Mukherjee S. Effect of Ag and Ti Addition on the Deformation and Tribological Behavior of Zr-Co-Al Bulk Metallic Glass. Metals. 2025; 15(2):213. https://doi.org/10.3390/met15020213
Chicago/Turabian StyleAlla, Siva Shankar, Mohammad Eskandari, Shristy Jha, Ziyu Pei, S. Vincent, Wook Ha Ryu, Eun Soo Park, and Sundeep Mukherjee. 2025. "Effect of Ag and Ti Addition on the Deformation and Tribological Behavior of Zr-Co-Al Bulk Metallic Glass" Metals 15, no. 2: 213. https://doi.org/10.3390/met15020213
APA StyleAlla, S. S., Eskandari, M., Jha, S., Pei, Z., Vincent, S., Ryu, W. H., Park, E. S., & Mukherjee, S. (2025). Effect of Ag and Ti Addition on the Deformation and Tribological Behavior of Zr-Co-Al Bulk Metallic Glass. Metals, 15(2), 213. https://doi.org/10.3390/met15020213









