Influence of Sulfide Concentration on the Properties of Cr3C2-25(Ni20Cr) Cermet Coating on Al7075 Substrate
Abstract
:1. Introduction
2. Material and Experimental Methods
3. Results and Discussion
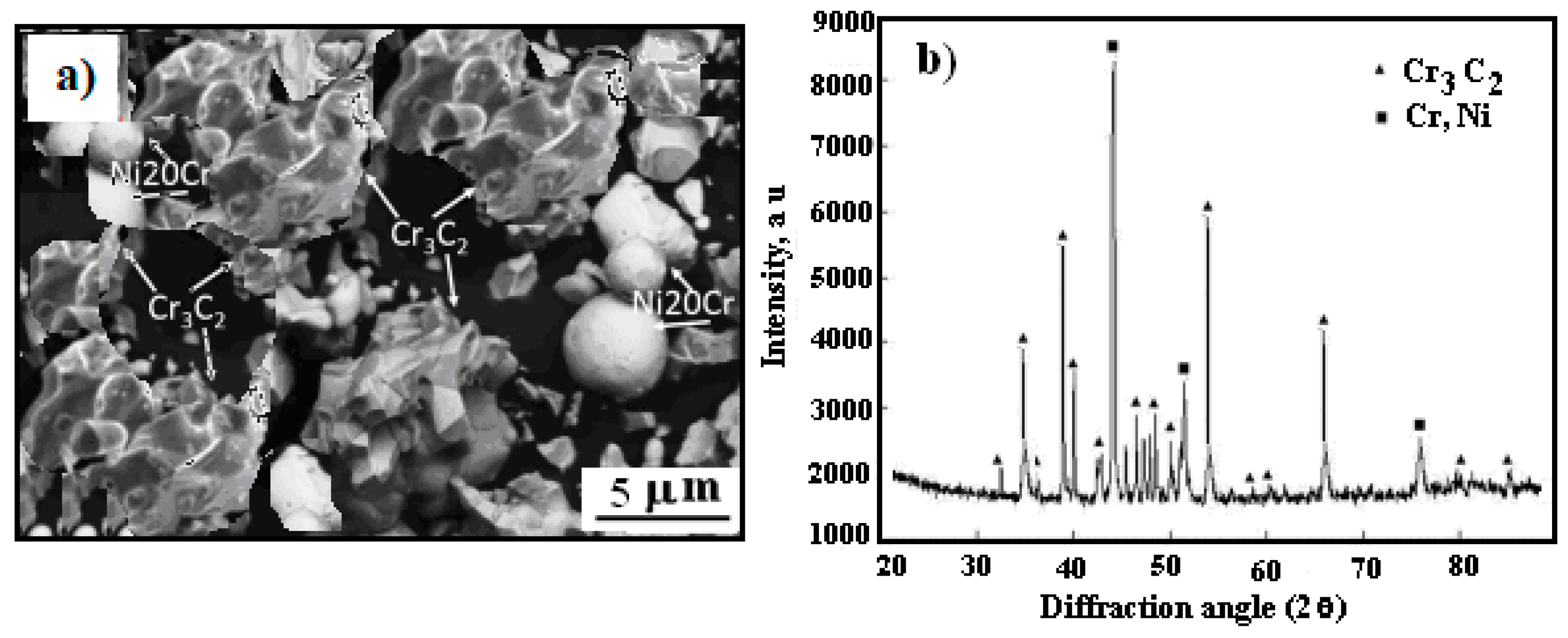
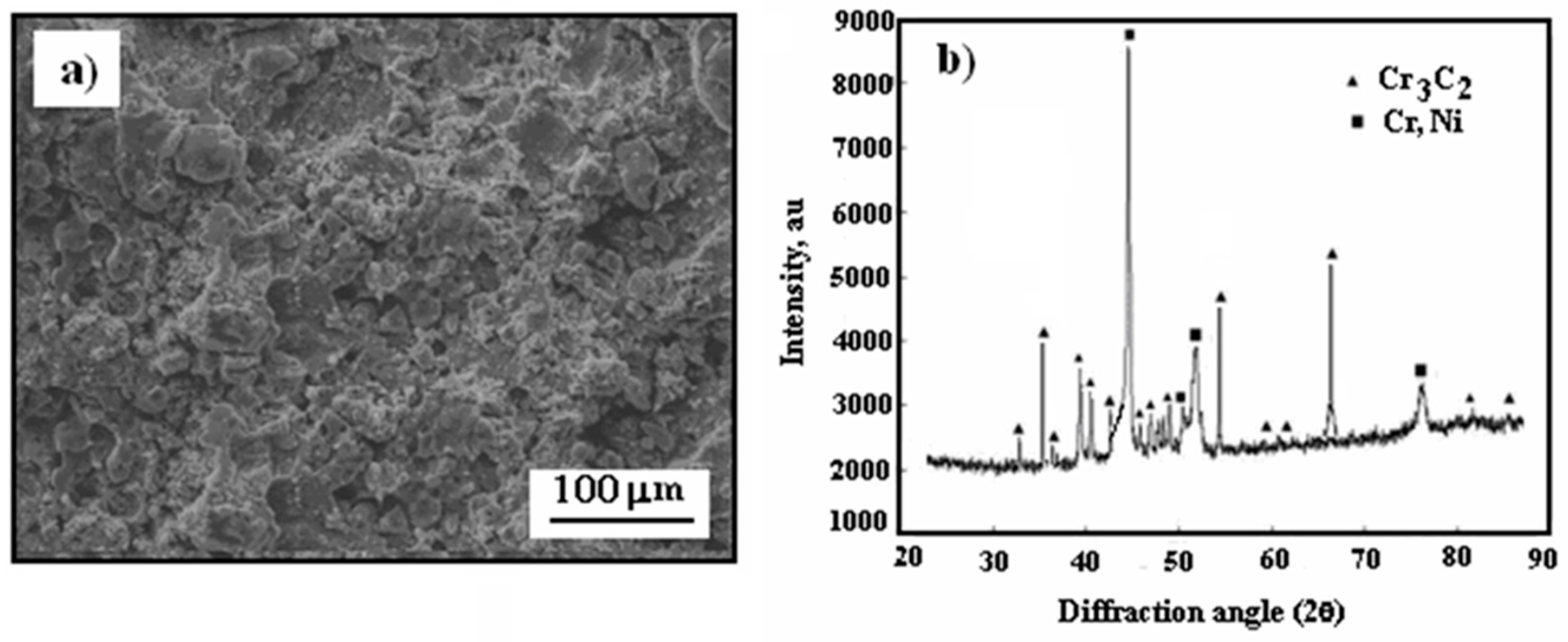
3.1. Surface Morphology
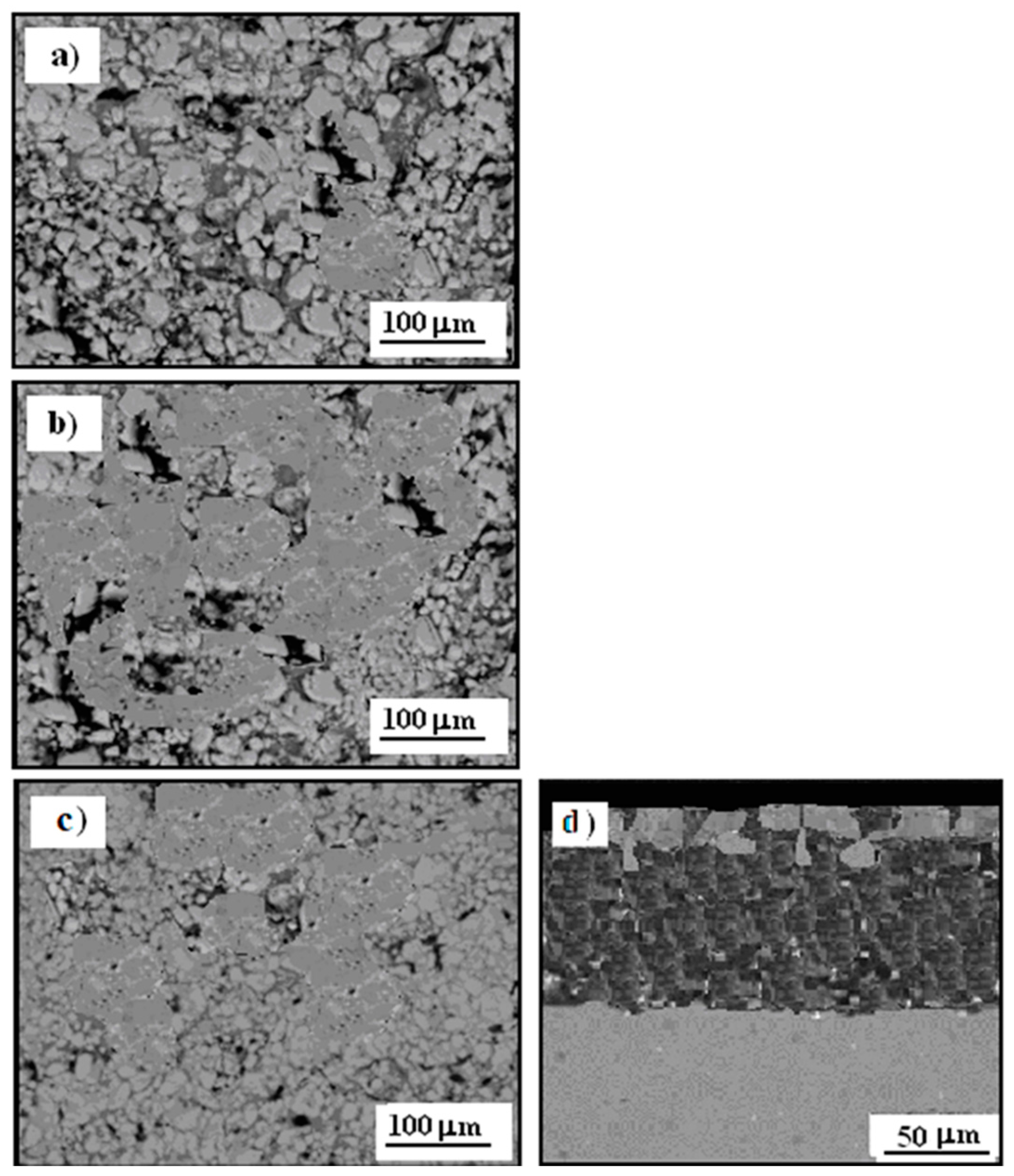
3.2. Microstructure Cermet Coating
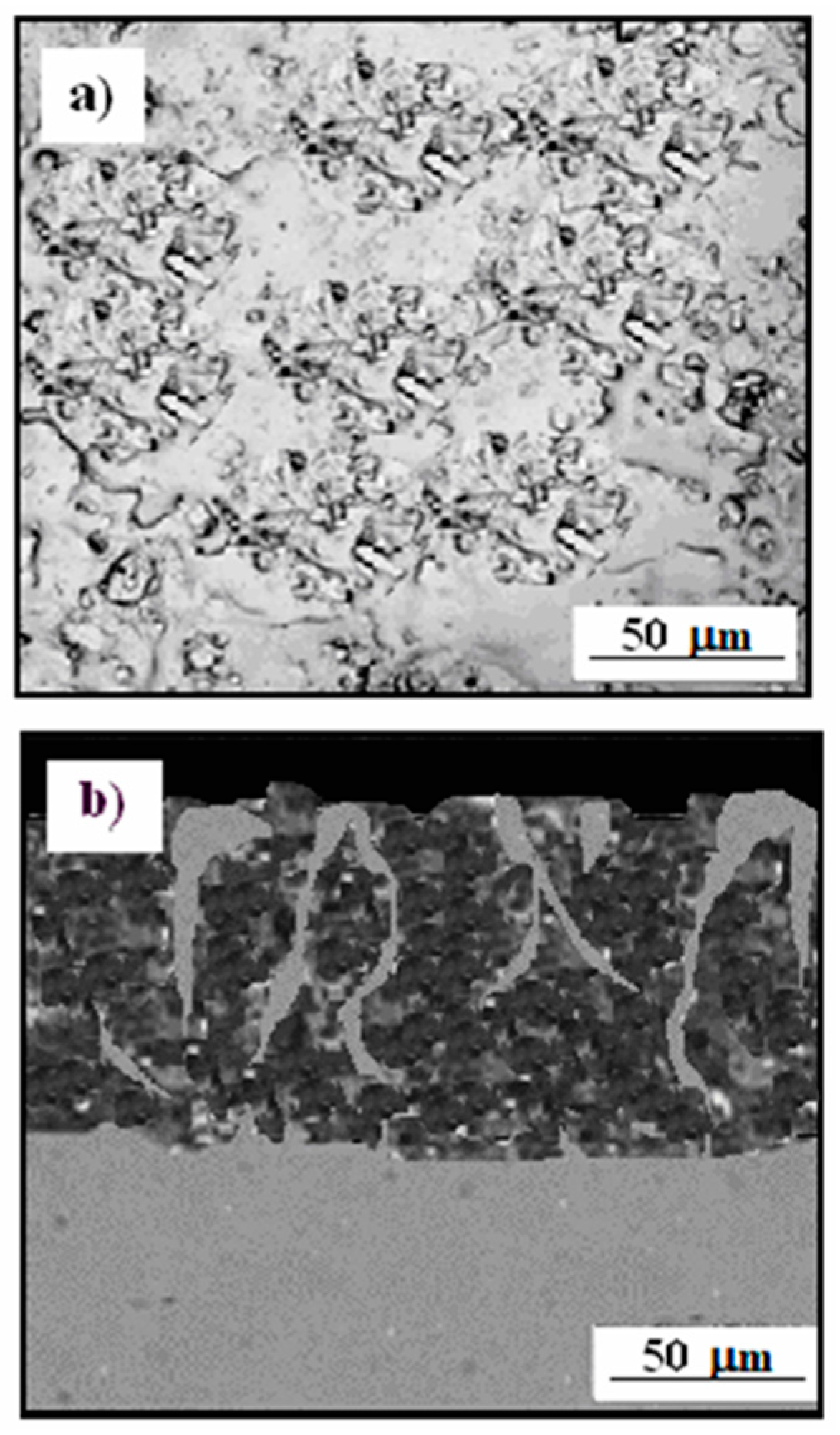
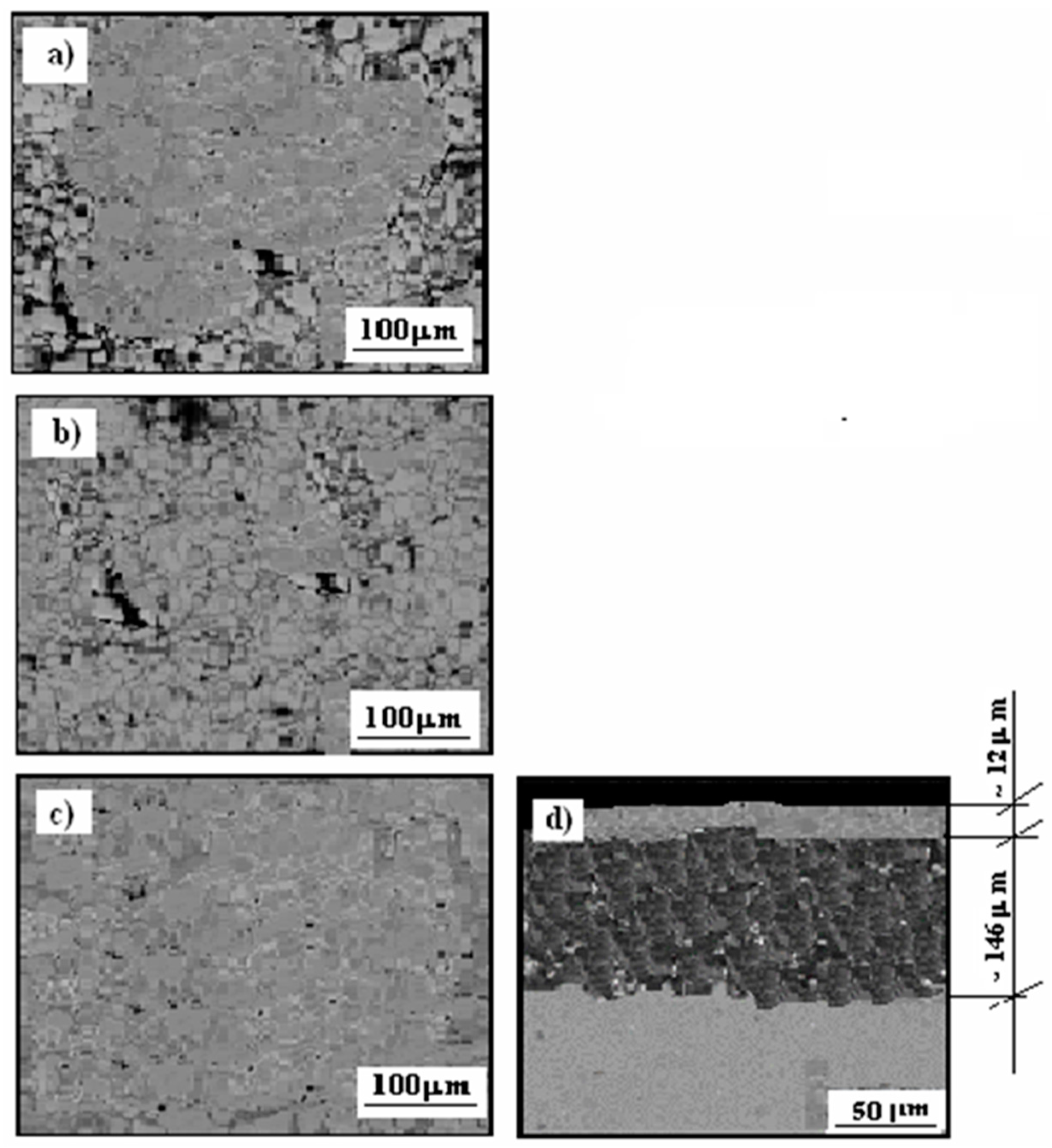
3.3. Microstructure Cermet Coating After Exposure to Corrosive Environment
3.4. Cermet Coating After Exposure in Thioacetic Acid Amide
3.5. Microhardness
3.6. Corrosion Test
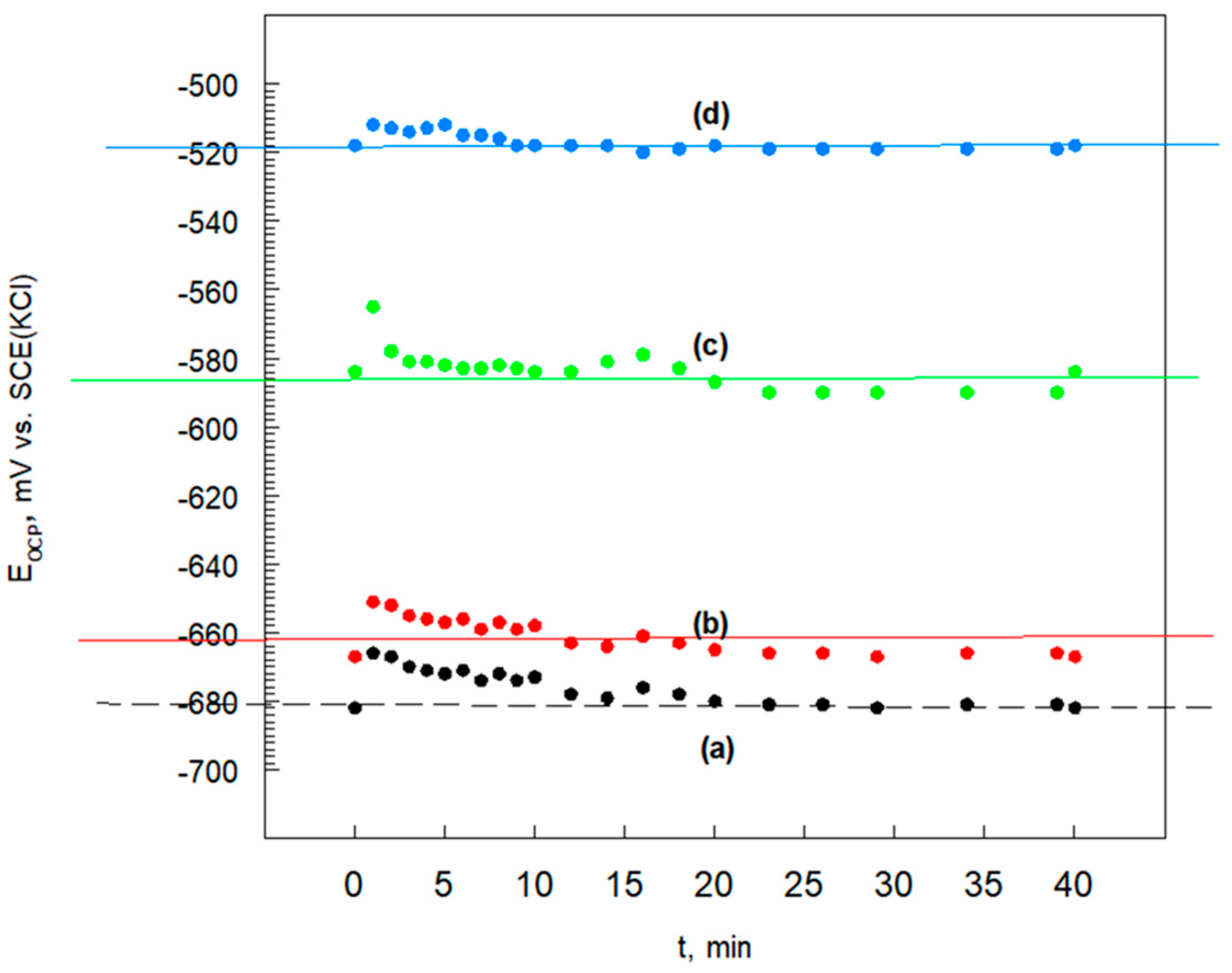
3.6.1. Open Circuit Potential
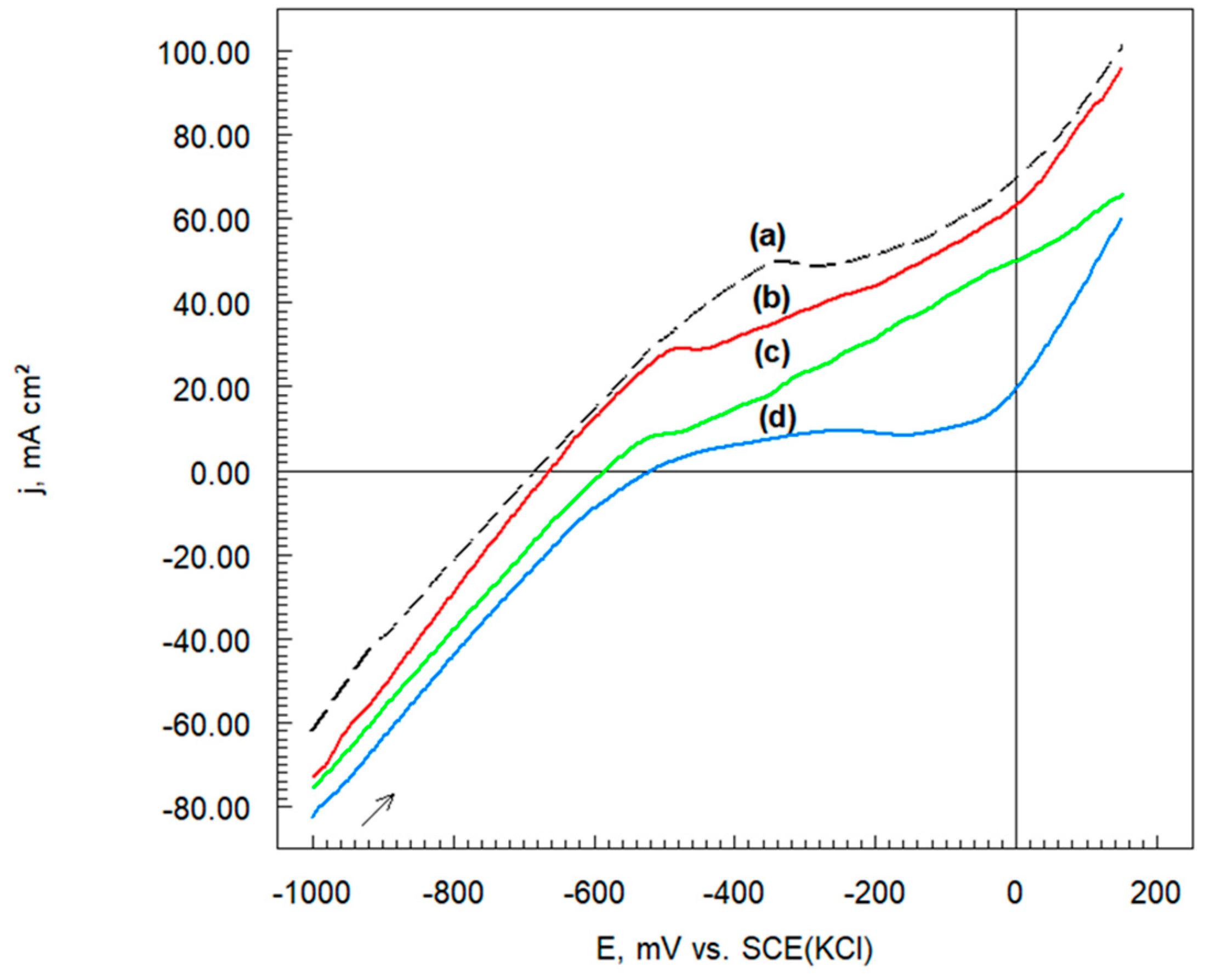
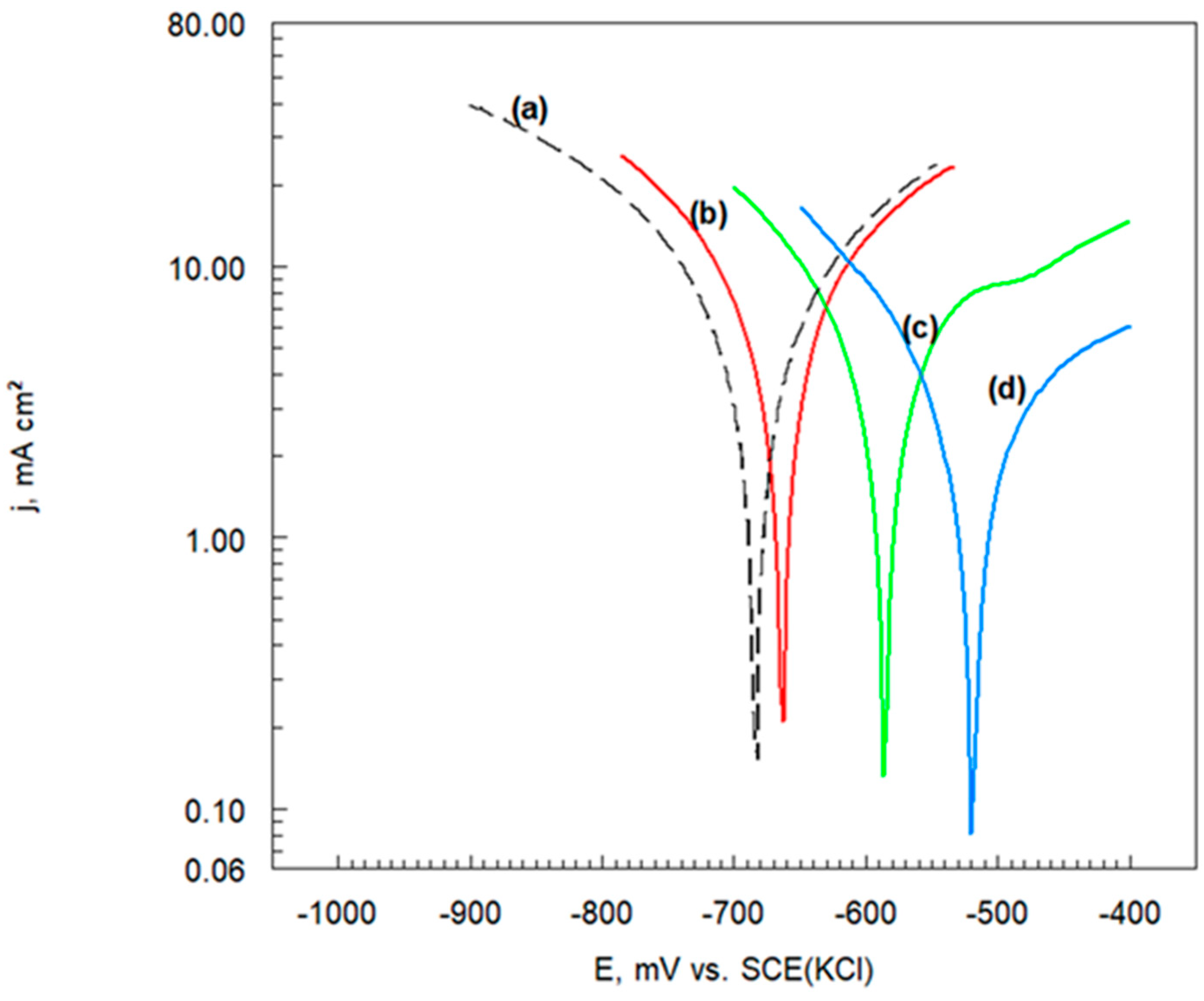
3.6.2. Potentiodynamic Polarization Curves
3.6.3. Chronoamperometric Curves
3.7. Corrosion Electrochemical Parameters
3.7.1. Polarization Resistance
3.7.2. Corrosion Rate
3.8. Cermet Coating After Corrosion Test
4. Conclusions
- The Cr3C2-25(Ni20Cr) cermet coating was deposited on the Al7075 substrate using the cold-sprayed method. The cermet coating surface was rough and wavy but adhered well to the substrate.
- The ceramic coatings were chemically modified in a thioacetic acid amide {TAA) environment. The cermet surfaces were covered with a thin, smooth, and well-adhered layer of sulfides (MemSn)ads.
- As the TAA concentration increases, the microhardness (HV10) of the Cr3C2-25(Ni20Cr)/Al7075 coatings increases slightly.
- The adsorbed (MemSn)ads layer effectively separates the Cr3C2-25(Ni20Cr)/Al7075 coating surface from contact with the aggressive corrosive environment.
- The sulfide coatings on the cermet surface were destroyed in the acidic chloride solution. The least destruction of the (MemSn)ads coating was observed for the Cr3C2-25(Ni20Cr)-0.15 sample after exposure in the solution containing 0.15 M TAA.
- The highest polarization resistance (Rp) and the lowest corrosion rate (CW) values were recorded for the cermet coating after exposure in the solution containing 0.15 M TAA.
- The (MemSn)ads layer on the Cr3C2-25(Ni20Cr)/Al7075 surface significantly impedes the exchange of mass and electric charge between the electrode and the electrolyte solution.
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Montero-Sistiaga, M.L.; Mertens, R.; Vrancken, B.; Wang, X.; Van Hooreweder, B.; Kruth, J.-P.; Van Humbeeck, J. Changing the alloy composition of Al7075 for better processability by selective laser melting. J. Mater. Process. Technol. 2016, 23B, 437–445. [Google Scholar] [CrossRef]
- Rocha, A.M.F.; Bastos, A.C.; Cardoso, J.P.; Rodrigues, F.; Fernandes, C.M.; Soares, F.; Sacramento, J.; Senos, A.M.R.; Ferreira, M.G.S. Corrosion behaviour of WC hardmetals with nickel-based binders. Corros. Sci. 2019, 147, 384–393. [Google Scholar] [CrossRef]
- Ang, A.S.M.; Berndt, C.C.; Sesso, M.L.; Anupam, A.S.P.; Kottada, R.S.; Murty, B.S. Plasma-sprayed high entropy alloys: Microstructure and properties of AlCoCrFeNi and MnCoCrFeNi. Metall. Mater. Trans. 2015, 46, 791–800. [Google Scholar] [CrossRef]
- Niu, X.; Singh, S.; Garg, A.; Singh, H.; Panda, B.; Peng, X.; Zhang, Q. Review of materials used in laser-aided additive manufacturing processes to produce metallic products. Front. Mech. Eng. 2019, 14, 282–298. [Google Scholar] [CrossRef]
- Mazurkiewicz, A.; Smolik, J. Comparative analysis of wear mechanism of different types of forging dies. Arch. Mater. Sci. Eng. 2011, 49, 40–45. [Google Scholar]
- Lin, L.; Li, G.-L.; Wang, H.-D.; Kang, J.-J.; Xu, Z.-L.; Wang, H.-J. Structure and wear behavior of NiCr–Cr3C2 coatings sprayed by supersonic plasma spraying and high velocity oxy-fuel technologies. Appl. Surf. Sci. 2015, 356, 383–390. [Google Scholar] [CrossRef]
- Bolelli, G.; Berger, L.-M.; Börner, T.; Koivuluoto, H.; Matikainen, V.; Lusvarghi, L.; Lyphout, C.; Markocsan, X.; Nylén, P.; Sassatelli, P.; et al. Sliding and abrasive wear behaviour of HVOF and HVAF-sprayed Cr3C2–NiCr hard metal coatings. Wear 2016, 358–359, 32–50. [Google Scholar] [CrossRef]
- Singh, H.; Sidhu, T.S.; Karthikeyan, J.; Kalsi, S.B.S. Development and characterization of Cr3C2–NiCr coated superalloy by novel cold spray process. Mater. Manuf. Process. 2015, 31, 1476–1482. [Google Scholar] [CrossRef]
- Vidaller, M.V.; List, A.; Gaertner, F.; Klassen, T.; Dosta, S.; Guilemany, J.M. Single impact bonding of cold sprayed Ti-6Al-4V powders on different substrates. J. Therm. Spray Technol. 2015, 24, 644–658. [Google Scholar] [CrossRef]
- Ziemian, C.W.; Wright, W.J.; Cipoletti, D.E. Influence of impact conditions on feedstock deposition behavior of cold-sprayed Fe-based metallic glass. J. Therm. Spray Technol. 2018, 27, 843–856. [Google Scholar] [CrossRef]
- Sevillano, F.; Poza, P.; Munez, C.J.; Vezzu, S.; Rech, S.; Trentin, A. Cold-sprayed Ni-Al2O3 coatings for applications in power generation industry. J. Therm. Spray Techn. 2013, 22, 772–782. [Google Scholar] [CrossRef]
- Luo, X.-T.; Li, Y.-J.; Li, C.-J. A comparison of cold spray deposition behavior between gas atomized and dendritic porous electrolytic Ni powders under the same spray conditions. Mater. Lett. 2016, 163, 58–60. [Google Scholar] [CrossRef]
- Souza, R.C.; Voorwald, H.J.C.; Cioffi, M.O.H. Fatigue strength of HVOF sprayed Cr3C2-25CrNi and WC-10Ni on AISI 4340 steel. Surf. Coat. Technol. 2008, 203, 191–198. [Google Scholar] [CrossRef]
- Varis, T.; Suhonen, T.; Calonius, O.; Cuban, J.; Pietola, M. Optimization of HVOF Cr3C2NiCr coating for increased fatigue performance. Surf. Coat. Technol. 2016, 305, 123–131. [Google Scholar] [CrossRef]
- Matikainen, V.; Koivuluoto, H.; Vuoristo, P. A study of Cr3C2-based HVOF- and HVAF-sprayed coatings: Abrasion, dry particle erosion and cavitation erosion resistance. Wear 2020, 446–447, 20318. [Google Scholar] [CrossRef]
- Luiz, L.A.; de Andrade, J.; Pesqueira, C.M.; Siqueira, I.B.D.A.F.; Sucharski, G.B.; de Sousa, M.J. Corrosion behavior and galvanic corrosion resistance of WC and Cr3C2 cermet coatings in Madeira river water. J. Therm. Spray Technol. 2021, 30, 205–221. [Google Scholar] [CrossRef]
- Scendo, M.; Zorawski, W.; Staszewska-Samson, K.; Goral, A. Influence of laser treatment on the corrosion resistance of Cr3C2-25(Ni20Cr) cermet coating. Materials 2021, 14, 4078. [Google Scholar] [CrossRef]
- Scendo, M.; Żórawski, W. Corrosion properties of cold-sprayed Cr3C2-25(Ni20Cr) coatings after heat treatment. Materials 2024, 17, 6289. [Google Scholar] [CrossRef]
- Bergant, Z.; Trdan, U.; Grum, J. Effect of high-temperature furnace treatment on the microstructure and corrosion behavior of NiCrBSi flame-sprayed coatings. Corros. Sci. 2014, 88, 372–386. [Google Scholar] [CrossRef]
- Zheng, Z.B.; Zheng, Y.G.; Sun, W.H.; Wang, J.Q. Erosion–corrosion of HVOF-sprayed Fe-based amorphous metallic coating under impingement by a sand-containing NaCl solution. Corros. Sci. 2013, 76, 337–347. [Google Scholar] [CrossRef]
- Sadeghimeresht, E.; Markocsan, N.; Nylén, P.; Björklund, S. Corrosion performance of bi-layer Ni/Cr2C3–NiCr HVAF thermal spray coating. Appl. Surf. Sci. 2016, 369, 470–481. [Google Scholar] [CrossRef]


| Sample Name | Microhardness HV10 |
|---|---|
| Cr3C2-25(Ni20Cr)-0.05 | 358 ± 2 |
| Cr3C2-25(Ni20Cr)-0.10 | 385 ± 1 |
| Cr3C2-25(Ni20Cr)-0.15 | 409 ± 3 |
| Sample Name | EOCP mV vs. SCE(KCl) |
|---|---|
| Cr3C2-25(Ni20Cr) | −682 |
| Cr3C2-25(Ni20Cr)-0.05 | −662 |
| Cr3C2-25(Ni20Cr)-0.10 | −586 |
| Cr3C2-25(Ni20Cr)-0.15 | −518 |
| Sample Name | Ecorr | jcorr | −bc | ba |
|---|---|---|---|---|
| mV vs. SCE | mA cm−2 | mV dec−1 | ||
| Cr3C2-25(Ni20Cr) | −682 | 8.0 | 260 | 270 |
| Cr3C2-25(Ni20Cr)-0.05 | −662 | 7.0 | 190 | 310 |
| Cr3C2-25(Ni20Cr)-0.10 | −586 | 5.5 | 180 | 350 |
| Cr3C2-25(Ni20Cr)-0.15 | −518 | 3.0 | 140 | 370 |
| Sample Name | Rp Ω cm2 |
|---|---|
| Cr3C2-25(Ni20Cr) | 7189 |
| Cr3C2-25(Ni20Cr)-0.05 | 7307 |
| Cr3C2-25(Ni20Cr)-0.10 | 9384 |
| Cr3C2-25(Ni20Cr)-0.15 | 14,701 |
| Sample Name | CW mg/Year |
|---|---|
| Cr3C2-25(Ni20Cr) | 452 |
| Cr3C2-25(Ni20Cr)-0.05 | 396 |
| Cr3C2-25(Ni20Cr)-0.10 | 311 |
| Cr3C2-25(Ni20Cr)-0.15 | 170 |
| Sample Name | DC |
|---|---|
| Cr3C2-25(Ni20Cr)-0.05 | 0.13 |
| Cr3C2-25(Ni20Cr)-0.10 | 0.31 |
| Cr3C2-25(Ni20Cr)-0.15 | 0.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scendo, M. Influence of Sulfide Concentration on the Properties of Cr3C2-25(Ni20Cr) Cermet Coating on Al7075 Substrate. Metals 2025, 15, 273. https://doi.org/10.3390/met15030273
Scendo M. Influence of Sulfide Concentration on the Properties of Cr3C2-25(Ni20Cr) Cermet Coating on Al7075 Substrate. Metals. 2025; 15(3):273. https://doi.org/10.3390/met15030273
Chicago/Turabian StyleScendo, Mieczyslaw. 2025. "Influence of Sulfide Concentration on the Properties of Cr3C2-25(Ni20Cr) Cermet Coating on Al7075 Substrate" Metals 15, no. 3: 273. https://doi.org/10.3390/met15030273
APA StyleScendo, M. (2025). Influence of Sulfide Concentration on the Properties of Cr3C2-25(Ni20Cr) Cermet Coating on Al7075 Substrate. Metals, 15(3), 273. https://doi.org/10.3390/met15030273






