Arc Erosion Properties of the Ag-Cr2AlC Contact Material
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Sample Preparation
2.2. Arc Erosion Test
3. Results and Discussion
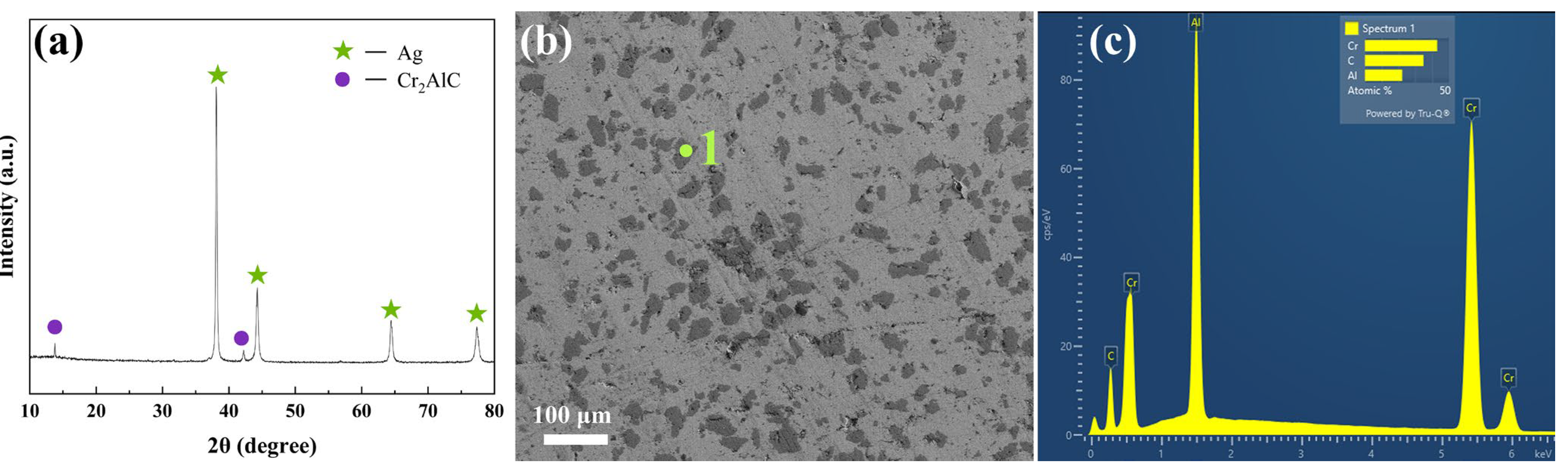
3.1. Physical Properties
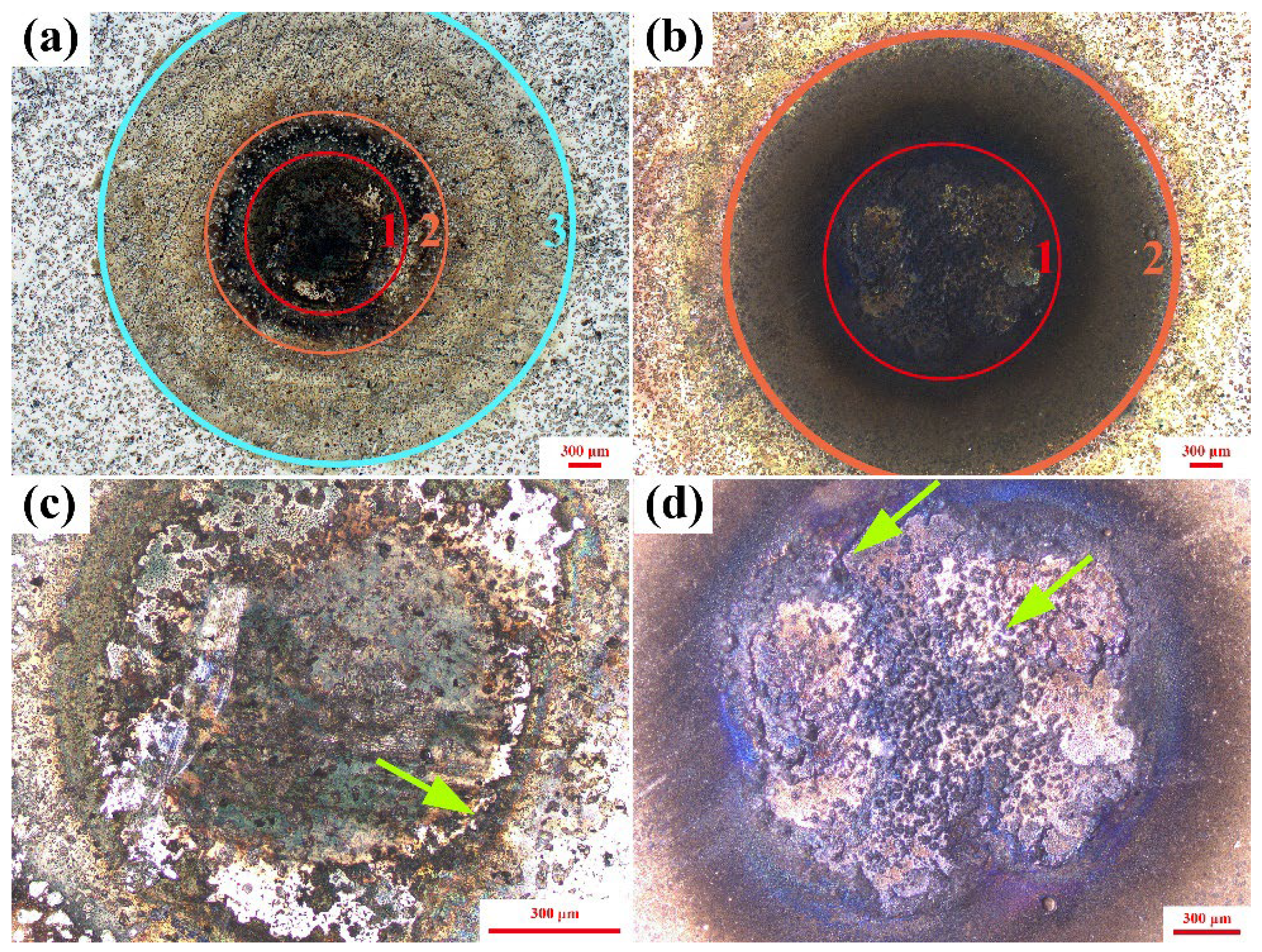
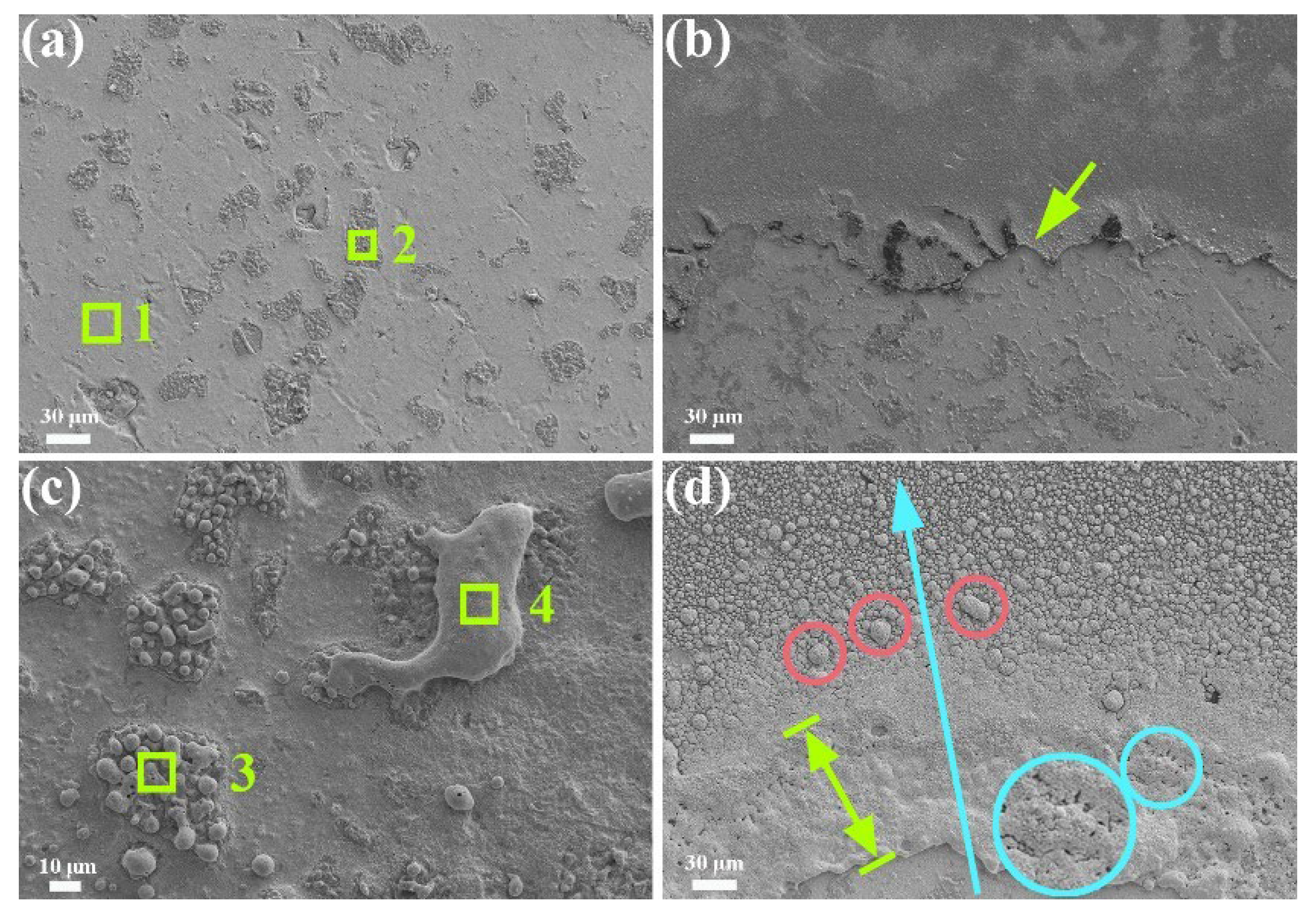
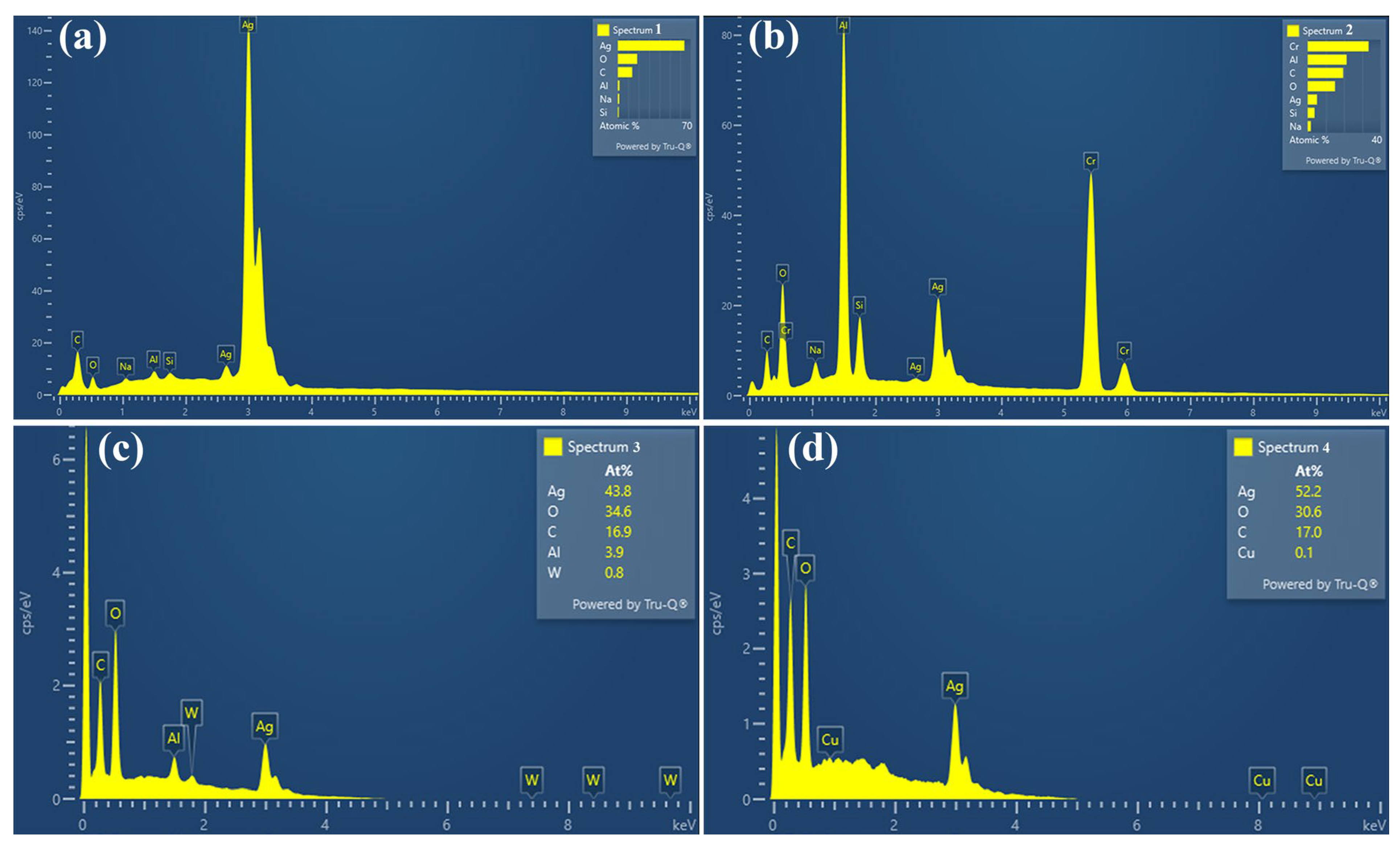
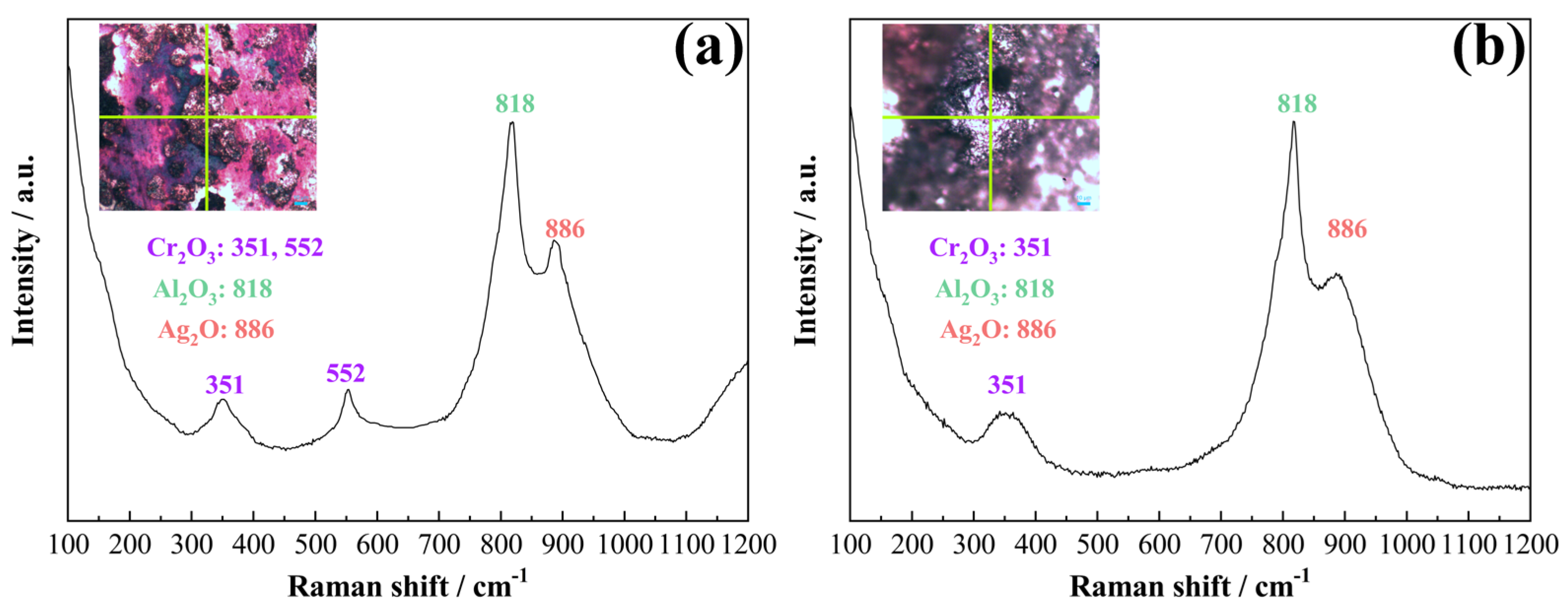
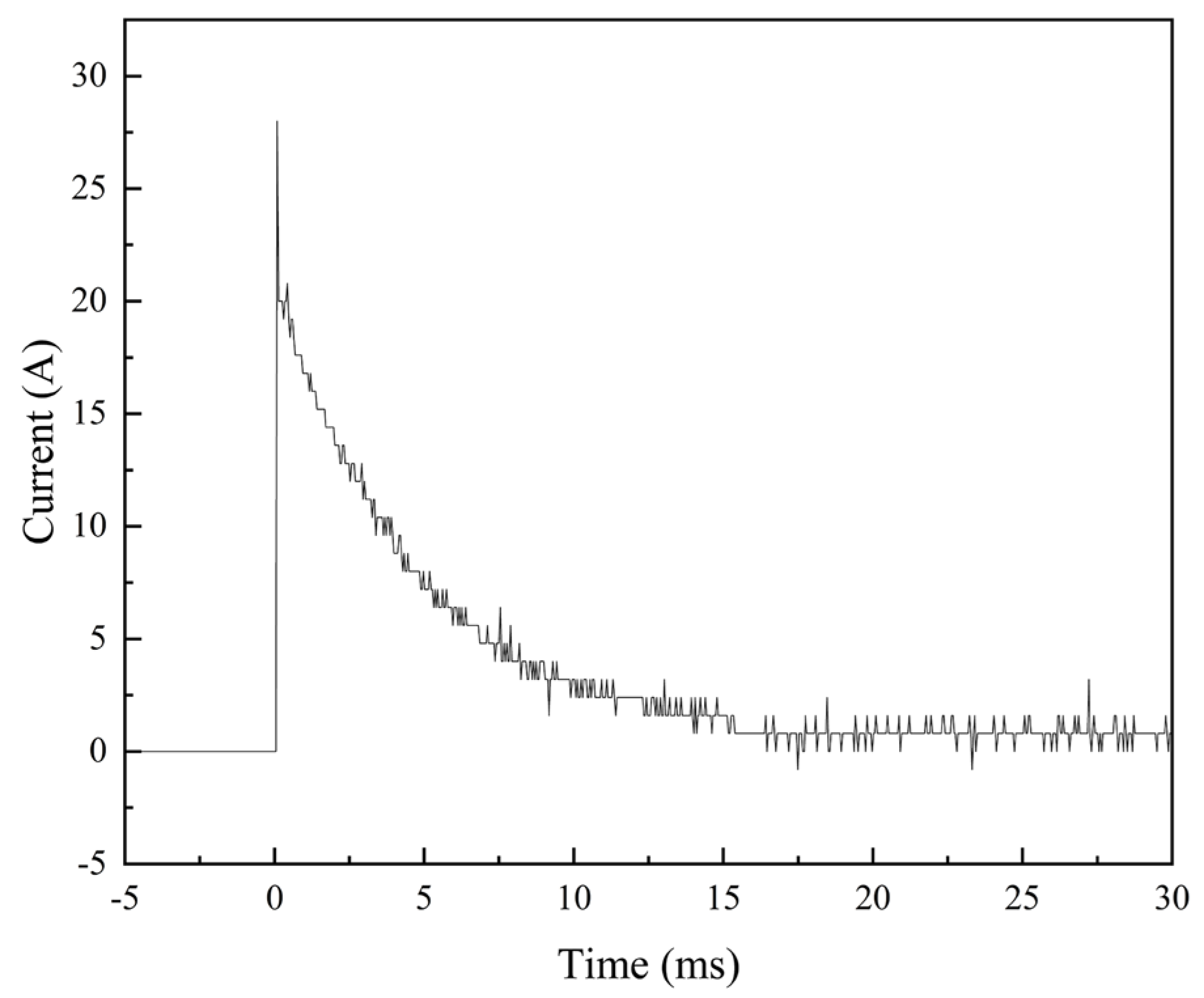
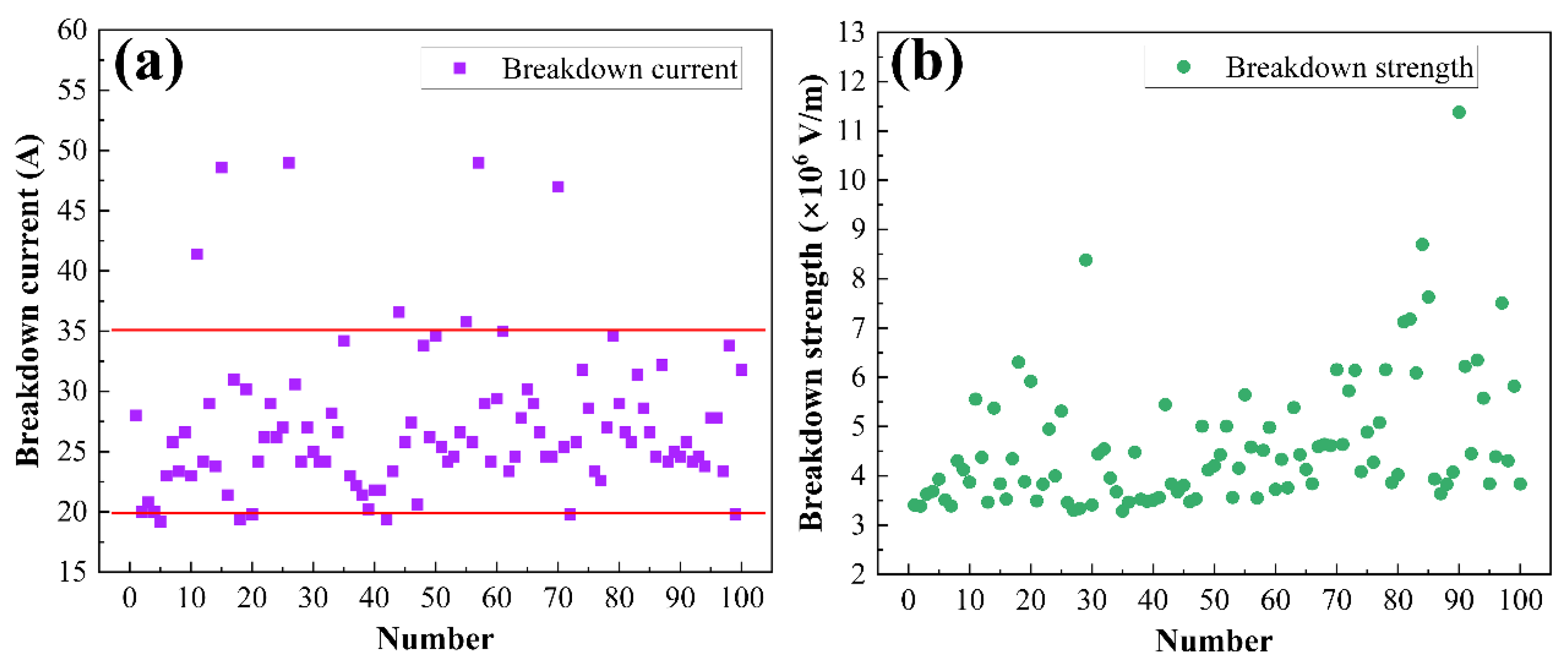
3.2. Arc Erosion Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nian, Y.H.; Zhang, Z.Y.; Liu, M.M.; Zhou, X.L. The electronic structure and stability of Ti2SnC/Ag interface studied by first-principles calculations. Adv. Theory Simul. 2023, 7, 2300649. [Google Scholar] [CrossRef]
- Ren, W.; Zheng, Z.; Zhang, C.; Liu, J.; Liu, X. Characterization method for electric arc erosion based on the in-situ measurement of electrode surface morphology. Measurement 2023, 221, 113501. [Google Scholar] [CrossRef]
- Sone, H.; Takagi, T. Role of the metallic phase arc discharge on arc erosion in Ag contacts. IEEE Trans. Compon. Hybrids. Manuf. Technol. 1990, 13, 13–19. [Google Scholar] [CrossRef]
- Pons, F.; Cherkaoui, M.; Ilali, I.; Dominiak, S. Evolution of the AgCdO contact material surface microstructure with the number of arcs. J. Electron. Mater. 2010, 39, 456–463. [Google Scholar] [CrossRef]
- Wang, H.; Cai, Q.; Wang, J.; Zhang, Y.; Hu, D.; Wang, Y. First-principles and experimental investigations on physical properties and arc erosion behavior of metal-doped AgSnO2 electrical contact materials. Ceram. Int. 2023, 49, 26033–26048. [Google Scholar] [CrossRef]
- Li, G.; Ma, Y.; Zhang, X.; Fang, X.; Feng, W. Interface strengthening and fracture characteristics of the Ag-based contact materials reinforced with nanoporous SnO2(Cu, CuO) phases. Appl. Surf. Sci. 2021, 543, 148812. [Google Scholar] [CrossRef]
- Feng, W.; Lu, H.; Li, G. Electrical contact performances of Ag-SnO2(CuO)/Cu-SnO2(CuO) heterogeneous materials prepared by hot-press sintering. Ceram. Int. 2025. [Google Scholar] [CrossRef]
- Yang, M.; Xie, M.; Ning, D.; Chen, S.; Yang, Y. Research progress of AgCu electrical contact materials. Precious Met. 2024, 45, 1–6. [Google Scholar]
- Li, H.; Cao, Q.; Zheng, J.; Sun, L.; Rong, W. Enhancing the electrical performance of AgNi contact material by doping Ag2WO4. Mater. Lett. 2024, 372, 137009. [Google Scholar] [CrossRef]
- Kesim, M.T.; Yu, H.; Sun, Y.; Aindow, M.; Alpay, S.P. Corrosion, oxidation, erosion and performance of Ag/W-based circuit breaker contacts: A review. Corros. Sci. 2018, 135, 12–34. [Google Scholar] [CrossRef]
- Wu, C.; Wu, X.; Ding, J.; Ding, K.; Zhang, P.; Ma, C.; Hu, D.; Liu, D.; Zhang, S.; Zheng, W.; et al. Achieving highly conductive Ag/Ti3AlC2 composite by inhibiting interdiffusion. Scripta. Mater. 2025, 255, 116343. [Google Scholar] [CrossRef]
- Radovic, M.; Barsoum, M.W. MAX phases: Bridging the gap between metals and ceramics. Am. Ceram. Soc. Bull. 2013, 92, 20–27. [Google Scholar]
- Barsoum, M.W. The MN+1AXN phases: A new class of solids: Thermodynamically stable nanolaminates. Prog. Solid. State. Chem. 2000, 28, 201–281. [Google Scholar] [CrossRef]
- Ding, J.; Tian, W.; Wang, D.; Zhang, P.; Chen, J.; Sun, Z. Arc erosion and degradation mechanism of Ag/Ti2AlC composite. Acta Metall. Sin. 2019, 55, 627–637. [Google Scholar]
- Wang, D.; Tian, W.; Ma, A.; Ding, J.; Wang, C.; You, Y.; Zhang, P.; Chen, J.; Zhang, Y.; Sun, Z. Anisotropic properties of Ag/Ti3AlC2 electrical contact materials prepared by equal channel angular pressing. J. Alloys Compd. 2019, 784, 431–438. [Google Scholar] [CrossRef]
- Wu, X.L.; Wu, C.Z.; Wei, X.P.; Sun, W.J.; Ma, C.J.; Zhang, Y.D.; Li, G.G.; Chen, L.M.; Wang, D.D.; Zhang, P.G.; et al. Influence of nano-mechanical evolution of Ti3AlC2 ceramic on the arc erosion resistance of Ag-based composite electrical contact material. J. Adv. Ceram. 2024, 13, 176–188. [Google Scholar] [CrossRef]
- Ding, K.; Ding, J.; Zhang, K.; Chen, L.; Ma, C.; Bai, Z.; Zhang, P.; Sun, Z. Micro/nano-mechanical properties evolution and degradation mechanism of Ti3AlC2 ceramic reinforced Ag-based composites under high-temperature arc corrosion. Ceram. Int. 2022, 48, 33670–33681. [Google Scholar] [CrossRef]
- Huang, X.C.; Feng, Y.; Qian, G.; Zhou, Z.J. Arc ablation properties of Ti3SiC2 material. Ceram. Int. 2019, 45, 20297–20306. [Google Scholar] [CrossRef]
- Zhou, Z.; Feng, Y.; Zhao, H.; Qian, G.; Zhang, J.; Zhang, X.; Huang, X. Arc erosion behavior and mechanism of Ag/Ti3SiC2 cathodes in different atmospheres. Ceram. Int. 2021, 47, 2319–2328. [Google Scholar] [CrossRef]
- Huang, X.; Li, L.; Ge, J.; Zhao, H.; Zhou, Z. Investigation on the arc erosion performance of Ag–Ta2AlC composite under air conditions. J. Adv. Ceram. 2024, 13, 1359–1368. [Google Scholar] [CrossRef]
- Gomes, A.S.O.; Yaghini, N.; Martinelli, A.; Ahlberg, E. A micro-Raman spectroscopic study of Cr(OH)3 and Cr2O3 nanoparticles obtained by the hydrothermal method. J. Raman Spectrosc. 2017, 48, 1256–1263. [Google Scholar] [CrossRef]
- Larbi, T.; Ouni, B.; Gantassi, A.; Doll, K.; Amlouk, M.; Manoubi, T. Structural, optical andvibrational properties of Cr2O3 with ferromagnetic and antiferromagnetic order: A combined experimental and density functional theory study. J. Magn. Magn. Mater. 2017, 444, 16–22. [Google Scholar] [CrossRef]
- Anjum, M.; Kumar, R.; Barakat, M.A. Visible light driven photocatalytic degradation of organic pollutants in wastewater and real sludge using ZnO–ZnS/Ag2O–Ag2S nanocomposite. J. Taiwan Inst. Chem. Eng. 2017, 77, 227–235. [Google Scholar] [CrossRef]
- Meek, J.M.; Craggs, J.D. Electrical Breakdown of Gases; Oxford University Press: Oxford, UK, 1953. [Google Scholar]
- Miller, H.C.; Farrall, G.A. Polarity effect in vacuum breakdown electrode conditioning. J. Appl. Phys. 1965, 36, 1338–1344. [Google Scholar] [CrossRef]
- Wang, X.; Liang, S.; Yang, P.; Fan, Z. Effect of milling time on electrical breakdown behavior of Al2O3/Cu composite. J. Mater. Eng. Perform. 2010, 19, 906–911. [Google Scholar] [CrossRef]
- Bingjun, D.; Zhimao, Y.; Xiaotian, W. Influence of microstructure on dielectric strength of CuCr contact materials in a vacuum. IEEE Trans. Compon. Packag. Manuf. Technol. Part A 1996, 19, 76–81. [Google Scholar] [CrossRef]
- Dong, L.; Chen, W.; Deng, N.; Song, J.; Wang, J. Investigation on arc erosion behaviors and mechanism of W70Cu30 electrical contact materials adding graphene. J. Alloys Compd. 2017, 696, 923–930. [Google Scholar] [CrossRef]
- Jensen, K.L. Electron emission theory and its application: Fowler–Nordheim equation and beyond. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. Process. Meas. Phenom. 2003, 21, 1528–1544. [Google Scholar] [CrossRef]
- Koneru, A.; Karmarkar, N.; Wang, Y.; Mohanty, P.S.; Li, B.Q. Evaluation of cold-sprayed CuCr coatings as contact materials for vacuum interrupters. Vacuum 2023, 216, 112384. [Google Scholar] [CrossRef]


| Test Point | d1 (μm) | d2 (μm) | d (μm) | Hardness (HV) |
|---|---|---|---|---|
| (a) | 55.346 | 55.994 | 55.67 | 59.8 |
| (b) | 55.188 | 55.404 | 55.296 | 60.6 |
| (c) | 54.972 | 54.109 | 54.5405 | 62.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Ge, J.; Zhou, Z.; Chen, B.; Zhuo, F.; Zhang, H. Arc Erosion Properties of the Ag-Cr2AlC Contact Material. Metals 2025, 15, 293. https://doi.org/10.3390/met15030293
Huang X, Ge J, Zhou Z, Chen B, Zhuo F, Zhang H. Arc Erosion Properties of the Ag-Cr2AlC Contact Material. Metals. 2025; 15(3):293. https://doi.org/10.3390/met15030293
Chicago/Turabian StyleHuang, Xiaochen, Jinlong Ge, Zijue Zhou, Bin Chen, Feng Zhuo, and Hongdi Zhang. 2025. "Arc Erosion Properties of the Ag-Cr2AlC Contact Material" Metals 15, no. 3: 293. https://doi.org/10.3390/met15030293
APA StyleHuang, X., Ge, J., Zhou, Z., Chen, B., Zhuo, F., & Zhang, H. (2025). Arc Erosion Properties of the Ag-Cr2AlC Contact Material. Metals, 15(3), 293. https://doi.org/10.3390/met15030293







