In Situ Observation by X-Ray Radioscopy of Liquid Decomposition During Directional Solidification of Al-Cu-Sn Alloys
Abstract
:1. Introduction
2. Materials and Methods
2.1. Thermodynamic Calculations
2.2. Differential Scanning Calorimetry
2.3. In Situ X-Ray Radioscopy Applied to Solidification Experiments
2.3.1. Experimental Procedure
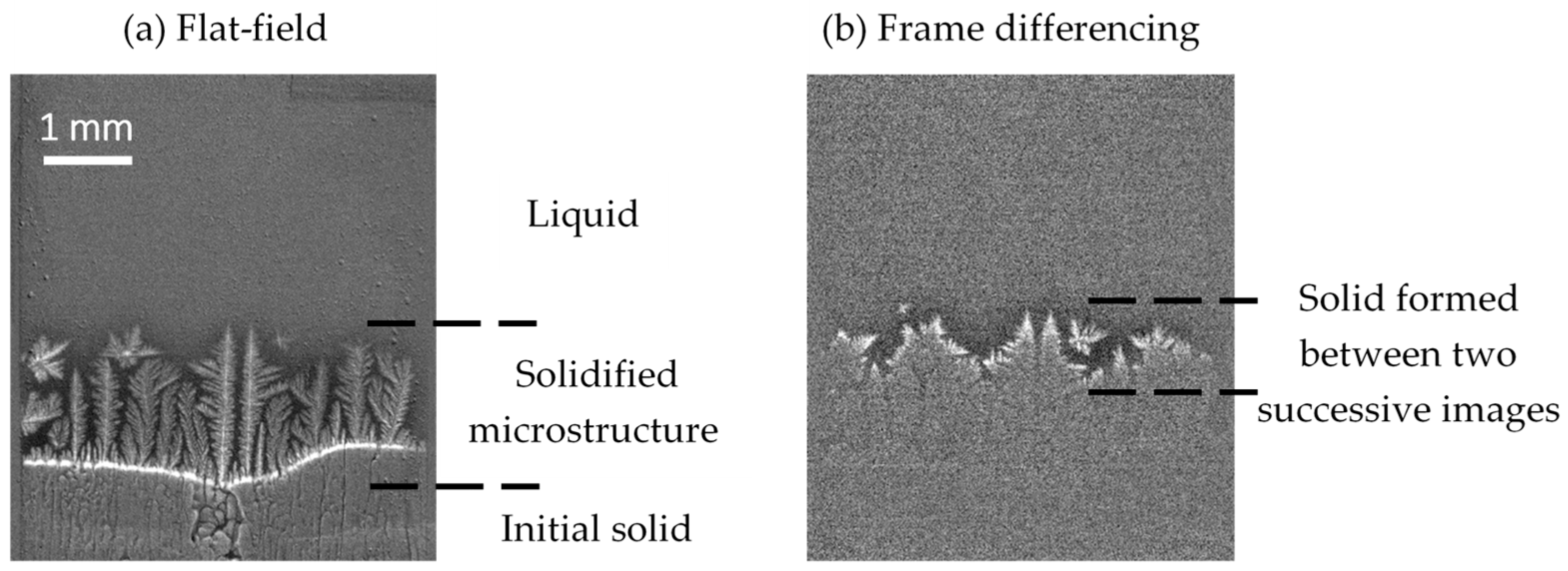
2.3.2. Image Processing
3. Results
3.1. Solidification Path Calculations
3.2. Solidification Sequence Determined by Differential Scanning Calorimetry
3.3. Dynamics of Al-Cu-Sn Solidification Processes
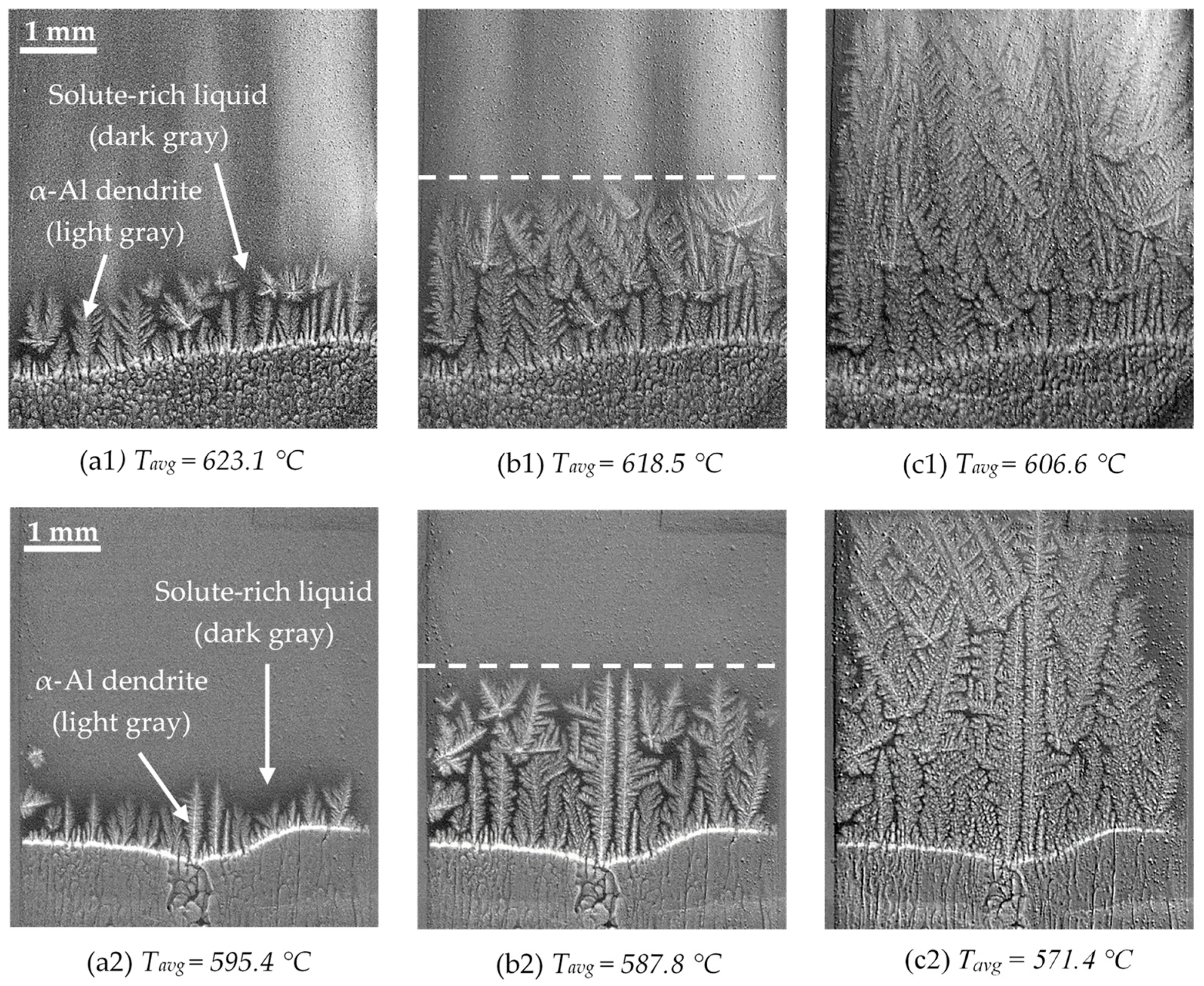
3.3.1. First Step: Columnar Growth of α-Al Dendrites
3.3.2. Second and Third Steps: Liquid-Phase Demixing and Monotectic Reaction
- Al-10Cu-10Sn alloy
- Al-10Cu-20Sn alloy
3.3.3. Third Step: Final Eutectic Reaction
4. Discussion
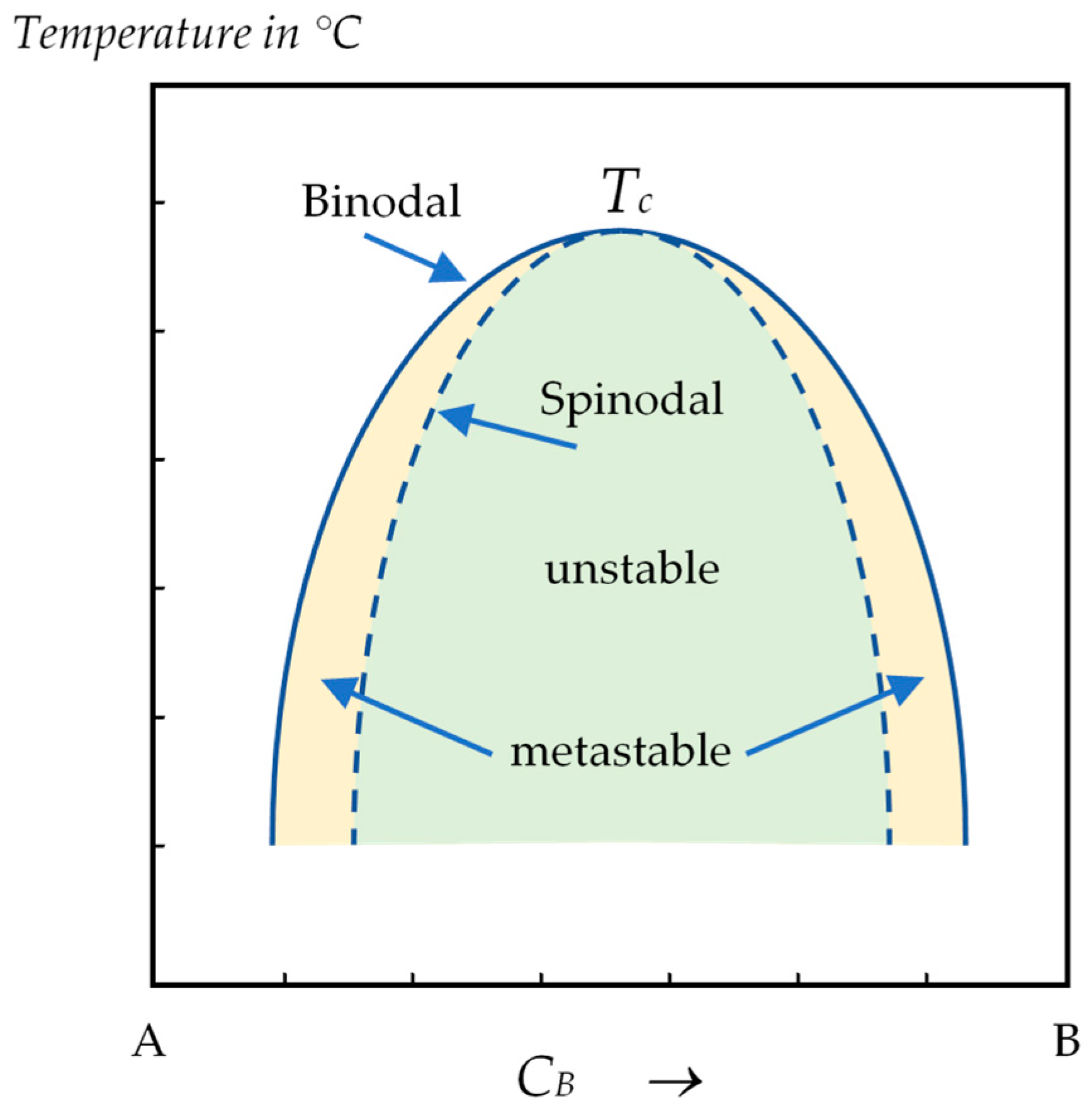
- In the region between the binodal and the spinodal curves, the uniform liquid is assumed to be metastable. Therefore, the formation of Sn droplets requires a certain amount of energy, which can be greatly reduced by the help of inhomogeneities or impurities in the system (heterogeneous nucleation). In our experiments, owing to the thickness of the sample, the internal oxide layer of the sample could provide some active sites, like for the nucleation and growth of equiaxed grains [27,28]. Nevertheless, the nucleation and growth of Sn droplets remains rare;
- For liquid compositions within the spinodal dome, the homogeneous solution is unstable against microscopic fluctuations in density or composition. Therefore, the liquid demixing will take place immediately, with no thermodynamic barrier to the growth of a new phase and the phase transformation is solely diffusion-controlled. In that case, the Sn-rich liquid may appear in the form of clouds and in large quantities.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ran, G.; Zhou, J.-E.; Xi, S.; Li, P. Microstructure and morphology of Al–Pb bearing alloy synthesized by mechanical alloying and hot extrusion. J. Alloys Compd. 2006, 419, 66–70. [Google Scholar] [CrossRef]
- Yang, Q.; Sun, Z.P.; Wang, Z.M. Effects of Sn, Si, and Cu addition on the microstructure and properties of hypermonotectic Al92Bi8 alloy. Mater. Sci. Eng. A 2019, 756, 71–81. [Google Scholar] [CrossRef]
- Lu, W.; Zhang, S.; Li, J. Segregation driven by collision and coagulation of minor droplets in Al–Bi immiscible alloys under aerodynamic levitation condition. Mater. Lett. 2013, 107, 340–343. [Google Scholar] [CrossRef]
- Lu, W.; Zhang, S.; Zhang, W.; Kaptay, G.; Yu, J.; Fu, Y.; Li, J. Direct observation of the segregation driven by bubble evolution and liquid phase separation in Al–10 wt.% Bi immiscible alloy. Scr. Mater. 2015, 102, 19–22. [Google Scholar] [CrossRef]
- Lu, Z.C.; Zeng, M.; Gao, Y.; Zhu, M. Significant improvement of wear properties by creating micro/nano dual-scale structure in Al-Sn alloys. Wear 2012, 296, 469–478. [Google Scholar] [CrossRef]
- Kotadia, H.R.; Patel., J.B.; Fan, Z.Y.; Doernberg, E.; Schmid-Fetzer, R. Processing of Al-45Sn-10Cu Based Immiscible Alloy by a Rheomixing Process. In Proceedings of the 10th International Conference on Semi-Solid Processing of Alloys and Composites, Aachen, Germany, 16–18 September 2008; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2008. [Google Scholar]
- Kotadia, H.R.; Das, A.; Doernberg, E.; Schmid-Fetzer, R. A comparative study of ternary Al-Sn-Cu immiscible alloys prepared by conventional casting and casting under high-intensity ultrasonic irradiation. Mater. Chem. Phys. 2011, 131, 241–249. [Google Scholar] [CrossRef]
- Bertelli, F.; Brito, C.; Ferreira, I.L.; Reinhart, G.; Nguyen-Thi, H.; Mangelinck-Noël, N.; Cheung, N.; Garcia, A. Cooling thermal parameters, microstructure, segregation and hardness in directionally solidified Al-Sn-(Si;Cu) alloys. Mater. Des. 2015, 72, 31–42. [Google Scholar] [CrossRef]
- Bertelli, F.; Freitas, E.S.; Cheung, N.; Arenas, M.A.; Conde, A.; de Damborenea, J.; Garcia, A. Microstructure, tensile properties and wear resistance correlations on directionally solidified Al-Sn-(Cu; Si) alloys. J. Alloys Compd. 2017, 695, 3621–3631. [Google Scholar] [CrossRef]
- Li, S.H.; Li, Y.; Guo, H.; Wen, Z.; Zhu, Z. Effect of electromagnetic stirring frequency on tribological performance and corrosion resistance of Al-Sn bearing alloy. Mater. Today Commun. 2022, 32, 14. [Google Scholar] [CrossRef]
- Xu, M.; Yin, Y.-G.; Li, C.-M.; Duan, C.-C. A comparative study on Sn macrosegregation behavior of ternary Al-Sn-Cu alloys prepared by gravity casting and squeeze casting. China Foundry 2023, 20, 63–70. [Google Scholar] [CrossRef]
- Bykov, P.A.; Kalashnikov, I.; Kobeleva, L.; Katin, I.; Mikheev, R. Mapping wear modes of composite materials with intermetallic reinforcing based on antifrictional alloy of system Al-Sn-Cu. Lett. Mater. 2021, 11, 181–186. [Google Scholar] [CrossRef]
- Karagadde, S.; Leung, C.L.A.; Lee, P.D. Progress on In Situ and Operando X-ray Imaging of Solidification Processes. Materials 2021, 14, 2374. [Google Scholar] [CrossRef] [PubMed]
- Xiang, K.; Qin, L.; Zhao, Y.; Huang, S.; Du, W.; Boller, E.; Rack, A.; Li, M.; Mi, J. Operando study of the dynamic evolution of multiple Fe-rich intermetallics of an Al recycled alloy in solidification by synchrotron X-ray and machine learning. Acta Mater. 2024, 279, 120267. [Google Scholar] [CrossRef]
- Han, I.; Feng, S.; Wilde, F.; Grant, P.S.; Liotti, E. Revealing hot tear formation dynamics in Al–Cu alloys with X-ray radiography. Acta Mater. 2024, 262, 119421. [Google Scholar] [CrossRef]
- Qin, L.; Du, W.; Cipiccia, S.; Bodey, A.J.; Rau, C.; Mi, J. Synchrotron X-ray operando study and multiphysics modelling of the solidification dynamics of intermetallic phases under electromagnetic pulses. Acta Mater. 2024, 265, 119593. [Google Scholar] [CrossRef]
- Becker, M.; Klein, S.; Kargl, F. In-situ solute measurements with a laboratory polychromatic microfocus X-ray source during equiaxed solidification of an Al-Ge alloy. Scr. Mater. 2016, 124, 34–37. [Google Scholar] [CrossRef]
- Shevchenko, N.; Roshchupkina, O.; Sokolova, O.; Eckert, S. The effect of natural and forced melt convection on dendritic solidification in Ga-In alloys. J. Cryst. Growth 2015, 417, 1–8. [Google Scholar] [CrossRef]
- Reinhart, G.; Browne, D.J.; Kargl, F.; García-Moreno, F.; Becker, M.; Sondermann, E.; Binder, K.; Mullen, J.S.; Zimmermann, G.; Mathiesen, R.H.; et al. In-situ X-ray monitoring of solidification and related processes of metal alloys. Npj Microgravity 2023, 9, 70. [Google Scholar] [CrossRef]
- Werner, T.; Becker, M.; Baumann, J.; Xiao, X.; Pickmann, C.; Sturz, L.; Brillo, J.; Kargl, F. In situ observation of the cryophile migration of hydrogen bubbles in Al-alloys during directional melting and the impact of surface tension. Acta Mater. 2022, 224, 117503. [Google Scholar] [CrossRef]
- Schaffer, P.L.; Mathiesen, R.H.; Arnberg, L.; Di Sabatino, M.; Snigirev, A. In situ investigation of spinodal decomposition in hypermonotectic Al–Bi and Al–Bi–Zn alloys. New J. Phys. 2008, 10, 053001. [Google Scholar] [CrossRef]
- Schaffer, P.L.; Mathiesen, R.H.; Arnberg, L. L-2 droplet interaction with alpha-Al during solidification of hypermonotectic Al-8 wt.% Bi alloys. Acta Mater. 2009, 57, 2887–2895. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Y.; Tang, Y.; Ma, J.; Mao, B.; Xue, Y.; Xing, H.; Zhang, J.; Sun, B. In situ study on the oscillation of mobile droplets and force analysis during the directional solidification of Al-Bi alloy. J. Mater. Res. Technol. 2024, 177, 1–9. [Google Scholar] [CrossRef]
- De Albuquerque, S.; Reinhart, G.; Magalhães, D.C.C.; Spinelli, J.E.; Soltani, H.; Boumechta, W.; Bertelli, F.; Nguyen-Thi, H. Solidification paths of Al-Cu-Sn alloys: Comparison of thermodynamic analyses and solidification experiments using in situ X-radiography. Mater. Today Commun. 2024, 40, 109545. [Google Scholar] [CrossRef]
- Thermo-Calc Software: Computational Materials Engineering. Available online: https://thermocalc.com (accessed on 3 March 2025).
- Jain, S.; Patel, M.; Kumar, V.; Samal, S. Effect of Si on Phase Equilibria, Mechanical Properties and Tribological Behaviour of Al-Cu Alloy. Silicon 2023, 15, 1807–1820. [Google Scholar] [CrossRef]
- Soltani, H.; Ngomesse, F.; Reinhart, G.; Benoudia, M.C.; Zahzouh, M.; Nguyen-Thi, H. Impact of gravity on directional solidification of refined Al-20wt.%Cu alloy investigated by in situ X-radiography. J. Alloys Compd. 2021, 862, 158028. [Google Scholar] [CrossRef]
- Soltani, H.; Reinhart, G.; Benoudia, M.C.; Zahzouh, M.; Nguyen-Thi, H. Impact of gravity-related phenomena on the grain structure formation: Comparative study between horizontal and vertical solidification of a refined Al-20wt.%Cu alloy. IOP Conf. Ser. Mater. Sci. Eng. 2019, 529, 012019. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Hecht, U.; Gránásy, L.; Pusztai, T.; Böttger, B.; Apel, M.; Witusiewicz, V.; Ratke, L.; De Wilde, J.; Froyen, L.; Camel, D.; et al. Multiphase solidification in multicomponent alloys. Mat. Sci. Eng. R 2004, 46, 1–49. [Google Scholar] [CrossRef]
- Zhang, D.; Atkinson, H.V.; Dong, H.; Zhu, Q. Differential Scanning Calorimetry (DSC) and Thermodynamic Prediction of Liquid Fraction vs Temperature for Two High-Performance Alloys for Semi-Solid Processing (Al-Si-Cu-Mg (319s) and Al-Cu-Ag (201)). Metall. Mater. Trans. A 2017, 48, 4701–4712. [Google Scholar] [CrossRef]
- Kočí, V.; Fort, J.; Madera, J.; Scheinherrova, L.; Trnik, A.; Cerny, R. Correction of Errors in DSC Measurements Using Detailed Modeling of Thermal Phenomena in Calorimeter-Sample System. IEEE Trans. Instrum. Meas. 2020, 69, 8178–8186. [Google Scholar] [CrossRef]
- Höhne, G.W.H.; Cammenga, H.; Eysel, W.; Gmelin, E.; Hemminger, W. The temperature calibration of scanning calorimeters. Thermochim. Acta 1990, 160, 1–12. [Google Scholar] [CrossRef]
- Ghanbari, E.; Picken, S.J.; van Esch, J.H. Analysis of differential scanning calorimetry (DSC): Determining the transition temperatures, and enthalpy and heat capacity changes in multicomponent systems by analytical model fitting. J. Therm. Anal. Calorim. 2023, 148, 12393–12409. [Google Scholar] [CrossRef]
- Ratke, L.; Diefenbach, S. Liquid immiscible alloys. Mat. Sci. Eng. R 1995, 15, 263–347. [Google Scholar] [CrossRef]



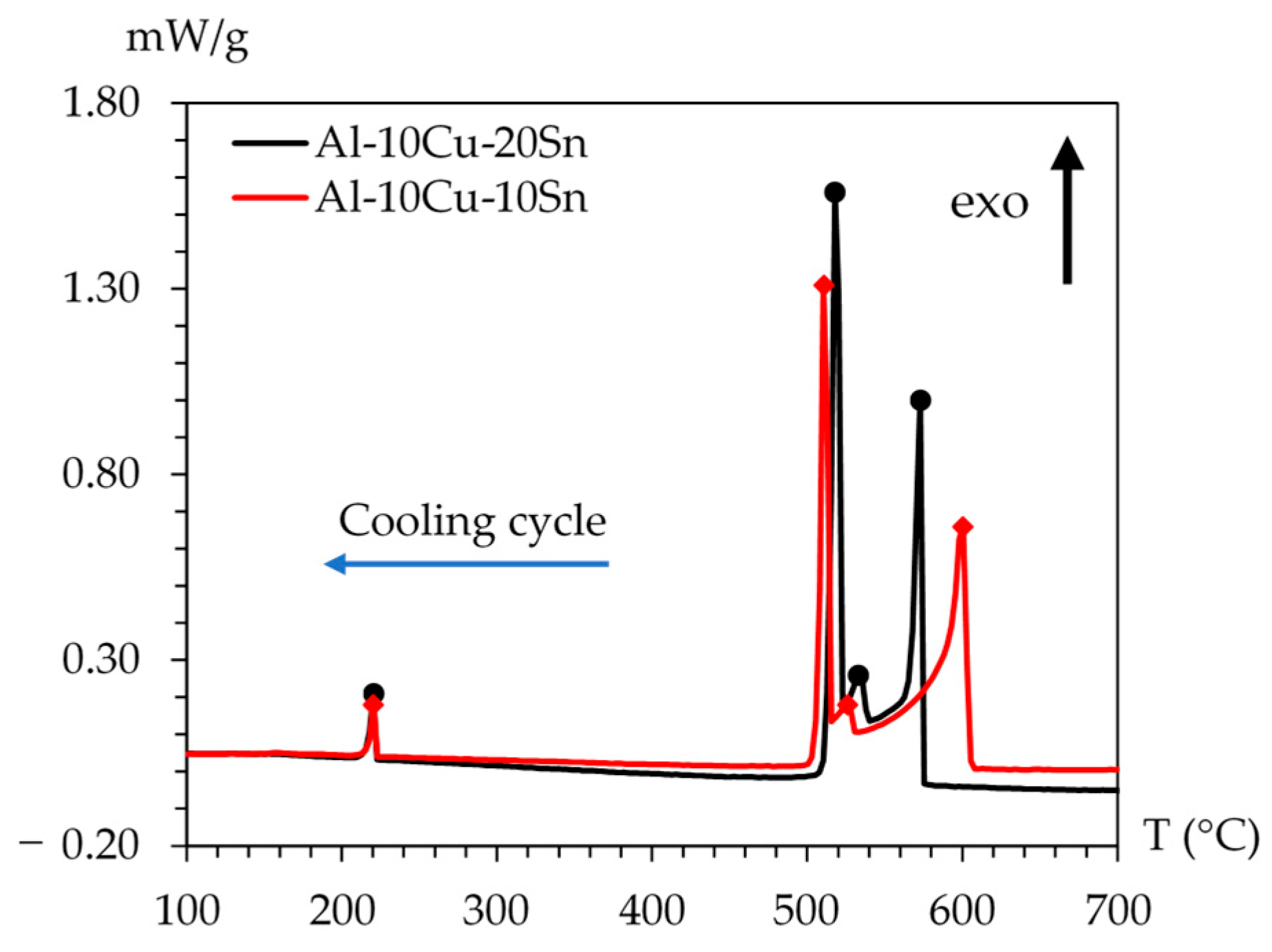



| Phase Reactions | Al-10Cu-10Sn | Al-10Cu-20Sn |
|---|---|---|
| Liquid L | >615 °C | >597.5 °C |
| L → L′ + α-Al (dendrites) | 615 → 537.5 °C | 597.5 → 550.6 °C |
| L′ → α-Al + L′ + L″ (Sn-rich) | 537.5 → 529.4 °C | 550.6 → 529.4 °C |
| L′ → α-Al + L″ + θ-Al2Cu | Monotectic reaction at 529.4 °C | |
| L′ + α-Al → θ-Al2Cu | 529.4 → 229.5 °C | 529.4 → 229.5 °C |
| L″ → α-Al + θ-Al2Cu + β-Sn | Eutectic reaction at 229.5 °C | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Albuquerque, S.; Reinhart, G.; Soltani, H.; Magalhães, D.C.C.; Spinelli, J.E.; Nguyen-Thi, H. In Situ Observation by X-Ray Radioscopy of Liquid Decomposition During Directional Solidification of Al-Cu-Sn Alloys. Metals 2025, 15, 296. https://doi.org/10.3390/met15030296
De Albuquerque S, Reinhart G, Soltani H, Magalhães DCC, Spinelli JE, Nguyen-Thi H. In Situ Observation by X-Ray Radioscopy of Liquid Decomposition During Directional Solidification of Al-Cu-Sn Alloys. Metals. 2025; 15(3):296. https://doi.org/10.3390/met15030296
Chicago/Turabian StyleDe Albuquerque, Sarah, Guillaume Reinhart, Hadjer Soltani, Danielle Cristina Camilo Magalhães, José Eduardo Spinelli, and Henri Nguyen-Thi. 2025. "In Situ Observation by X-Ray Radioscopy of Liquid Decomposition During Directional Solidification of Al-Cu-Sn Alloys" Metals 15, no. 3: 296. https://doi.org/10.3390/met15030296
APA StyleDe Albuquerque, S., Reinhart, G., Soltani, H., Magalhães, D. C. C., Spinelli, J. E., & Nguyen-Thi, H. (2025). In Situ Observation by X-Ray Radioscopy of Liquid Decomposition During Directional Solidification of Al-Cu-Sn Alloys. Metals, 15(3), 296. https://doi.org/10.3390/met15030296








