Achieving High Strength and Plasticity by Controlling the Volume Fractions of Martensite and Ferrite in Rare Earth, Micro-Alloyed Dual-Phase Steel
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Material and Heat Treatment Methods
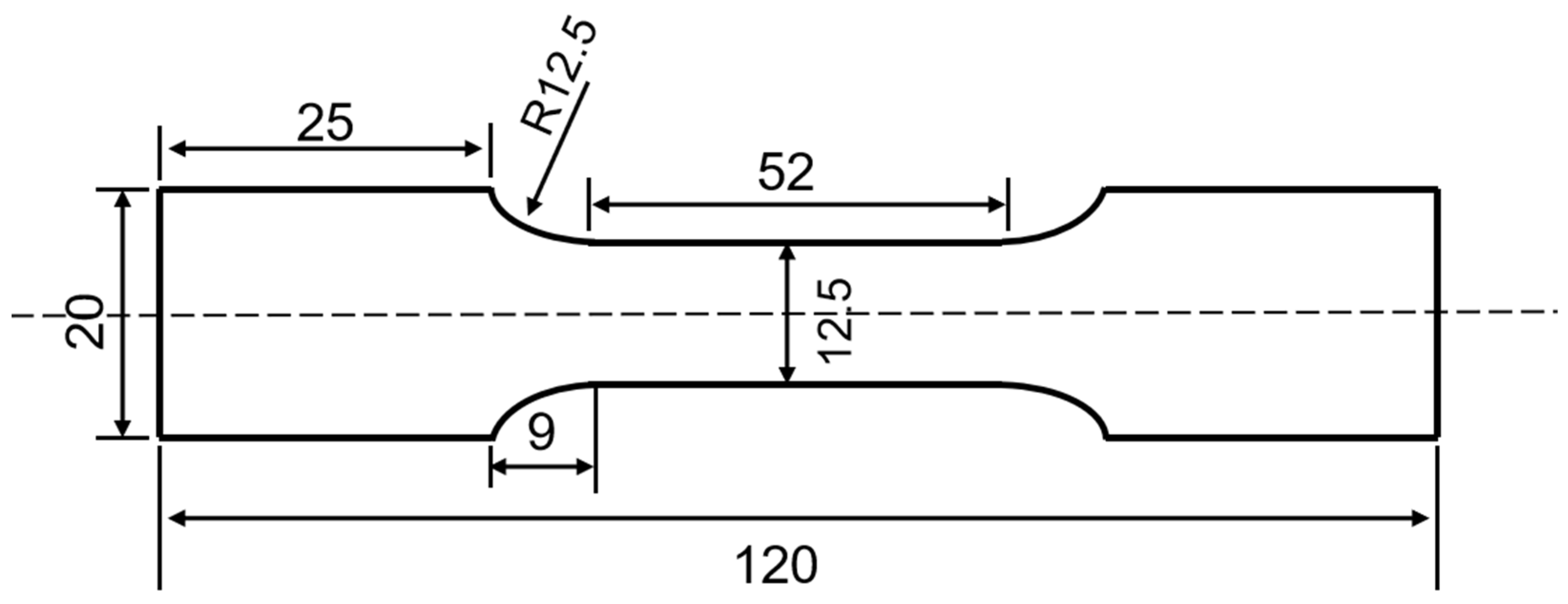
2.2. Tensile Tests
2.3. Microstructure Characterization
3. Results
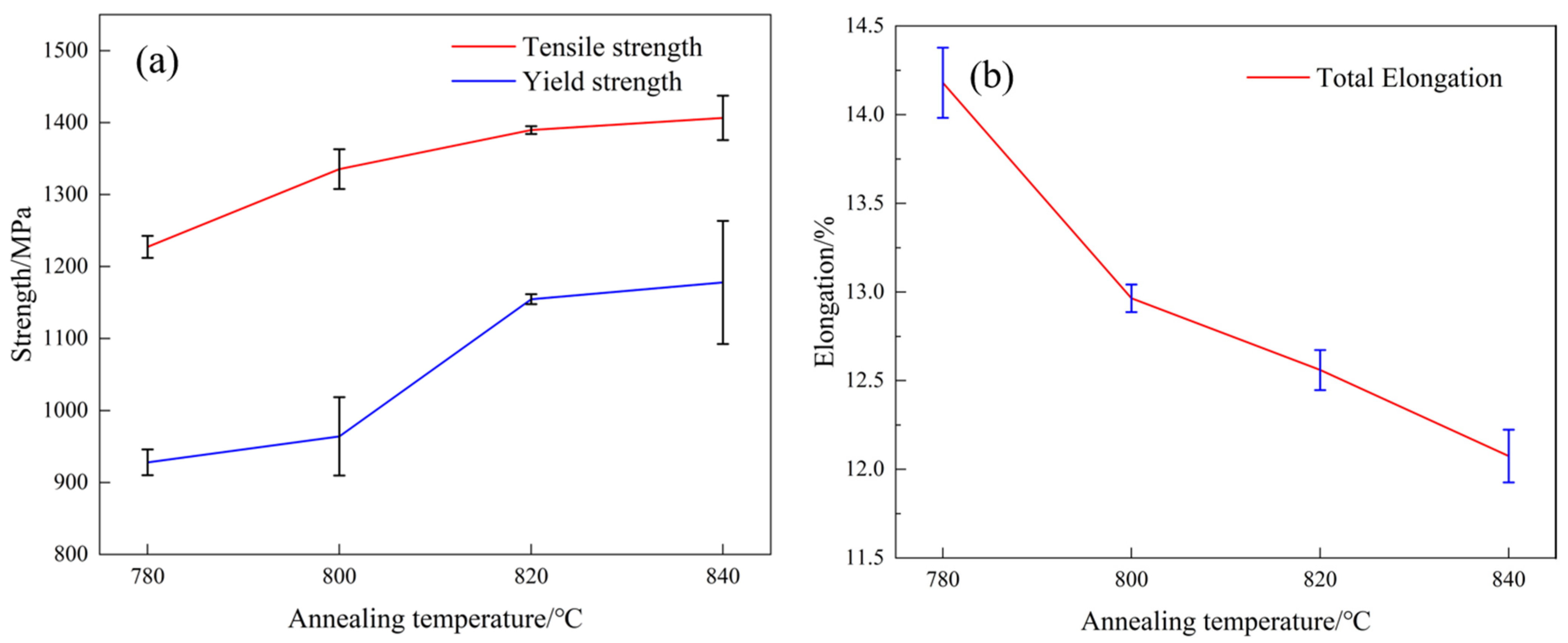
3.1. Mechanical Properties
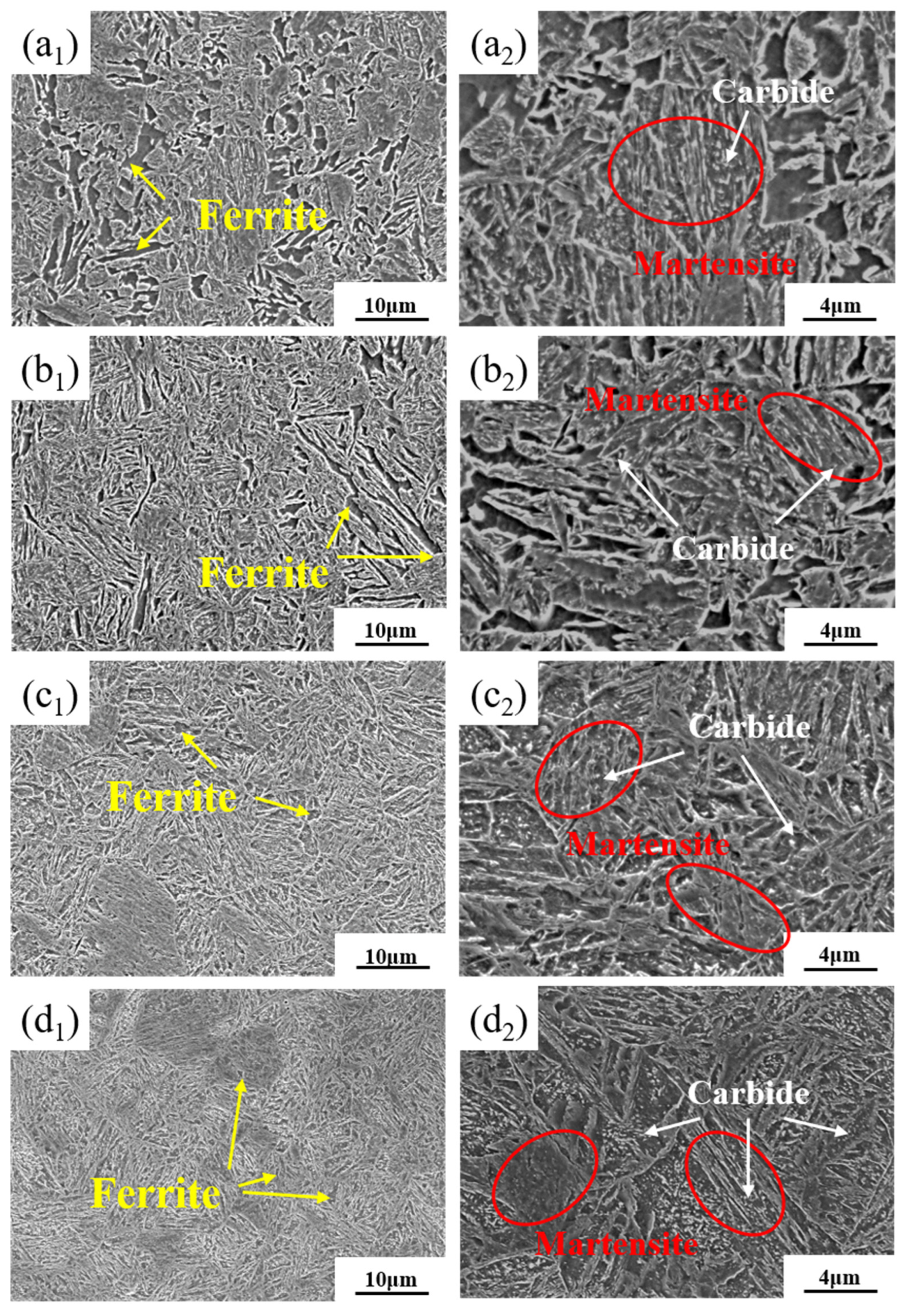
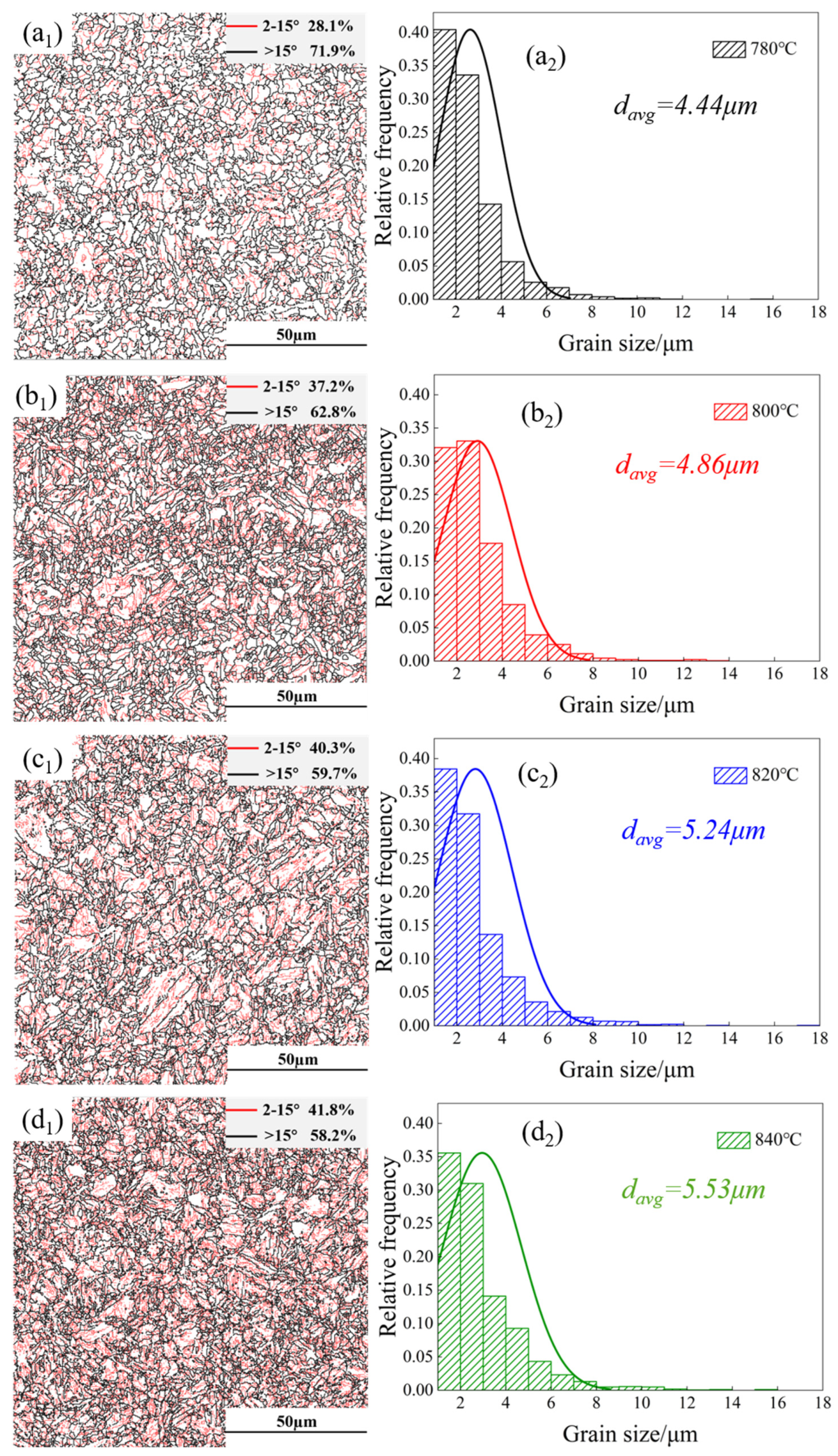
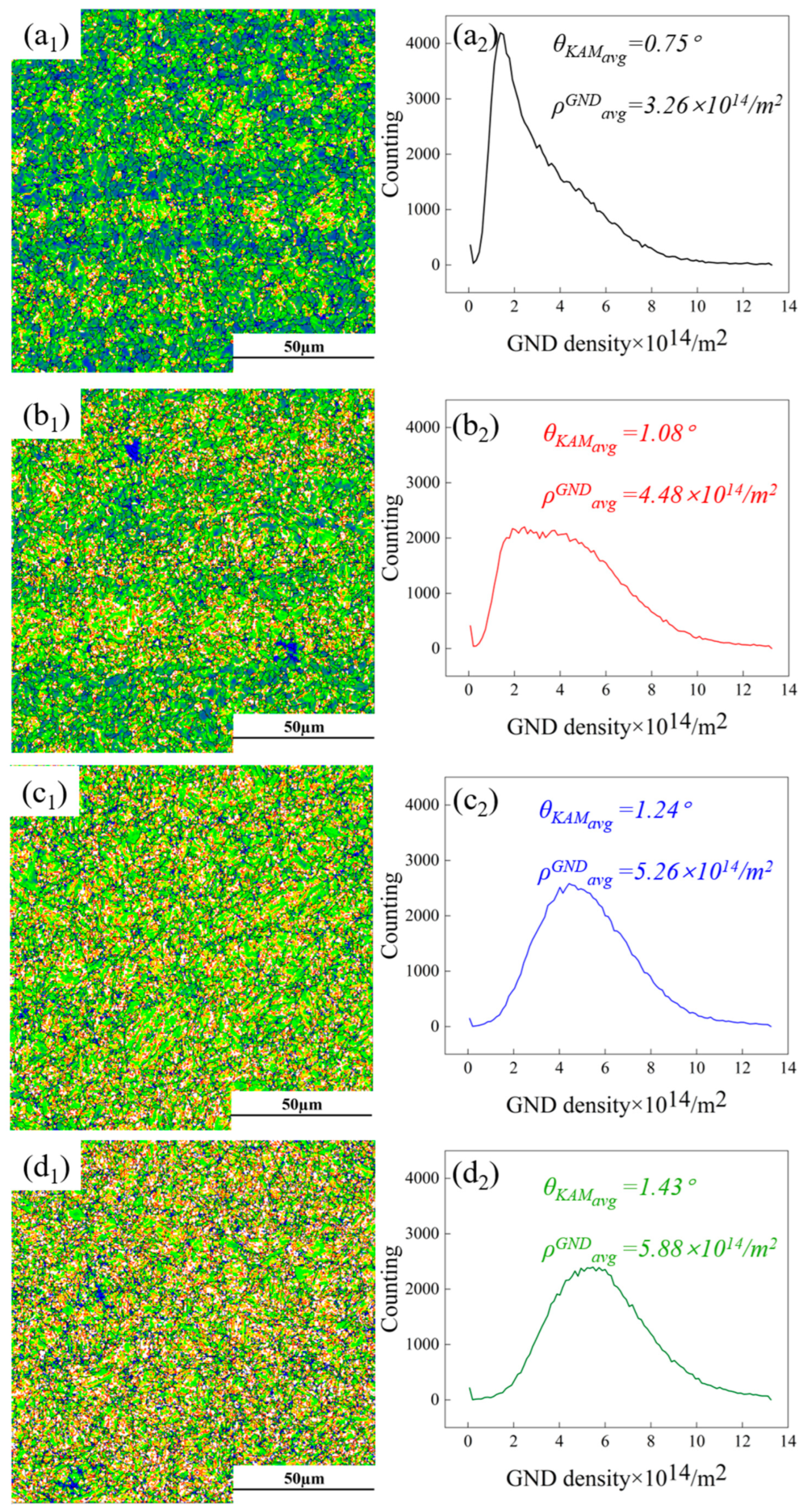
3.2. Microstructural Distribution
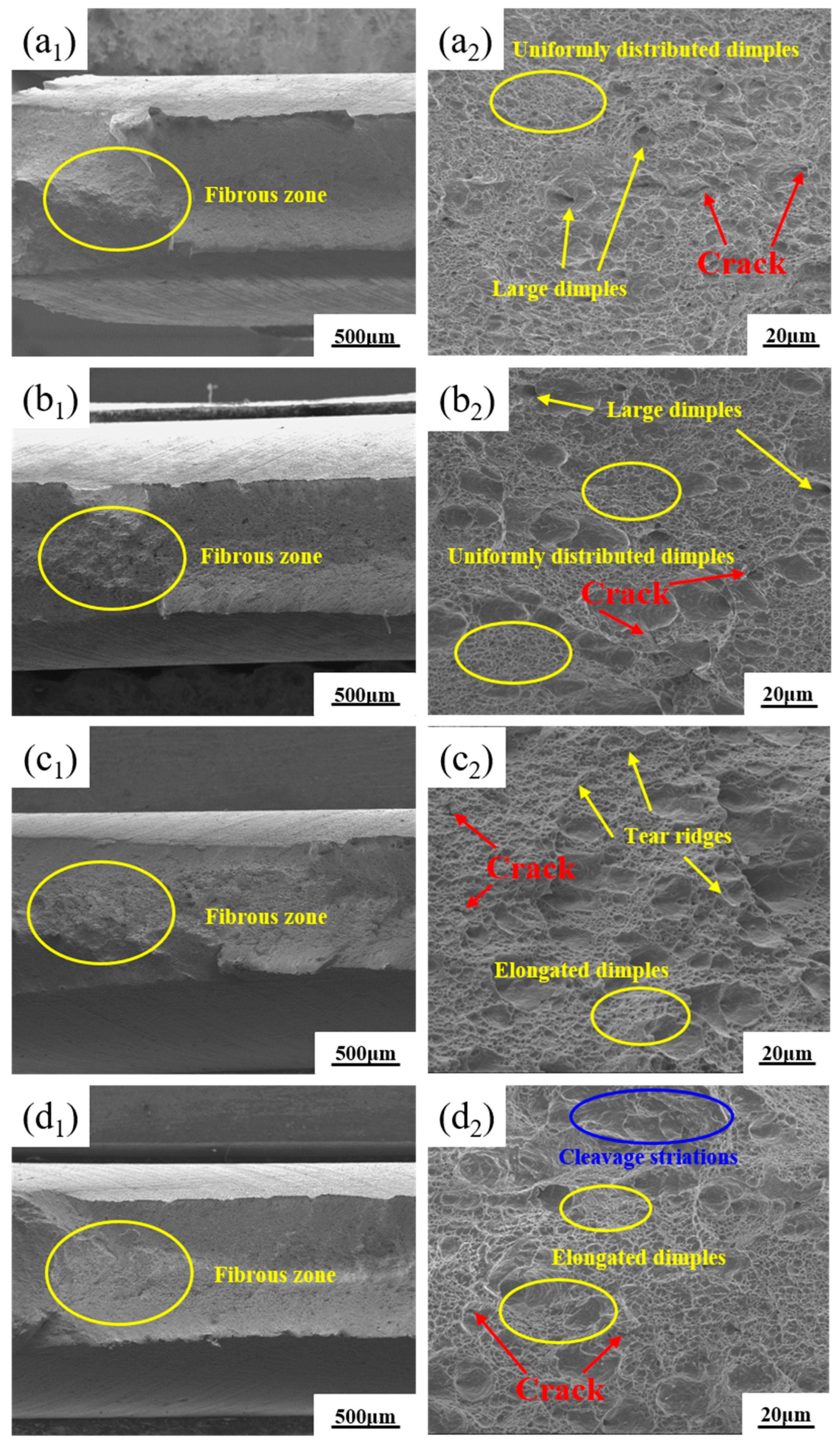
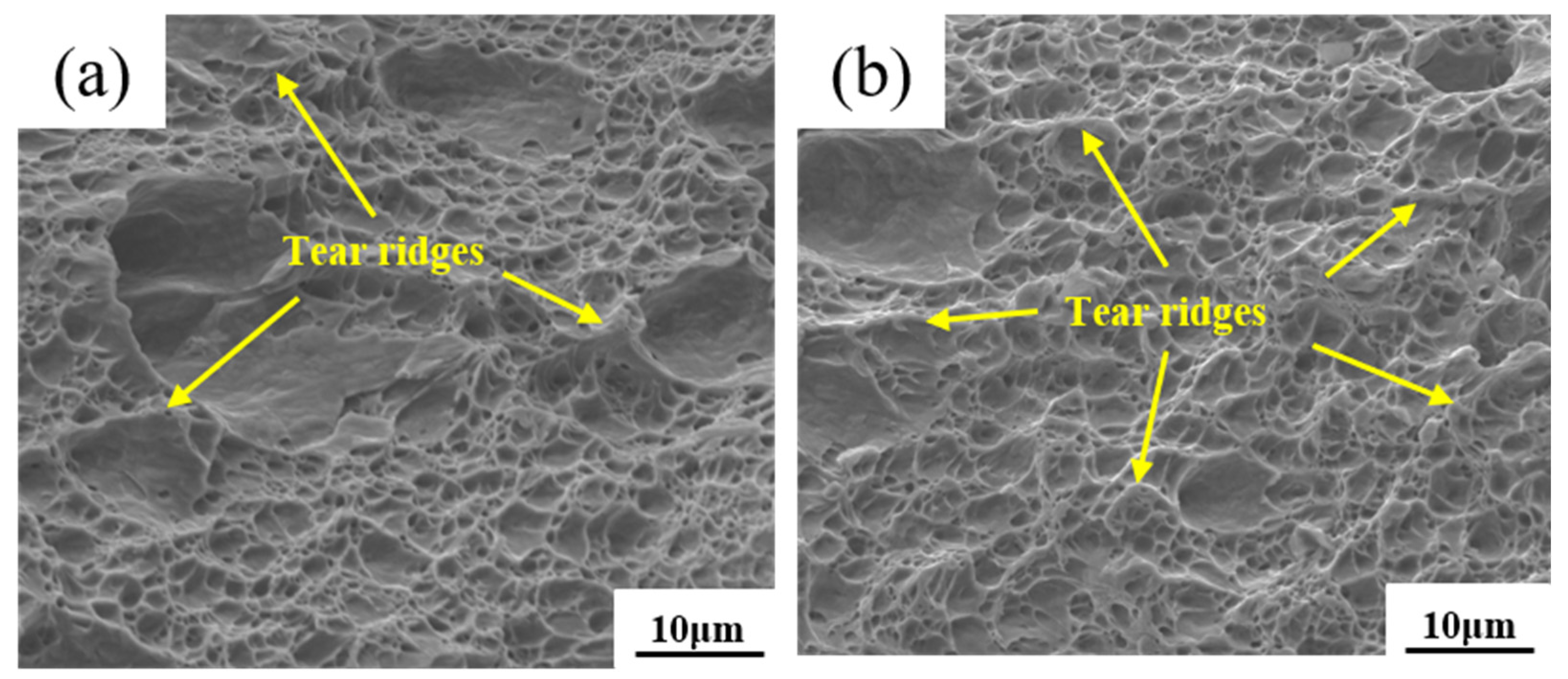
3.3. Fracture Morphology of the Experimental Steel
4. Discussion
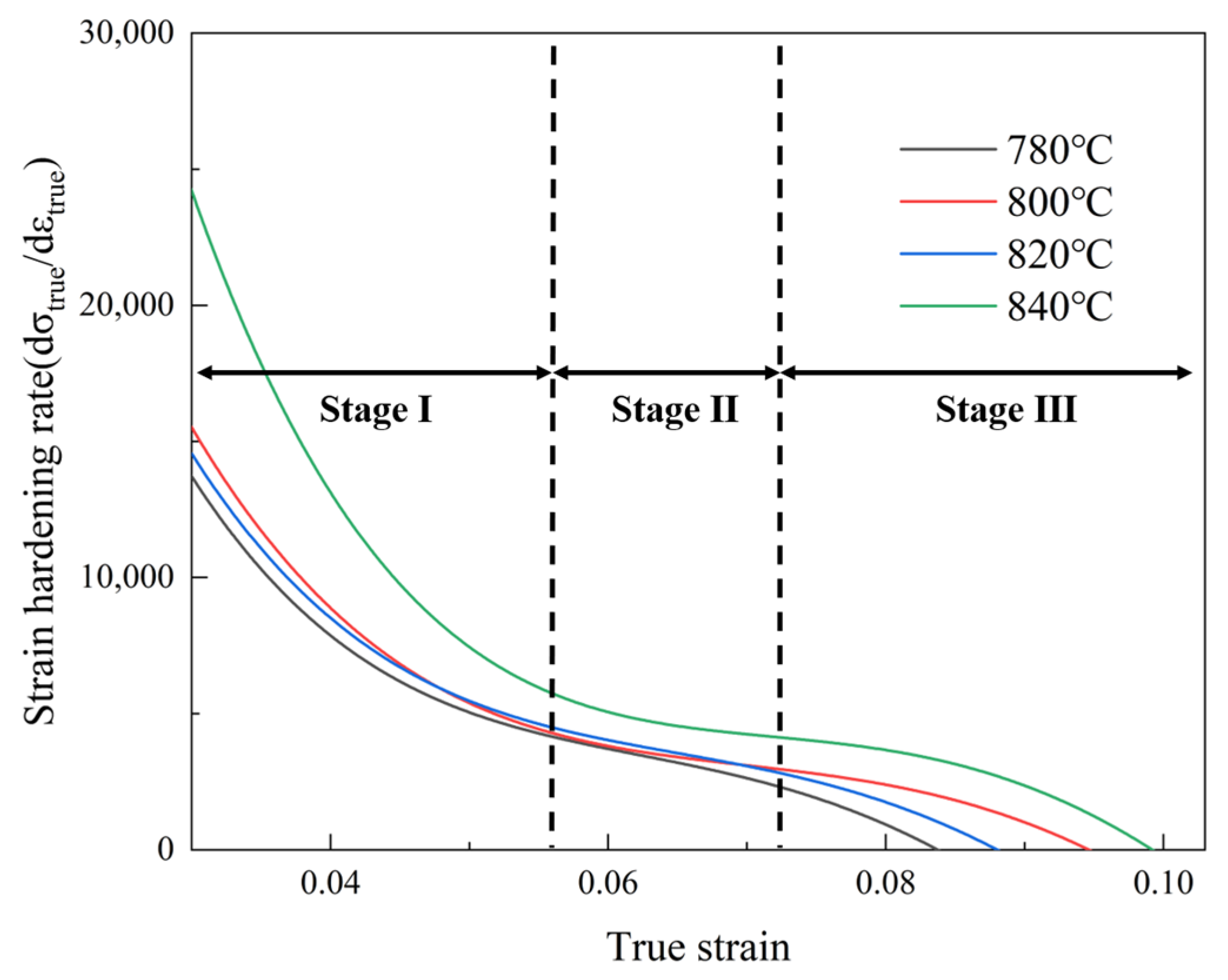
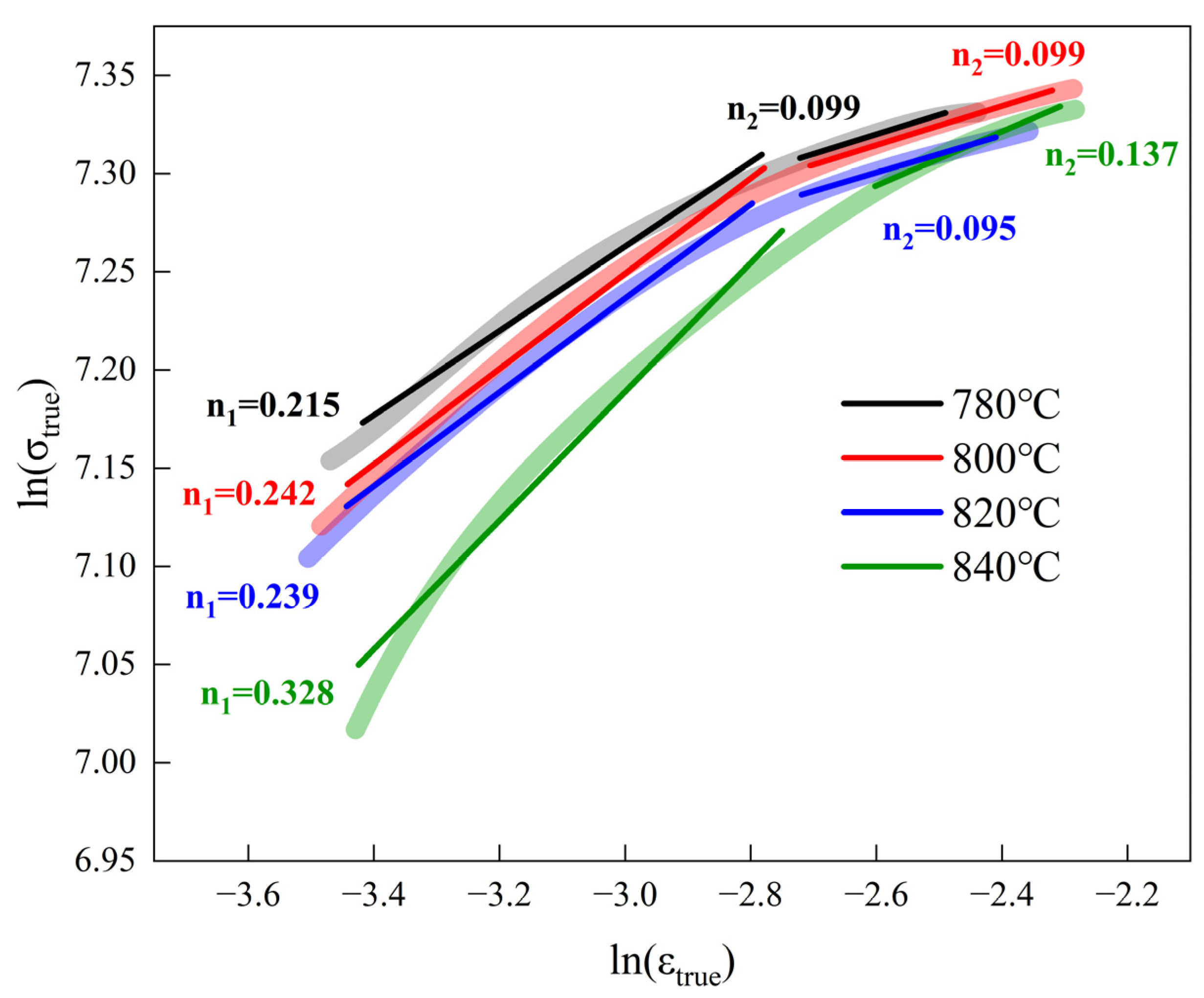
4.1. The Strain-Hardening Ability of the Experimental Steel
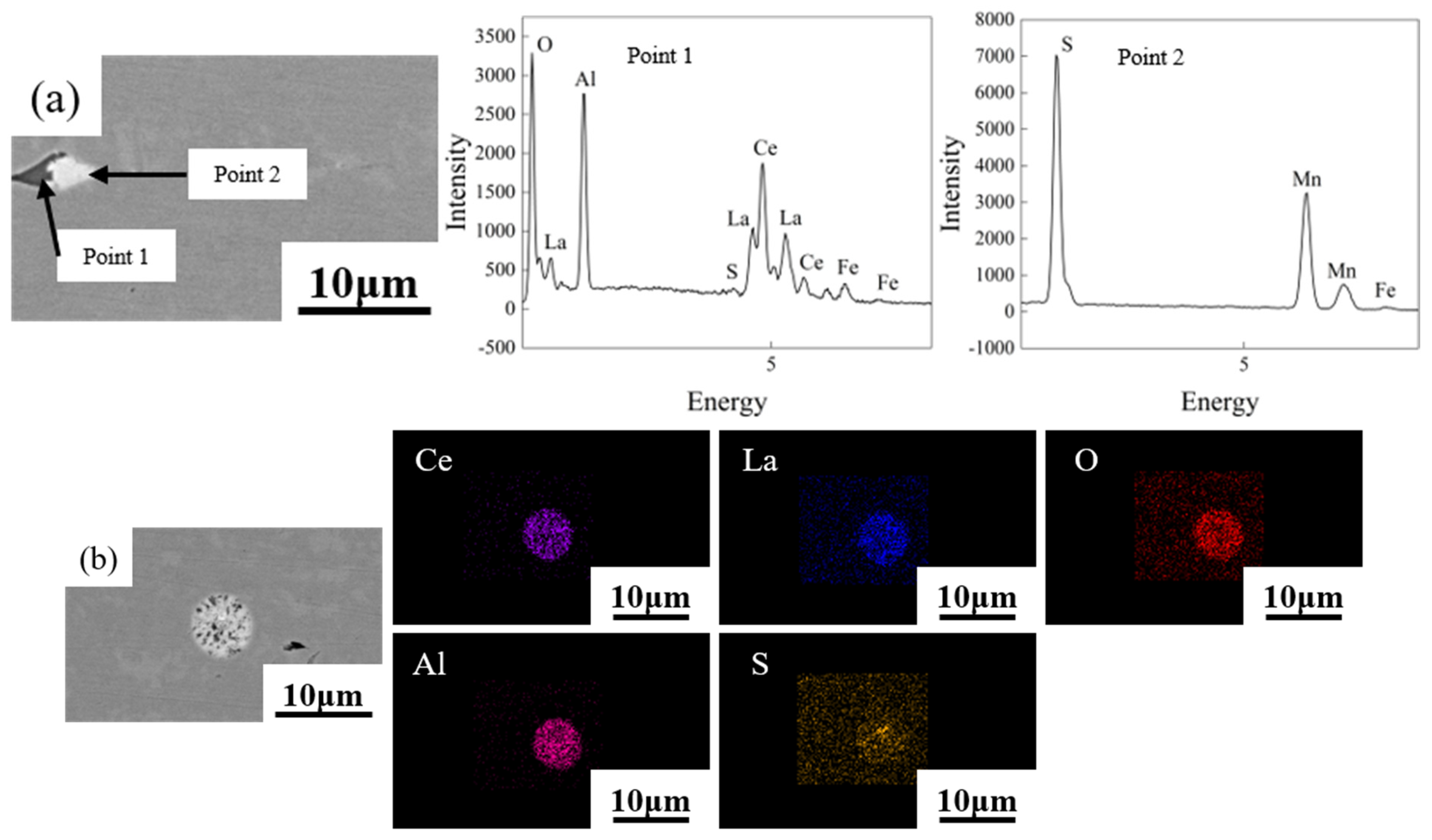
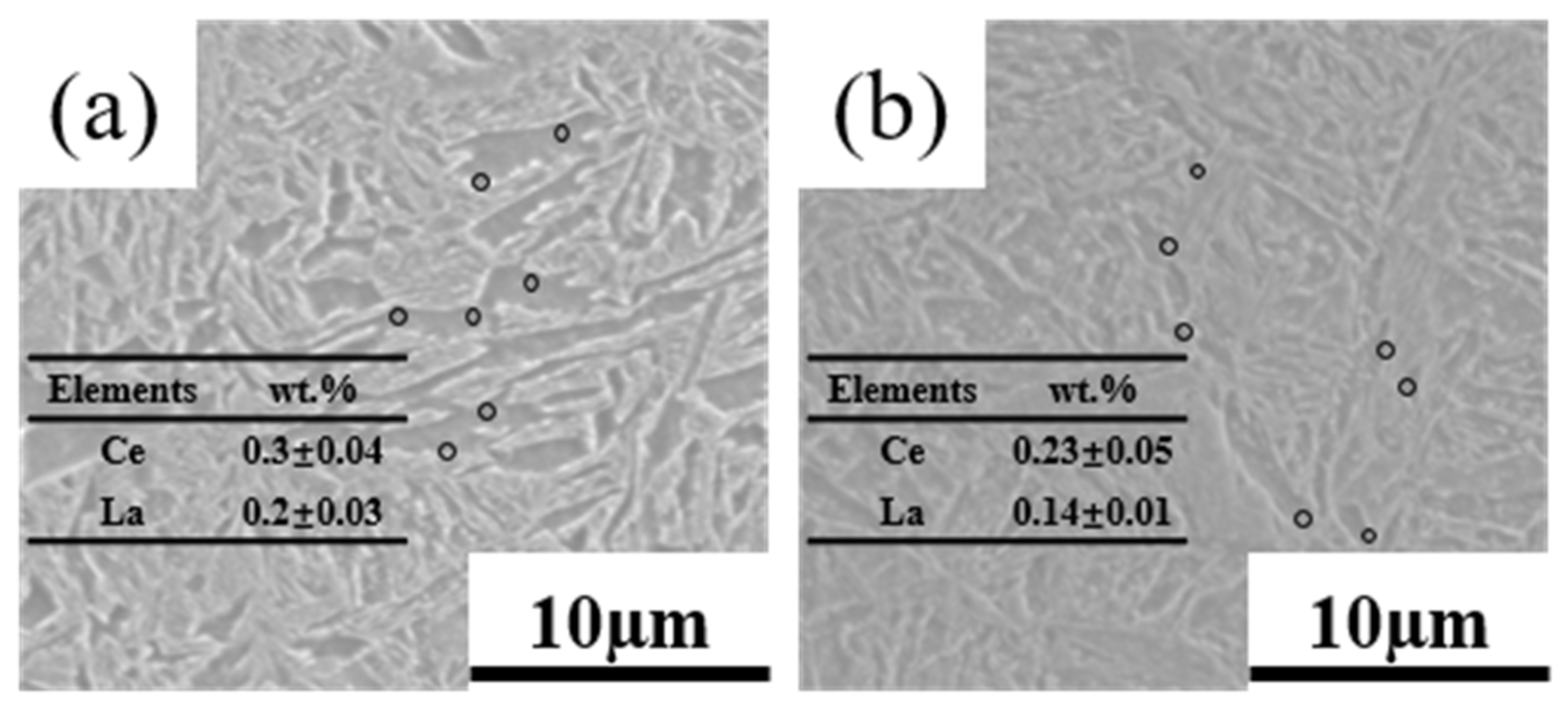
4.2. Effects of Rare Earth Elements
4.3. Microstructure Analysis
5. Conclusions
- After complete quenching and intercritical quenching and tempering, the microstructure of the DP steel consisted of martensite and ferrite, with most martensite appearing in the lath form, and only a small amount of blocky martensite was visible. The alternating and uniform distribution of ferrite and martensite confirmed that quenched martensite laths served as effective nucleation sites. The small EGS of all four groups of DP steel also indicated that performing complete quenching before critical annealing helped refine the microstructure.
- The rare earth elements readily adsorbed impurity elements, forming composite inclusions that were nearly ellipsoidal. That reduced the detrimental effect of inclusions on the mechanical properties of the DP steel. The segregation of rare earth elements at GBs helped refine the grains and enhanced the DP steel’s strength, toughness, and strain-hardening capacity. Due to the diffusion ability of alloying elements, the segregation of rare earth elements at GBs was more pronounced at lower annealing temperatures.
- As the critical annealing temperature rose, the martensite content rose from 60% to 93%. The UTS of the experimental steel increased from 1227 MPa to 1406 MPa, the yield strength increased from 928 MPa to 1177 MPa, the TE decreased from 14.18% to 12.07%, and the strain-hardening index increased from 0.215 to 0.328. The mechanical properties of the DP steel were strongly related to the martensite content. The dislocation density in the martensite was much higher than in the ferrite, and the corresponding plasticity and toughness decreased. All four groups of DP steel had excellent strength–toughness synergy, with a strength–plasticity accumulation of approximately 17 GPa∙% and better performance after annealing at 780 and 820 °C.
- The fracture mode of the DP steel was typically a ductile fracture. At lower annealing temperatures, it had better plasticity and toughness. The refinement of the DP steel microstructure, the uniform distribution of both phases, the modification of inclusions, dislocation behaviour in the ferrite phase, and low-temperature tempering that weakened the martensite phase’s hardness collectively resulted in the improved coordination of deformation between the martensite and the ferrite. That prevented significant phase separation, enhancing the overall mechanical performance.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bouaziz, O.; Zurob, H.; Huang, M. Driving Force and Logic of Development of Advanced High Strength Steels for Automotive Applications. Steel Res. Int. 2013, 84, 937–947. [Google Scholar] [CrossRef]
- Soleimani, M.; Kalhor, A.; Mirzadeh, H. Transformation-Induced Plasticity (TRIP) in Advanced Steels: A Review. Mater. Sci. Eng. A 2020, 795, 140023. [Google Scholar] [CrossRef]
- Liu, L.; Maresca, F.; Hoefnagels, J.P.M.; Vermeij, T.; Geers, M.G.D.; Kouznetsova, V.G. Revisiting the Martensite/Ferrite Interface Damage Initiation Mechanism: The Key Role of Substructure Boundary Sliding. Acta Mater. 2021, 205, 116533. [Google Scholar] [CrossRef]
- Hofmann, H.; Mattissen, D.; Schaumann, T.W. Advanced Cold Rolled Steels for Automotive Applications. Steel Res. Int. 2009, 80, 22–28. [Google Scholar] [CrossRef]
- Asadipoor, M.; Kadkhodapour, J.; Pourkamali Anaraki, A.; Sharifi, S.M.H.; Darabi, A.C.; Barnoush, A. Experimental and Numerical Investigation of Hydrogen Embrittlement Effect on Microdamage Evolution of Advanced High-Strength Dual-Phase Steel. Met. Mater. Int. 2021, 27, 2276–2291. [Google Scholar] [CrossRef]
- Prahl, U.; Papaefthymiou, S.; Uthaisangsuk, V.; Bleck, W.; Sietsma, J.; Van Der Zwaag, S. Micromechanics-Based Modelling of Properties and Failure of Multiphase Steels. Comput. Mater. Sci. 2007, 39, 17–22. [Google Scholar] [CrossRef]
- Frómeta, D.; Cuadrado, N.; Rehrl, J.; Suppan, C.; Dieudonné, T.; Dietsch, P.; Calvo, J.; Casellas, D. Microstructural Effects on Fracture Toughness of Ultra-High Strength Dual Phase Sheet Steels. Mater. Sci. Eng. A 2021, 802, 140631. [Google Scholar] [CrossRef]
- Soliman, M.; Palkowski, H. Strain Hardening Dependence on the Structure in Dual-Phase Steels. Steel Res. Int. 2021, 92, 2000518. [Google Scholar] [CrossRef]
- Mostaan, H.; Saeedpour, P.; Ahmadi, H.; Nouri, A. Laser Welding of Dual-Phase Steels with Different Silicon Contents: Phase Evolutions, Microstructural Observations, Mechanical Properties, and Fracture Behavior. Mater. Sci. Eng. A 2021, 811, 140974. [Google Scholar] [CrossRef]
- Mazaheri, Y.; Jahanara, A.H.; Sheikhi, M.; Kalashami, A.G. High Strength-Elongation Balance in Ultrafine Grained Ferrite-Martensite Dual Phase Steels Developed by Thermomechanical Processing. Mater. Sci. Eng. A 2019, 761, 138021. [Google Scholar] [CrossRef]
- Xu, X.; Van Der Zwaag, S.; Xu, W. The Effect of Ferrite–Martensite Morphology on the Scratch and Abrasive Wear Behaviour of a Dual Phase Construction Steel. Wear 2016, 348–349, 148–157. [Google Scholar] [CrossRef]
- Chakraborti, P.C.; Mitra, M.K. Microstructure and Tensile Properties of High Strength Duplex Ferrite–Martensite (DFM) Steels. Mater. Sci. Eng. A 2007, 466, 123–133. [Google Scholar] [CrossRef]
- Bag, A.; Ray, K.K.; Dwarakadasa, E.S. Influence of Martensite Content and Morphology on the Toughness and Fatigue Behavior of High-Martensite Dual-Phase Steels. Metall. Mater. Trans. A 2001, 32, 2207–2217. [Google Scholar] [CrossRef]
- Rosenberg, G.; Sinaiová, I.; Juhar, Ľ. Effect of Microstructure on Mechanical Properties of Dual Phase Steels in the Presence of Stress Concentrators. Mater. Sci. Eng. A 2013, 582, 347–358. [Google Scholar] [CrossRef]
- Pinard, P.T.; Schwedt, A.; Ramazani, A.; Prahl, U.; Richter, S. Characterization of Dual-Phase Steel Microstructure by Combined Submicrometer EBSD and EPMA Carbon Measurements. Microsc. Microanal. 2013, 19, 996–1006. [Google Scholar] [CrossRef]
- Han, J.; Wang, K.; Wang, Z.; Yu, H. Tailoring the Strength and Low-Temperature Toughness of HSLA Structural Steel by Adding Trace Ce. Mater. Today Commun. 2024, 40, 109789. [Google Scholar] [CrossRef]
- Zhang, J.; Di, H.; Deng, Y.; Misra, R.D.K. Effect of Martensite Morphology and Volume Fraction on Strain Hardening and Fracture Behavior of Martensite–Ferrite Dual Phase Steel. Mater. Sci. Eng. A 2015, 627, 230–240. [Google Scholar] [CrossRef]
- Yaghoobi, F.; Jamaati, R.; Jamshidi Aval, H. Simultaneous Enhancement of Strength and Ductility in Ferrite-Martensite Steel via Increasing the Martensite Fraction. Mater. Chem. Phys. 2021, 259, 124204. [Google Scholar] [CrossRef]
- Park, K.-T.; Lee, Y.K.; Shin, D.H. Fabrication of Ultrafine Grained Ferrite/Martensite Dual Phase Steel by Severe Plastic Deformation. ISIJ Int. 2005, 45, 750–755. [Google Scholar] [CrossRef]
- Ashrafi, H.; Shamanian, M.; Emadi, R.; Saeidi, N. A Novel and Simple Technique for Development of Dual Phase Steels with Excellent Ductility. Mater. Sci. Eng. A 2017, 680, 197–202. [Google Scholar] [CrossRef]
- Papa Rao, M.; Subramanya Sarma, V.; Sankaran, S. Processing of Bimodal Grain-Sized Ultrafine-Grained Dual Phase Microalloyed V-Nb Steel with 1370 MPa Strength and 16 Pct Uniform Elongation Through Warm Rolling and Intercritical Annealing. Metall. Mater. Trans. A 2014, 45, 5313–5317. [Google Scholar] [CrossRef]
- Alibeyki, M.; Mirzadeh, H.; Najafi, M. Fine-Grained Dual Phase Steel via Intercritical Annealing of Cold-Rolled Martensite. Vacuum 2018, 155, 147–152. [Google Scholar] [CrossRef]
- Yin, C.-C.; Cheng, L.; Wang, Z.-H.; Zhao, T.-L.; Cheng, S.; Hu, S.-E.; Liu, Z.-C.; Luo, D.; Xiao, D.-H.; Jin, X.; et al. Local Corrosion Behaviors in the Coarse-Grained Heat-Affected Zone in a Newly Developed Zr–Ti–Al–RE Deoxidized High-Strength Low-Alloy Steel. Materials 2023, 16, 876. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Z.; Li, B.; Chen, W.; Zhang, J.; Mao, J. Inclusions Modification by Rare Earth in Steel and the Resulting Properties: A Review. J. Rare Earths 2024, 42, 431–445. [Google Scholar] [CrossRef]
- Torkamani, H.; Raygan, S.; Garcia Mateo, C.; Rassizadehghani, J.; Palizdar, Y.; San-Martin, D. Contributions of Rare Earth Element (La,Ce) Addition to the Impact Toughness of Low Carbon Cast Niobium Microalloyed Steels. Met. Mater. Int. 2018, 24, 773–788. [Google Scholar] [CrossRef]
- Zhang, S.; Yu, J.; Li, H.; Jiang, Z.; Geng, Y.; Feng, H.; Zhang, B.; Zhu, H. Refinement Mechanism of Cerium Addition on Solidification Structure and Sigma Phase of Super Austenitic Stainless Steel S32654. J. Mater. Sci. Technol. 2022, 102, 105–114. [Google Scholar] [CrossRef]
- Liu, P.; Hou, X.; Yang, C.; Luan, Y.; Zheng, C.; Li, D. Synergic Evolution of Microstructure-Texture-Stored Energy in Rare-Earth-Added Interstitial-Free Steels Undergoing Static Recrystallization. Acta Metall. Sin. (Engl. Lett.) 2023, 36, 661–680. [Google Scholar] [CrossRef]
- Liu, P.; Hou, X.; Yang, C.; Luan, Y.; Zheng, C.; Li, D.; Ma, G. Tailoring Microstructure Evolution and Austenite Stability of TRIP Steels by Rare-Earth Micro-Alloying. Mater. Charact. 2023, 203, 113035. [Google Scholar] [CrossRef]
- Cheng, S.; Hou, T.; Zheng, Y.; Yin, C.; Wu, K. Effect of Rare Earth Elements on Microstructure and Tensile Behavior of Nb-Containing Microalloyed Steels. Materials 2024, 17, 1701. [Google Scholar] [CrossRef]
- Zhao, J.; Guo, Y.; Fan, X.; Yan, B.; Lu, X. Tensile Properties and Prediction of DP780 Treated with Rare Earth Ce Based on DE-SVM-II. Mater. Today Commun. 2022, 33, 104771. [Google Scholar] [CrossRef]
- Ayyandurai, A.; Uzair Ul Haque, M. Influence of Rare Earth Metals on Inclusion Modification of Dual Phase Steel: Influence Des Métaux de Terres Rares Sur La Modification Des Inclusions de l’acier Biphasé. Can. Metall. Q. 2024, 63, 1030–1039. [Google Scholar] [CrossRef]
- Zhao, J.; He, K.; Guo, Y.; Fan, X.; Yan, B.; Lu, X.; Zhang, X. Evolution of Microstructure and Mechanical Properties in Dual-Phase Steel Containing Ce and Nb. J. Mater. Eng. Perform. 2024, 33, 9829–9839. [Google Scholar] [CrossRef]
- ASTM E8/E8M-21; Standard Test Methods for Tension Testing of Metallic Materials. ASTM International: West Conshohocken, PA, USA, 2021.
- Kang, S.; Speer, J.G.; Krizan, D.; Matlock, D.K.; De Moor, E. Prediction of Tensile Properties of Intercritically Annealed Al-Containing 0.19C–4.5Mn (Wt%) TRIP Steels. Mater. Des. 2016, 97, 138–146. [Google Scholar] [CrossRef]
- Soliman, M.; Palkowski, H. On Factors Affecting the Phase Transformation and Mechanical Properties of Cold-Rolled Transformation-Induced-Plasticity–Aided Steel. Metall. Mater. Trans. A 2008, 39, 2513–2527. [Google Scholar] [CrossRef]
- Mirzadeh, H.; Alibeyki, M.; Najafi, M. Unraveling the Initial Microstructure Effects on Mechanical Properties and Work-Hardening Capacity of Dual-Phase Steel. Metall. Mater. Trans. A 2017, 48, 4565–4573. [Google Scholar] [CrossRef]
- Krauss, G. Steels: Processing, Structure, and Performance. Choice Rev. Online 2006, 43, 43-6550. [Google Scholar] [CrossRef]
- Nouroozi, M.; Mirzadeh, H.; Zamani, M. Effect of Microstructural Refinement and Intercritical Annealing Time on Mechanical Properties of High-Formability Dual Phase Steel. Mater. Sci. Eng. A 2018, 736, 22–26. [Google Scholar] [CrossRef]
- Azizi-Alizamini, H.; Militzer, M.; Poole, W.J. Formation of Ultrafine Grained Dual Phase Steels through Rapid Heating. ISIJ Int. 2011, 51, 958–964. [Google Scholar] [CrossRef]
- Barik, R.K.; Ghosh, A.; Sk, M.B.; Biswal, S.; Dutta, A.; Chakrabarti, D. Bridging Microstructure and Crystallography with the Micromechanics of Cleavage Fracture in a Lamellar Pearlitic Steel. Acta Mater. 2021, 214, 116988. [Google Scholar] [CrossRef]
- Xi, X.; Wang, J.; Chen, L.; Wang, Z. On the Microstructural Strengthening and Toughening of Heat-Affected Zone in a Low-Carbon High-Strength Cu-Bearing Steel. Acta Metall. Sin. (Engl. Lett.) 2021, 34, 617–627. [Google Scholar] [CrossRef]
- Geng, R.; Li, J.; Shi, C.; Zhi, J.; Lu, B. Effect of Ce on Microstructures, Carbides and Mechanical Properties in Simulated Coarse-Grained Heat-Affected Zone of 800-MPa High-Strength Low-Alloy Steel. Mater. Sci. Eng. A 2022, 840, 142919. [Google Scholar] [CrossRef]
- Luo, H.; Wang, X.; Liu, Z.; Yang, Z. Influence of Refined Hierarchical Martensitic Microstructures on Yield Strength and Impact Toughness of Ultra-High Strength Stainless Steel. J. Mater. Sci. Technol. 2020, 51, 130–136. [Google Scholar] [CrossRef]
- Kubin, L.P.; Mortensen, A. Geometrically Necessary Dislocations and Strain-Gradient Plasticity: A Few Critical Issues. Scr. Mater. 2003, 48, 119–125. [Google Scholar] [CrossRef]
- Ma, X.; Huang, C.; Moering, J.; Ruppert, M.; Höppel, H.W.; Göken, M.; Narayan, J.; Zhu, Y. Mechanical Properties of Copper/Bronze Laminates: Role of Interfaces. Acta Mater. 2016, 116, 43–52. [Google Scholar] [CrossRef]
- Kadkhodapour, J.; Butz, A.; Ziaei Rad, S. Mechanisms of Void Formation during Tensile Testing in a Commercial, Dual-Phase Steel. Acta Mater. 2011, 59, 2575–2588. [Google Scholar] [CrossRef]
- Samei, J.; Green, D.E.; Cheng, J.; De Carvalho Lima, M.S. Influence of Strain Path on Nucleation and Growth of Voids in Dual Phase Steel Sheets. Mater. Des. 2016, 92, 1028–1037. [Google Scholar] [CrossRef]
- Hosseini-Toudeshky, H.; Anbarlooie, B.; Kadkhodapour, J.; Shadalooyi, G. Microstructural Deformation Pattern and Mechanical Behavior Analyses of DP600 Dual Phase Steel. Mater. Sci. Eng. A 2014, 600, 108–121. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Li, C.-H.; Tsao, T.-C.; Chiu, P.-H.; Tsai, S.-P.; Yang, J.-R.; Chiang, L.-J.; Wang, S.-H. A Novel Technique for Developing a Dual-Phase Steel with a Lower Strength Difference between Ferrite and Martensite. Mater. Today Commun. 2020, 23, 100895. [Google Scholar] [CrossRef]
- Balliger, N.K.; Gladman, T. Work Hardening of Dual-Phase Steels. Met. Sci. 1981, 15, 95–108. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.; Li, C.; Jiang, M. A Coupled Thermodynamic Model for Prediction of Inclusions Precipitation during Solidification of Heat-Resistant Steel Containing Cerium. J. Iron Steel Res. Int. 2015, 22, 457–463. [Google Scholar] [CrossRef]
- Ren, Q.; Zhang, L.; Liu, Y.; Cui, L.; Yang, W. Transformation of Cerium-Containing Inclusions in Ultra-Low-Carbon Aluminum-Killed Steels during Solidification and Cooling. J. Mater. Res. Technol. 2020, 9, 8197–8206. [Google Scholar] [CrossRef]
- Wang, H.; Bao, Y.; Zhi, J.; Duan, C.; Gao, S.; Wang, M. Effect of Rare Earth Ce on the Morphology and Distribution of Al2O3 Inclusions in High Strength IF Steel Containing Phosphorus during Continuous Casting and Rolling Process. ISIJ Int. 2021, 61, 657–666. [Google Scholar] [CrossRef]
- Geng, R.; Li, J.; Shi, C. Evolution of Inclusions with Ce Addition and Ca Treatment in Al-Killed Steel during RH Refining Process. ISIJ Int. 2021, 61, 1506–1513. [Google Scholar] [CrossRef]
- Cai, G.-J.; Li, C.-S. Effects of Ce on Inclusions and Corrosion Resistance of Low-Nickel Austenite Stainless Steel. Mater. Corros. 2015, 66, 1445–1455. [Google Scholar] [CrossRef]
- Liu, X.; Yang, J.; Yang, L.; Gao, X. Effect of Ce on Inclusions and Impact Property of 2Cr13 Stainless Steel. J. Iron Steel Res. Int. 2010, 17, 59–64. [Google Scholar] [CrossRef]
- Su, C.; Feng, G.; Zhi, J.; Zhao, B.; Wu, W. The Effect of Rare Earth Cerium on Microstructure and Properties of Low Alloy Wear-Resistant Steel. Metals 2022, 12, 1358. [Google Scholar] [CrossRef]
- Yin, T.W.; Shen, Y.F.; Xue, W.Y.; Jia, N.; Zuo, L. Ce Addition Enabling Superior Strength and Ductility Combination of a Low-Carbon Low-Manganese Transformation-Induced Plasticity Steel. Mater. Sci. Eng. A 2022, 849, 143474. [Google Scholar] [CrossRef]
- Calcagnotto, M.; Adachi, Y.; Ponge, D.; Raabe, D. Deformation and Fracture Mechanisms in Fine- and Ultrafine-Grained Ferrite/Martensite Dual-Phase Steels and the Effect of Aging. Acta Mater. 2011, 59, 658–670. [Google Scholar] [CrossRef]
- Ebrahimian, A.; Ghasemi Banadkouki, S.S. Mutual Mechanical Effects of Ferrite and Martensite in a Low Alloy Ferrite-Martensite Dual Phase Steel. J. Alloys Compd. 2017, 708, 43–54. [Google Scholar] [CrossRef]
- Calcagnotto, M.; Ponge, D.; Demir, E.; Raabe, D. Orientation Gradients and Geometrically Necessary Dislocations in Ultrafine Grained Dual-Phase Steels Studied by 2D and 3D EBSD. Mater. Sci. Eng. A 2010, 527, 2738–2746. [Google Scholar] [CrossRef]
- Ghassemi-Armaki, H.; Maaß, R.; Bhat, S.P.; Sriram, S.; Greer, J.R.; Kumar, K.S. Deformation Response of Ferrite and Martensite in a Dual-Phase Steel. Acta Mater. 2014, 62, 197–211. [Google Scholar] [CrossRef]
- Sodjit, S.; Uthaisangsuk, V. Microstructure Based Prediction of Strain Hardening Behavior of Dual Phase Steels. Mater. Des. 2012, 41, 370–379. [Google Scholar] [CrossRef]







| Element | Mass Percentage |
|---|---|
| C | 0.19 |
| Si | 0.40 |
| Mn | 1.60 |
| Cr | 0.15 |
| Mo | 0.05 |
| B | 0.0012 |
| Nb | 0.02 |
| Al | 0.035 |
| P | <0.010 |
| S | <0.0050 |
| Ce + La | 0.015 |
| Fe | Bal. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Song, X.; Yu, J.; Geng, W.; You, X.; Jia, J. Achieving High Strength and Plasticity by Controlling the Volume Fractions of Martensite and Ferrite in Rare Earth, Micro-Alloyed Dual-Phase Steel. Metals 2025, 15, 310. https://doi.org/10.3390/met15030310
Li Z, Song X, Yu J, Geng W, You X, Jia J. Achieving High Strength and Plasticity by Controlling the Volume Fractions of Martensite and Ferrite in Rare Earth, Micro-Alloyed Dual-Phase Steel. Metals. 2025; 15(3):310. https://doi.org/10.3390/met15030310
Chicago/Turabian StyleLi, Zhishen, Xinli Song, Jin Yu, Wei Geng, Xuewen You, and Juan Jia. 2025. "Achieving High Strength and Plasticity by Controlling the Volume Fractions of Martensite and Ferrite in Rare Earth, Micro-Alloyed Dual-Phase Steel" Metals 15, no. 3: 310. https://doi.org/10.3390/met15030310
APA StyleLi, Z., Song, X., Yu, J., Geng, W., You, X., & Jia, J. (2025). Achieving High Strength and Plasticity by Controlling the Volume Fractions of Martensite and Ferrite in Rare Earth, Micro-Alloyed Dual-Phase Steel. Metals, 15(3), 310. https://doi.org/10.3390/met15030310





