Recycling Potential of Copper-Bearing Waelz Slag via Oxidative Sulfuric Acid Leaching
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
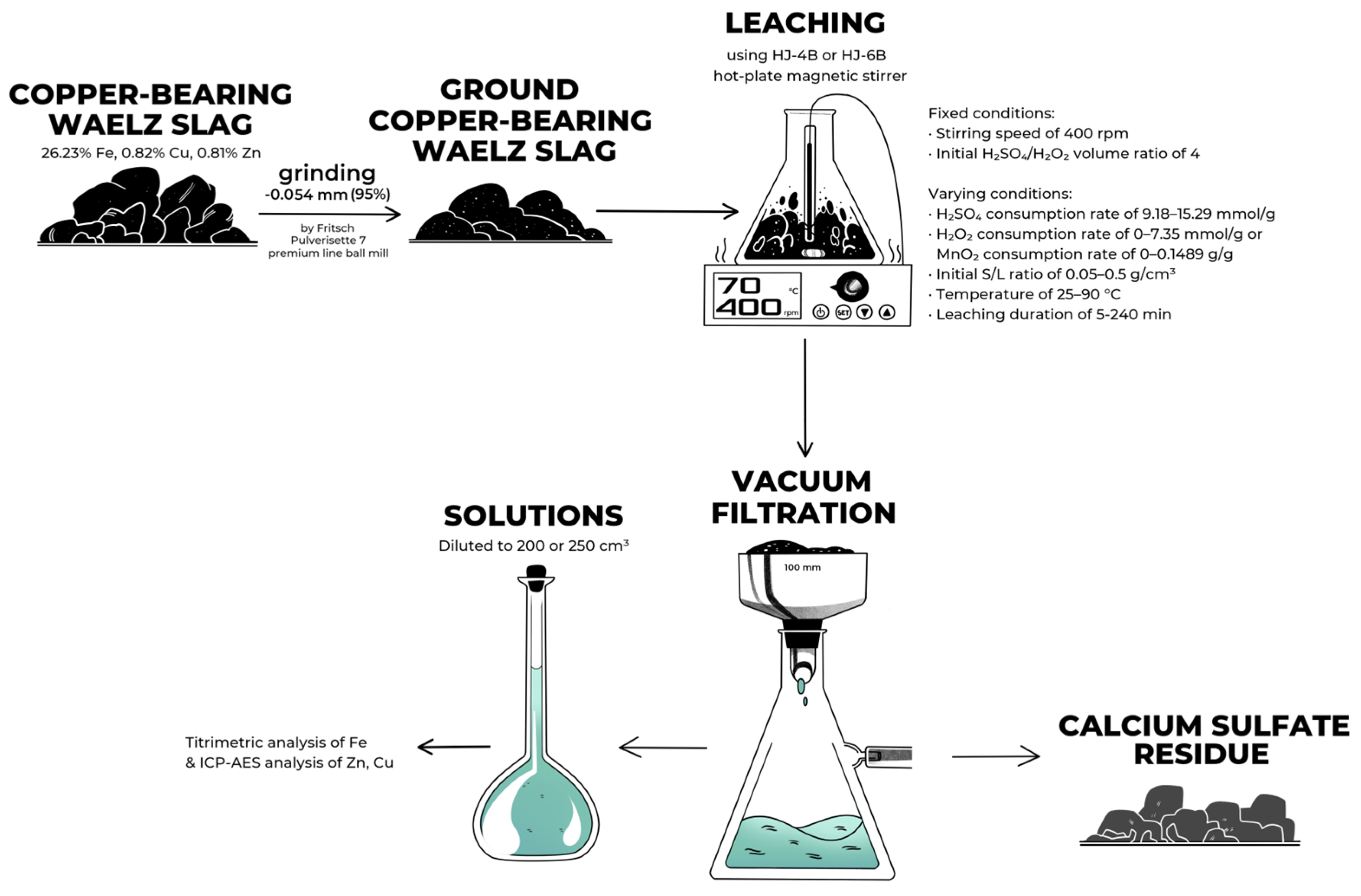
2.2. Experiments
2.3. Analysis Methods
3. Results
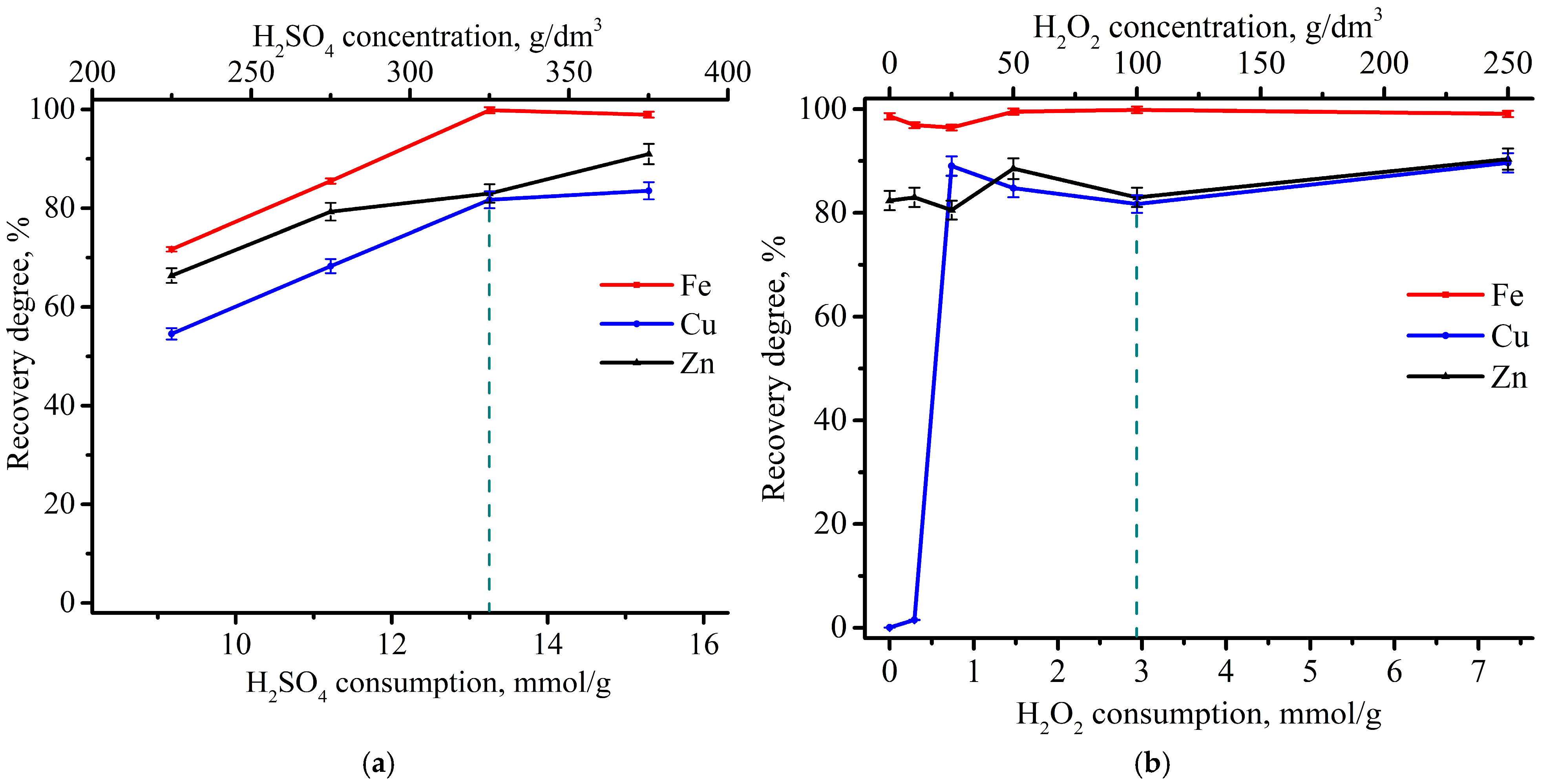
3.1. Determination of the Sulfuric Acid and Hydrogen Peroxide Consumption
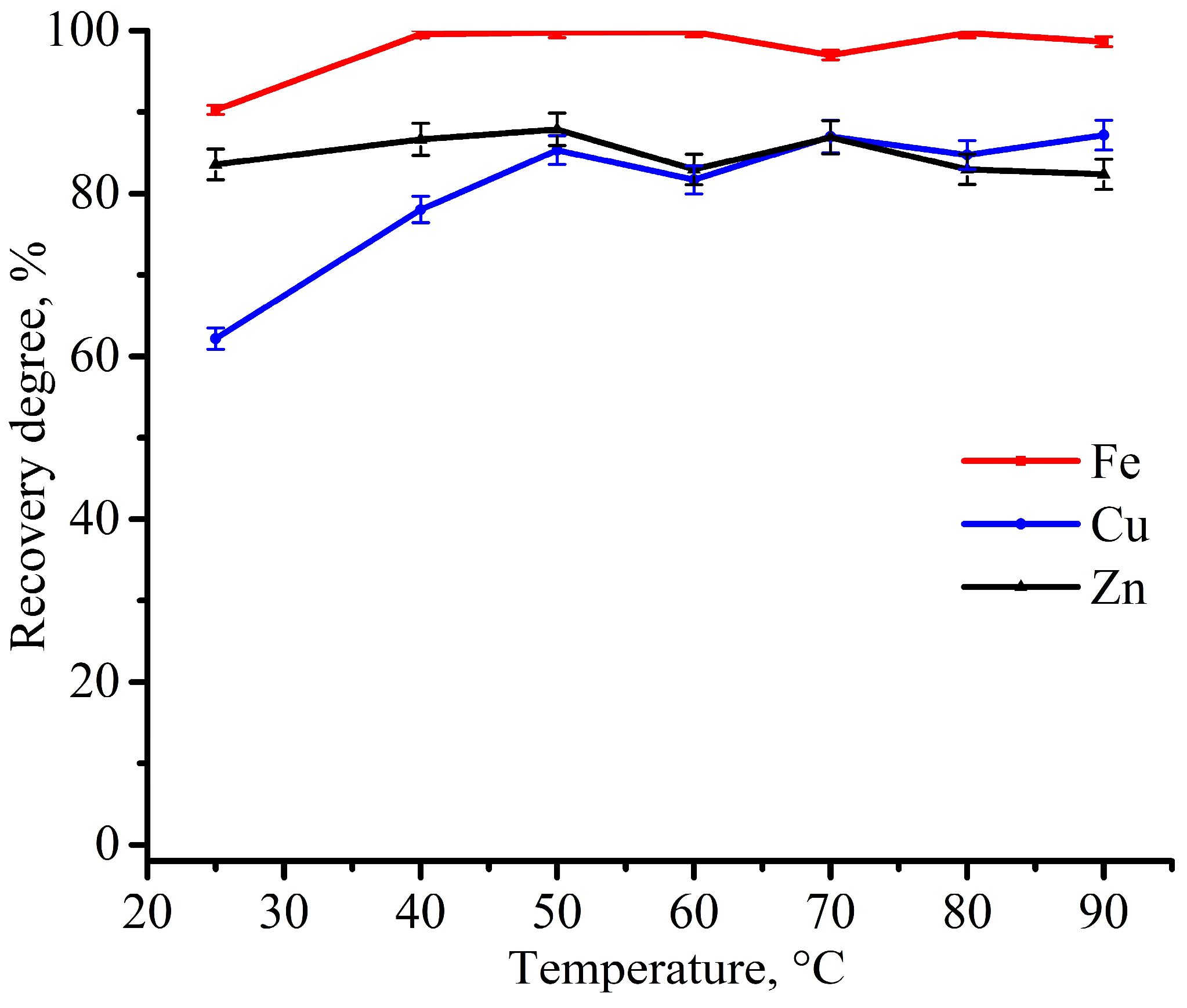
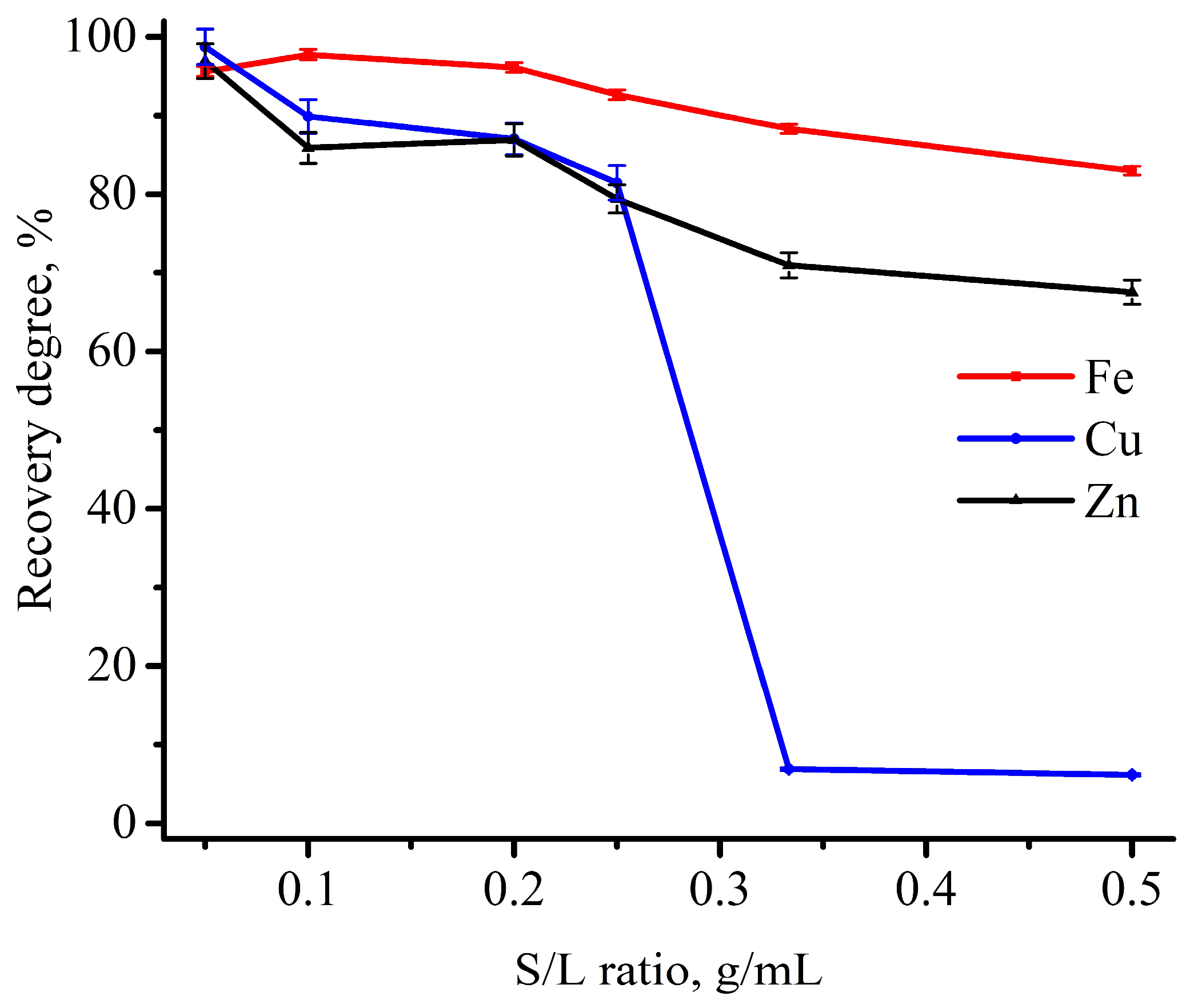
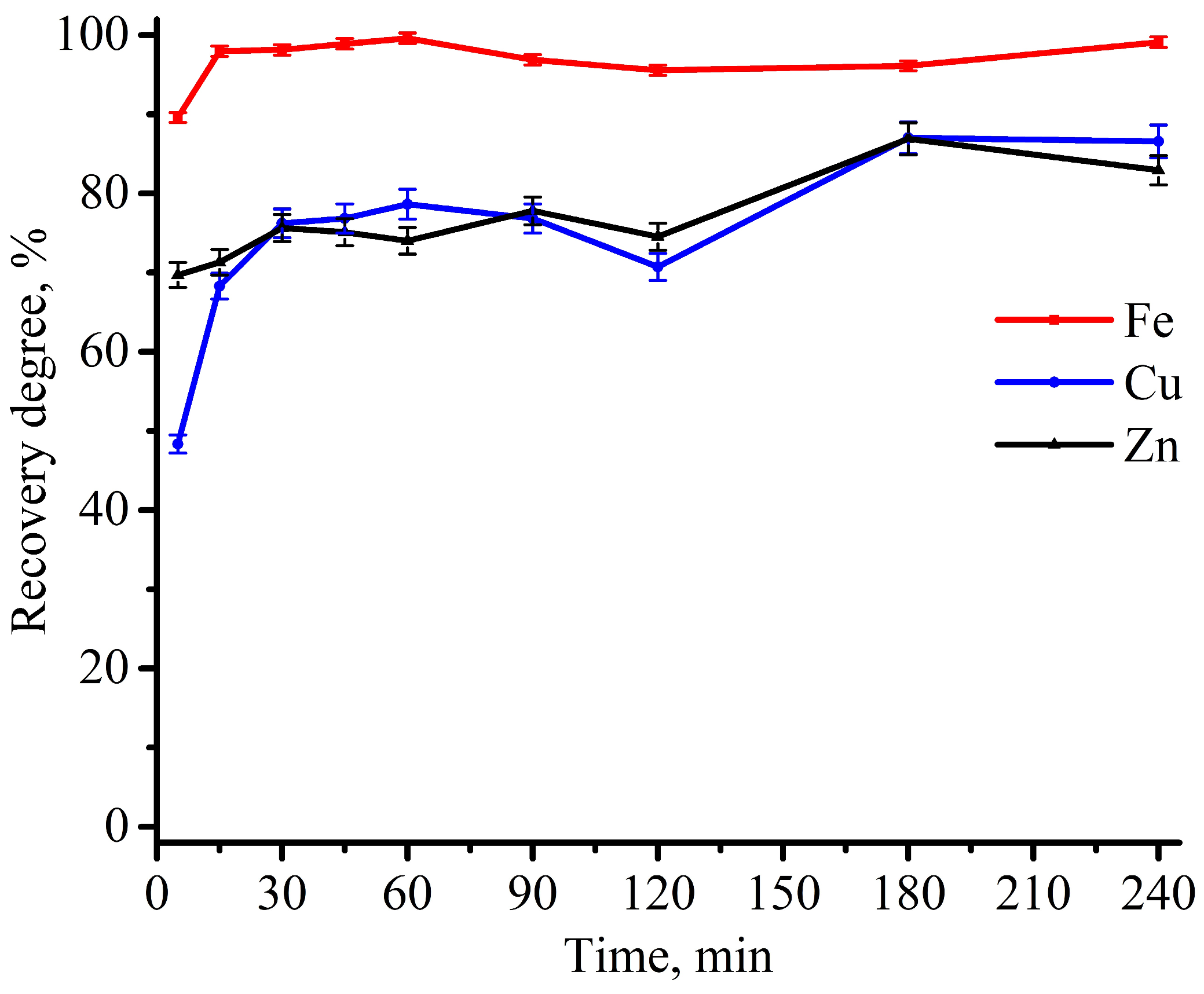
3.2. Experimental Optimization of Leaching Conditions
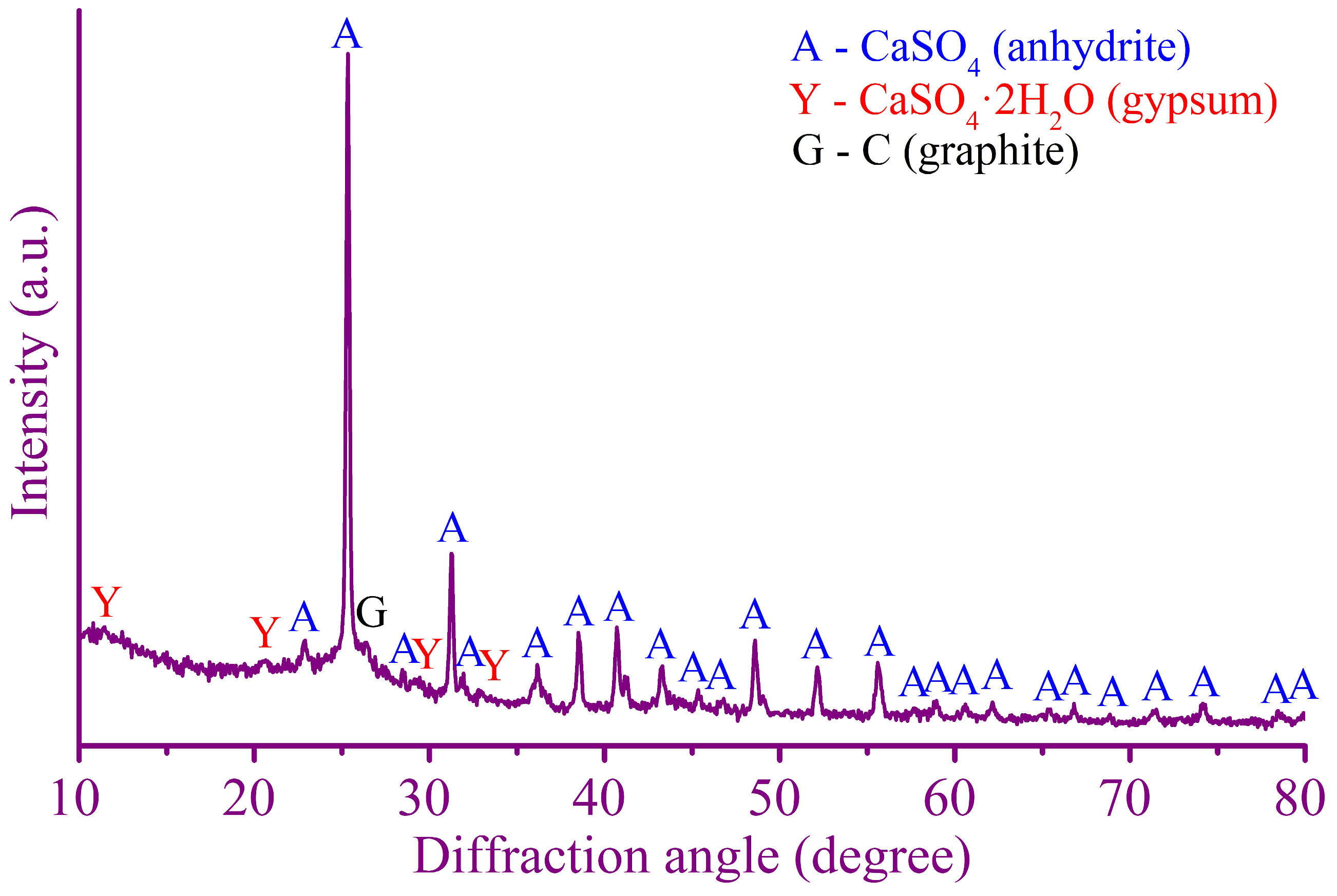
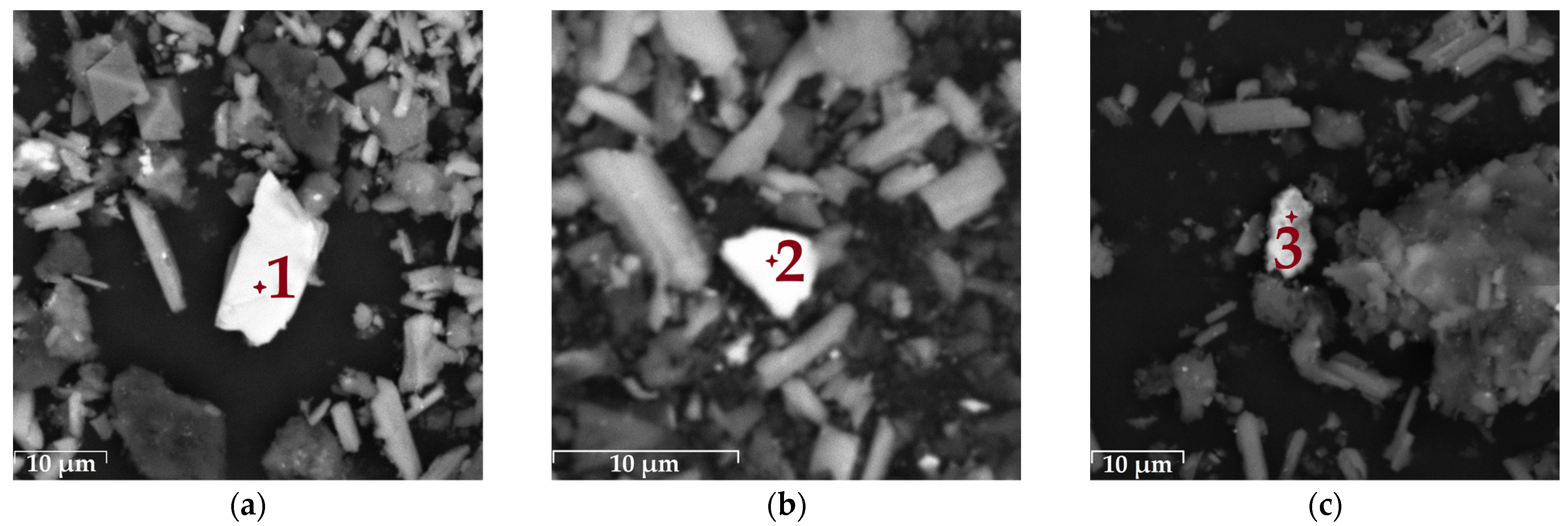
3.3. Characterization of the Leaching Residue
3.4. Possibility of Oxidant Substitution
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CBWS | Copper-bearing Waelz slag |
| S/L ratio | Solid-to-liquid ratio |
| XRD | X-ray diffraction |
| SEM | Scanning electron microscopy |
| EDX detector | Energy-dispersive X-ray detector |
References
- Norgate, T.; Jahanshahi, S. Low Grade Ores—Smelt, Leach or Concentrate? Miner. Eng. 2010, 23, 65–73. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Z.; Wu, Y.; Li, L.; Li, B.; Pan, D.; Zuo, T. Environmental Benefits of Secondary Copper from Primary Copper Based on Life Cycle Assessment in China. Resour. Conserv. Recycl. 2019, 146, 35–44. [Google Scholar] [CrossRef]
- Northey, S.; Haque, N.; Mudd, G. Using Sustainability Reporting to Assess the Environmental Footprint of Copper Mining. J. Clean. Prod. 2013, 40, 118–128. [Google Scholar] [CrossRef]
- World Copper Factbook. 2024. Available online: https://icsg.org/download/2024-09-23-the-world-copper-factbook-2024/?wpdmdl=8185&refresh=66f165d4bdad41727096276&ind=66f165bba8103&filename=Factbook2024.pdf (accessed on 15 January 2025).
- Zhou, W.; Liu, X.; Lyu, X.; Gao, W.; Su, H.; Li, C. Extraction and Separation of Copper and Iron from Copper Smelting Slag: A Review. J. Clean. Prod. 2022, 368, 133095. [Google Scholar] [CrossRef]
- Feng, S.; Che, J.; Zhang, W.; Zuo, Y.; Wang, C.; Ma, B.; Chen, Y. A Sustainable Approach for Recovering Copper and Zinc from Copper Smelting Flue Dust: Paving the Path for Waste Reduction. Sep. Purif. Technol. 2024, 342, 127037. [Google Scholar] [CrossRef]
- Sun, P.-P.; Kim, T.-Y.; Seo, H.; Cho, S.-Y. Separation and Recovery of Cu from Industrial Dust via a Solvometallurgical Process. Metals 2022, 12, 1723. [Google Scholar] [CrossRef]
- Sethurajan, M.; Huguenot, D.; Lens, P.N.L.; Horn, H.A.; Figueiredo, L.H.A.; van Hullebusch, E.D. Leaching and Selective Copper Recovery from Acidic Leachates of Três Marias Zinc Plant (MG, Brazil) Metallurgical Purification Residues. J. Environ. Manag. 2016, 177, 26–35. [Google Scholar] [CrossRef]
- Falagán, C.; Grail, B.M.; Johnson, D.B. New Approaches for Extracting and Recovering Metals from Mine Tailings. Miner. Eng. 2017, 106, 71–78. [Google Scholar] [CrossRef]
- Valderrama, L.; Tapia, J.; Pavez, O.; Santander, M.; Rivera, V.; Gonzalez, M. Recovery of Copper from Slags Through Flotation at the Hernán Videla Lira Smelter. Minerals 2024, 14, 1228. [Google Scholar] [CrossRef]
- Behera, S.S.; Panda, S.K.; Das, D.; Mohapatra, R.K.; Kim, H.I.; Lee, J.Y.; Jyothi, R.K.; Parhi, P.K. Microwave Assisted Leaching Investigation for the Extraction of Copper(II) and Chromium(III) from Spent Catalyst. Sep. Purif. Technol. 2020, 244, 116842. [Google Scholar] [CrossRef]
- Rao, M.D.; Singh, K.K.; Morrison, C.A.; Love, J.B. Recycling Copper and Gold from E-Waste by a Two-Stage Leaching and Solvent Extraction Process. Sep. Purif. Technol. 2021, 263, 118400. [Google Scholar] [CrossRef]
- Agrawal, A.; Sahu, K.K. Problems, Prospects and Current Trends of Copper Recycling in India: An Overview. Resour. Conserv. Recycl. 2010, 54, 401–416. [Google Scholar] [CrossRef]
- Phiri, T.C.; Singh, P.; Nikoloski, A.N. The Potential for Copper Slag Waste as a Resource for a Circular Economy: A Review—Part II. Miner. Eng. 2021, 172, 107150. [Google Scholar] [CrossRef]
- Grudinsky, P.; Yurtaeva, A.; Pankratov, D.; Pasechnik, L.; Musaelyan, R.; Dyubanov, V. The Waelz Slag from Electric Arc Furnace Dust Processing: Characterization and Magnetic Separation Studies. Materials 2024, 17, 2224. [Google Scholar] [CrossRef] [PubMed]
- Barna, R.; Bae, H.R.; Méhu, J.; Van der Sloot, H.; Moszkowicz, P.; Desnoyers, C. Assessment of Chemical Sensitivity of Waelz Slag. Waste Manag. 2000, 20, 115–124. [Google Scholar] [CrossRef]
- Kozlov, P.A. The Waelz Process; Ore and Metals PH: Moscow, Russia, 2003; 160p. [Google Scholar]
- Onuk, P.; Melcher, F. Mineralogical and Chemical Quantification of Waelz Slag. Int. J. Miner. Process. Extr. Metall. 2022, 7, 50–60. [Google Scholar] [CrossRef]
- Lobanov, V.G.; Savelyev, S.M.; Nechvoglod, O.V.; Kolmachikhina, O.B.; Makovskaya, O.Y. Study of the Phase Composition and Metal Speciation of Aged Elektrotsink Clinker. Metallurgist 2025, 68, 1389–1396. [Google Scholar] [CrossRef]
- Kozlov, P.A.; Vyatkin, V.N.; Reshetnikov, Y.V. Research and Development of Pyrometallurgical Technology of Processing of Copper Industry Wastes with Extraction of Zinc, Lead, and Tin. Tsvetnye Met. 2015, 5, 46–50. [Google Scholar] [CrossRef]
- Stoychev, S.; Minchev, E.; Kyurkchiev, A.; Radonov, G. Technologies for Treatment of Zinc-Containing Waste from Metallurgy in KCM AD. In PbZn 2020: 9th International Symposium on Lead and Zinc Processing; Siegmund, A., Alam, S., Grogan, J., Kerney, U., Shibata, E., Eds.; Springer: Cham, Switzerland, 2020; pp. 799–809. [Google Scholar]
- Jiang, Y.; Sun, L.-D.; Li, N.; Gao, L.; Chattopadhyay, K. Metal-Doped ZnFe2O4 Nanoparticles Derived from Fe-Bearing Slag with Enhanced Visible-Light Photoactivity. Ceram. Int. 2020, 46, 28828–28834. [Google Scholar] [CrossRef]
- Yoon, J.; Yoon, C.; Sugimoto, H.; Honjo, A. A Study on the Resource Recovery of Fe-Clinker Generated in the Recycling Process of Electric Arc Furnace Dust. Resour. Recycl. 2023, 32, 50–59. [Google Scholar] [CrossRef]
- Kolesnikov, A.; Fediuk, R.; Amran, M.; Klyuev, S.; Klyuev, A.; Volokitina, I.; Naukenova, A.; Shapalov, S.; Utelbayeva, A.; Kolesnikova, O.; et al. Modeling of Non-Ferrous Metallurgy Waste Disposal with the Production of Iron Silicides and Zinc Distillation. Materials 2022, 15, 2542. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, D.; Mapelli, C.; Barella, S.; Gruttadauria, A.; Di Landro, U. Laboratory Investigation of Waelz Slag Stabilization. Process Saf. Environ. Prot. 2015, 94, 227–238. [Google Scholar] [CrossRef]
- Quijorna, N.; Miguel, G.S.; Andrés, A. Incorporation of Waelz Slag into Commercial Ceramic Bricks: A Practical Example of Industrial Ecology. Ind. Eng. Chem. Res. 2011, 50, 5806–5814. [Google Scholar] [CrossRef]
- Abbà, A.; Sorlini, S.; Collivignarelli, M.C. Research Experiences on the Reuse of Industrial Waste for Concrete Production. MATEC Web Conf. 2017, 121, 10001. [Google Scholar] [CrossRef]
- Vegas, I.; Ibañez, J.A.; San José, J.T.; Urzelai, A. Construction Demolition Wastes, Waelz Slag and MSWI Bottom Ash: A Comparative Technical Analysis as Material for Road Construction. Waste Manag. 2008, 28, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Cifrian, E.; Coronado, M.; Quijorna, N.; Alonso-Santurde, R.; Andrés, A. Waelz Slag-Based Construction Ceramics: Effect of the Trial Scale on Technological and Environmental Properties. J. Mater. Cycles Waste Manag. 2019, 21, 1437–1448. [Google Scholar] [CrossRef]
- Evdokimov, S.I.; Pan’shin, A.M. Selecting the Concentration Technology of Clinker Using the Waelz Process on Zinc Cakes. Russ. J. Non-Ferr. Met. 2009, 50, 81–88. [Google Scholar] [CrossRef]
- Orehkova, N.N.; Gorlova, O.E.; Glagoleva, I.V. Study of the Separation of Mineral Phases of Waelz Clinker for Its Disposal. IOP Conf. Ser. Mater. Sci. Eng. 2020, 962, 042030. [Google Scholar] [CrossRef]
- Ergasheva, M.S.; Mirsaotov, S.U.; Khojiev, S.T. Use of Zinc Plant Clinker as a Reducing Agent in the Processing of Copper Slags. Eur. Sch. J. 2021, 2, 218–222. [Google Scholar]
- Iliev, P.; Stefanova, V.; Iliev, P.; Stefanova, V.; Lucheva, B.; Kolev, D. Selective Autoclave Recovery of Copper and Silver from Waelz Clinker in Ammonia Medium. J. Chem. Technol. Metall. 2017, 52, 340–345. [Google Scholar]
- Lucheva, B.; Iliev, P.; Draganova, K.; Stefanova, V. Recovery of Copper and Silver from Waelz Clinker Wasted from Zinc Production. J. Chem. Technol. Metall. 2014, 49, 12–15. [Google Scholar]
- Free, M.L. Hydrometallurgy, 1st ed.; Wiley: Hoboken, NJ, USA, 2013; 444p. [Google Scholar]
- Crowson, P. Some Observations on Copper Yields and Ore Grades. Resour. Policy 2012, 37, 59–72. [Google Scholar] [CrossRef]
- Drobe, M.; Haubrich, F.; Gajardo, M.; Marbler, H. Processing Tests, Adjusted Cost Models and the Economies of Reprocessing Copper Mine Tailings in Chile. Metals 2021, 11, 103. [Google Scholar] [CrossRef]
- Cacciuttolo, C.; Atencio, E. Past, Present, and Future of Copper Mine Tailings Governance in Chile (1905–2022): A Review in One of the Leading Mining Countries in the World. Int. J. Environ. Res. Public Health 2022, 19, 13060. [Google Scholar] [CrossRef]
- Aleksandrova, T.N.; Orlova, A.V.; Taranov, V.A. Current Status of Copper-Ore Processing: A Review. Russ. J. Non-Ferr. Met. 2021, 62, 375–381. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, X.; Chen, W.; Geng, Y.; Wilson, J. Measuring Environmental Impacts from Primary and Secondary Copper Production under the Upgraded Technologies in Key Chinese Enterprises. Environ. Impact Assess. Rev. 2022, 96, 106855. [Google Scholar] [CrossRef]
- Roine, A. HSC Chemistry® Software, Version 9.9; Metso: Helsinki, Finland. Available online: https://www.metso.com/portfolio/hsc-chemistry/ (accessed on 3 March 2025).
- Hu, H.; Tang, Y.; Ying, H.; Wang, M.; Wan, P.; Jin Yang, X. The Effect of Copper on Iron Reduction and Its Application to the Determination of Total Iron Content in Iron and Copper Ores by Potassium Dichromate Titration. Talanta 2014, 125, 425–431. [Google Scholar] [CrossRef]
- Lidin, R.A.; Molochko, V.A.; Andreeva, L.L. Reactions of Inorganic Substances: Handbook, 2nd ed.; Lidin, R.A., Ed.; Drofa: Moscow, Russia, 2007; 637p. [Google Scholar]
- Zhou, L.; Peng, T.; Sun, H.; Wang, S. Thermodynamics Analysis and Experiments on Ti-Bearing Blast Furnace Slag Leaching Enhanced by Sulfuric Acid Roasting. RSC Adv. 2022, 12, 34990–35001. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Liu, W.; Zhang, G.; Tang, S.; Yue, H.; Liang, B.; Luo, D. Recovery of Titanium, Aluminum, Magnesium and Separating Silicon from Titanium-Bearing Blast Furnace Slag by Sulfuric Acid Curing—Leaching. Int. J. Miner. Metall. Mater. 2022, 29, 1705–1714. [Google Scholar] [CrossRef]
- Schlesinger, M.E.; Sole, K.C.; Davenport, W.G.; Alvear Flores, G.R.F. Hydrometallurgical Copper Extraction: Introduction and Leaching. In Extractive Metallurgy of Copper; Elsevier: Amsterdam, The Netherlands, 2022; pp. 361–406. [Google Scholar]
- Lucchesi, P.J. Oscillometric Investigation of Precipitation and Dissolution Rates of Sparingly Soluble Sulfates. J. Colloid. Sci. 1956, 11, 113–123. [Google Scholar] [CrossRef]
- De Schepper, M.; Van den Heede, P.; Arvaniti, E.C.; De Buysser, K.; Van Driessche, I.; De Belie, N. Sulfates in Completely Recyclable Concrete and the Effect of CaSO4 on the Clinker Mineralogy. Constr. Build. Mater. 2017, 137, 300–306. [Google Scholar] [CrossRef]
- Sinyoung, S.; Songsiriritthigul, P.; Asavapisit, S.; Kajitvichyanukul, P. Chromium Behavior during Cement-Production Processes: A Clinkerization, Hydration, and Leaching Study. J. Hazard. Mater. 2011, 191, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Adhikary, S.K.; D’Angelo, A.; Viola, V.; Catauro, M.; Perumal, P. Alternative Construction Materials from Industrial Side Streams: Are They Safe? Energy Ecol. Environ. 2024, 9, 206–214. [Google Scholar] [CrossRef]
- Koralegedara, N.H.; Pinto, P.X.; Dionysiou, D.D.; Al-Abed, S.R. Recent Advances in Flue Gas Desulfurization Gypsum Processes and Applications—A Review. J. Environ. Manag. 2019, 251, 109572. [Google Scholar] [CrossRef]
- Aakriti; Maiti, S.; Jain, N.; Malik, J. A Comprehensive Review of Flue Gas Desulphurized Gypsum: Production, Properties, and Applications. Constr. Build. Mater. 2023, 393, 131918. [Google Scholar] [CrossRef]
- Xu, W.; Liu, C.; Du, K.; Gao, Q.; Liu, Z.; Wang, W. A Brief Review on Flue Gas Desulfurization Gypsum Recovery toward Calcium Carbonate Preparation. Environ. Sci. Adv. 2024, 3, 1351–1363. [Google Scholar] [CrossRef]
- Antonijević, M.M.; Dimitrijević, M.; Janković, Z. Leaching of Pyrite with Hydrogen Peroxide in Sulphuric Acid. Hydrometallurgy 1997, 46, 71–83. [Google Scholar] [CrossRef]
- Bogdanović, G.D.; Petrović, S.; Sokić, M.; Antonijević, M.M. Chalcopyrite Leaching in Acid Media: A Review. Metall. Mater. Eng. 2020, 26, 177–198. [Google Scholar] [CrossRef]
- Pecina, T.; Franco, T.; Castillo, P.; Orrantia, E. Leaching of a Zinc Concentrate in H2SO4 Solutions Containing H2O2 and Complexing Agents. Miner. Eng. 2008, 21, 23–30. [Google Scholar] [CrossRef]
- Gupta, C.K.; Mukherjee, T.K. Hydrometallurgy in Extraction Processes, 1st ed.; Routledge: New York, NY, USA, 2019; Volume 1, 248p. [Google Scholar]
- Nicol, M.J. The Role and Use of Hydrogen Peroxide as an Oxidant in the Leaching of Minerals. 1. Acid Solutions. Hydrometallurgy 2020, 193, 105328. [Google Scholar] [CrossRef]
- Kazanbaev, L.A.; Kozlov, P.A.; Kubasov, V.L.; Kolesnikov, A.V. Zinc Hydrometallurgy (Cleaning of Solutions and Electrolysis); Ore and Metals PH: Moscow, Russia, 2006; 176p. [Google Scholar]
- Sinclair, R.J. The Extractive Metallurgy of Zinc, 1st ed.; Australasian Institute of Mining and Metallurgy: Carlton, VIC, Australia, 2005; 294p. [Google Scholar]
- Antrekowitsch, J.; Steinlechner, S.; Unger, A.; Rösler, G.; Pichler, C.; Rumpold, R. Zinc and Residue Recycling. In Handbook of Recycling: State-of-the-Art for Practitioners, Analysts, and Scientists; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 113–124. [Google Scholar]
- Ehsani, I.; Ehsani, A.; Çavuşlu, F.; Kantarci, S. Characterization and Leaching Behavior of Mixed Oxide-Sulfide Zinc Ore from Hakkari-Türkiye. In Proceedings of the 18th International Mineral Processing Symposium IMPS2024, Eskişehir, Türkiye, 16–18 October 2024; Volkan Bozkurt, M.M., Ipek, H., Serkan Gökçen, H., Bilir, K., Eds.; Department of Mining Engineering, Eskişehir Osmangazi University: Eskişehir, Türkiye, 2024; pp. 283–290. [Google Scholar]
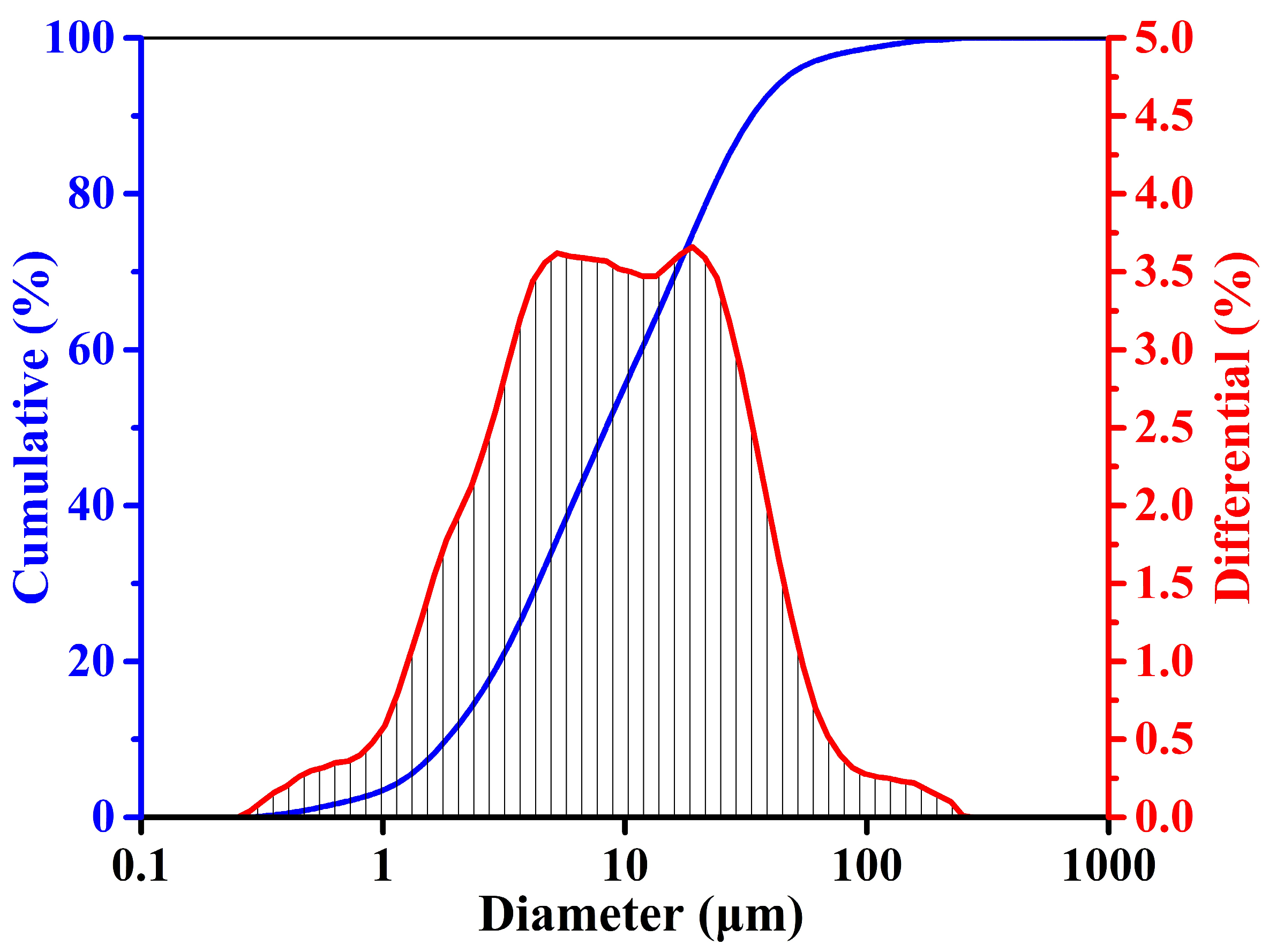



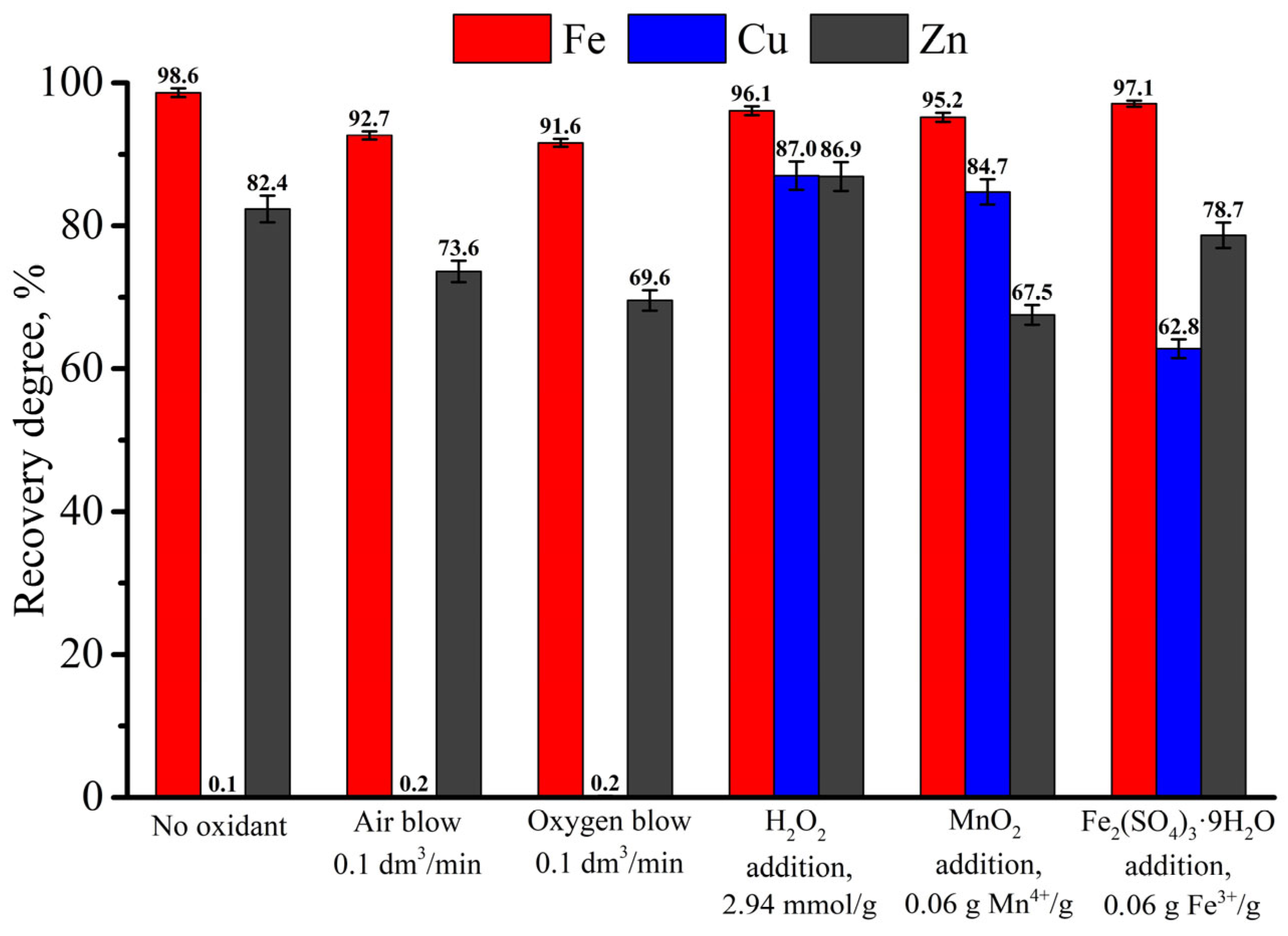
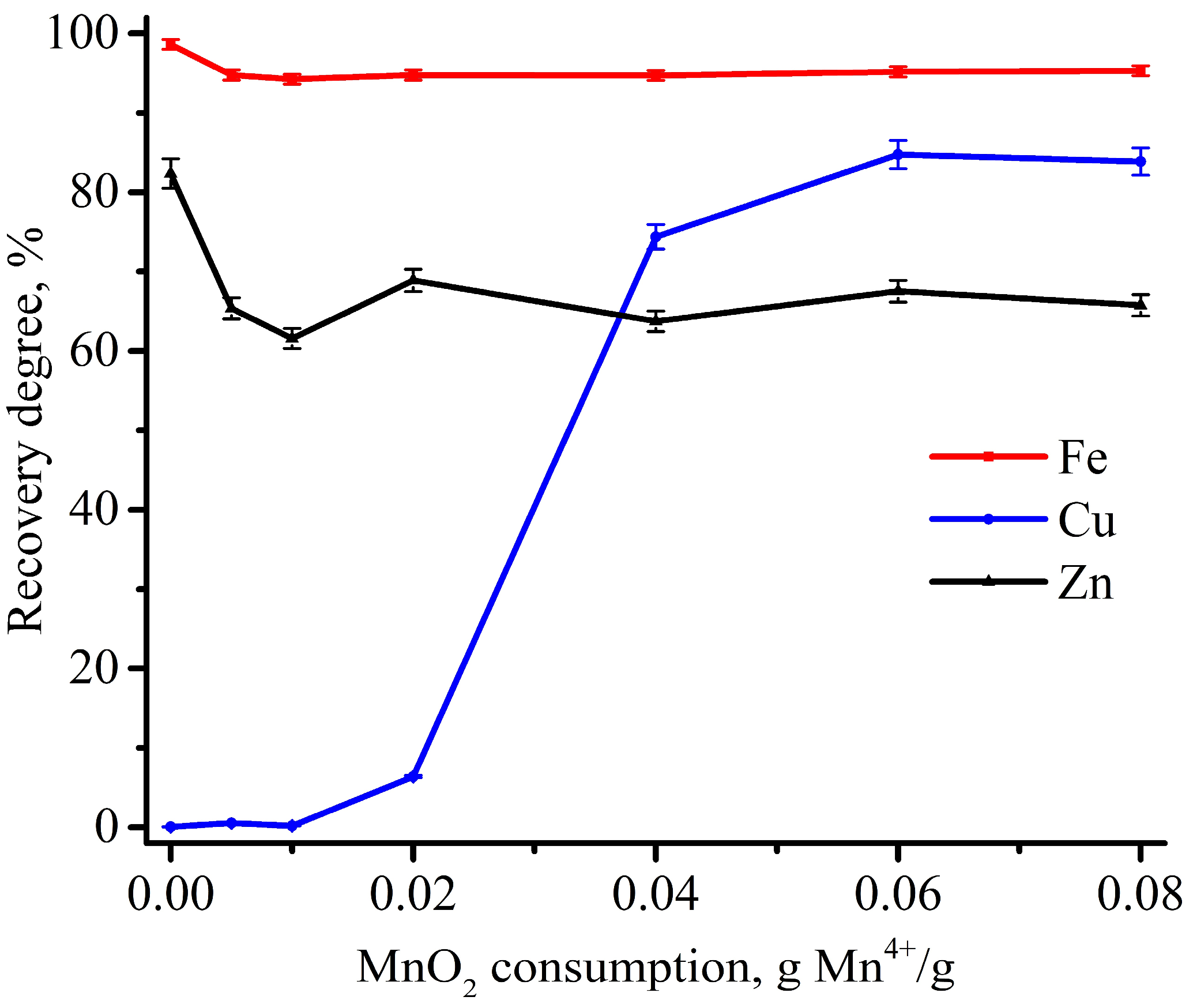
| Fe | Cu | Zn | C | Ca | Si | Al | Cr | Mg | Mn | Ni | Cd | As | P | S | Pb | Sb | Ba | Ti |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 26.23 | 0.82 | 0.81 | 17.1 | 8.99 | 5.40 | 2.80 | 0.40 | 4.40 | 2.00 | 0.066 | 0.028 | 0.34 | 0.16 | 2.20 | 0.30 | 0.06 | 0.20 | 0.14 |
| α-Fe | FeO | γ-Fe2O3 | FeOOH | C | Ca2Al2SiO7 | Ca2MgSi2O7 | Mg2SiO4 | CaMgSiO4 | MgO | CaS | MnS | FeAs | CaTiO3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 16.00 | 2.02 | 3.07 | 9.62 | 17.1 | 10.27 | 9.79 | 3.00 | 4.10 | 2.83 | 2.18 | 3.17 | 0.59 | 0.40 | |
| ZnO | ZnS | Zn2SiO4 | ZnFe2O4 | Cu | NaAlSiO4 | Ca3MgSi2O8 | Pb2SiO4 | Ni | Cr | PbO | BaO | P2O5 | Sb2O3 | KAlSi2O6 |
| 0.55 | 0.20 | 0.19 | 0.46 | 0.82 | 3.34 | 2.00 | 0.33 | 0.063 | 0.40 | 0.03 | 0.22 | 0.37 | 0.07 | 1.17 |
| Elements | Fe | Cu | Zn | Ca | Si | Al | Mg | Mn | Ba | Cd | Cr | Ni | Pb | Sb | P | Ti |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration, mg/dm3 | 4072.4 | 125 | 117 | 194 | 4.1 | 194 | 573 | 290 | 0.031 | 4.1 | 25.3 | 8.0 | 2.9 | 1.6 | 19.1 | 15.8 |
| Recovery degree, % | 96.1 | 87.0 | 86.9 | 20.0 | 0.5 | 80.5 | 93.2 | 92.8 | 0.1 | 91.0 | 48.3 | 70.6 | 6.1 | 34.3 | 90.6 | 71.4 |
| Ca | S | Si | C | Fe | Cu | Zn | Al | Ba | Cd | Cr | Ni | Mg | Mn | P | Pb | Sb | Ti |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 11.49 | 12.7 | 6.00 | 25.9 | 1.63 | 0.17 | 0.17 | 0.87 | 0.32 | 0.004 | 0.33 | 0.031 | 0.48 | 0.23 | 0.024 | 0.45 | 0.063 | 0.064 |
| No. | Phase | Composition, at. % | ||||||
|---|---|---|---|---|---|---|---|---|
| Fe | S | Zn | Cu | Ca | Si | O | ||
| 1 | Iron sulfide | 30.6 | 69.4 | - | - | - | - | - |
| 2 | Iron–copper sulfide | 26.6 | 45.3 | 0.5 | 11.5 | 0.4 | 0.8 | 14.9 |
| 3 | Zinc sulfide | 2.2 | 52.6 | 42.3 | - | 0.9 | 2.0 | - |
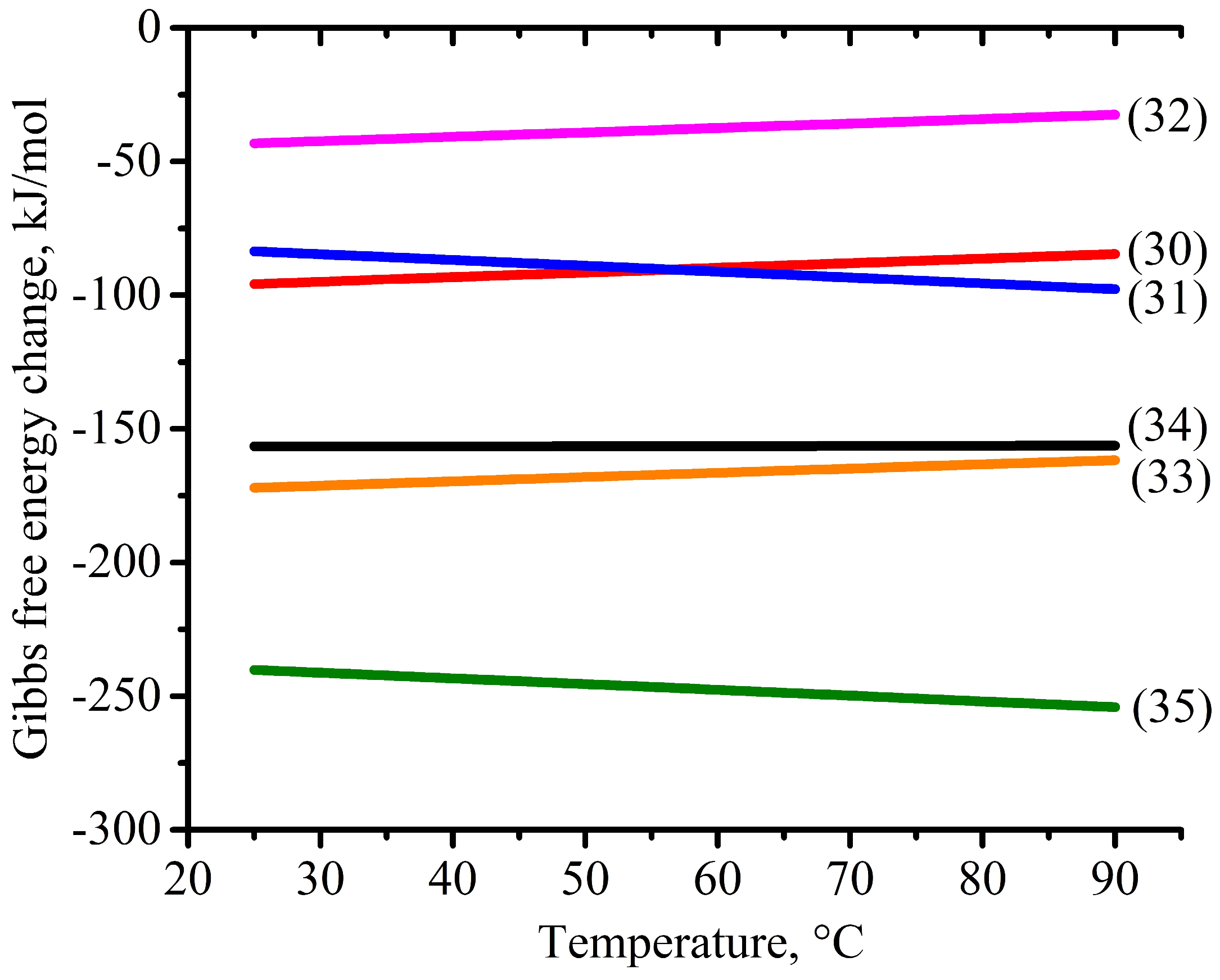
| Reaction Number | Reaction | Temperature (K) Dependence of ΔG, kJ/mol |
|---|---|---|
| (30) | Cu + 2Fe3+ = Cu2+ + 2Fe2+ | ΔG = −0.218∙T − 78.132 |
| (31) | Fe2+ + 0.5H2O2 + H+ = Fe3+ + H2O | ΔG = 0.173∙T − 100.131 |
| (32) | Fe2+ + 0.5MnO2 + 2H+ = Fe3+ + 0.5Mn2+ + H2O | ΔG = 0.165∙T − 47.358 |
| (33) | Cu + 0.5O2(g) + 2H+ = Cu2+ + H2O | ΔG = 0.160∙T − 176.077 |
| (34) | Fe + Cu2+ = Fe2+ + Cu | ΔG = 0.004∙T − 156.698 |
| (35) | Fe + 2Fe3+ = 3Fe2+ | ΔG = −0.214∙T − 234.830 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grudinsky, P.; Vasileva, E.; Dyubanov, V. Recycling Potential of Copper-Bearing Waelz Slag via Oxidative Sulfuric Acid Leaching. Metals 2025, 15, 330. https://doi.org/10.3390/met15030330
Grudinsky P, Vasileva E, Dyubanov V. Recycling Potential of Copper-Bearing Waelz Slag via Oxidative Sulfuric Acid Leaching. Metals. 2025; 15(3):330. https://doi.org/10.3390/met15030330
Chicago/Turabian StyleGrudinsky, Pavel, Ekaterina Vasileva, and Valery Dyubanov. 2025. "Recycling Potential of Copper-Bearing Waelz Slag via Oxidative Sulfuric Acid Leaching" Metals 15, no. 3: 330. https://doi.org/10.3390/met15030330
APA StyleGrudinsky, P., Vasileva, E., & Dyubanov, V. (2025). Recycling Potential of Copper-Bearing Waelz Slag via Oxidative Sulfuric Acid Leaching. Metals, 15(3), 330. https://doi.org/10.3390/met15030330







