Advances in Hydrolysis of Magnesium and Alloys: A Conceptual Review on Parameters Optimization for Sustainable Hydrogen Production
Abstract
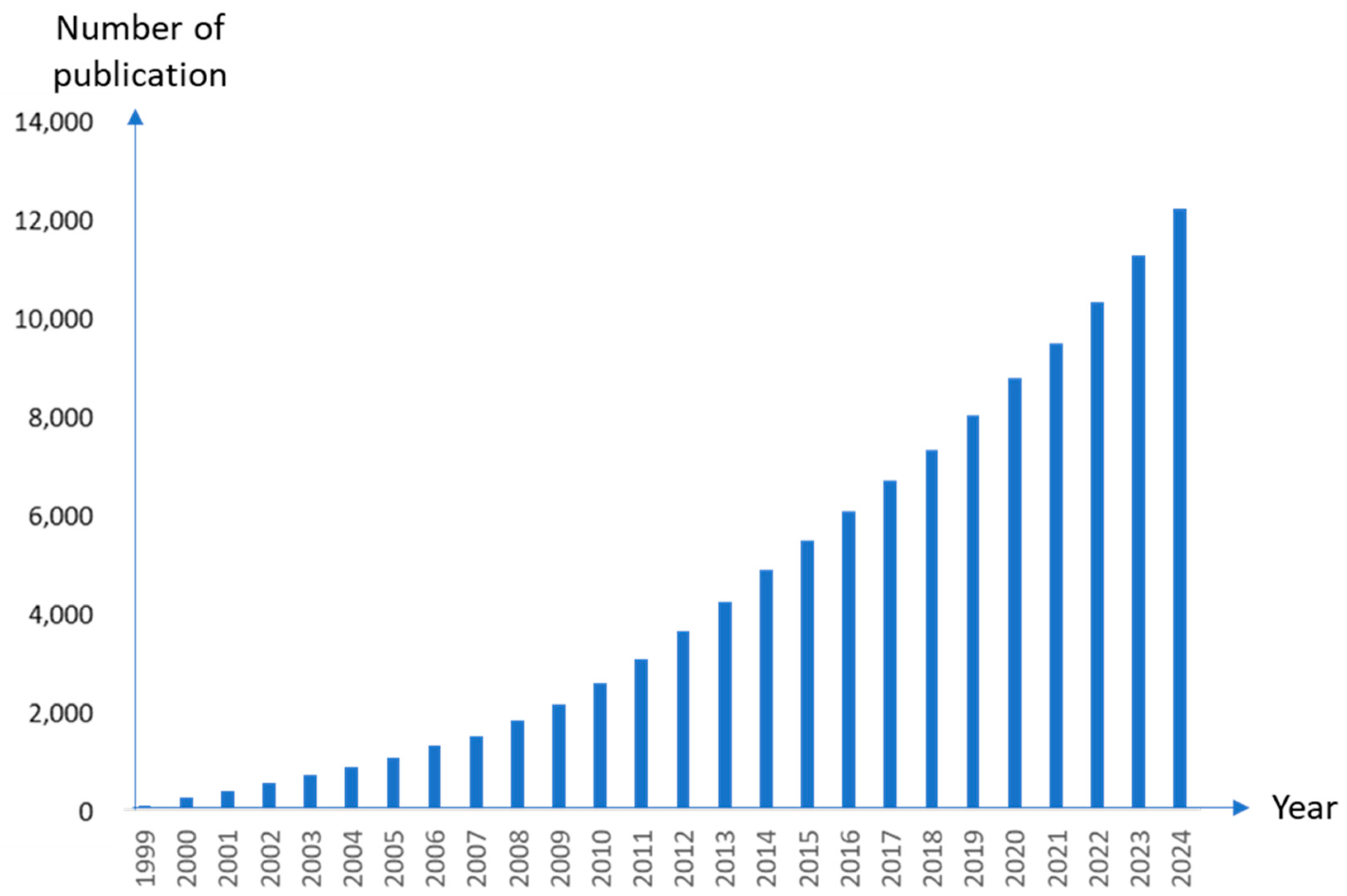
:1. Introduction
2. Various Additives Used
2.1. Metals and Their Oxides and Hydrides
2.2. Borohydrides
2.3. Halide (MClx and MFx) Additives
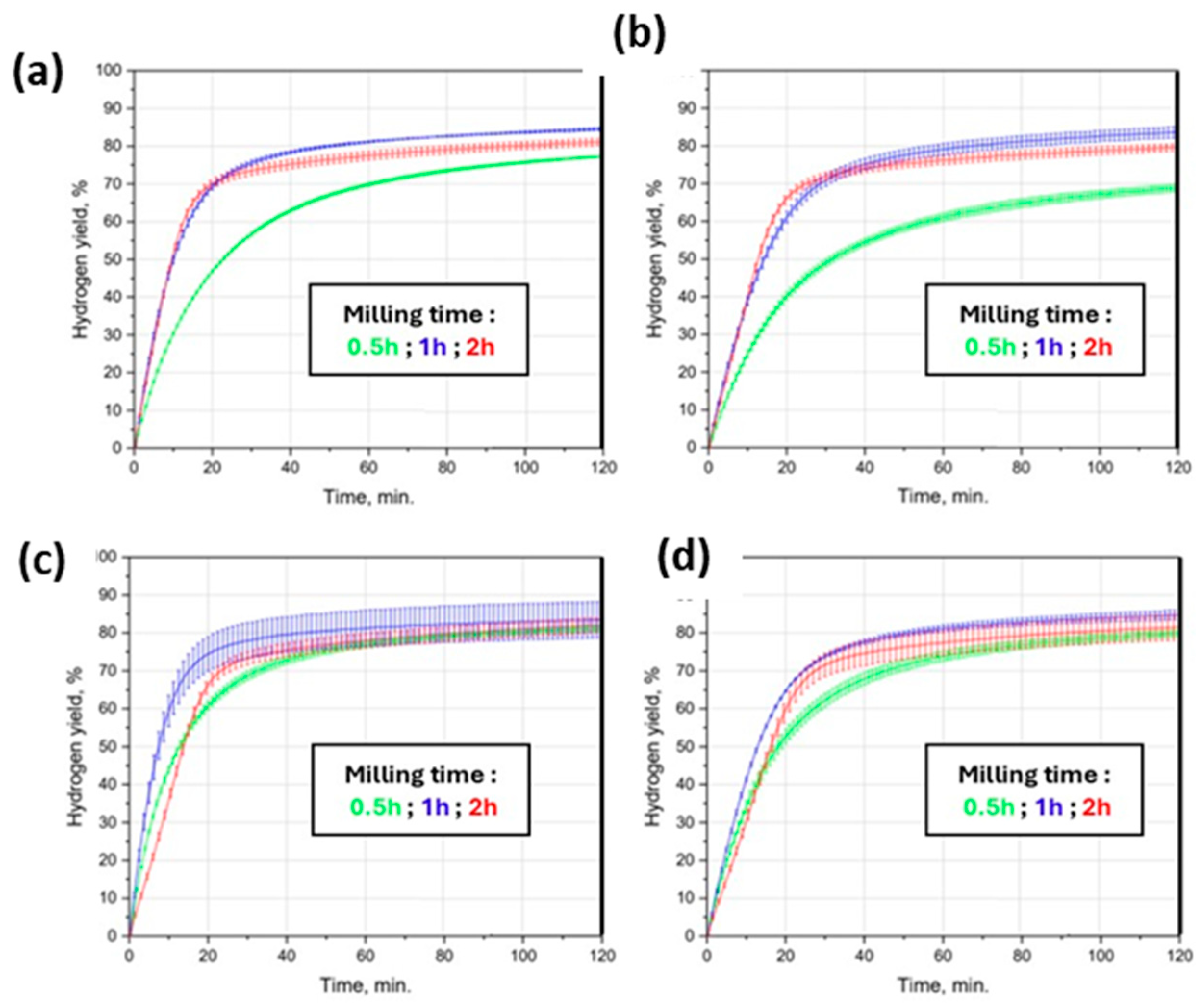
2.4. Carbon and Carbon-Based Additives
2.5. Mg-Al Mixtures and Alloys
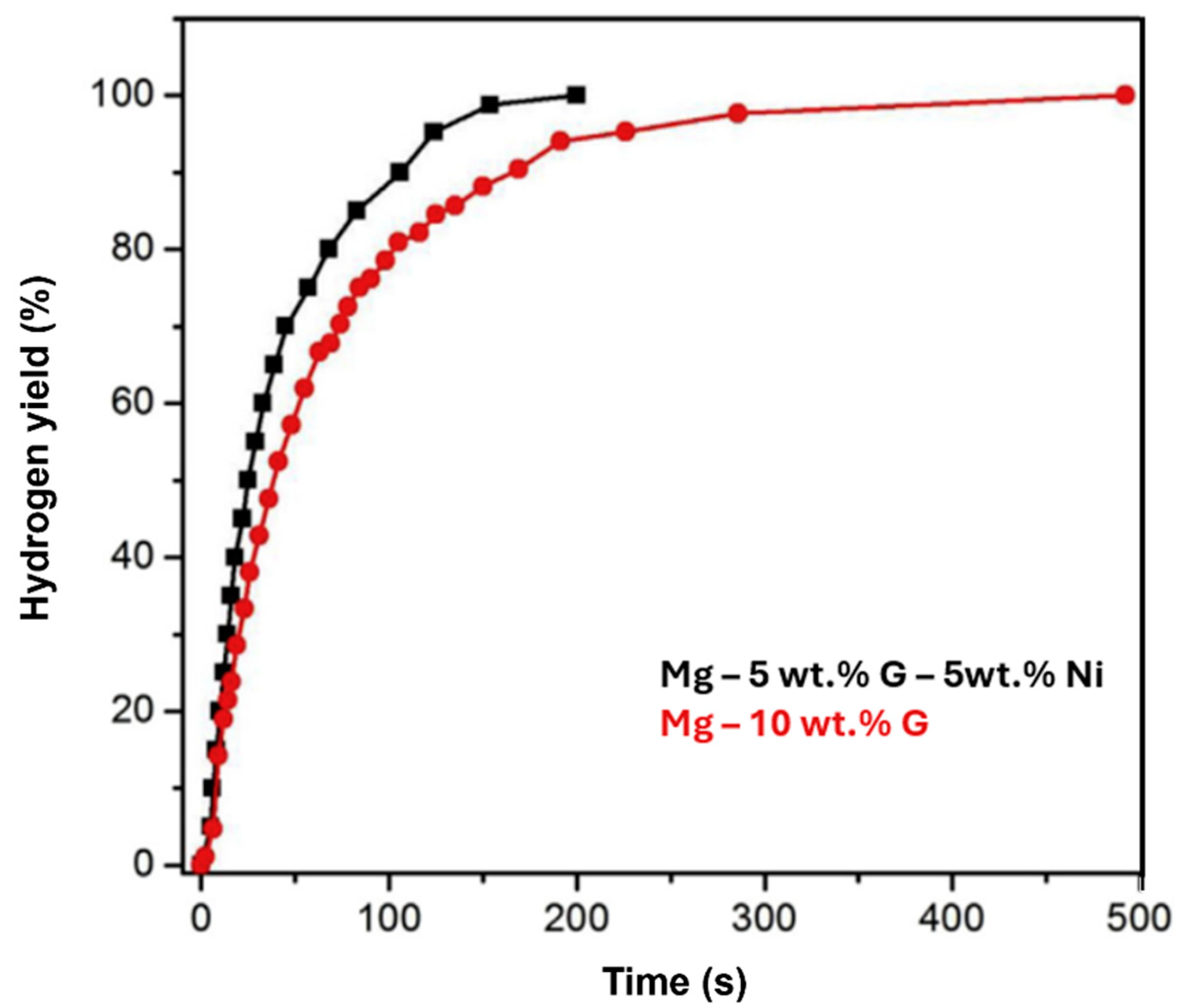
2.6. Mg-Metals Alloys
2.6.1. Mg-Ni Alloys
2.6.2. Mg-Ca Alloys
2.6.3. Mg-Cu Alloys
2.6.4. Mg-Li Alloys
2.6.5. Mg-Mg-Low Melting Point Metallic Element Alloy
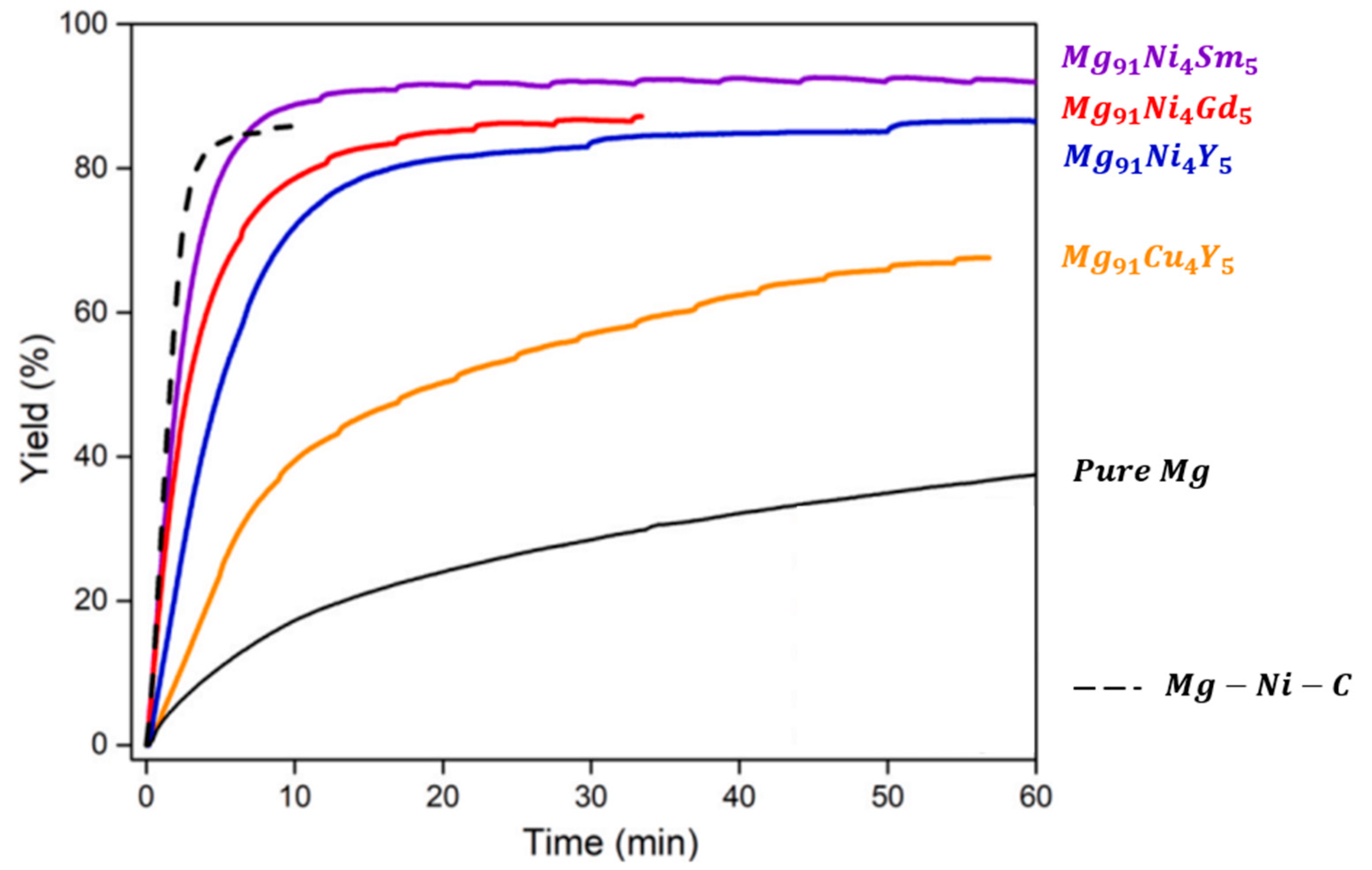
2.6.6. Mg-RE Alloys
2.7. Mg2X-Based Alloys (X = Ni, Cu, Sn, Si)
2.8. Mg3M-Based Alloys (M = La, Ce, Pr, Nd, Mm, In) and Ternary (and More) Alloys
3. Environment Enhancing Hydrogen Generation
3.1. Chloride Salt
3.1.1. Sodium Chloride (NaCl)
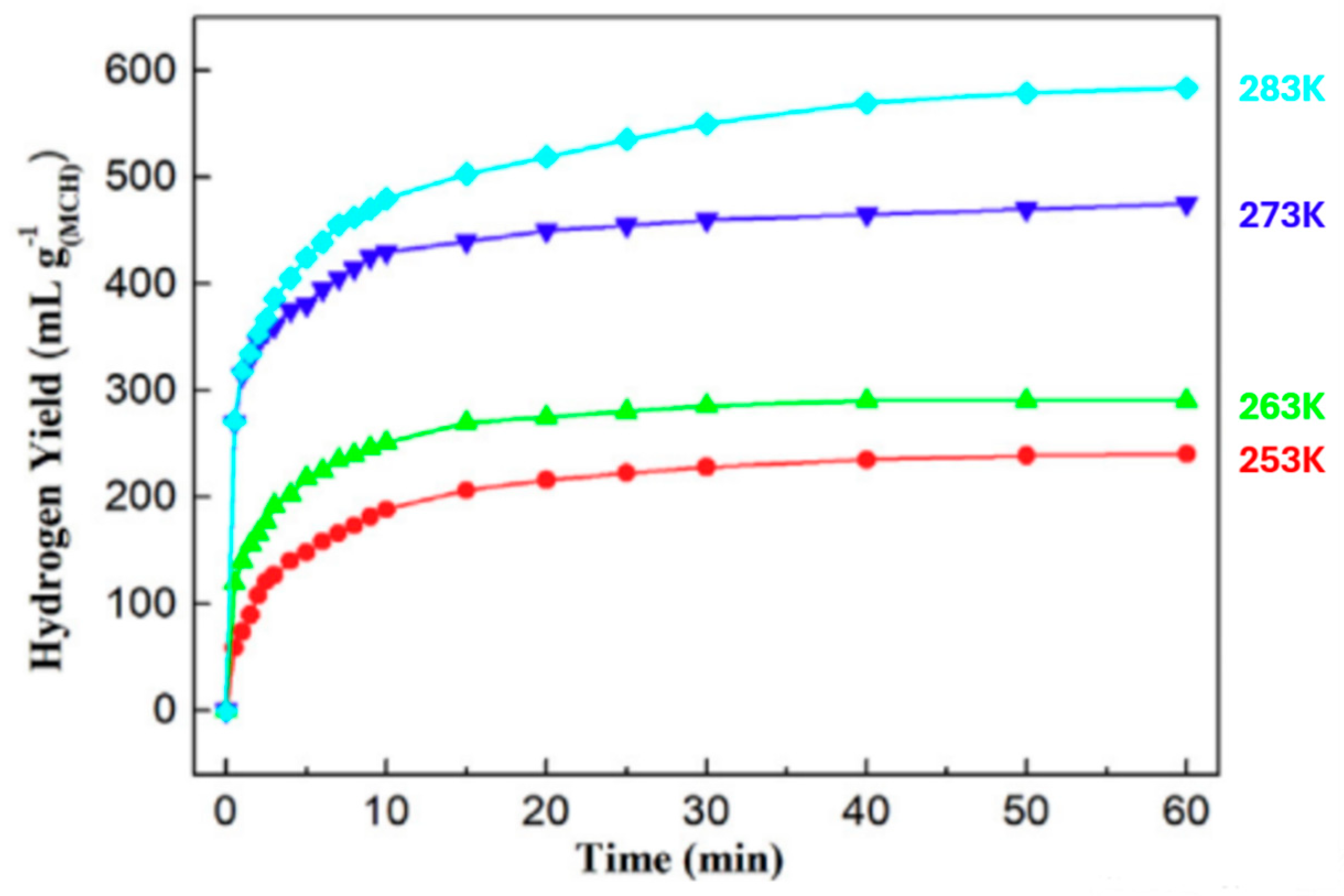
3.1.2. Magnesium Chloride (MgCl2)
3.1.3. Calcium Chloride (CaCl2)
3.1.4. Potassium Chloride (KCl)/Lithium Chloride (LiCl)
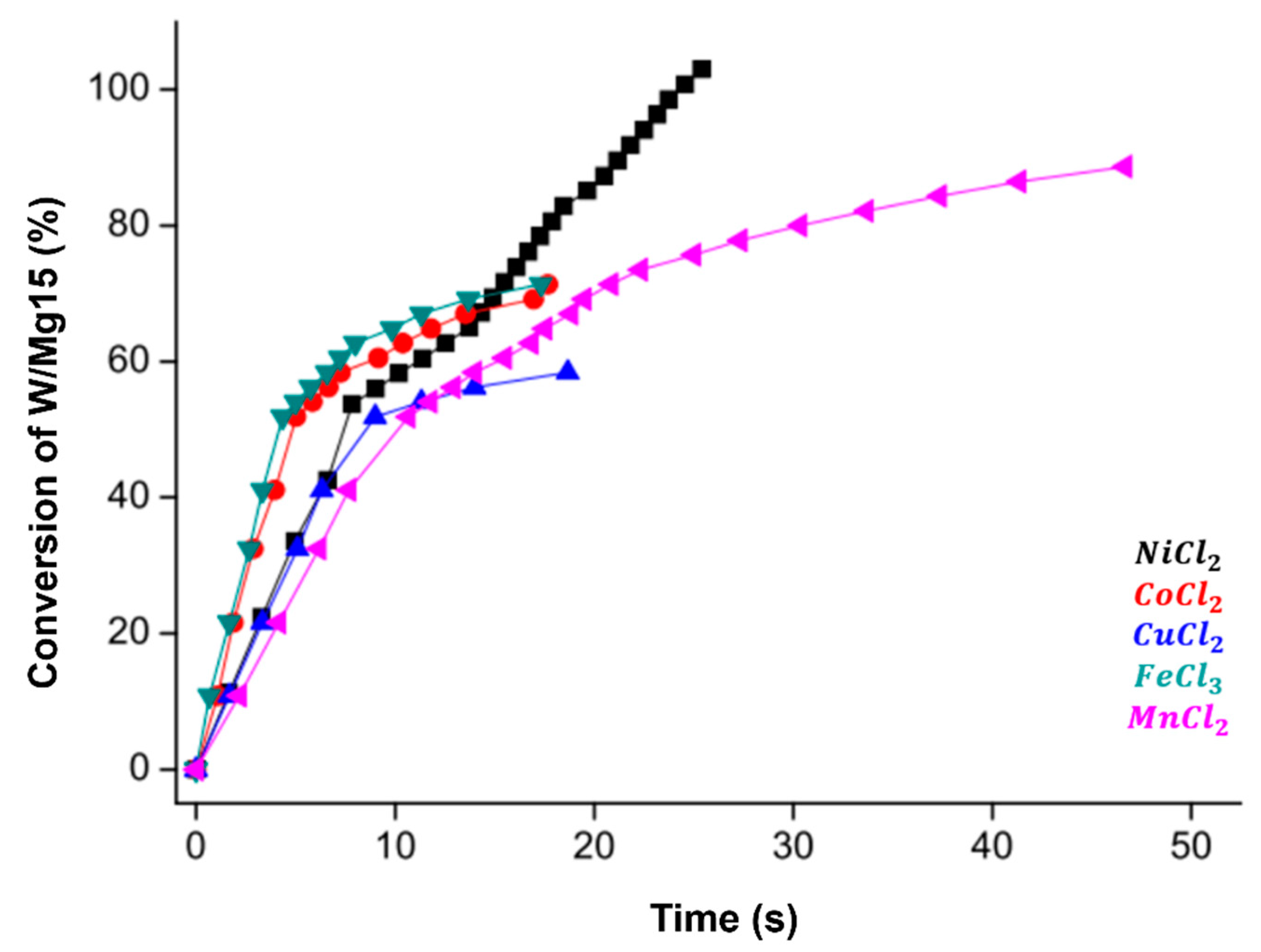
3.1.5. Transition Metal Chlorides (NiCl2, CoCl2, CuCl2, FeCl2, MnCl2)
3.1.6. Aluminum Chloride (AlCl3)
3.1.7. Ammonium Chloride (NH4Cl)
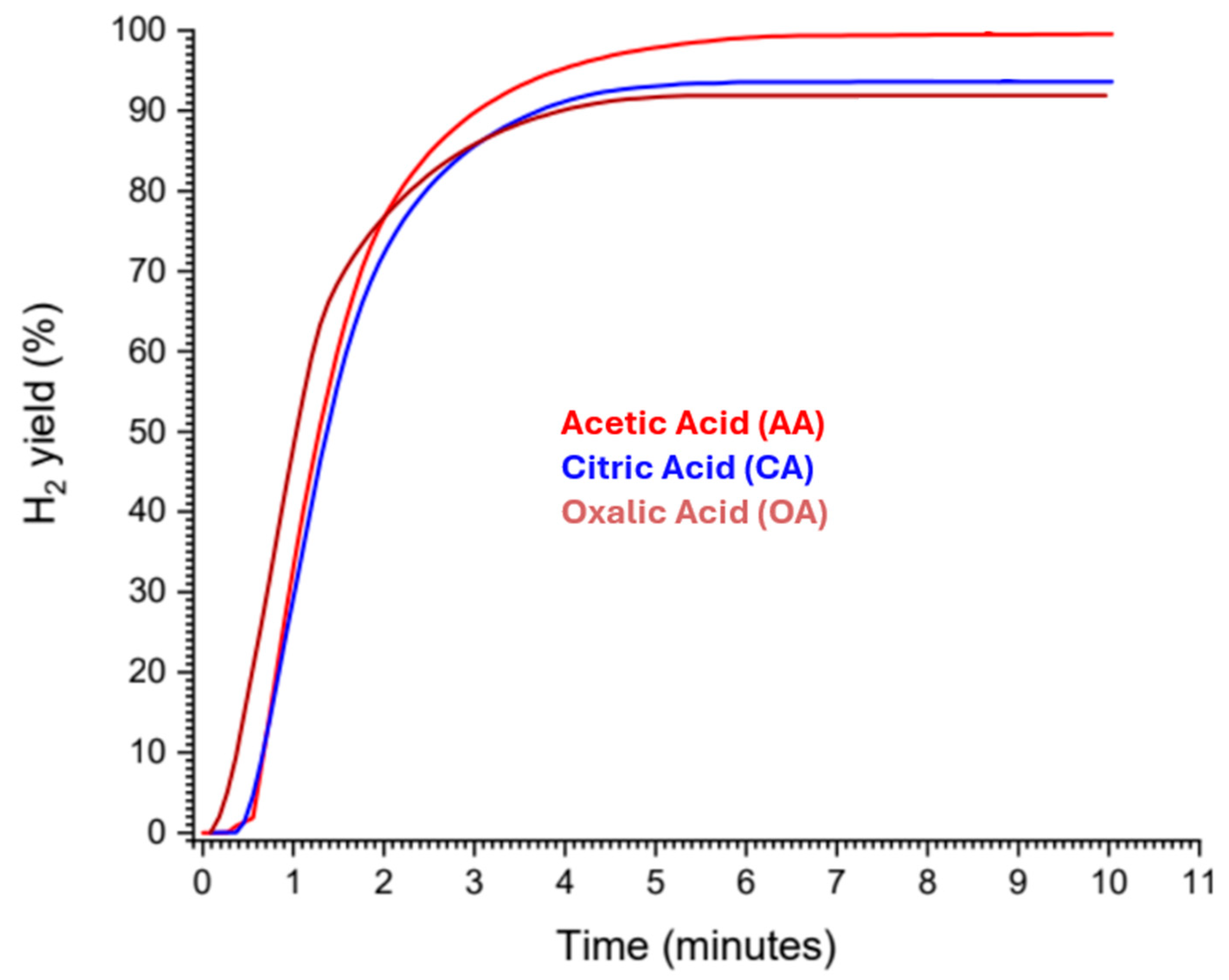
3.2. Organic Acids (Citric, Acetic and Oxalic Acids)
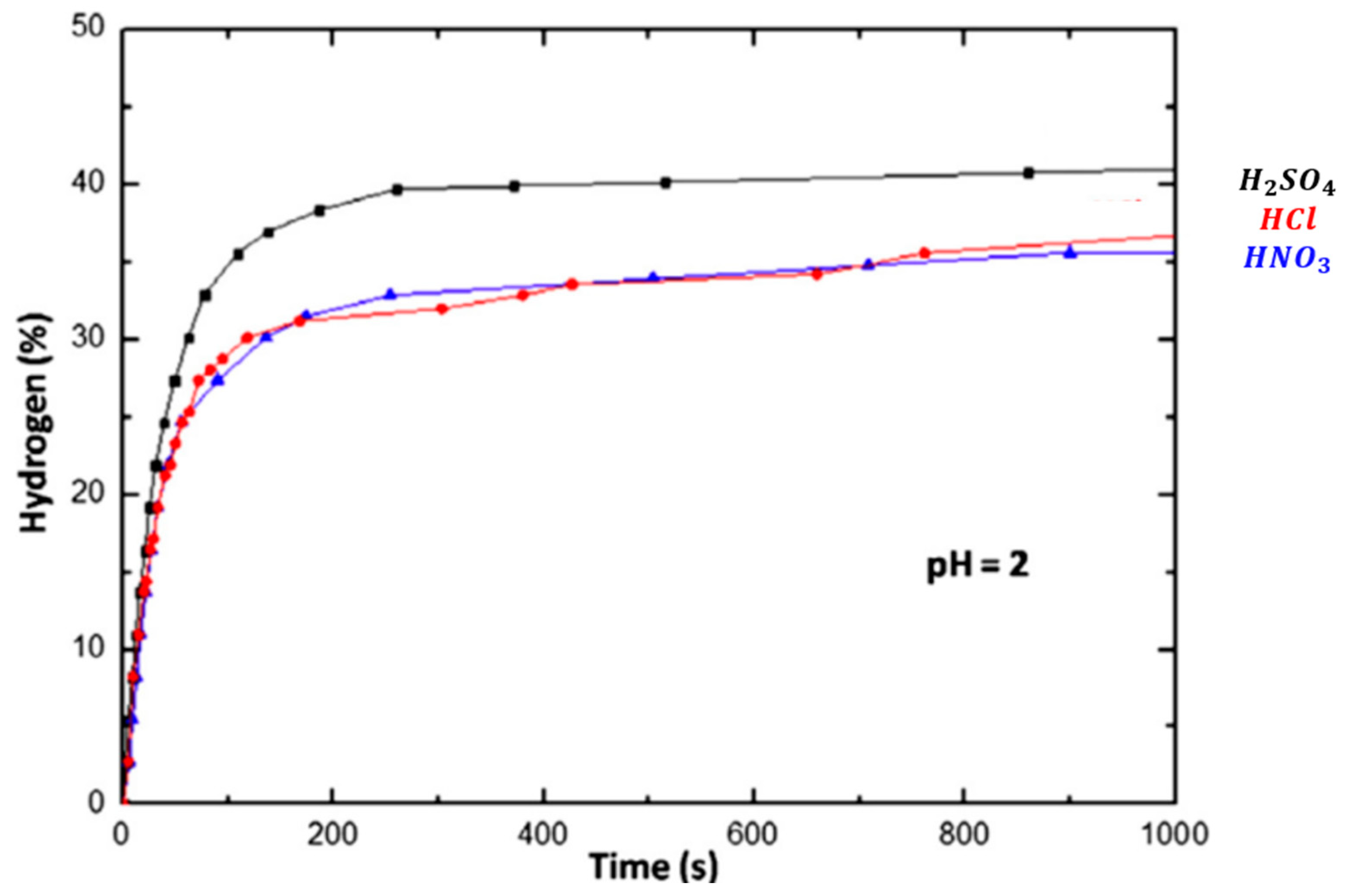
3.3. Inorganic Acids
Hydrochloric Acid (HCl), Sulfuric Acid (H2SO4), Nitric Acid (HNO3)
3.4. Nitrogen-Based Inorganic Salts
Ammonium Nitrate (NH4NO3)
3.5. Brewery Wastewater
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, J.; Xu, T.; Zhang, Z.; Zhang, J.; Huang, H.; Liu, B.; Li, Y.; Yuan, J.; Zhang, B.; Wu, Y. Utlra-Fast Hydrolysis Performance of MgH2 Catalyzed by Ti-Zr-Fe-Mn-Cr-V High-Entropy Alloys. J. Energy Chem. 2024, 98, 77–86. [Google Scholar] [CrossRef]
- Xiao, F.; Wu, T.; Yang, Y. Research Progress in Hydrogen Production by Hydrolysis of Magnesium-Based Materials. Int. J. Hydrogen Energy 2024, 49, 696–718. [Google Scholar] [CrossRef]
- Huang, H.; Xu, T.; Chen, J.; Zhao, Y.; Lv, Y.; Liu, B.; Zhang, B.; Yuan, J.; Wu, Y. Efficient Nanocatalysis of Ni/Sc2O3@FLG for Magnesium Hydrolysis of Hydrogen Generation. J. Mater. Sci. Technol. 2024, 175, 235–243. [Google Scholar] [CrossRef]
- Rodríguez, M.; Urretavizcaya, G.; Bobet, J.-L.; Castro, F.J. Effective Hydrogen Production by Hydrolysis of Mg Wastes Reprocessed by Mechanical Milling with Iron and Graphite. J. Alloys Compd. 2023, 946, 169352. [Google Scholar] [CrossRef]
- Zhang, L.; Yong, H.; Wang, S.; Yan, Z.; Xu, Z.; Li, Y.; Liu, B.; Hu, J.; Zhang, Y. Investigating of Hydrolysis Kinetics and Catalytic Mechanism of Mg–Ce–Ni Hydrogen Storage Alloy Catalyzed by Ni/Co/Zn-Based MOF. Int. J. Hydrogen Energy 2024, 71, 1416–1427. [Google Scholar] [CrossRef]
- Naseem, K.; Zhang, J.; Fayyaz, A.; Hayat, W.; Ahmed, S.; Khursheed, S. Enhanced Generation of Hydrogen through Hydrolysis of Biochar-Coupled Magnesium: Analysis of the Performance of Biochar-Support and the Effect of Metallic Coating on Biochar. J. Environ. Chem. Eng. 2024, 12, 111770. [Google Scholar] [CrossRef]
- Wu, D.; Li, R.; Zhou, Q.; Tang, R.; Xiao, F. Light-Activated Hydrolysis Properties of Mg-Based Materials. RSC Adv. 2022, 12, 6533–6539. [Google Scholar] [CrossRef]
- Bai, J.; Yun, H.; Wang, X.; Dai, X.; Hou, X.; Xu, Y. Hydrolytic Hydrogen Production Behavior in NaCl Solution of Mg and Mg-GO Composite Synthesized by Mechanochemical Reaction. J. Alloys Compd. 2023, 969, 172473. [Google Scholar] [CrossRef]
- Ge, L.; Zhu, Y.; Qiu, M.; Yang, S.; Sun, N.; Wei, W.; Li, J.; Chen, X. A Highly Active Pd Clusters Hosted by Magnesium Hydroxide Nanosheets Promoting Hydrogen Storage. Appl. Catal. B Environ. 2023, 333, 122793. [Google Scholar] [CrossRef]
- Qiu, H.; Yang, G.; Lv, L.; Han, X.; Zang, S.; Liu, W.; Wang, X. Hydrogen Generation from the Hydrolysis of LaMg12H27 Ball-Milled with LiH. Int. J. Hydrogen Energy 2023, 48, 20216–20224. [Google Scholar] [CrossRef]
- Varnagiris, S.; Urbonavicius, M.; Knoks, A.; Milcius, D. Enhanced Hydrogen Generation in Low-Range Acidic Solutions Using Mg2NiH4 Powders and Their Mixtures Ball-Milled with NaCl and Fused Silica. Int. J. Hydrogen Energy 2024, 50, 614–626. [Google Scholar] [CrossRef]
- Buryakovskaya, O.A.; Ambaryan, G.N.; Suleimanov, M.Z.; Tarasenko, A.B.; Vlaskin, M.S. Enhanced Hydrogen Generation from Magnesium–Aluminum Scrap Ball Milled with Low Melting Point Solder Alloy. Materials 2023, 16, 4450. [Google Scholar] [CrossRef] [PubMed]
- Buryakovskaya, O.A.; Ambaryan, G.N.; Tarasenko, A.B.; Suleimanov, M.Z.; Vlaskin, M.S. Effects of Bi–Sn–Pb Alloy and Ball-Milling Duration on the Reactivity of Magnesium–Aluminum Waste-Based Materials for Hydrogen Production. Materials 2023, 16, 4745. [Google Scholar] [CrossRef]
- Grosjean, M.-H.; Roué, L. Hydrolysis of Mg–Salt and MgH2–Salt Mixtures Prepared by Ball Milling for Hydrogen Production. J. Alloys Compd. 2006, 416, 296–302. [Google Scholar] [CrossRef]
- Al Bacha, S.; Zakhour, M.; Nakhl, M.; Bobet, J.-L. Effect of Ball Milling in Presence of Additives (Graphite, AlCl3, MgCl2 and NaCl) on the Hydrolysis Performances of Mg17Al12. Int. J. Hydrogen Energy 2020, 45, 6102–6109. [Google Scholar] [CrossRef]
- Zhong, S.; Wu, C.; Li, J.; Chen, Y.; Wang, Y.; Yan, Y. Effect of Cations in Chloride Solutions on Low-Temperature Hydrolysis Performances and Mechanisms of Mg–Ca-Based Hydride. J. Alloys Compd. 2021, 851, 156762. [Google Scholar] [CrossRef]
- Zou, M.-S.; Guo, X.-Y.; Huang, H.-T.; Yang, R.-J.; Zhang, P. Preparation and Characterization of Hydro-Reactive Mg–Al Mechanical Alloy Materials for Hydrogen Production in Seawater. J. Power Sources 2012, 219, 60–64. [Google Scholar] [CrossRef]
- Legrée, M.; Charbonnier, V.; Al Bacha, S.; Asano, K.; Sakaki, K.; Aubert, I.; Mauvy, F.; Bobet, J.-L. Hydrogen Generation by Hydrolysis Reaction Using Magnesium Alloys with Long Period Stacking Ordered Structure. Int. J. Hydrogen Energy 2021, 46, 35161–35171. [Google Scholar] [CrossRef]
- Pugazhendhi, B.S.; Kar, A.; Sinnaeruvadi, K.; Suwas, S. Effect of Aluminium on Microstructure, Mechanical Property and Texture Evolution of Dual Phase Mg-8Li Alloy in Different Processing Conditions. Arch. Civ. Mech. Eng. 2018, 18, 1332–1344. [Google Scholar] [CrossRef]
- Hong, S.-H.; Kim, H.-J.; Song, M.Y. Rate Enhancement of Hydrogen Generation through the Reaction of Magnesium Hydride with Water by MgO Addition and Ball Milling. J. Ind. Eng. Chem. 2012, 18, 405–408. [Google Scholar] [CrossRef]
- Awad, A.; El-Asmar, E.; Tayeh, T.; Mauvy, F.; Nakhl, M.; Zakhour, M.; Bobet, J.-L. Effect of carbons (G and CFs), TM (Ni, Fe and Al) and oxides (Nb2O5 and V2O5) on hydrogen generation from ball milled Mg-based hydrolysis reaction for fuel cell. Energy 2016, 95, 175–186. [Google Scholar] [CrossRef]
- Yang, B.; Zou, J.; Huang, T.; Mao, J.; Zeng, X.; Ding, W. Enhanced Hydrogenation and Hydrolysis Properties of Core-Shell Structured Mg-MOx (M = Al, Ti and Fe) Nanocomposites Prepared by Arc Plasma Method. Chem. Eng. J. 2019, 371, 233–243. [Google Scholar] [CrossRef]
- Huang, M.; Ouyang, L.; Chen, Z.; Peng, C.; Zhu, X.; Zhu, M. Hydrogen Production via Hydrolysis of Mg-Oxide Composites. Int. J. Hydrogen Energy 2017, 42, 22305–22311. [Google Scholar] [CrossRef]
- Huang, M.; Ouyang, L.; Ye, J.; Liu, J.; Yao, X.; Wang, H.; Shao, H.; Zhu, M. Hydrogen Generation via Hydrolysis of Magnesium with Seawater Using Mo, MoO2, MoO3 and MoS2 as Catalysts. J. Mater. Chem. A 2017, 5, 8566–8575. [Google Scholar] [CrossRef]
- Huang, M.; Ouyang, L.; Liu, J.; Wang, H.; Shao, H.; Zhu, M. Enhanced Hydrogen Generation by Hydrolysis of Mg Doped with Flower-like MoS2 for Fuel Cell Applications. J. Power Sources 2017, 365, 273–281. [Google Scholar] [CrossRef]
- Zhu, Y.; Luo, S.; Lin, H.; Liu, Y.; Zhu, Y.; Zhang, Y.; Li, L. Enhanced hydriding kinetics of Mg-10 at% Al composite by forming Al 12 Mg 17 during hydriding combustion synthesis. J. Alloys Compd. 2017, 712, 44–49. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, M.; Guan, F.; Yu, R.; Zhang, Y.; Qin, H.; Chen, X.; Fu, Q.; Wang, Z. Study on Hydrolysis of Magnesium Hydride by Interface Control. Int. J. Photoenergy 2020, 2020, 8859770. [Google Scholar] [CrossRef]
- Liu, Z.; Zhong, J.; Leng, H.; Xia, G.; Yu, X. Hydrolysis of Mg-Based Alloys and Their Hydrides for Efficient Hydrogen Generation. Int. J. Hydrogen Energy 2021, 46, 18988–19000. [Google Scholar] [CrossRef]
- Ouyang, L.Z.; Xu, Y.J.; Dong, H.W.; Sun, L.X.; Zhu, M. Production of Hydrogen via Hydrolysis of Hydrides in Mg–La System. Int. J. Hydrogen Energy 2009, 34, 9671–9676. [Google Scholar] [CrossRef]
- Tessier, J.-P.; Palau, P.; Huot, J.; Schulz, R.; Guay, D. Hydrogen Production and Crystal Structure of Ball-Milled MgH2–Ca and MgH2–CaH2 Mixtures. J. Alloys Compd. 2004, 376, 180–185. [Google Scholar] [CrossRef]
- Liu, P.; Wu, H.; Wu, C.; Chen, Y.; Xu, Y.; Wang, X.; Zhang, Y. Microstructure Characteristics and Hydrolysis Mechanism of Mg–Ca Alloy Hydrides for Hydrogen Generation. Int. J. Hydrogen Energy 2015, 40, 3806–3812. [Google Scholar] [CrossRef]
- Jiang, J.; Ouyang, L.; Wang, H.; Liu, J.; Shao, H.; Zhu, M. Controllable Hydrolysis Performance of MgLi Alloys and Their Hydrides. ChemPhysChem 2019, 20, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, J.; Liu, J.W.; Ouyang, L.Z.; Zhu, M. Catalysis and Hydrolysis Properties of Perovskite Hydride NaMgH3. J. Alloys Compd. 2013, 580, S197–S201. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Liu, H.; Dong, Z.; Cao, G.; Yan, M. Hydrogen Generation from Mg–LiBH4 Hydrolysis Improved by AlCl3 Addition. Energy 2014, 68, 548–554. [Google Scholar] [CrossRef]
- Li, S.-L.; Song, J.-M.; Uan, J.-Y. Mg-Mg2X (X=Cu, Sn) Eutectic Alloy for the Mg2X Nano-Lamellar Compounds to Catalyze Hydrolysis Reaction for H2 Generation and the Recycling of Pure X Metals from the Reaction Wastes. J. Alloys Compd. 2019, 772, 489–498. [Google Scholar] [CrossRef]
- Ouyang, L.Z.; Huang, J.M.; Wang, H.; Wen, Y.J.; Zhang, Q.A.; Sun, D.L.; Zhu, M. Excellent Hydrolysis Performances of Mg3RE Hydrides. Int. J. Hydrogen Energy 2013, 38, 2973–2978. [Google Scholar] [CrossRef]
- Ouyang, L.; Ma, M.; Huang, M.; Duan, R.; Wang, H.; Sun, L.; Zhu, M. Enhanced Hydrogen Generation Properties of MgH2-Based Hydrides by Breaking the Magnesium Hydroxide Passivation Layer. Energies 2015, 8, 4237–4252. [Google Scholar] [CrossRef]
- Zhong, H.; Wang, H.; Liu, J.; Sun, D.; Fang, F.; Zhang, Q.; Ouyang, L.; Zhu, M. Enhanced hydrolysis properties and energy efficiency of MgH2-base hydrides. J. Alloys Compd. 2016, 680, 419–426. [Google Scholar] [CrossRef]
- Ma, M.; Duan, R.; Ouyang, L.; Zhu, X.; Peng, C.; Zhu, M. Hydrogen generation via hydrolysis of H-CaMg2 and H-CaMg1.9Ni0.1. Int. J. Hydrogen Energy 2017, 42, 22312–22317. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, Y.; Liu, X.; Wu, H.; Liu, B. Hydrolytic Properties of Hydrogenated Mg–Li-Gd-Zn-Zr Alloys. J. Phys. Chem. Solids 2025, 199, 112518. [Google Scholar] [CrossRef]
- Yun, H.; Bai, J.; Wang, X.; Dai, X.; Hou, X.; Xu, Y. Hydrolytic H2 production behavior and mechanism of surface activated AZ91D alloy waste by borohydrides in neutral solution at room temperature. J. Alloys Compd. 2024, 980, 173612. [Google Scholar] [CrossRef]
- Grosjean, M.-H.; Zidoune, M.; Roué, L.; Huot, J.-Y. Hydrogen production via hydrolysis reaction from ball-milled Mg-based materials. Int. J. Hydrogen Energy 2006, 31, 109–119. [Google Scholar] [CrossRef]
- Mao, J.; Zou, J.; Lu, C.; Zeng, X.; Ding, W. Hydrogen storage and hydrolysis properties of core-shell structured Mg-MFx (M=V, Ni, La and Ce) nano-composites prepared by arc plasma method. J. Power Sources 2017, 366, 131–142. [Google Scholar] [CrossRef]
- Wang, X.; Guo, X.; Zhu, L.; Wang, S.; Zou, M.; Li, X.; Zhang, X.; Song, T. The Activation of Mg Powder Promoted by Chloride and Activation Mechanism. Metals 2021, 11, 1435. [Google Scholar] [CrossRef]
- Shetty, T.; Szpunar, J.A.; Faye, O.; Eduok, U. A Comparative Study of Hydrogen Generation by Reaction of Ball Milled Mixture of Magnesium Powder with Two Water-Soluble Salts (NaCl and KCl) in Hot Water. Int. J. Hydrogen Energy 2020, 45, 25890–25899. [Google Scholar] [CrossRef]
- Al Bacha, S.; Pighin, S.A.; Urretavizcaya, G.; Zakhour, M.; Nakhl, M.; Castro, F.J.; Bobet, J.-L. Effect of Ball Milling Strategy (Milling Device for Scaling-up) on the Hydrolysis Performance of Mg Alloy Waste. Int. J. Hydrogen Energy 2020, 45, 20883–20893. [Google Scholar] [CrossRef]
- Sun, Q.; Zou, M.; Guo, X.; Yang, R.; Huang, H.; Huang, P.; He, X. A Study of Hydrogen Generation by Reaction of an Activated Mg–CoCl2 (Magnesium–Cobalt Chloride) Composite with Pure Water for Portable Applications. Energy 2015, 79, 310–314. [Google Scholar] [CrossRef]
- Bai, J.; Liu, J.; Yun, H.; Li, D.; Cao, Q.; Hou, X.; Xu, Y. Hydrolysis Hydrogen Generation Behavior of Mechanico-Chemical Reaction Mg-M (M = Ni, Ce, and La) Binary Alloys—A Feasible Strategy for Activating and Improving Environmental Stability. J. Mater. Res. Technol. 2022, 21, 363–372. [Google Scholar] [CrossRef]
- Hou, K.; Hou, X.; Ye, X.; Li, D.; Suo, G.; Xie, L.; Shu, Q.; Cao, Q.; Bai, J. Carbon Nanotubes and (Mg10Ni)85Ce15 Synergistically Activate Mg-Al Alloy Waste for Efficiently Hydrolysis Hydrogen Generation. Fuel 2022, 324, 124829. [Google Scholar] [CrossRef]
- Imamura, H.; Tabata, S.; Shigetomi, N.; Takesue, Y.; Sakata, Y. Composites for Hydrogen Storage by Mechanical Grinding of Graphite Carbon and Magnesium. J. Alloys Compd. 2002, 330–332, 579–583. [Google Scholar] [CrossRef]
- Huang, Z.G.; Guo, Z.P.; Calka, A.; Wexler, D.; Liu, H.K. Effects of Carbon Black, Graphite and Carbon Nanotube Additives on Hydrogen Storage Properties of Magnesium. J. Alloys Compd. 2007, 427, 94–100. [Google Scholar] [CrossRef]
- Ma, M.; Yang, L.; Ouyang, L.; Shao, H.; Zhu, M. Promoting Hydrogen Generation from the Hydrolysis of Mg-Graphite Composites by Plasma-Assisted Milling. Energy 2019, 167, 1205–1211. [Google Scholar] [CrossRef]
- Al Bacha, S.; Pighin, S.A.; Urretavizcaya, G.; Zakhour, M.; Castro, F.J.; Nakhl, M.; Bobet, J.-L. Hydrogen Generation from Ball Milled Mg Alloy Waste by Hydrolysis Reaction. J. Power Sources 2020, 479, 228711. [Google Scholar] [CrossRef]
- Chen, K.; Jiang, J.; Ouyang, L.; Wang, H.; Liu, J.; Shao, H.; Zhu, M. Enhanced Hydrogen Generation from Hydrolysis of MgLi Doped with Expanded Graphite. J. Magnes. Alloys 2021, 9, 2185–2193. [Google Scholar] [CrossRef]
- Hou, X.; Shi, H.; Yang, L.; Hou, K.; Wang, Y.; Feng, L.; Suo, G.; Ye, X.; Zhang, L.; Yang, Y. H2 generation kinetics/thermodynamics and hydrolysis mechanism of high-performance La-doped Mg-Ni alloys in NaCl solution—A large-scale and quick strategy to get hydrogen. J. Magnes. Alloy. 2021, 9, 1068–1083. [Google Scholar] [CrossRef]
- Ghali, E.; Dietzel, W.; Kainer, K.-U. General and Localized Corrosion of Magnesium Alloys: A Critical Review. J. Mater. Eng. Perform. 2004, 13, 7–23. [Google Scholar] [CrossRef]
- Zhong, H.; Ouyang, L.; Zeng, M.; Liu, J.; Wang, H.; Shao, H.; Felderhoff, M.; Zhu, M. Realizing facile regeneration of spent NaBH4 with Mg–Al alloy. J. Mater. Chem. A 2019, 7, 10723–10728. [Google Scholar] [CrossRef]
- Qin, C.; Ouyang, L.; Wang, H.; Liu, J.; Shao, H.; Zhu, M. Regulation of high-efficient regeneration of sodium borohydride by magnesium-aluminum alloy. Int. J. Hydrogen Energy 2019, 44, 29108–29115. [Google Scholar] [CrossRef]
- Al Bacha, S.; Aubert, I.; Zakhour, M.; Nakhl, M.; Bobet, J.-L. Hydrolysis properties, corrosion behavior and microhardness of AZ91 “model” alloys. J. Alloys Compd. 2020, 845, 156283. [Google Scholar] [CrossRef]
- Al Bacha, S.; Aubert, I.; Zakhour, M.; Nakhl, M.; Bobet, J. Valorization of AZ91 by the hydrolysis reaction for hydrogen production (Electrochemical approach). J. Magnes. Alloys 2021, 9, 1942–1953. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Q.; Li, Z.; Yuan, W.; Zheng, Y.; Cui, Z.; Yang, X.; Yeung, K.W.; Wu, S. A combined coating strategy based on atomic layer deposition for enhancement of corrosion resistance of AZ31 magnesium alloy. Appl. Surf. Sci. 2018, 434, 1101–1111. [Google Scholar] [CrossRef]
- Ahmadi, S.; Alimirzaloo, V.; Faraji, G.; Doniavi, A. Properties inhomogeneity of AM60 magnesium alloy processed by cyclic extrusion compression angular pressing followed by extrusion. Trans. Nonferrous Met. Soc. China 2021, 31, 655–665. [Google Scholar] [CrossRef]
- Yang, R.; Chen, K.; Wen, S.; Zhu, S.; Qin, H.; Wu, X.; Zhou, Y.; Che, Y.; Shi, Y.; He, J. Enhanced Strength and Hardness of AS41 Magnesium Alloy Fabricated by Selective Laser Melting. Materials 2022, 15, 5863. [Google Scholar] [CrossRef]
- Yang, W.; Liu, X.; Liu, J.; Wang, Z.; Zhou, J.; Cen, K. Thermogravimetric analysis of hydrogen production of Al–Mg–Li particles and water. Int. J. Hydrogen Energy 2016, 41, 7927–7934. [Google Scholar] [CrossRef]
- Zheng, T.; Zhang, J.; Tang, Y.; Wan, P.; Yuan, Q.; Hu, H.; Coulon, F.; Hu, Q.; Yang, X.J. Production of High-Purity Hydrogen and Layered Doubled Hydroxide by Hydrolysis of Mg-Al Alloys. Chem. Eng. Technol. 2021, 44, 797–803. [Google Scholar] [CrossRef]
- Oh, S.; Kim, M.; Eom, K.; Kyung, J.; Kim, D.; Cho, E.; Kwon, H. Design of Mg–Ni alloys for fast hydrogen generation from seawater and their application in polymer electrolyte membrane fuel cells. Int. J. Hydrogen Energy 2016, 41, 5296–5303. [Google Scholar] [CrossRef]
- Liu, M.; Yao, Z.; Gu, J.; Li, C.; Huang, X.; Zhang, L.; Huang, Z.; Fan, M. Issues and opportunities facing hydrolytic hydrogen production materials. Chem. Eng. J. 2023, 461, 141918. [Google Scholar] [CrossRef]
- Tan, W.; Yang, Y.-E.; Fang, Y.-X. Isothermal hydrogen production behavior and kinetics of bulk eutectic Mg–Ni-based alloys in NaCl solution. J. Alloys Compd. 2020, 826, 152363. [Google Scholar] [CrossRef]
- Grosjean, M.-H.; Zidoune, M.; Roué, L. Hydrogen production from highly corroding Mg-based materials elaborated by ball milling. J. Alloys Compd. 2005, 404–406, 712–715. [Google Scholar] [CrossRef]
- Li, J.; Liu, P.; Wu, C.; Chen, Y. Common ion effect in the hydrolysis reaction of Mg Ca alloy hydride-salt composites. Int. J. Hydrogen Energy 2017, 42, 1429–1435. [Google Scholar] [CrossRef]
- Hou, X.; Yang, L.; Hou, K.; Shu, Q.; Cao, Q.; Liu, Y.; Feng, L.; Suo, G.; Ye, X.; Zhang, L.; et al. Hydrolysis Hydrogen Generation Medium Regulated by Alkali Metal Cations for Mg-Based Alloy-Green Seawater Modification Strategy. J. Power Sources 2021, 509, 230364. [Google Scholar] [CrossRef]
- Oh, S.; Kim, H.; Kim, M.; Eom, K.; Kyung, J.; Kim, D.; Cho, E.; Kwon, H. Design of Mg-Cu alloys for fast hydrogen production, and its application to PEM fuel cell. J. Alloys Compd. 2018, 741, 590–596. [Google Scholar] [CrossRef]
- Wang, T.; Tang, B.; Yuan, S.; Zhao, S.; Wang, E.; Yao, C. Study on hydrolysis property of Mg-8Li alloy for hydrogen production. IOP Conf. Ser. Earth Environ. Sci. 2019, 242, 042008. [Google Scholar] [CrossRef]
- Xiao, F.; Guo, Y.; Yang, R.; Li, J. Hydrogen generation from hydrolysis of activated magnesium/low-melting-point metals alloys. Int. J. Hydrogen Energy 2019, 44, 1366–1373. [Google Scholar] [CrossRef]
- Bai, J.; Li, D.; Cao, Q.; Hou, X.; Xu, Y. Hydrolysis H2 Generation Behavior of AM50 Alloy Waste Coactivated by Mg-Based Master Alloys. Int. J. Hydrogen Energy 2022, 47, 31191–31201. [Google Scholar] [CrossRef]
- Al Bacha, S.; Awad, A.; El Asmar, E.; Tayeh, T.; Bobet, J.-L.; Nakhl, M.; Zakhour, M. Hydrogen generation via hydrolysis of ball milled WE43 magnesium waste. Int. J. Hydrogen Energy 2019, 44, 17515–17524. [Google Scholar] [CrossRef]
- Xie, X.; Ni, C.; Wang, B.; Zhang, Y.; Zhao, X.; Liu, L.; Wang, B.; Du, W. Recent advances in hydrogen generation process via hydrolysis of Mg-based materials: A short review. J. Alloys Compd. 2020, 816, 152634. [Google Scholar] [CrossRef]
- Wang, H.; Northwood, D.O. Synthesis of Ni nanoparticles by hydrolysis of Mg2Ni. J. Mater. Sci. 2008, 43, 1050–1056. [Google Scholar] [CrossRef]
- Wang, H.; Han, L.; Hu, H.; Northwood, D.O. The hydrolysis behaviour of Mg2Ni and Mg2NiH4 in water or a 6M KOH solution and its application to Ni nanoparticles synthesis. J. Alloys Compd. 2009, 470, 539–543. [Google Scholar] [CrossRef]
- Varnagiris, S.; Urbonavicius, M. Hydrogen generation kinetics via hydrolysis of Mg2Ni and Mg2NiH4 powders. Int. J. Hydrogen Energy 2021, 46, 36323–36335. [Google Scholar] [CrossRef]
- Chang, K.; Zhu, J.; Ma, N.; Sun, J.; Han, P. Characterization and Hydrolysis Mechanism of Soluble Al-Mg-Ga-Sn Alloys in Hydraulic Fracturing Applications. J. Alloys Compd. 2024, 998, 174891. [Google Scholar] [CrossRef]
- Tan, Z.; Ouyang, L.; Huang, J.; Liu, J.; Wang, H.; Shao, H.; Zhu, M. Hydrogen generation via hydrolysis of Mg2Si. J. Alloys Compd. 2019, 770, 108–115. [Google Scholar] [CrossRef]
- Cuzacq, L.; Polido, C.; Silvain, J.-F.; Bobet, J.-L. Hydrogen Production Properties of Aluminum–Magnesium Alloy Presenting β-Phase Al3Mg2. Metals 2023, 13, 1868. [Google Scholar] [CrossRef]
- Uchiyama, T.; Ando, D.; Sutou, Y. Catalyze hydrolysis reaction for hydrogen generation by Mg/Mg2Ca nanolamellar structure in Mg–Ca alloys. J. Alloys Compd. 2022, 919, 165767. [Google Scholar] [CrossRef]
- Akbarzadeh, R.; Adeniran, J.A.; Lototskyy, M.; Asadi, A. Simultaneous Brewery Wastewater Treatment and Hydrogen Generation via Hydrolysis Using Mg Waste Scraps. J. Clean. Prod. 2020, 276, 123198. [Google Scholar] [CrossRef]
- Xu, X.; Yong, H.; Zhao, Y.; Wang, S.; Wang, Y.; Liu, B.; Hu, J.; Zhang, Y. Distinct hydrolysis kinetics and catalytic mechanism of CaMg2, CaMg2X0.1(X = Ni, Zn, Ti) ternary alloys. Int. J. Hydrogen Energy 2024, 50, 247–260. [Google Scholar] [CrossRef]
- Oh, S.; Cho, T.; Kim, M.; Lim, J.; Eom, K.; Kim, D.; Cho, E.; Kwon, H. Fabrication of Mg–Ni–Sn alloys for fast hydrogen generation in seawater. Int. J. Hydrogen Energy 2017, 42, 7761–7769. [Google Scholar] [CrossRef]
- Alasmar, E.; Awad, A.; Hachem, D.; Tayeh, T.; Nakhl, M.; Zakhour, M.; Gaudin, E.; Bobet, J.-L. Hydrogen generation from Nd-Ni-Mg system by hydrolysis reaction. J. Alloys Compd. 2018, 740, 52–60. [Google Scholar] [CrossRef]
- Hou, X.; Wang, Y.; Yang, Y.; Hu, R.; Yang, G.; Feng, L.; Suo, G.; Ye, X.; Zhang, L.; Shi, H.; et al. Enhanced hydrogen generation behaviors and hydrolysis thermodynamics of as-cast Mg–Ni–Ce magnesium-rich alloys in simulate seawater. Int. J. Hydrogen Energy 2019, 44, 24086–24097. [Google Scholar] [CrossRef]
- Legrée, M.; Charbonnier, V.; Al Bacha, S.; Asano, K.; Sakaki, K.; Aubert, I.; Mauvy, F.; Sabatier, J.; Bobet, J.-L. Hydrogen generation performances and electrochemical properties of Mg alloys with 14H long period stacking ordered structure. J. Alloys Compd. 2023, 937, 168154. [Google Scholar] [CrossRef]
- He, T.; Chen, W.; Wang, W.; Du, S.; Deng, S. Microstructure and hydrogen production of the rapidly solidified Al–Mg-Ga-In-Sn alloy. J. Alloys Compd. 2020, 827, 154290. [Google Scholar] [CrossRef]
- Yu, S.-H.; Uan, J.-Y.; Hsu, T.-L. Effects of Concentrations of NaCl and Organic Acid on Generation of Hydrogen from Magnesium Metal Scrap. Int. J. Hydrogen Energy 2012, 37, 3033–3040. [Google Scholar] [CrossRef]
- Buryakovskaya, O.A.; Kurbatova, A.I.; Vlaskin, M.S.; Valyano, G.E.; Grigorenko, A.V.; Ambaryan, G.N.; Dudoladov, A.O. Waste to Hydrogen: Elaboration of Hydroreactive Materials from Magnesium-Aluminum Scrap. Sustainability 2022, 14, 4496. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Yuan, Q.; Song, X. A Novel Material of Nanoporous Magnesium for Hydrogen Generation with Salt Water. J. Power Sources 2018, 395, 8–15. [Google Scholar] [CrossRef]
- Hou, X.; Li, D.; Hou, K.; Zhao, D.; Wang, C.; Ye, X.; Suo, G.; Hu, P.; Xu, G.; Yang, G. Anions Regulation Strategy in Simulated Seawater: A Sustainable and Efficient Approach for on-Site Hydrolysis Hydrogen Generation from Mg Alloy Waste. J. Power Sources 2024, 612, 234782. [Google Scholar] [CrossRef]
- Buryakovskaya, O.A.; Vlaskin, M.S.; Ryzhkova, S.S. Hydrogen Production Properties of Magnesium and Magnesium-Based Materials at Low Temperatures in Reaction with Aqueous Solutions. J. Alloys Compd. 2019, 785, 136–145. [Google Scholar] [CrossRef]
- Kantürk Figen, A.; Coşkuner, B.; Pişkin, S. Hydrogen Generation from Waste Mg Based Material in Various Saline Solutions (NiCl2, CoCl2, CuCl2, FeCl3, MnCl2). Int. J. Hydrogen Energy 2015, 40, 7483–7489. [Google Scholar] [CrossRef]
- Kantürk Figen, A.; Coşkuner Filiz, B. Hydrogen Production by the Hydrolysis of Milled Waste Magnesium Scraps in Nickel Chloride Solutions and Nickel Chloride Added in Marmara Sea and Aegean Sea Water. Int. J. Hydrogen Energy 2015, 40, 16169–16177. [Google Scholar] [CrossRef]
- Sevastyanova, L.G.; Genchel, V.K.; Klyamkin, S.N.; Larionova, P.A.; Bulychev, B.M. Hydrogen Generation by Oxidation of “Mechanical Alloys” of Magnesium with Iron and Copper in Aqueous Salt Solutions. Int. J. Hydrogen Energy 2017, 42, 16961–16967. [Google Scholar] [CrossRef]
- Tan, Y.; Yang, H.; Cheng, J.; Hu, J.; Tian, G.; Yu, X. Preparation of Hydrogen from Metals and Water without CO2 Emissions. Int. J. Hydrogen Energy 2022, 47, 38134–38154. [Google Scholar] [CrossRef]
- Uan, J.-Y.; Yu, S.-H.; Lin, M.-C.; Chen, L.-F.; Lin, H.-I. Evolution of Hydrogen from Magnesium Alloy Scraps in Citric Acid-Added Seawater without Catalyst. Int. J. Hydrogen Energy 2009, 34, 6137–6142. [Google Scholar] [CrossRef]
- Lototskyy, M.V.; Davids, M.W.; Sekgobela, T.K.; Arbuzov, A.A.; Mozhzhukhin, S.A.; Zhu, Y.; Tang, R.; Tarasov, B.P. Tailoring of Hydrogen Generation by Hydrolysis of Magnesium Hydride in Organic Acids Solutions and Development of Generator of the Pressurised H2 Based on This Process. Inorganics 2023, 11, 319. [Google Scholar] [CrossRef]
- Tayeh, T.; Awad, A.S.; Nakhl, M.; Zakhour, M.; Silvain, J.-F.; Bobet, J.-L. Production of Hydrogen from Magnesium Hydrides Hydrolysis. Int. J. Hydrogen Energy 2014, 39, 3109–3117. [Google Scholar] [CrossRef]
- Cui, Z.; Ge, F.; Lin, Y.; Wang, L.; Lei, L.; Tian, H.; Yu, M.; Wang, X. Corrosion Behavior of AZ31 Magnesium Alloy in the Chloride Solution Containing Ammonium Nitrate. Electrochim. Acta 2018, 278, 421–437. [Google Scholar] [CrossRef]


| Materials | H2 Production Kinetic | Total H2 Production (wt.%) |
|---|---|---|
| H-CaMg2 | 800 mL/g in 50 s | 7.14 wt.% |
| H-CaMg1.9 Ni0.1 | 968 mL/g in 5 min | 9.4 wt.% |
| H-30 wt.% Ca-Mg | 755.7 mL/g in 1 h | 6.7 wt.% |
| H-Mg3La | 474 mL/g in 5 min | 8.47 wt.% |
| H-Mg3LaNi0.1 | 446 mL/g in 5 min | 9.05 wt.% |
| H-La2Mg17 | 653 mL/g in 10 min | 11.2 wt.% |
| H-Mg3CeNi0.1 | 725 mL/g in 10 min | 9.71 wt.% |
| H-Mg17Al12 | 1389 mL/g in 1 h | 12.4 wt.% |
| H-MgLi | 1263 mL/g in 5 min | 15.8 wt.% |
| Sample (0.5 g) | Hydrogen Generation Volume (L) | Conversion Yield (%) | Reaction Duration (s) |
|---|---|---|---|
| Mg | 0.002 | 4.28 | 5 |
| Mg-6% NiCl2 | 0.347 | 79.2 | 276 |
| Mg-6% CoCl2 | 0.423 | 96.6 | 325 |
| Mg-6% CuCl2 | 0.141 | 32.2 | 830 |
| Mg-6% FeCl3 | 0.258 | 58.9 | 1684 |
| Sample | Mill Gas | H2 Generation in 0.6 M MgCl2 After 5 min | Yield (%) | H2 Generation in 0.6 M MgCl2 After 60 min | Yield (%) | H2 (wt.%) Generation in 0.5 M HCl |
|---|---|---|---|---|---|---|
| Mg alloy | 0.03 ± 0.03 | 0.5 | 1.1 ± 0.1 | 17.2 | 6.7 ± 0.2 | |
| Mg alloy 35 h Mg alloy 50 h Mg alloy 100 h | H2 H2 H2 | 3.5 ± 0.1 3.5 ± 0.1 3.2 ± 0.1 | 43.8 42.4 32.6 | 6.8 ± 0.2 6.3 ± 0.2 5.1 ± 0.1 | 83.8 75.3 52.2 | 8.1 ± 0.3 8.3 ± 0.3 9.7 ± 0.3 |
| Mg alloy + G 20 h Mg alloy + G 50 h | Ar Ar | 6.4 ± 0.2 6.0 ± 0.2 | 97.9 96.6 | 6.4 ± 0.2 6.1 ± 0.2 | 98.4 98.3 | 6.5 ± 0.3 6.2 ± 0.3 |
| Mg alloy + G 20 h + AlCl3 20 h Mg alloy + G 50 h + AlCl3 20 h | Ar Ar | 5.9 ± 0.2 6.0 ± 0.2 | 94.9 92.0 | 5.9 ± 0.2 6.1 ± 0.2 | 95.5 93.0 | 6.2 ± 0.3 6.6 ± 0.3 |
| Alloys | Hydrogen Generation Volume (mL/g) at RT | Conversion Yield (%) |
|---|---|---|
| Mg + 10 wt.% Zn | 875 | 95.1 |
| Mg + 10 wt.% In | 856 | 93.0 |
| Mg + 10 wt.% Sn | 810 | 88.0 |
| Mg + 10 wt.% Bi | 775 | 84.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarlet, L.; Kabongo, L.; Marques, D.; Bobet, J.-L. Advances in Hydrolysis of Magnesium and Alloys: A Conceptual Review on Parameters Optimization for Sustainable Hydrogen Production. Metals 2025, 15, 363. https://doi.org/10.3390/met15040363
Jarlet L, Kabongo L, Marques D, Bobet J-L. Advances in Hydrolysis of Magnesium and Alloys: A Conceptual Review on Parameters Optimization for Sustainable Hydrogen Production. Metals. 2025; 15(4):363. https://doi.org/10.3390/met15040363
Chicago/Turabian StyleJarlet, Léa, Lumière Kabongo, Dylan Marques, and Jean-Louis Bobet. 2025. "Advances in Hydrolysis of Magnesium and Alloys: A Conceptual Review on Parameters Optimization for Sustainable Hydrogen Production" Metals 15, no. 4: 363. https://doi.org/10.3390/met15040363
APA StyleJarlet, L., Kabongo, L., Marques, D., & Bobet, J.-L. (2025). Advances in Hydrolysis of Magnesium and Alloys: A Conceptual Review on Parameters Optimization for Sustainable Hydrogen Production. Metals, 15(4), 363. https://doi.org/10.3390/met15040363








