Abstract
Silicon stands out as an exceptionally viable anode material, distinguished by its substantial capacity, plentiful natural reserves, eco-friendliness, and favorable low working potential. Nonetheless, the material’s pronounced volume fluctuations readily induce particle fragmentation, detachment of active components, and repeated disruption of the solid electrolyte interphase (SEI) layer. These factors contribute to a shortened cycle life and rapid capacity fading, thus hindering its practical application. The carbon composite approach can efficiently counteract these issues by capitalizing on silicon’s high capacity and employing carbon as a cushioning agent to diminish volume swelling, thus enhancing the deployment of silicon-based anode materials. This paper offers an exhaustive examination of the lithiation processes involved in Si/C anodes and delves into the strategic utilization of diverse carbon materials, including graphite, graphene, graphdiyne, carbon nanotubes, carbon fibers, MXenes, pitch, heteroatom-doped polymers, biomass-derived carbon, carbon-containing gas-derived carbon, MOFs, and g-C3N4 to advance the application of silicon in lithium-ion battery (LIB) anodes. Overall, this paper concentrates on summarizing the current research status and technological advancement and juxtaposes the merits and demerits of various carbon sources in Si/C anodes, thus providing a comprehensive assessment and forward-looking perspective on their future development.
1. Introduction
Lithium-ion batteries (LIBs) garnered widespread adoption in electric vehicles and portable electronics owing to their outstanding electrochemical properties [1]. With the development of the energy storage materials market, higher energy density is required for LIBs, and the currently widely used commercialized graphitic carbon anode is gradually unable to meet this demand due to its low specific capacity (only 372 mAh/g in theory) [2]. Hence, silicon (Si) is considered a prime contender as an anode material for next-generation LIBs, which is attributed to its exceptionally high specific discharge capacity (up to 4200 mAh/g), superior safety characteristics, and plentiful availability [3]. The substantial volume change will continuously trigger frequent rupture and reconstruction processes at the solid electrolyte interface (SEI) [4], accompanied by a significant electrolyte consumption [5]. Consequently, this phenomenon gives rise to significant instability during the electrochemical cycling process of Si anode materials, thereby hindering the full exploitation of Si with the high theoretical specific capacity and resulting in poor cycling stability and the coulombic efficiency [6].
Starting from the perspective of structural design, nanoparticles, nanosheets, and nanoporous structures have proven to be effective to mitigate the volume expansion issues associated with Si anode [4]. Furthermore, incorporating Si nanostructures demonstrated significant improvements in the cycle life when compared to larger-size Si counterparts [7]. Nonetheless, Si NPS exhibits a substantial specific surface area, thereby augmenting the interfacial contact between the electrode and the electrolyte, which consequently leads to an initial coulombic efficiency [8]. In addition, the low electronic conductivity of Si can adversely affect the electrochemical process [9]. It has been found that carbon coating can improve the structural stability of Si and SEI, thus prompting extensive research on the development of Si/C composites, which has become a new research hotspot in scientific investigations [10].
In lithium-ion batteries, the selection of carbon sources for Si/C composite anodes holds profound significance [11]. Serving as a “buffering scaffold” for the Si anode, carbon materials, with their unique structures and properties, effectively mitigate the substantial volume expansion of Si during lithiation/delithiation processes [12]. By incorporating suitable carbon sources, stable support is provided to Si particles [13], preventing them from fracturing or detaching due to volume changes [14], thereby significantly enhancing the battery’s cycle stability [15]. This crucial role of carbon sources lays a solid foundation for the feasibility of Si anode materials in practical applications [16].
Carbon sources not only play a structural supporting role in Si/C composite anodes, but also greatly enhance the composite’s electrical conductivity. Carbon materials are excellent conductors in themselves, and their outstanding electron conduction capability makes the composite anode material more efficient during charging and discharging processes [17]. By introducing carbon sources, the internal resistance of the battery can be reduced, and the charging and discharging rates can be increased, thereby comprehensively improving the overall performance of the battery [18]. Furthermore, carbon sources facilitate the formation of a stable and uniform SEI film, further enhancing the battery’s cycle stability and coulombic efficiency [19]. Therefore, the selection and optimization of carbon sources have a decisive impact on the electrochemical performance of Si/carbon composite anode materials [20].
The paper provides a comprehensive review of the lithiation mechanism of Si/C composite anode materials and provides insights into the diverse carbon sources that make up this anode, including, but not limited to, classical carbon materials such as graphite, graphene, graphene-alkyne, carbon nanotubes, and carbon fibers, as well as emerging high-performance options such as MXene, asphaltene-based carbons, heteroatom-doped polymers, biomass-converted carbons, carbon-containing gas pyrolysis carbons, metal-organic frameworks (MOFs)-derived carbon, and the two-dimensional layered material g-C3N4. On this basis, the article further provides an exhaustive comparative analysis of the advantages and disadvantages of the selection of different carbon sources in Si/C anode, aiming to provide a theoretical basis for optimizing the anode performance and looking forward to the potential future development direction in material design and application. This review not only deepens the understanding of the lithiation process of Si/C anode materials, but also provides valuable insights for advancing the technology of high-performance lithium-ion batteries.
2. Lithiation Mechanism
In Si/C anode materials, the individual lithiation and delithiation characteristics of Si and C components have been highlighted [9]. For instance, Cheng et al. [21] conducted an in-depth study on the lithiation and delithiation processes of nanosilica-filled carbon nanotubes (CNTs). During the lithiation process, the migration of Li+ is divided into three steps. Firstly, the CNT undergoes lithiation and a SEI layer is formed on the outer surface of the CNT. Subsequently, Li+ penetrates the carbon layer into the interior of the carbon nanotube. Finally, facilitated by surface diffusion, Li+ is easily transported to the Si NP through the CNT/Si NPS interface. In addition, Wang et al. [22] conducted an investigation on the lithiation behavior of Si NP that was attached to and embedded within carbon nanofibers. The lithiation of Si NP embedded in carbon was observed to be delayed compared to that of the surface-attached Si NP. The alloying process involving Si and Li was characterized as follows:
Si + xLi+ + xe+ ⇔ LixSi.
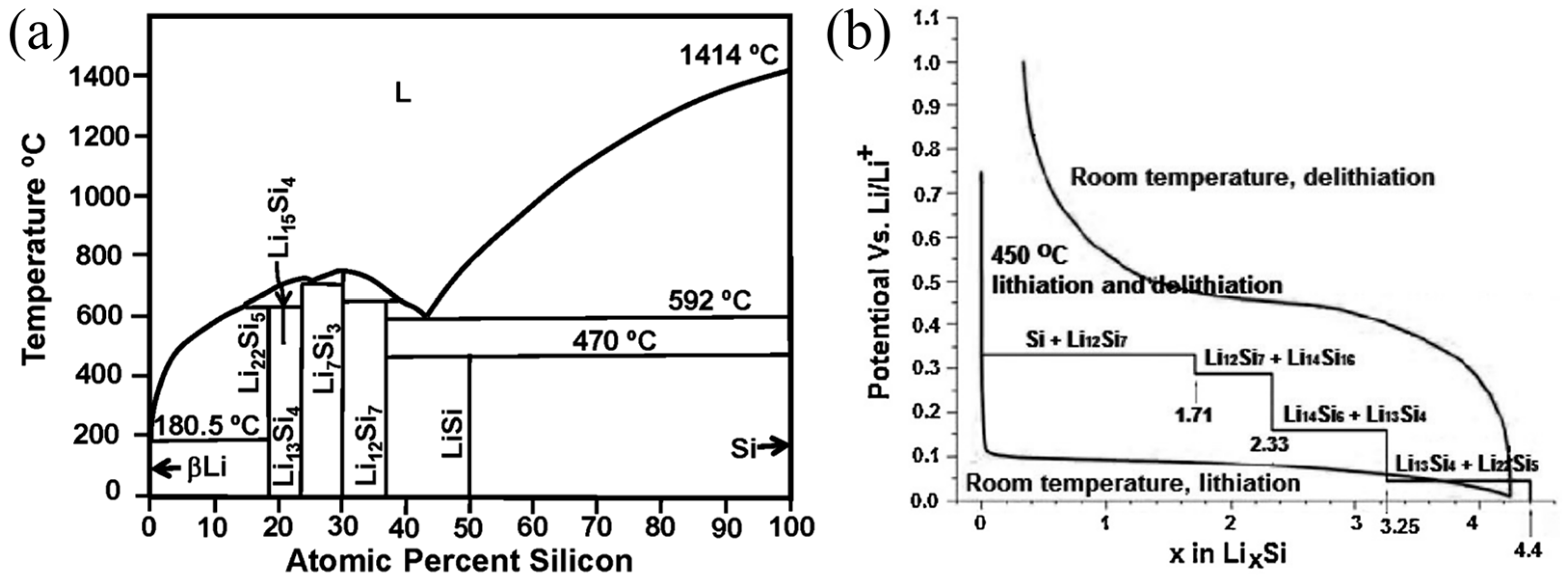
The electrochemical synthesis of Si and Li alloys at a temperature of 450 °C aligns with the phase diagram of the Li-Si system, as depicted in Figure 1a,b. This process led to the emergence of four unique phases: Li12Si7, Li7S3, Li15Si4, and Li22Si5. These phases are associated with voltage plateaus at 0.33 V, 0.29 V, 0.16 V, and 0.04 V, as reported in reference [23]. At room temperature, the lithiation of Si diverges from equilibrium conditions. This deviation is influenced by the coexistence of crystalline silicon (c-Si) and amorphous lithium-silicon alloys (a-LixSi), resulting in a low voltage plateau of approximately 0.1 V during the initial discharge curve. As the voltage declines below 50 mV, a gradual transition from a-LixSi to crystalline lithium-rich silicon (c-Li3.75Si) is evident. Furthermore, during subsequent cycling, the lithiation curve lacks a voltage plateau and exhibits a cyclic pattern. During the delithiation process, c-Li3.75Si reverts to a-LixSi and then transforms back into amorphous silicon (a-Si). In the subsequent charging cycle, a-Si persists in its amorphous state [24]. The behavior of silicon nanowires (Si NWs) during lithiation and delithiation was examined through in situ lithium-ion (Li+) nuclear magnetic resonance (NMR) spectroscopy. Upon discharging the Si NWs at a current rate of C/25 within a voltage range spanning from 0 to 2 V, four distinct processes were discernible. The first process primarily unfolds between 300 and 250 mV, where the amorphous silicon (a-Si) lattice undergoes gradual lithiation to form Li2.0Si, encompassing Si networks and Si-Si clusters. The second process takes place at 100 mV, involving further lithiation of the a-Si lattice and Li2.0Si to form Li3.5Si. This transformation is attributed to the cleavage of Si-Si bonds, the emergence of smaller Si clusters, and ultimately the creation of isolated Si anions. The third process, occurring at 50 mV, entails the transformation of a-LixSi into crystalline lithium-rich silicon (c-Li3.75Si). Lastly, the fourth process, observed at 30 mV, involves the presence of an excess per lithium phase in a-LixSi, predominantly converting to c-Li3.75+xSi, where x ranges from 0.2 to 0.3.
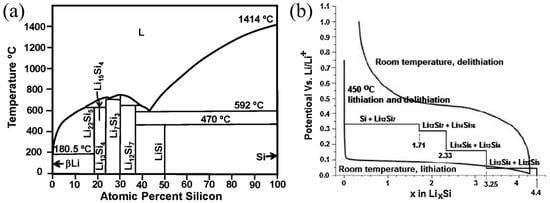
Figure 1.
(a) Phase diagram of Li-Si system Reprinted with permission from ref. [23]. Copyright 2025 Elsevier. (b) Si electrochemical lithiation/delithiation curves Reprinted with permission from ref. [24]. Copyright 2025 Elsevier.
3. Carbon Sources in Si/C Anode
The exceptional electrochemical characteristics of Si/C composites can be ascribed to the rich variety of carbon sources and the diverse methodologies employed in their preparation [9]. This document outlines the prevalent carbon sources incorporated within Si/C composites, encompassing graphite [25], graphene [26], graphdiyne [27], carbon nanotubes, carbon fibers, MXene, pitch, heteroatomic polymers, biomass-derived carbon, carbon-containing gas-derived carbon, MOFs, and g-C3N4.
3.1. Graphite
Graphite found extensive application as an anode material in LIBs, primarily due to its low cost, excellent stability, and high electrical conductivity. During charging, graphite absorbs lithium ions to store electrical energy, and its layered structure facilitates the free intercalation and deintercalation of lithium ions, thereby enhancing charge–discharge efficiency [17]. Further improvements in battery energy density and cycle life can be achieved through modifications such as doping or nanostructuring. Additionally, the stability of graphite anodes mitigates expansion and contraction during battery operation, reducing the risks of thermal runaway and short circuits. However, graphite anodes exhibit limitations, primarily stemming from their limited theoretical capacity of only 372 mAh/g, which is insufficient to meet the demands for high energy density. Furthermore, the formation of a SEI layer on the graphite surface during the initial charge–discharge cycle results in an irreversible loss of initial capacity [18]. Natural graphite also requires spheroidization and surface modification to address issues such as anisotropic kinetic characteristics, volume expansion, and interfacial instability [28].
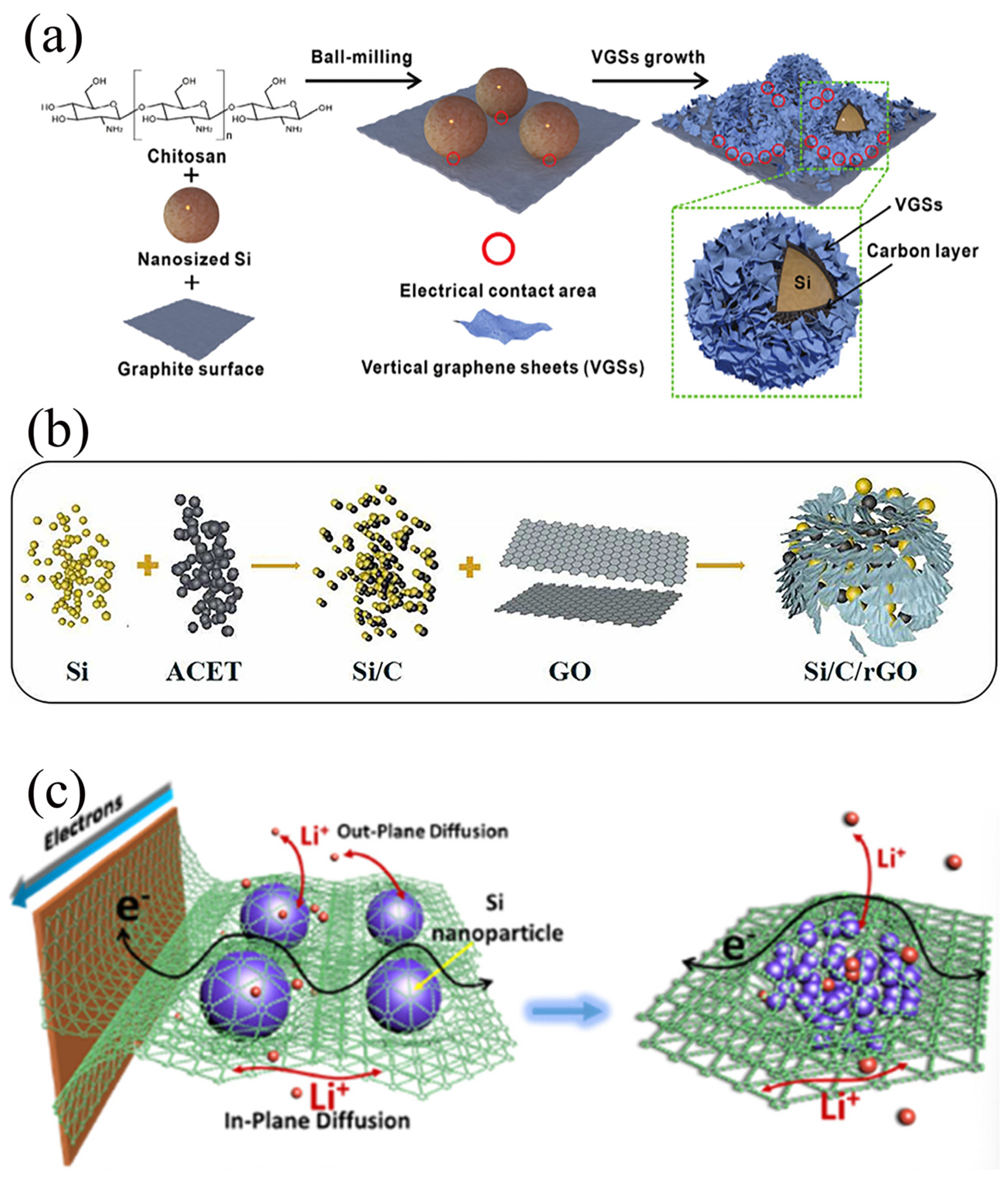
Composite materials formed from graphite and Si present promising application prospects in LIB anodes. However, significant volume expansion of Si during charging and discharging leads to instability in the electrode structure, impacting the battery’s cycle life [16]. Secondly, the relatively low electrical conductivity of Si limits the electrochemical performance of the composites. In addition, the weak interfacial interaction between Si and graphite poses difficulties in forming a stable composite structure [9]. Lastly, ensuring uniform distribution of Si within the composites is challenging, affecting the overall performance of the material [29]. Jannes et al. [30] successfully synthesized a Si-graphite composite (Si@Gr/C) containing 10 wt% Si as the anode for lithium-ion batteries, aiming to enhance cyclic stability. This was accomplished by employing Si and graphite as the primary materials, utilizing fluidized bed granulation technology, and applying a carbon coating methodology. The crux of their approach lies in the uniform dispersion of Si particles within the graphite matrix through fluidized bed technology and the reduction in Si particle surface area via carbon coating. These strategies collectively stabilize the composite structure and augment electrical conductivity. Furthermore, the superior performance of the Si@Gr/C material was validated at a pilot scale. Electrochemical tests demonstrated a substantial improvement in capacity retention after 125 cycles, along with good adaptability to calendaring processing. Compared to the uncoated Si@Gr material, Si@Gr/C exhibited milder performance degradation following calendaring. As shown in Figure 2a, Yu et al. [25] used chitosan as a binder to uniformly attach Si nanoparticles onto the graphite surface by utilizing the ball milling method, followed by in situ growth of vertical graphene sheets via thermal CVD, ultimately yielding vertical graphene sheet/Si/carbon/graphite (VGS@Si/C/G) composites. VGS@Si/TC/G composite material exhibits a significantly high ICE of 86.9%, a remarkable reversible capacity of 1341.2 mAh/g, and excellent cycling stability even after undergoing 1000 cycles at a current density of 0.5 C. The lithium-ion full cell, using the VGS@Si/C/G sample as the negative electrode and NCM 811 as the positive electrode, exhibited a remarkable weight energy density of 343.6 Wh kg−1. Moreover, even after undergoing 500 cycles, the full cell still maintained an excellent capacity retention of 91.5% and a high coulombic efficiency of 99.9%. After 500 cycles, the full cell still maintained an excellent capacity retention rate of 91.5% and a remarkably high coulombic efficiency of 99.9%.
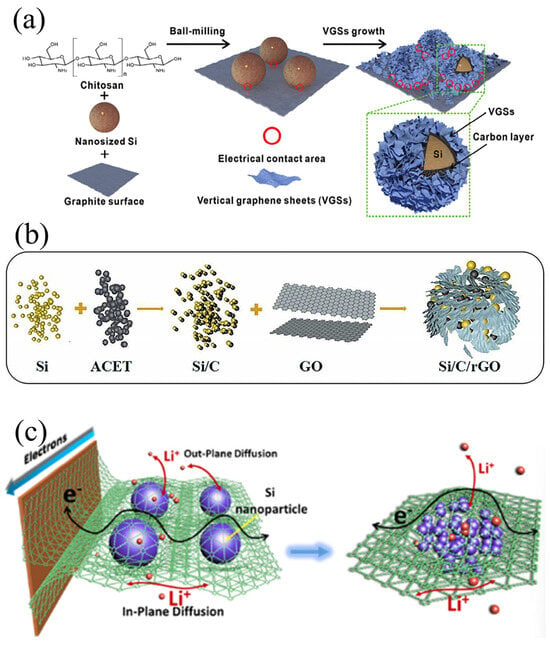
Figure 2.
(a) Schematic illustration of the preparation process of VGSs@Si/C/G composites Reprinted with permission from ref. [25]. Copyright 2025 John Wiley and Sons. (b) Schematic of the process for fabricating Si/C/rGO composite Reprinted with permission from ref. [26]. Copyright 2025 Elsevier. (c) The interaction between 3D seamless GDY, Si NP, and current collector, as well as the lithiation mechanism Reprinted with permission from ref. [27]. Copyright 2025 Elsevier.
Si/graphite electrodes, currently the most advanced high-energy-density anode solution nearing commercialization, achieved preliminary applications in the field of traction batteries. However, achieving a balance between performance and cost necessitates innovative breakthroughs through the synergistic integration of materials, structures, and processes. Over the next five years, with the maturation of Si-based material modification, interface engineering, and pre-lithiation technologies, silicon/graphite electrodes are poised to propel LIBs energy densities beyond 400 Wh kg−1, making them a pivotal choice for electric vehicles and energy storage systems.
3.2. Graphene
Graphene, a two-dimensional material composed of a single layer of carbon atoms arranged in a tightly packed hexagonal lattice, garnered significant attention in the field of materials science due to its exceptional electrical conductivity, high thermal conductivity, remarkable mechanical strength, and enormous specific surface area [31]. The integration of graphene with Si materials to form silicon/graphene (Si@G) anodes represents an innovation in the realm of lithium-ion batteries, aiming to combine the high electrical conductivity of graphene with the high theoretical specific capacity of Si, thereby substantially enhancing the energy density and cyclic stability of the batteries [32].
In Si@G anodes, the role of graphene is indispensable. It not only establishes efficient electron transport pathways, accelerating the charging and discharging processes of the battery [33], but also leverages its unique flexibility and high specific surface area to effectively mitigate the volume expansion of Si during charging and discharging, thereby maintaining the structural stability of the electrode [34]. Furthermore, graphene can encapsulate Si particles, reducing direct contact between Si and the electrolyte, minimizing the occurrence of side reactions, stabilizing the SEI film, and further extending the cycle life of the battery [35]. As depicted in Figure 2b, Zhang et al. [36] reported a facile approach for the preparation of Si/carbon/reduced graphene oxide (Si/C/rGO) composites. Initially, ACET and Si were mechanically ball-milled to create an intermediate composite, designated as i-Si/C in this study. Subsequently, the i-Si/C suspension was subjected to modification with aqueous ammonia, and subsequently self-assembled with a GO suspension through electrostatic attraction, resulting in the formation of Si/C/GO composites. Finally, the GO was reduced using N2H4∙H2O, yielding the desired Si/C/rGO composite. The Si/C/rGO composite exhibits a honeycomb structure with a large specific surface area, abundant pore channels, and exceptional stability. When employed as an electrode in LIBs, it initially demonstrates an ICE exceeding 85%, with the ICE for the second cycle surpassing 94%. Secondly, it possesses remarkable cycling performance, maintaining a discharge-specific capacity of 1004 mAh/g even after undergoing 270 cycles at a current density of 1 A/g. Furthermore, it displays exceptional rate capability, manifesting a reversible capacity of approximately 445 mAh/g even under a high current density of 5 A/g, underscoring the promising potential of the Si/C/rGO electrode.
The Si@G composite, comprised of graphene and Si, exhibits significant potential in significantly enhancing the electrochemical performance and cycling stability of LIB anode materials. However, numerous challenges persist in effectively controlling the number of graphene layers, addressing surface defects, and designing oxygen-containing functional groups on the surface. Additionally, the complexity of preparation methods, high production costs, and concerns over experimental safety associated with Si@G composites pose barriers to their industrial-scale adoption, necessitating further research and design endeavors.
3.3. Graphdiyne
The specific structure of graphyne is characterized by an all-carbon network structure, wherein alkyne bonds are periodically linked to double bonds within a two-dimensional plane. This arrangement and combination of single chemical bonds deviates significantly from conventional carbon materials [36]. The precise design of graphyne based on different molecular structures confirms that graphyne is a large carbon family with numerous family members [37]. In recent years, various graphitic alkynes, such as graphitic monoalkyne, graphitic dialkyne, and graphitic tetraalkyne, have been successfully synthesized, demonstrating their excellent edibility and versatility [38]. As shown in Figure 2c, Li et al. [27] successfully developed an innovative preparation strategy for Si anodes, which involves in situ growth of graphdiyne (GDY) nanosheets encapsulating Si particles to construct a 3D all-carbon conductive and mechanical network. This approach effectively addresses the volume change challenges faced by Si anodes during alloying/dealloying processes, preventing the disintegration of the conductive and mechanical network and enhancing the interfacial contact between Si particles and the current collector. The resultant high-performance Si anode material exhibits exceptional capacity (2300 mAh/g) and high energy density (1343 W h L−1), along with significant improvements in long-term cycling stability. More importantly, the in situ growth method is readily scalable and highly extensible, making it well-suited for practical applications. This breakthrough not only opens up new avenues for the commercialization of Si anodes, but also provides fresh insights into enhancing the performance of other types of electrode materials.
Graphyne has the advantages of low preparation temperature, controllable size and shape, and good electrical conductivity and mechanical properties, which render it a highly promising material for various applications. However, its preparation method is complicated, and the cost of raw materials is high. Furthermore, the research is still in the laboratory stage, and there is still a certain distance on the road to industrialization.
3.4. Carbon Nanotubes
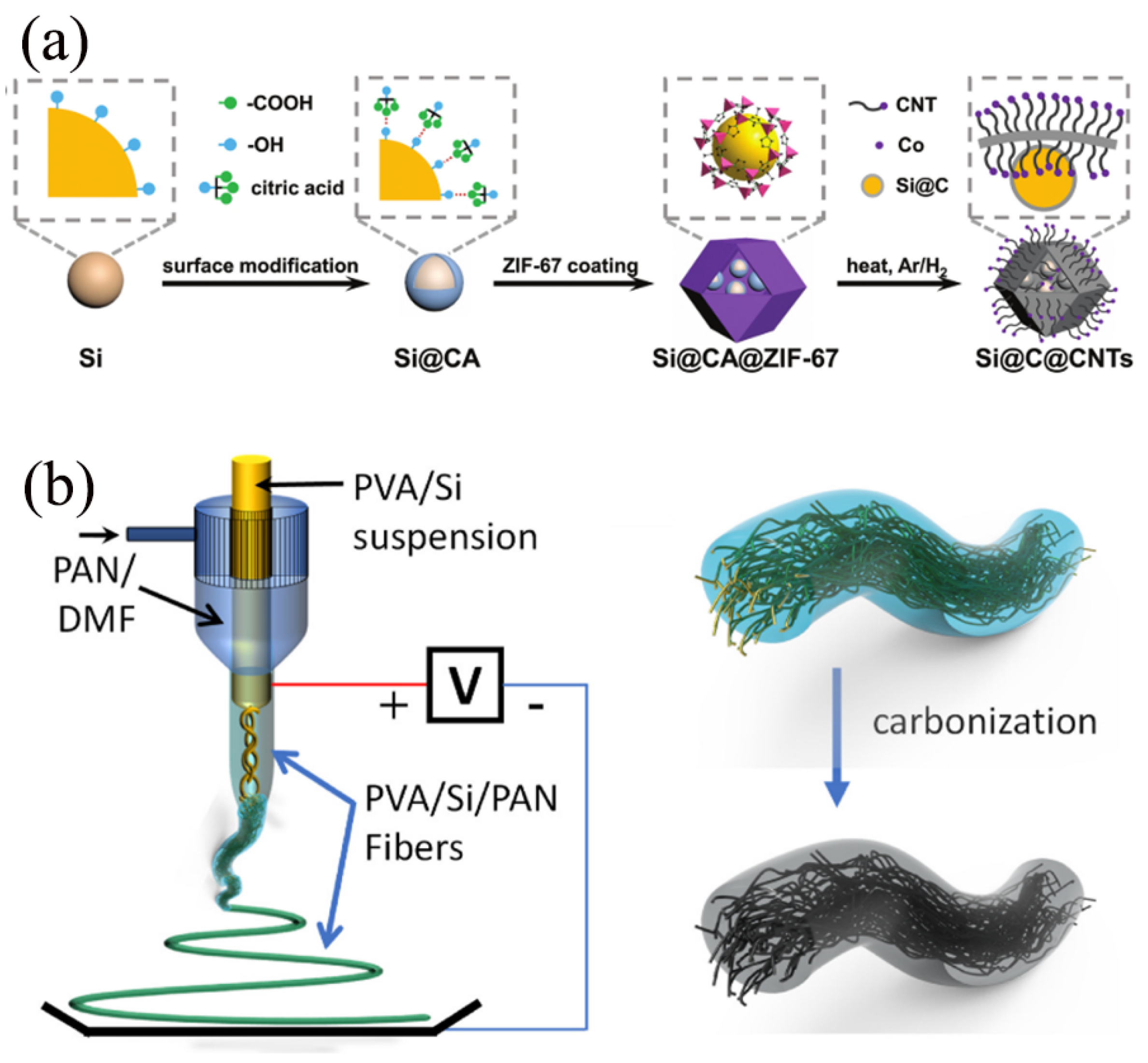
Carbon nanotubes (CNTs), formed by coaxially arranged hexagonal carbon atoms into one-dimensional tubular nanostructures [8], emerged as stars in the field of materials science due to their high strength, high modulus, high thermal conductivity, and exceptional electrical conductivity [15]. In the realm of LIBs technology, CNTs have been ingeniously applied to construct CNTs/Si anode composites [39]. This composite material combines the high theoretical lithium storage capacity of Si with the conductivity and structural stability advantages of CNTs, aiming to address the degradation of cycling performance in Si anodes caused by significant volume changes during charging and discharging [19]. Serving as a carrier, CNTs not only enhance the electrical conductivity of the Si anode, but also effectively mitigate Si’s volume expansion through their unique structure, thereby significantly improving the battery’s cycling stability and energy density. Therefore, combining carbon nanotubes with Si presents a reasonable strategy. As shown in Figure 3a, Si/C composites were synthesized by Fan et al. [40] as anode materials, wherein multifunctional carbon frameworks derived from MOFs (ZIF-67) reinforced with CNTs acted as an external carbon layer to encapsulate the internal carbon-covered Si NPs. The surface modification of Si NPS by citric acid (CA) was employed, resulting in the growth of homogeneous ZIF-67 on the surface of Si NPS, and serving as a carbon precursor for the internal carbon layer of Si NPS. The unique Si/C composites exhibit feature CNT-enhanced dual-carbon stress buffers, which are generated by internal citric acid and external ZIF-67, to accommodate volume expansion and relieve the stress of lithiated Si. A finite element model was developed to simulate the mechanical stresses and demonstrate the superiority of the dual stress buffer structure. In addition, the ion diffusion kinetics and electron transport in the electrode were enhanced by the incorporation of an external carbon framework derived from ZIF-67. Based on these advantages, the obtained Si/C composites with a novel structure are capable of achieving superior multiplication performance and excellent cycling stability, with a high specific capacity of 680 mAh/g (based on the mass of the Si/C composite) at a high multiplication rate of 1 A g−1 for 1000 cycles. Li et al. [41] synthesized the Si/CNTs/G@C-1 composite material using CNTs, Si, and graphite (G) as the core components through an innovative combination of ball milling and spray drying techniques. This method not only enhances the structural stability of the composite, but also markedly improves its electrical conductivity. The incorporation of CNTs establishes a three-dimensional conductive network, effectively mitigating the volume expansion of Si during charging and discharging processes. Meanwhile, the coating of amorphous carbon serves as a “lubricant”, further improving the interfacial compatibility between Si and graphite and reducing interfacial stress. Consequently, the Si/CNTs/G@C-1 composite exhibits exceptional electrochemical performance, including high specific capacity, improved cycling stability, and superior rate capability. After 500 cycles at a current density of 1 A/g, the reversible capacity remains as high as 465 mAh/g, and at a higher current density of 2 A/g, the rate performance reaches an impressive 523 mAh/g. Furthermore, the composite maintains a reversible capacity of 355 mAh/g after 1000 cycles at 1 A/g, demonstrating its long-term cycling stability. This preparation strategy is not only straightforward and scalable, suitable for large-scale production, but also holds practical significance for the industrial application of Si-based nanocomposite anodes in lithium-ion batteries, providing novel insights for the development of high-performance lithium-ion batteries.
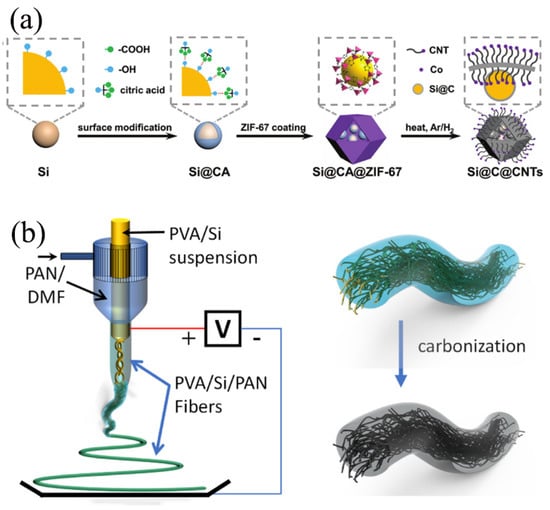
Figure 3.
(a) Graphical representation of the synthetic process of the Si@C@CNTs Reprinted with permission from ref. [40]. Copyright 2025 John Wiley and Sons. (b) Schematic diagram of PVA/Si/PAN fibers prepared by coaxial electrostatic spinning Reprinted with permission from ref. [42]. Copyright 2025 Elsevier.
The excellent mechanical properties and excellent flexibility of CNTs enable them to effectively accommodate the significant volume strain induced by lithiation and delithiation processes in Si NPS, making CNTs the most excellent carbon sources for Si/C anode. Furthermore, with the improvement of the CNT process, large-scale preparation can be fully realized, which greatly reduces its manufacturing cost. However, the simple mechanical mixing approach fails to fully utilize the excellent synergistic effect between carbon nanotubes and Si NPS. Moreover, implementing complex in situ generation such as the CVD method poses significant challenges on a large scale. Therefore, how to simplify the experiments and reduce the cost is the top priority.
3.5. Carbon Fiber
Carbon fiber represents a class of high-strength, high-modulus fibrous material derived from the high-temperature carbonization of organic fibers [22], which is extensively utilized as a reinforcing agent to enhance the overall performance of composite materials [43]. The integration of carbon fiber with Si materials to form Si-C anodes emerged as a significant research direction in the field of LIB anode materials [44]. Carbon fiber/Si anodes combine the high theoretical lithium storage capacity of Si with the favorable electrical conductivity and structural stability of carbon materials, aiming to elevate the energy density, power density, and cycling life of lithium-ion batteries. As shown in Figure 3b, Pei et al. [42] prepared a novel nanofiber-wrapped microfiber, Si-rich composite negative electrode by using the coaxial electrostatic spinning technique, using polyvinyl alcohol (PVA)/Si suspension as the intermediate fluid stream and polyacrylonitrile (PAN)/dimethylformamide (DMF) solution as the sheath stream. High-quality non-woven carbon microfibers derived from PAN shells offer structural protection to the internally embedded short Si-rich nanofibers, mitigating further oxidation of Si during fiber processing. In conjunction with the incorporation of thin carbon skins onto the internal Si/carbon fiber bundles, the microfiber mats form a conductive network serving as a 3D collector to facilitate lithium-ion transportation and charge transfer during charging/discharging processes. Meanwhile, the short Si nanofibers and the mesoporous buffer space between them effectively mitigate the induced stresses of Si nanoparticles initialized by volume changes during lithium insertion and extraction. The incorporation of these microfibers-in-nanofibers composite anode features effectively achieves a delicate balance between electrode crushing and high reversible capacity, thereby leading to promising electrochemical performance and enhancing capacity retention of the Si-rich anode, thus advancing its commercialization. This unique fiber anode material, with a Si content of 40%, exhibits exceptional characteristics by effectively balancing some of the major challenges associated with Si-rich anodes. It demonstrates a specific capacity of approximately 900 mAh/g and maintains a remarkable capacity retention of approximately 90% from the 50th cycle to the 250th cycle.
The electrostatic spinning method, initially inefficient in early experiments, evolved over time to enable large-scale electrostatic spinning production of carbon fibers. The controllable structure as well as the excellent performance of carbon fibers have great advantages, making them a powerful carbon sources to realize the industrialization of Si-C negative electrodes.
3.6. MXene
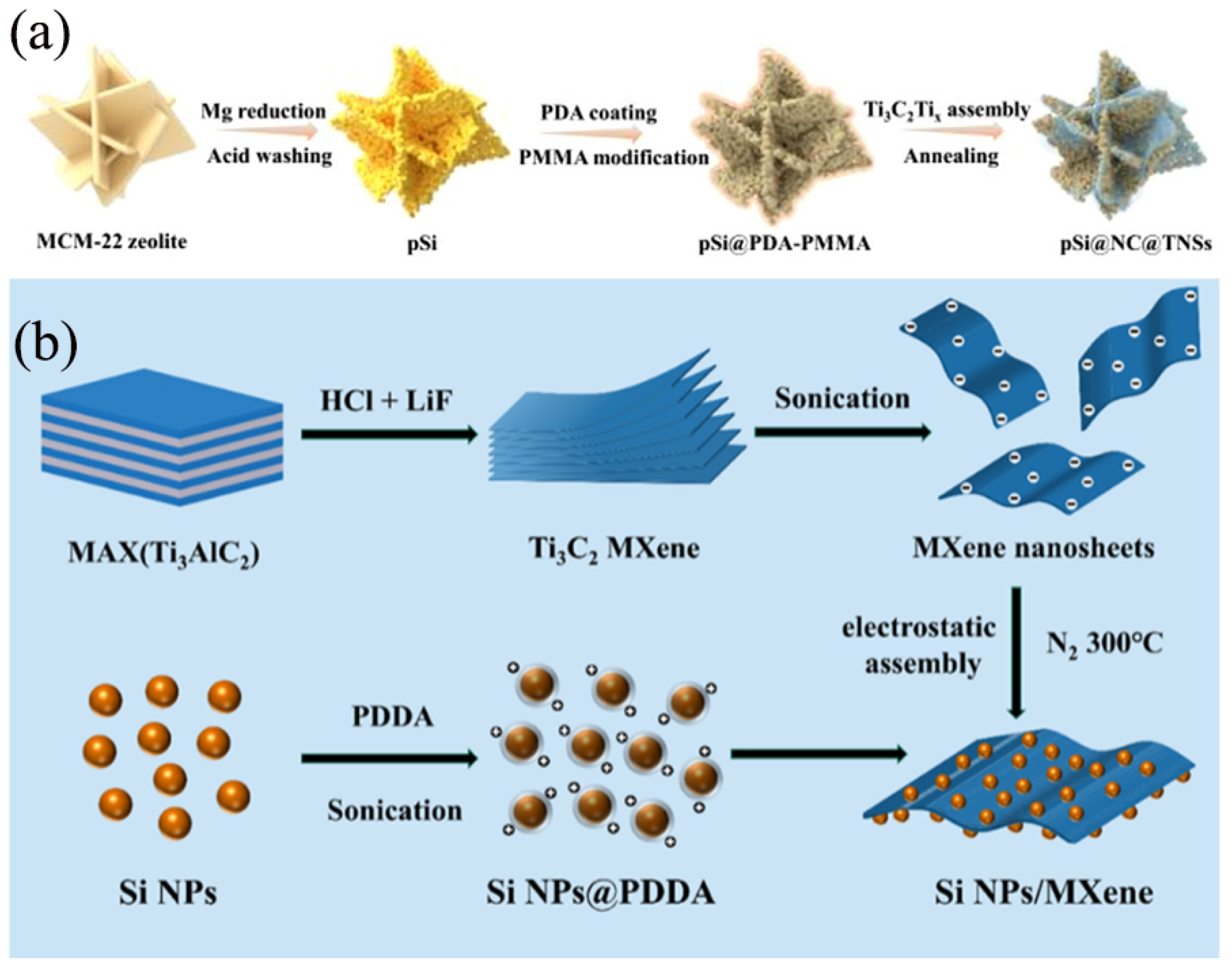
MXene represents a novel class of two-dimensional (2D) inorganic compounds comprising transition metal carbides, nitrides, or carbonitrides with a thickness of just a few atomic layers [45], belonging to the Mn+1AXn phase materials [46], commonly referred to as MAX phases. It is distinguished by its metallic conductivity, large surface aspect ratio, abundant surface chemistry, robust mechanical strength, and exceptional flexibility [47]. Maxenes, as novel anode material, feature a layered structure that facilitates rapid intercalation and deintercalation of lithium ions, thus holding promise for enhancing the charging and discharging rates of batteries [12]. Additionally, the high electrical conductivity of maxenes helps reduce the internal resistance of batteries, thereby improving energy efficiency [26]. Furthermore, surface modification or compositing with other materials can further enhance the cycling stability and rate performance of maxenes. Specifically, maxenes can serve as the active material for the anode in lithium-ion batteries, being directly coated onto the current collector to form the anode electrode [13]. During the charging and discharging process, lithium ions intercalate and deintercalate between the layers of maxenes, enabling the storage and release of electrical energy. Owing to their layered structure and rich surface chemistry, maxenes can be composited with nano-silicon to form a composite anode material with synergistic effects, further boosting battery performance [6]. When employed as the conductive matrix, MXene can effectively mitigate the volume expansion of Si during the lithiation process, enhance the electronic conductivity of the composite material, and facilitate the transport of electrons and ions [48]. Consequently, MXene/Si composites exhibit outstanding anode performance in secondary batteries. As depicted in Figure 4a, Cheng et al. [49] utilized microporous MCM-22 zeolite as a unique Si source to synthesize porous silicon (pSi) sheets via a straightforward magnesiothermic reduction process. Subsequently, these pSi sheets underwent carbon coating and further assembled with Ti3C2Tx MXene, resulting in a ternary pSi@NC@TNS composite. In this design, the porous Si sheets offer an abundance of active sites, thereby shortening the diffusion pathways for lithium-ion during electrochemical reactions. The N-doped carbon (NC) layer serves as a bonding interface, effectively facilitating the coupling of the pSi with Ti3C2Tx. The conductive network formed by the 2D Ti3C2Tx sheets and the intervening NC layer significantly enhances both the overall charge transport within the electrode material and contributes to the stabilization of the electrode structure. Consequently, the fabricated pSi@NC@TNS anode exhibits improved lithium storage performance, manifesting a high reversible capacity of 925 mAh/g at 0.5 A/g after undergoing 100 cycles. As shown in Figure 4b, Zhou et al. [50] reported a facile and scalable electrostatic assembly method to obtain Si NPs/MXene composites as LIB anode materials, which was based on the mutual attraction of negatively charged monolayer MXene nanosheets and positively charged Si NPs. The uniform attachment of Si NPs onto the MXene surface effectively prevented the self-restacking of MXene nanosheets. The MXene nanosheet matrix provides an excellent platform for facilitating Li+ ion diffusion while it eliminates the swelling effect of the Si negative electrode. The designed Si NP/MXene electrodes exhibited significantly improved cycling stability and multiplicity performance compared to the pristine Si and Si/Ti3C2Tx MXene. The composite maintained a capacity of 1917.9 mAh/g after undergoing 300 charge/discharge cycles at a current density of 0.5 A g−1.
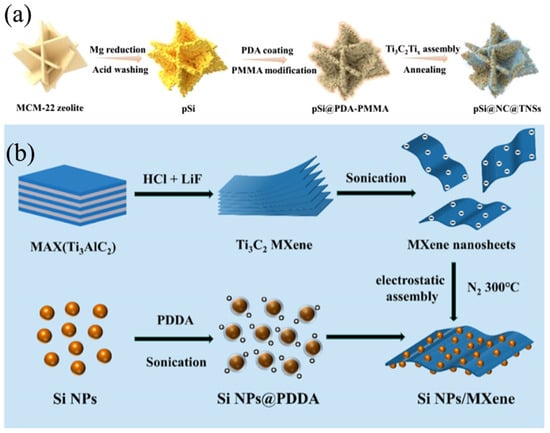
Figure 4.
(a) Schematic illustration of the typical preparation procedure of pSi@NC@TNSs Reprinted with permission from ref. [49]. Copyright 2025 Elsevier. (b) Synthesis process of the Si NPs/MXene composite Reprinted with permission from ref. [50]. Copyright 2023 American Chemical Society.
The sandwich structure formed by MXene material-coated nanosilica can well protect the volumetric strain caused by Si lithiation. Furthermore, its excellent mechanical properties and flexible structure significantly improve the cyclic stability of the composite material. However, the production cost of MXene materials remains high and their current stage is limited to laboratory research, thus impeding their commercialization.
3.7. Pitch
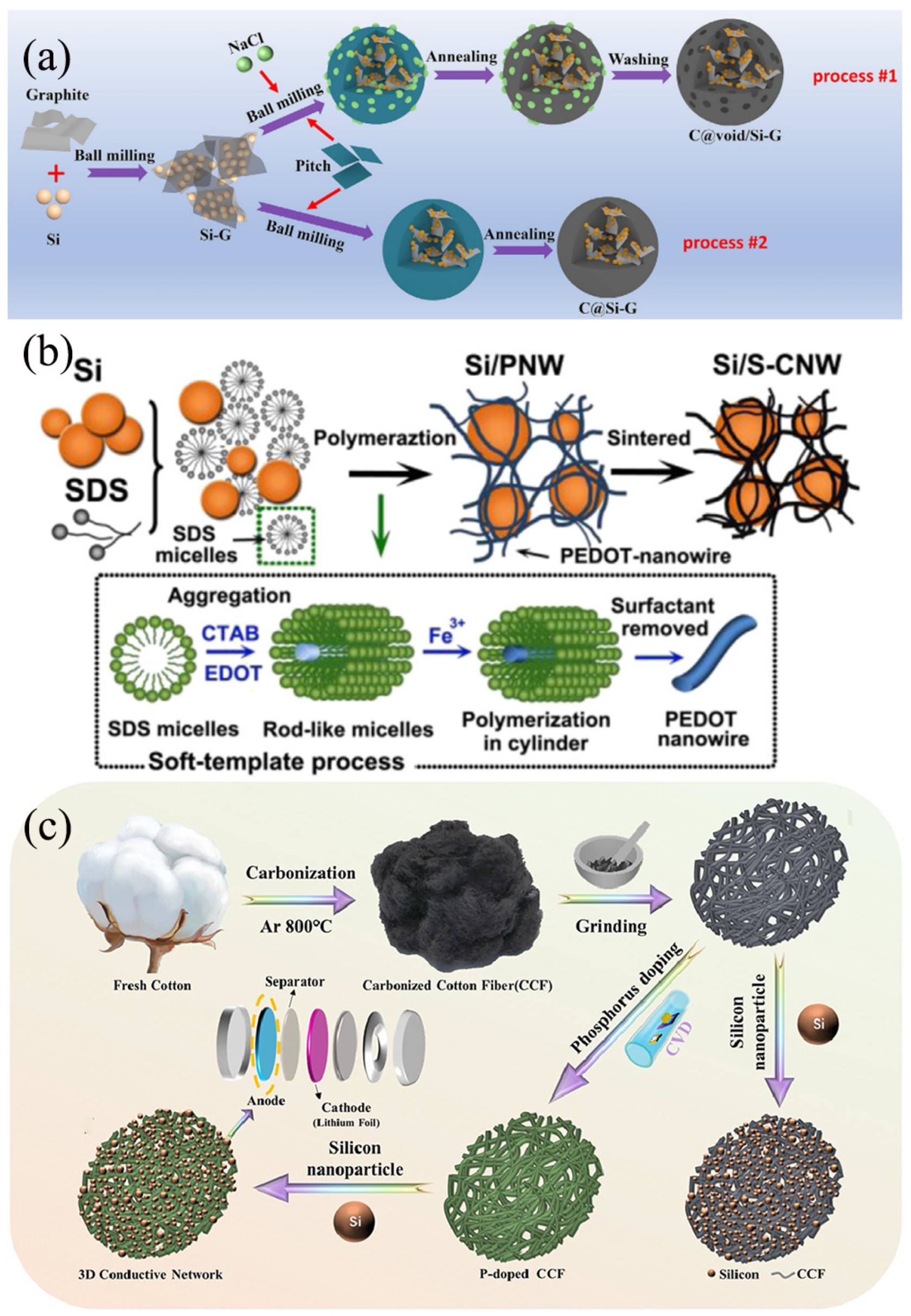
Pitch-derived carbon (PDC) is a type of carbon material produced from natural pitch or coal tar pitch through a series of processing steps, including high-temperature treatment and purification [51]. It boasts abundant sources, relatively low costs, and excellent processability. PDC/Si anode materials integrate the high lithium storage capacity of Si with the superior electrical conductivity and structural stability of PDC, aiming to enhance the energy density and cycling performance of lithium-ion batteries [29]. Specifically, Si serves as the active material providing high capacity, while PDC functions as the matrix material, offering conductive pathways and structural support [52]. As shown in Figure 5a, Shi et al. [53] reported a simple and cost-effective method for the large-scale production of Sie/carbon composites (C@void/Si-G) with a porous structure from waste silica powder and pitch. The C@void/Si-G composites were prepared by ball milling, heat treatment, and washing processes using sodium chloride (NaCl) as a templating agent. This structural design has multiple advantages: (1) Graphite is used as a support to inlay Si nanoparticles, which helps to enhance the electrical conductivity. (2) NaCl is removed in situ during the washing process, allowing a porous space to be obtained between the Si/graphite and pyrolyzed carbon. This porous space can effectively buffer the volume change of Si, greatly shorten the Li+ transport pathway, and importantly, facilitates easy removal of NaCl by washing. (3) Tightly wrapping the pyrolytic carbon around the Si/graphite composites ensures the formation of a strong SEI film on the carbon shell, thereby preventing the depletion of the electrolyte, and effectively accommodating the volume expansion strain to avoid the chalking process during the cycling process. As a result, the C@void/Si-G negative electrode with extensive porous space shows a remarkable cycling capacity of 1390.3 mAh/g at 0.1 C, demonstrates durable capacity retention of 1082.7 mAh/g after undergoing 200 cycles at 0.2 C, and maintains a capacity of 706.6 mAh/g after undergoing 500 cycles at 0.5 C. In addition, after cycling with the LiNi0.3Co0.3Mn0.3O2 (NCM 111) as the cathode, an ideal energy density of 446 Wh kg−1 and desired cycling stability (90.1% capacity retention after 100 cycles) were obtained in the full cell. Nyamtara et al. [54] employed a two-step pyrolysis synthesis approach to fabricate carbon-coated Si NPS composites. The carbon source was derived from coal tar pitch, while the Si nanoparticles were sourced from recycled industrial solar cells and mixed with 30% graphite during the ball milling process. The incorporation of 30% graphite within the Si nanoparticles enhanced the internal Li+ transportation within the composite material. Furthermore, the robust mechanical strength of the Si nanoparticles encapsulated within PDC exhibited a synergistic effect while undergoing repeated lithium-ion de/alloying reactions. The pyrolyzed composite exhibited excellent cycling and rate capabilities as a negative electrode material for lithium-ion batteries. When charged at a rate of 500 mA g−1 over more than 200 cycles, it demonstrated a high discharge capacity of 1524 mAh/g, accompanied by an average coulombic efficiency of 99.8% and a discharge capacity retention rate of 75.7%.
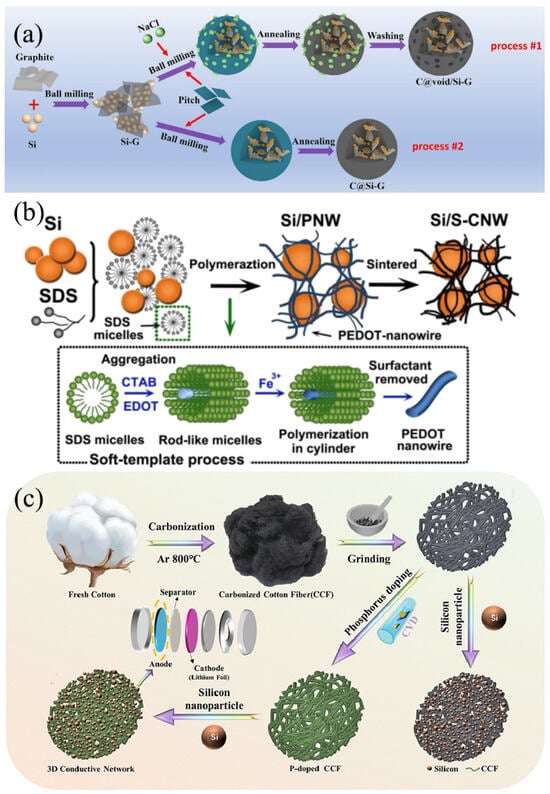
Figure 5.
(a) Formation procedures for C@Si-G and C@void/Si-G composites Reprinted with permission from ref. [53]. Copyright 2025 Elsevier. (b) The synthesis of Si/S-CNW Reprinted with permission from ref. [55]. Copyright 2025 Elsevier. (c) Synthesis principle of Si@PCCF composites Reprinted with permission from ref. [56]. Copyright 2025 Elsevier.
PDC/Si anodes, as a promising new type of anode material, are receiving increasing attention and research. By optimizing the preparation processes, improving the composite structure, and incorporating doping modifications, their performance can be further enhanced, and costs reduced. In the future, asphalt carbon/Si anodes are expected to play a significant role in electric vehicles, energy storage systems, and other fields, driving the continuous progress and development of LIBs technology.
3.8. Heteroatom Polymer
Heteroatom-containing polymer-derived carbons (HPCs) represent a class of porous carbon materials obtained from polymers containing heteroatoms (such as nitrogen, oxygen, sulfur, etc.) through pyrolysis or carbonization at high temperatures [57]. These materials not only retain the fundamental structural characteristics of the precursor polymers, but also exhibit unique physicochemical properties due to the introduction of heteroatoms, including high specific surface area [51], abundant pore structure, excellent electrical performance, and catalytic activity [58]. These properties endow HPCs with tremendous potential for applications in energy storage and conversion systems. The integration of HPCs with Si anodes aims to combine the high energy density of Si with the stability and catalytic activity of HPCs, thereby enhancing the overall battery performance. Through rational structural design, HPC/Si anodes hold promise in addressing issues such as volume expansion and poor cycling stability of Si anodes during charging and discharging processes [59]. Huang et al. [60] prepared Si/WPU composites through a straightforward physical mixing method using waterborne polyurethane (WPU) and Si NPS, leveraging the hydrogen bonding between carbamate groups and hydroxyl groups on the nano-sized Si. Subsequent pyrolysis yielded Si@NC with a core–shell structure featuring an N-doped carbon layer, which was employed as an anode material for lithium-ion batteries. The N-doped carbon layer facilitates the formation of a stable SEI film during charging and discharging cycles, thereby mitigating excessive loss of Si nanoparticles and lithium and buffering the volume changes of Si nanoparticles to prevent structural degradation of the anode. The resultant Si@NC anode maintained a high discharge specific capacity of 945.63 mAh/g after 300 cycles at a current density of 0.5 A/g, demonstrating significant improvements in rate capability and cycling stability. As shown in Figure 5b, Shao et al. [55] successfully developed an innovative Si/S-doped carbon nanowire composite (Si/S-CNW) through a meticulously designed two-step process. Initially, with the assistance of sodium dodecyl sulfate (SDS), a unique nanowire network-structured Si/PNW composite was synthesized via the soft template polymerization technique of 3,4-ethylenedioxythiophene (EDOT) in the presence of Si nanoparticles. Subsequently, the Si/PNW was converted into Si/S-CNW with significantly enhanced conductivity through carbonization treatment. Benefiting from the robust conductive bridges established by the highly conductive S-doped carbon nanowire network, the Si/S-CNW exhibited exceptional cycling stability and rate capability when utilized as an anode material for lithium-ion batteries.
HPC/Si anodes exhibit unique advantages and possess substantial potential for development in the field of lithium-ion batteries, albeit accompanied by certain challenges. With the continuous optimization of preparation processes and the ongoing innovation of doping strategies, it is believed that the performance of HPC anodes will be further enhanced in the future, thereby injecting new vitality into the development of the LIBs industry.
3.9. Biomass Derived Nanostructured Carbon
Biomass-derived carbons garnered significant academic and industrial interest due to their natural abundance, ease of processing, and inherently porous or hierarchical structures [61]. Incorporating biomass-derived carbons into Si/C composites holds great potential for the development of cost-effective and environmentally friendly preparation methodologies. Rice husk (RH), an abundant agricultural waste rich in Si, has been reported as a precursor for synthesizing Si NPS [62]. However, further investigation into the potential applications of RH in Si-based materials remains to be explored. Ren et al. [63] harnessed RH, Si NPs, and 1-butyl-3-methylimidazolium acetate (BMIMAcO) as precursors to fabricate Si@SiOx@C-800 composites through a one-step carbonization process at 800 °C. The irregularly shaped carbon blocks and SiOx layers formed by calcining rice husks at high temperatures are coated onto Si NPs. The Si@SiOx@C800 exhibits exceptional reversible capacity and rate capability, which can be attributed to the inhibition of volume expansion by the SiOx layer and the enhancement of Li+ diffusion coefficient facilitated by Si-N bond coupling. As depicted in Figure 5c, Cao et al. [56] established a comprehensive and enduring conductive network by adopting cost-effective and eco-friendly biochar cotton fibers, while further enhancing the overall conductivity through phosphorus doping. Additionally, this network serves as a robust scaffold, effectively preserving the integrity of electrodes and mitigating their degradation. First-principles calculations, based on density functional theory, confirmed that the electronic structure and charge rearrangement of the Si@PCCF electrode are the primary contributors to its improved conductivity and superior performance. The electrode demonstrates robust cycling performance, retaining a capacity of 1777.15 mAh/g at 0.3 C after undergoing 150 cycles, exceeding 47% of its initial capacity, and demonstrating a remarkable rate capability of 1244.24 mAh/g at 2.0 C.
Biomass carbon has a special biomass structure and renewability, making it highly suitable for forming composites with Si NPS to exhibit exceptional performance. Currently, relatively few studies have been conducted on the preparation of Si-based composites using biomass as a carbon source, mainly attributed to the inherent defects and unsatisfactory mechanical properties associated with biomass carbon. The agglomeration tendency of Si NPS hinders its uniform distribution in biomass carbon. From the above discussion, it is evident that extensive research is necessary to exploit the unique natural structure and composition of biomass to construct Si composites.
3.10. Carbon-Containing Gas-Derived Carbon
Carbon-containing gas-derived carbon refers to carbon-based materials synthesized through the decomposition of carbon-containing gases (such as C2H2, CO2, etc.) during high-temperature pyrolysis or chemical vapor deposition (CVD) processes. These materials exhibit high electrical conductivity, chemical stability, and tunable microstructures, making them widely applicable in the field of energy storage.
3.10.1. Acetylene (C2H2)
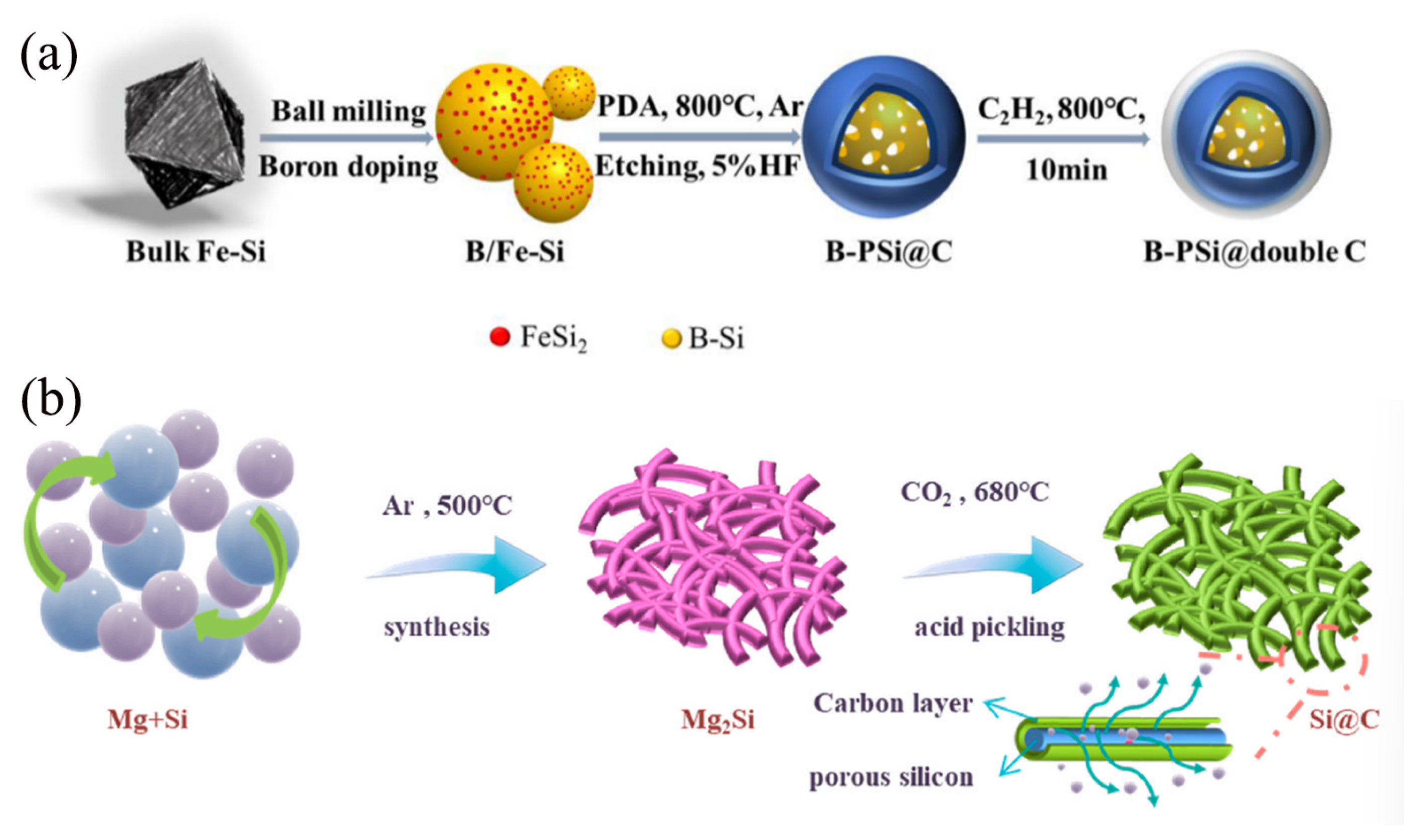
Under specific conditions, C2H2 gas can undergo pyrolysis or catalytic conversion to form carbonaceous materials with diverse structures and properties, such as graphitic carbon and amorphous carbon. Due to their unique physicochemical properties, these derived carbon materials hold broad application prospects in various fields, including electrode materials, catalyst supports, and energy storage. As shown in Figure 6a, Li et al. [64] prepared a novel composite of boron-doped porous silicon (B-PSi@double C) coated with a double carbon shell by using a modified ball milling method combined with the self-polymerization of dopamine hydrochloride and CVD of C2H2, using inexpensive industrial ferrosilicon alloys as raw materials. In this work, the boron-modified porous Si structure facilitates both improved electronic and Li+ transport dynamics. The internal flexible carbon effectively modulates the volume change of Si during lithiation and significantly enhances the electrical connectivity among the particles. In addition, the incorporation of external rigid carbon promotes the formation of a stable SEI formation and maintains structural integrity. This unique rigid-flexible dual-carbon structure combined with high-capacity modified porous Si particles enables the composite to exhibit excellent electrochemical performance in terms of lithium storage capacity and cycling stability. As a result, the B-PSi@double C electrode shows a reversible capacity of 1105.8 mAh/g and demonstrates excellent cycling stability over 300 cycles at a current density of 0.5 A g−1.
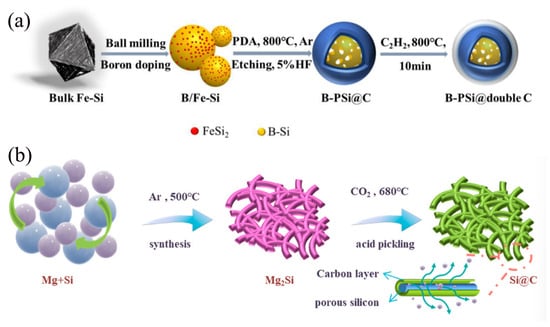
Figure 6.
(a) The fabrication process of B-PSi@double C composite Reprinted with permission from ref. [64]. Copyright 2025 Elsevier. (b) Schematic illustrations of the synthesis of CO2-derived carbon/Si composites Reprinted with permission from ref. [65]. Copyright 2025 Elsevier.
3.10.2. Carbon Dioxide (CO2)
CO2-derived carbon refers to carbon materials converted or derived from CO2 gas through specific technologies or processes. This type of carbon material can exhibit a variety of possible morphologies and properties, depending on its preparation method and subsequent treatment processes. In recent years, with the increasing emphasis on CO2 emission reduction and utilization, researchers have been exploring methods to convert CO2 into useful materials [66], including the preparation of various CO2-derived carbon materials [67]. As shown in Figure 6b, Li et al. [65] fabricated a carbon-coated porous Si composite material by utilizing magnesium silicide as a precursor and employing an innovative dealloying method with carbon dioxide serving as the gas-phase carbon source. The crux of this method lies in the reaction between magnesium and real-time-introduced carbon dioxide during the process, which fixes carbon elements and forms a controllable-thickness carbon coating encapsulating the porous Si surface. Compared to traditional Si anode materials, this carbon-coated porous Si composite exhibits remarkable advantages in lithium-ion batteries, including a high specific capacity of up to 2302.5 mAh/g, good rate capability, and excellent cycle stability. This not only addresses the severe volume changes experienced by Si anodes during lithiation/delithiation processes, but also enhances the battery’s capacity retention and coulombic efficiency. Furthermore, the composite demonstrates good performance in full-cell tests, offering significant scientific and practical implications for the recycling of carbon dioxide and the development of large-scale energy storage technologies.
In the research field of carbon-containing gas-derived carbon/Si anode materials, C2H2 and CO2 garnered significant attention from researchers as potential carbon sources [68]. Despite the relatively limited application of C2H2 in this domain and the scarcity of widespread reports on its specific development status and latest research progress, the rapid advancement of renewable energy technologies and the urgent demand for high-performance energy storage technologies hold promise for further exploring the potential of C2H2 as a carbon source in the preparation of Si anode materials. In contrast, CO2 achieved certain progress in its application within carbon-containing gas-derived carbon/Si anode materials. Researchers successfully converted CO2 into carbon materials through electrochemical reduction and other methods and explored their application in Si anode materials. The introduction of these carbon materials effectively improved the cycle stability and rate capability of Si anodes, demonstrating favorable electrochemical performance. In the future, with continuous technological advancements and in-depth research, the application prospects of carbon-containing gases such as C2H2 and CO2 in the preparation of Si anode materials will broaden, injecting new vitality into the development of the renewable energy industry.
3.11. Metal–Organic Frameworks (MOFs)
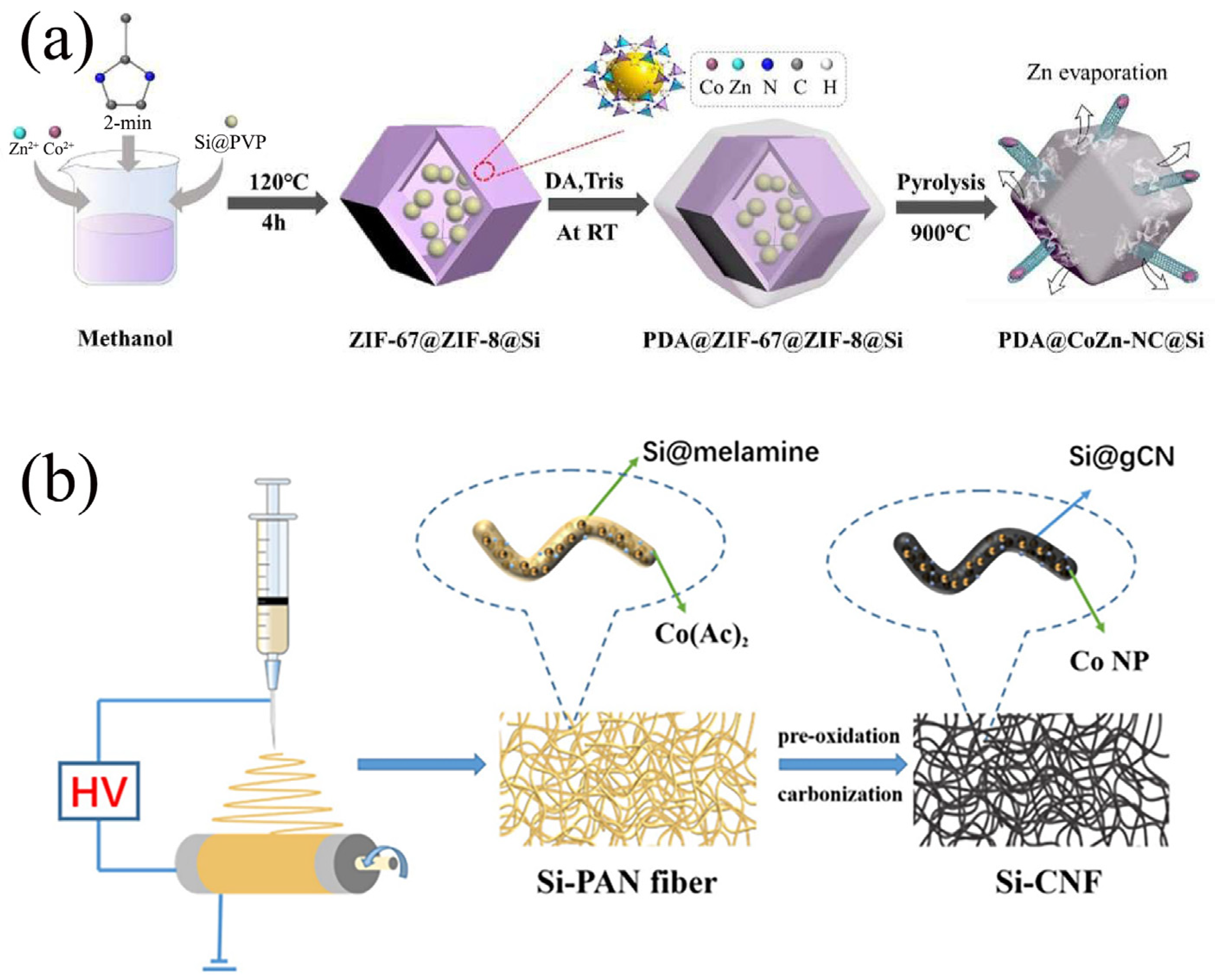
MOFs are porous crystalline materials self-assembled through coordination bonds between metal ions or clusters and organic ligands [69]. They are characterized by high specific surface areas, tunable pore structures, and chemical functionalization properties, making them widely applicable in catalysis, gas storage, and energy fields [40]. MOF-derived carbons are porous carbon materials prepared through high-temperature pyrolysis of MOF precursors (such as the ZIF and MIL series) [70]. These materials inherit the regular pore structures of the MOFs and form highly conductive and stable graphitized carbon skeletons or nanocomposites. As shown in Figure 7a, Niu et al. [71] successfully synthesized a novel carbon shell structure with hierarchical CNT dispersion for a stable PDA@CoZn-NC@Si anode through dopamine-mediated bimetallic ZIF-triggered surface polymerization. The CoZn-ZIF-derived N-doped C skeleton, intertwined with interconnected multi-core shell units, provided efficient and stable pathways for both electrons and Li+ ions. Simultaneously, the CoZn-NC matrix with induced voids imparted deformability to the anode, further mitigating stresses arising from Si volume expansion. The hierarchical carbon shell, serving as a dual protective layer, significantly prevented the formation of excessive SEI films. Based on these merits, the PDA@CoZn-NC@Si anode exhibited exceptional rate capability with specific capacities of 1037 mAh/g at 2 C and 1353 mAh/g at 1 C, respectively. Moreover, it maintained a reversible capacity of 814 mAh/g after undergoing 400 cycles at 0.5 C, representing a mere 0.14% capacity loss per cycle. Furthermore, through a solution-based chemical pre-lithiation approach, the full cell demonstrated improved ICE of 88.3% and a high energy density of 419.8 Wh/kg. Su et al. [72] fabricated Si@Co-NC composites consisting of Si nanoparticle cores encapsulated within N-doped/Co-incorporated carbon shells via the carbonization of zeolitic imidazolate frameworks (ZIFs) and Si nanoparticles. Due to the N doping and the introduction of Co particles to improve the electrical conductivity of the carbon shell, while the hollow structure can effectively mitigate the volume change, the Si@Co-NC composites have stable cycling performance. This innovative Si-based design and pre-lithiation method holds promise for advancing research on highly stable Si composite anodes for lithium-ion batteries.
MOF-derived carbon/Si anodes emerged as one of the research hotspots in the field of lithium-ion batteries. Researchers enhanced the performance and stability of MOF-derived carbon/Si anodes by continually improving preparation techniques and optimizing material structures. For instance, methods such as in situ synthesis and coating modification effectively mitigated the volume expansion issue of Si, thus improving the cycle stability and capacity of the batteries. Additionally, some studies combined MOF-derived carbon with other materials to form composite anode materials with special structures and properties, further expanding the application scope of MOF-derived carbon in lithium-ion batteries.
3.12. g-C3N4
Graphitic carbon nitride (g-C3N4), a polymeric semiconductor material with a unique electronic structure and exceptional stability, finds extensive applications in fields such as photocatalysis, energy storage, and sensing [40]. Its distinctive CN conjugation system enables it to conduct efficient photocatalytic reactions under visible light while exhibiting good chemical and thermal stability. By coating the surface of Si anodes with g-C3N4 layers, a composite material is formed [73]. This design combines the high stability of g-C3N4 with the high lithium storage capacity of Si, aiming to enhance the energy density and cycle stability of lithium-ion batteries. As shown in Figure 7b, Zhong et al. [74] utilized electrospinning technology to in situ synthesize nitrogen-rich g-C3N4 with a graphene-like structure from melamine precursors within CNFs, and combined it with Si nanoparticles to prepare hierarchical Si@g-C3N4/CNF composite anode materials. The prepared Si@g-C3N4/CNF anode materials exhibited exceptional performance in lithium-ion batteries, maintaining high reversible capacity and ultra-long cycle stability at high current densities. Molecular dynamics simulations validated that g-C3N4 can reduce the activation energy for lithium-ion diffusion and enhance the diffusion coefficient. These findings provide practical insights for the development of high-performance lithium-ion batteries, particularly for those utilizing Si-based carbon anode materials, offering a promising approach for the industrial application of Si anodes.
Currently, the preparation process of g-C3N4/Si anodes is relatively complex, requiring precise control of synthesis conditions, and the raw material costs are high, leading to a relatively high cost of g-C3N4/Si anodes. However, with the rapid advancement of renewable energy technologies and the urgent demand for high-performance energy storage technologies, research on g-C3N4/Si anode materials is gradually increasing. Researchers are continuously improving material performance and reducing costs by refining preparation techniques and optimizing material structures. Additionally, addressing the issues of complex preparation processes and high costs, researchers are actively exploring new synthesis methods and raw material sources, aiming to achieve large-scale production and commercialization of g-C3N4/Si anodes.
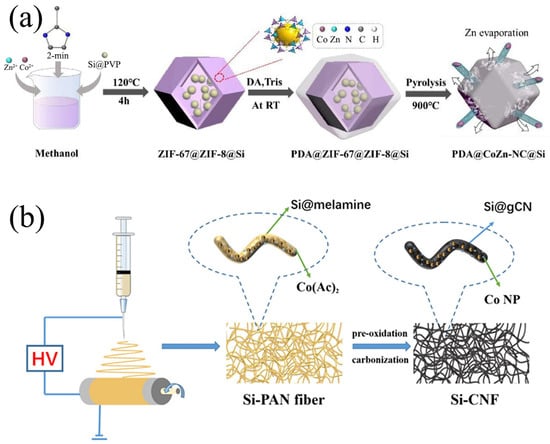
Figure 7.
(a) Synthesis process of PDA@CoZn-NC@Si Reprinted with permission from ref. [71]. Copyright 2025 Elsevier. (b) The schematic diagram of the formation and carbonization of Si@g-CN/CNF composite material Reprinted with permission from ref. [74]. Copyright 2025 Springer Nature.
Figure 7.
(a) Synthesis process of PDA@CoZn-NC@Si Reprinted with permission from ref. [71]. Copyright 2025 Elsevier. (b) The schematic diagram of the formation and carbonization of Si@g-CN/CNF composite material Reprinted with permission from ref. [74]. Copyright 2025 Springer Nature.
3.13. Future Outlook and Overview of Prospects
Table 1 provides a detailed comparison of the current performance of silicon–carbon anode materials. From the perspective of carbon source types, traditional carbon materials such as graphite, graphene, graphdiyne, and carbon nanotubes still occupy a core position [16]. The composites formed by combining these materials with silicon exhibit particularly outstanding electrochemical performance, especially in terms of capacity retention and cycling stability [75]. However, with the continuous advancement of science and technology and the deepening of scientific research [76], some new carbon sources, such as biomass-derived nanostructured carbon [53], carbon-containing gas-derived carbon [64], and MOFs [71], have also begun to emerge [7]. These new carbon sources, with their unique structures and excellent properties, opened up new pathways for further enhancing the electrochemical performance of silicon–carbon anode materials.

Table 1.
Comparison of Li Storage Properties of Si/C Negative Electrodes.
In terms of preparation methods, current research trends focus more on the innovativeness and practical applicability of methods [21]. For example, silicon–carbon anode materials prepared through a series of advanced processes, such as wet granulation and carbonization [74], high-speed fusion and low-temperature heating [75], and electrostatic assembly [50], achieved significant improvements in electrochemical performance. At the same time, new preparation methods, such as in situ growth and hydrothermal synthesis, are also being continuously explored and refined. The emergence of these innovative methods has not only greatly improved the overall performance of the materials, but also provided more diverse options for future industrial production [54].
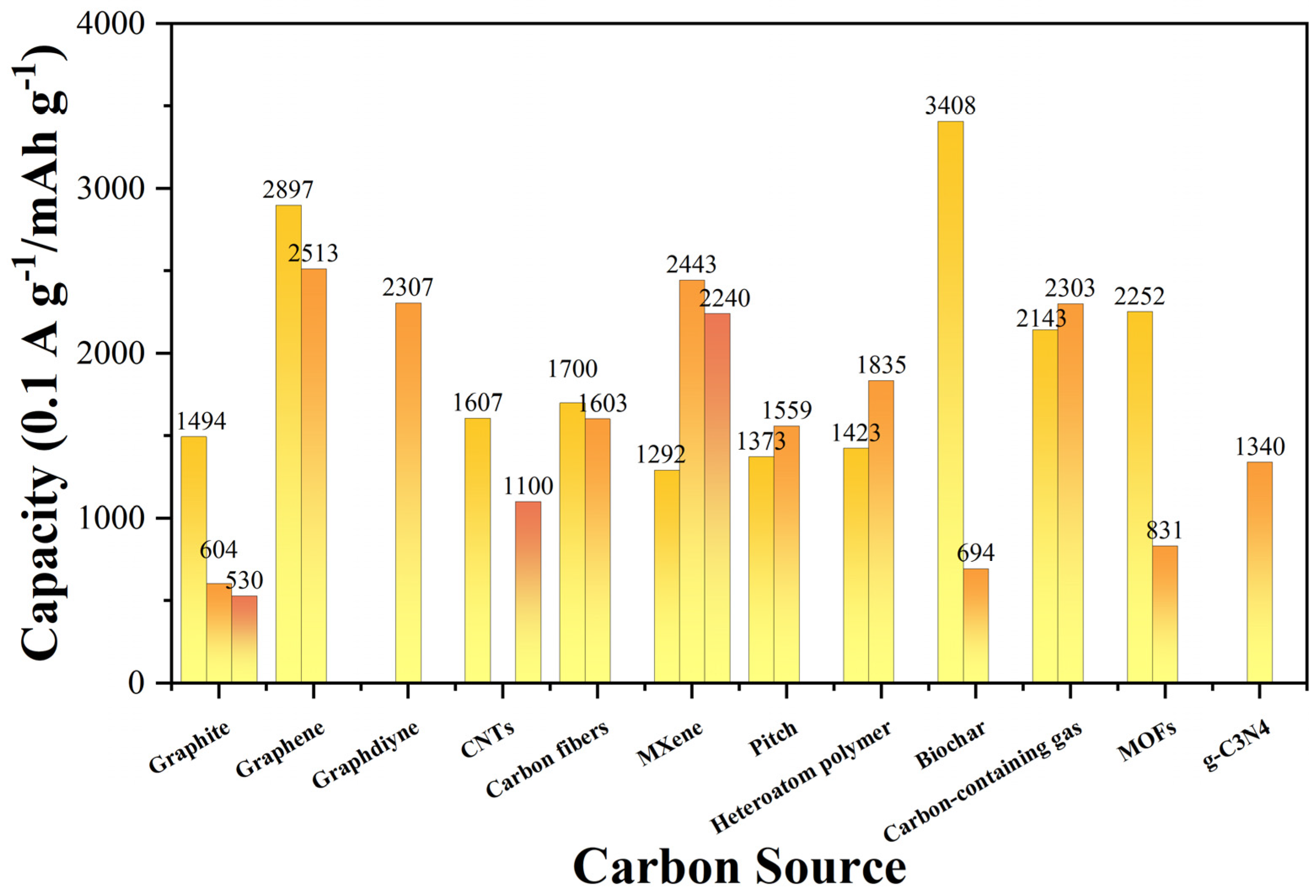
From the perspective of electrochemical performance, the development of current silicon–carbon anode materials achieved remarkable results. In terms of capacity, rate capability, and cycling stability, some materials demonstrated extremely outstanding performance [37]. Figure 8 and Table 1 unequivocally demonstrate that when graphene (such as Si/C/rGO [26], SiNPs@MC [77]) is used as the carbon source, the anode materials exhibit extremely high capacities (approaching or exceeding 2500 mAh g−1) and outstanding rate capabilities (able to maintain high capacities even at higher current densities). Graphdiyne (GDY-Si) and biomass-derived nanostructured carbon (such as Si@P-doped carbonized cotton fiber [56]) also showcase impressive capacity levels and rate capabilities. However, despite their advantages in initial capacity and rate performance, their cyclic stability varies, with some materials experiencing significant capacity decay after multiple cycles. In contrast, although some anode materials prepared from carbon sources such as SGHGs [75], SiG@B2O3-C [76], and Si@CNFs [79] may be slightly inferior in terms of initial capacity and rate performance, they exhibit better cyclic stability, maintaining relatively high capacities after multiple cycles. Furthermore, it can be observed that different preparation methods can significantly affect the electrochemical performance of the same carbon source material.
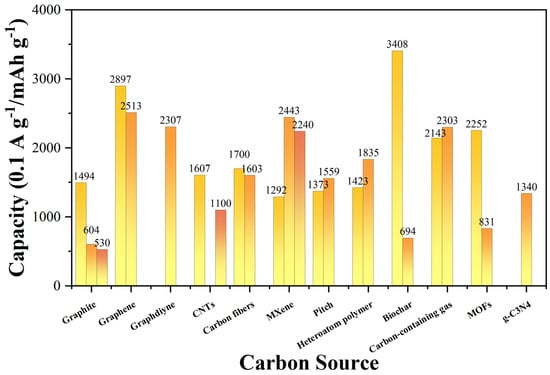
Figure 8.
Comparison of electrochemical performance of Si/C anodes with various carbon sources Reprinted from Refs. [25,26,27,40,41,42,49,50,53,54,55,56,63,64,65,71,74,75,76,77,78,79,80,81].
However, with the rapid development of emerging fields such as electric vehicles and energy storage systems, the performance requirements for silicon–carbon anode materials are also increasingly high. Therefore, future research will focus more on improving the comprehensive performance of materials and reducing production costs. For example, by optimizing the composition and preparation process of materials [76], the capacity and rate capability of materials can be further improved [77]; at the same time, by improving production processes and reducing raw material costs [54], silicon–carbon anode materials can become more competitive in the market.
In addition, it can be clearly seen from the table that current research has begun to pay attention to the thermodynamic conditions and anode chemical reactions of silicon–carbon anode materials. This is of crucial importance for deeply understanding the electrochemical performance and reaction mechanism of the materials. Future research will focus more on in-depth exploration and research in this field in order to provide more solid theoretical support and rich experimental evidence for the performance improvement of silicon–carbon anode materials and the development of new materials.
4. Conclusions and Future Prospective
In the development of Si/C composite materials, the selection of carbon sources is of paramount importance, necessitating comprehensive consideration of electrochemical performance, mechanical strength, thermal stability, cost-effectiveness, and sustainability. The ideal carbon source should facilitate stable charge transport and enhance the properties of the composite material, while simultaneously reducing costs and ensuring environmental compliance. Furthermore, the compatibilities between the carbon source and silicon, synthetic conditions, and large-scale production capabilities are also crucial for market application.
4.1. Conclusions
Graphite, with its stable physicochemical properties, low cost, and good electrical and thermal conductivity, found widespread application in the electronic and electrical fields [82]. However, its insufficient mechanical strength limits its development in high-end applications [83]. Graphene, as one of the thinnest and most robust materials, brings innovation to the manufacturing of transparent electronics and ultra-micro transistors due to its high transparency and excellent electrical conductivity. Nevertheless, high production costs and immature large-scale production techniques pose major obstacles to its market commercialization. Graphdiyne, on the other hand, exhibits tremendous potential in the superconducting, electronic, and energy fields, owing to its unique electronic structure and semiconducting properties [84], yet complex preparation processes and cost issues similarly hinder its commercialization progress.
The growth of CNTs and carbon fibers necessitates high-temperature conditions, posing challenges for the in situ construction of CNTs or carbon fibers–Si composites. The carbonization process imparts a certain degree of elasticity to asphalt and polymers that effectively limits the volume expansion of Si [85]. However, it should be noted that these materials possess certain toxicity and carcinogenic risk. Biomass-derived carbon boasts advantages in terms of low cost, environmental friendliness, and renewability. However, it requires improvements in performance stability and process complexity, with cost control and technological maturity being crucial for its market commercialization. Carbon materials derived from carbon-containing gases are abundant in raw materials and possess mature preparation processes, but their high costs and potential environmental pollution risks pose obstacles to their promotion. MOFs exhibit unique advantages in performance optimization due to their diverse structures and high specific surface areas. Nevertheless, issues related to preparation complexity and stability limit their widespread applications, with technological maturity and cost-effectiveness balance representing core challenges for market commercialization. C3N4 attracts attention due to its high hardness and excellent electrical conductivity, but the main problems currently faced are difficulties in preparation and high costs. Breakthroughs in technological bottlenecks and the expansion of market demand are key pathways for its market commercialization.
4.2. Future Prospective
Presently, biomass, petroleum-derived materials, and polymers serve as prevalent carbon matrices in the manufacture of commercial Si/C materials. These matrices undergo physical or chemical activation to acquire a well-defined porous structure, subsequently welcoming the deposition of silicon through the CVD process to forge Si/C anode materials. The CVD approach to synthesizing Si/C composite anode materials stands out as a promising route for the large-scale production of high-capacity Si/C anodes, owing to its swift production cycle, straightforward operation, and theoretically minimal cost. Consequently, it garnered substantial interest from both academic circles and industries. Notably, Group 14 in the United States cemented its industry footprint in Si/C anode batteries by unveiling a novel generation of vapor-deposited Si/C materials. However, the fabrication of Si/C anode materials via the CVD method is not devoid of challenges. During the silane decomposition phase, the growth mechanism of Si atoms can be bifurcated into homogeneous and heterogeneous nucleation. Homogeneous nucleation of Si atoms results in the emergence of a certain proportion of free Si nanoparticles, ultimately degrading the electrochemical performance. Hence, addressing the pressing need to foster heterogeneous nucleation during homogeneous nucleation of Si atoms is paramount. As for carbon deposition, maintaining the integrity of the carbon coating is crucial to hinder persistent reactions between Si and the electrolyte. In the scenario of simultaneous Si and carbon deposition, the specific capacity of existing materials remains comparatively low, suggesting an ample scope for enhancement.
Author Contributions
Conceptualization, X.S. and Z.M.; methodology, B.J. and X.S.; validation, L.L. (Liying Liang), Q.D. and B.L.; investigation, B.J., X.S. and Z.M.; resources, L.L. (Liying Liang), Q.D., L.L. (Liwei Liao), B.L. and J.D.; data curation, J.D.; data curation, B.J and Z.M.; writing—original draft preparation, B.J.; writing—review and editing, L.L.; visualization, B.L.; supervision, Q.D., L.L. (Liying Liang) and J.D.; project administration, J.D.; funding acquisition, J.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by Guangxi Science and Technology Major Program (grant NO.: AA24206029, AA24206037), Project for Enhancing Young and Middle-aged Teacher’s Research Basis Ability in Colleges of Guangxi, (Three-dimensional flexible structure design and electrochemical properties of carbon nanotube reinforced silicon–carbon anode materials: 2024KY0182), Introduction Talent Research Project (2022KJQD30, 2021KJQD30).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yang, Y.; Dong, R.; Cheng, H.; Wang, L.; Tu, J.; Zhang, S.; Zhao, S.; Zhang, B.; Pan, H.; Lu, Y. 2D Layered Materials for Fast-Charging Lithium-Ion Battery Anodes. Small 2023, 19, e2301574. [Google Scholar] [CrossRef]
- Chen, H.; Ke, G.; Wu, X.; Li, W.; Mi, H.; Li, Y.; Sun, L.; Zhang, Q.; He, C.; Ren, X. Carbon nanotubes coupled with layered graphite to support SnTe nanodots as high-rate and ultra-stable lithium-ion battery anodes. Nanoscale 2021, 13, 3782–3789. [Google Scholar] [CrossRef] [PubMed]
- Muraleedharan, P.M.; Kalidas, N.; Zhao, X.; Lehto, V.P. Biomass-Based Silicon and Carbon for Lithium-Ion Battery Anodes. Front. Chem. 2022, 10, 882081. [Google Scholar] [CrossRef]
- Zhang, T.; Li, C.; Wang, F.; Noori, A.; Mousavi, M.F.; Xia, X.; Zhang, Y. Recent Advances in Carbon Anodes for Sodium-Ion Batteries. Chem. Rec. 2022, 22, e202200083. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Ji, S.; Cho, H.; Park, J. Highly Flexible Triboelectric Nanogenerator Using Porous Carbon Nanotube Composites. Polymers 2023, 15, 1135. [Google Scholar] [CrossRef]
- Shi, Q.; Cheng, Y.; Wang, J.; Zhou, J.; Ta, H.Q.; Lian, X.; Kurtyka, K.; Trzebicka, B.; Gemming, T.; Rummeli, M.H. Strain Regulating and Kinetics Accelerating of Micro-Sized Silicon Anodes via Dual-Size Hollow Graphitic Carbons Conductive Additives. Small 2023, 19, e2205284. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Feng, X.; Zheng, R.; Lv, Y.; Wang, Z.; Zhao, Y.; Shi, L.; Yuan, S. Binary Network of Conductive Elastic Polymer Constraining Nanosilicon for a High-Performance Lithium-Ion Battery. ACS Nano 2021, 15, 14570–14579. [Google Scholar] [CrossRef]
- Tzeng, Y.; Jhan, C.Y.; Wu, Y.C.; Chen, G.Y.; Chiu, K.M.; Guu, S.Y. High-ICE and High-Capacity Retention Silicon-Based Anode for Lithium-Ion Battery. Nanomaterials 2022, 12, 1387. [Google Scholar] [CrossRef]
- Branchi, M.; Maresca, G.; Tsurumaki, A.; Suzuki, N.; Croce, F.; Panero, S.; Voje, J.; Aihara, Y.; Navarra, M.A. Silicon-Based Composite Anodes for All-Solid-State Lithium-Ion Batteries Conceived by a Mixture Design Approach. ChemSusChem 2023, 16, e202202235. [Google Scholar] [CrossRef]
- Wu, S.C.; Lin, C.W.; Chang, P.C.; Yang, T.Y.; Tang, S.Y.; Wu, D.C.; Liao, C.R.; Wang, Y.C.; Lee, L.; Yu, Y.J.; et al. Ecofriendly Synthesis of Waste-Tire-Derived Graphite Nanoflakes by a Low-Temperature Electrochemical Graphitization Process toward a Silicon-Based Anode with a High-Performance Lithium-Ion Battery. ACS Appl. Mater. Interfaces 2023, 15, 15279–15289. [Google Scholar] [CrossRef]
- Zhao, P.; Liu, C.; Liu, B.; Lu, K.; Jing, H.; Xia, X.; Xia, M.; Han, S.; Mandler, D.; Lei, W.; et al. Theoretical mechanisms and experimental validation of hard vs soft carbon coatings for enhanced silicon anode performance. Chem. Eng. J. 2025, 509, 161385. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, K.; Du, X.; Liu, G.; Du, Y.; Li, J. Wood-derived closed pore hard carbon encapsulated micro-sized silicon anode design for long-term practical lithium-ion battery. Chem. Eng. J. 2025, 508, 160846. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, R.; Wang, D.; Li, J.; Yao, X.; Chen, L.; Li, X.; Jiang, Z.; Liu, H.; Hou, Y.; et al. Orchestrating a Controllable Engineering of Dual-Model Carbon Structure in Si/C Anodes. Adv. Funct. Mater. 2025, 2423700. [Google Scholar] [CrossRef]
- Sun, Z.; Yin, Q.; Zhou, S.; Chen, H.; Wen, S.; Yang, H.; Wu, X.; Pan, J.; Han, J.; Yang, H.; et al. High-Performance Silicon Anodes Enabled by Multifunctional Ultrafine Silica Nanoparticle-Embedded Carbon Coatings for Lithium-Ion Batteries. Adv. Energy Mater. 2025, 2500189. [Google Scholar] [CrossRef]
- He, W.; Ji, H.; Platonova, M.; Chometon, R.; Dugas, R.; Tarascon, J.-M. Extending Si/C Anode Longevity through the Electrode Structure and Composition Design for All-Solid-State Batteries. ACS Appl. Mater. Interfaces 2025, 17, 12125–12135. [Google Scholar] [CrossRef]
- Yang, L.; Li, S.; Zhang, Y.; Feng, H.; Li, J.; Zhang, X.; Guan, H.; Kong, L.; Chen, Z. Multi-scale design of silicon/carbon composite anode materials for lithium-ion batteries: A review. J. Energy Chem. 2024, 97, 30–45. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, B.; Zhan, Z.; Hu, M.; Cai, F.; Świerczek, K.; Yang, K.; Ren, J.; Guo, Z.; Wang, Z. Boron-doped three-dimensional porous carbon framework/carbon shell encapsulated silicon composites for high-performance lithium-ion battery anodes. J. Colloid Interface Sci. 2024, 664, 790–800. [Google Scholar] [CrossRef]
- Yu, P.; Li, Z.; Zhang, D.; Xiong, Q.; Yu, J.; Zhi, C. Hierarchical Yolk-Shell Silicon/Carbon Anode Materials Enhanced by Vertical Graphene Sheets for Commercial Lithium-Ion Battery Applications. Adv. Funct. Mater. 2025, 35, 2413081. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, C.; Jiang, Y.; Shang, Z.; Zhu, J.; Zhang, J.; Jiang, M. Robust Nitrogen/Sulfur Co-Doped Carbon Frameworks as Multifunctional Coating Layer on Si Anodes Toward Superior Lithium Storage. Adv. Energy Mater. 2025, 15, 2403086. [Google Scholar] [CrossRef]
- Qin, G.; Jia, Z.; Sun, S.; Wu, H.; Hu, K.; Liu, D.; Gao, Y.; Chen, J. Carbon-Coated Si Nanosheets as Anode Materials for High-Performance Lithium-Ion Batteries. ACS Appl. Nano Mater. 2024, 7, 7595–7604. [Google Scholar] [CrossRef]
- Yu, W.-J.; Liu, C.; Hou, P.-X.; Zhang, L.; Shan, X.-Y.; Li, F.; Cheng, H.-M. Lithiation of Silicon Nanoparticles Confined in Carbon Nanotubes. ACS Nano 2015, 9, 5063–5071. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Li, Y.; Li, X.; Hu, S.; Zhang, X.; Xu, W.; Thevuthasan, S.; Baer, D.R.; Zhang, J.-G.; Liu, J.; et al. In Situ TEM Study of Lithiation Behavior of Silicon Nanoparticles Attached to and Embedded in a Carbon Matrix. ACS Nano 2012, 6, 8439–8447. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Cui, Y. Designing nanostructured Si anodes for high energy lithium ion batteries. Nano Today 2012, 7, 414–429. [Google Scholar] [CrossRef]
- Park, C.-M.; Kim, J.-H.; Kim, H.; Sohn, H.-J. Li-alloy based anode materials for Li secondary batteries. Chem. Soc. Rev. 2010, 39, 3115–3141. [Google Scholar] [CrossRef]
- Yu, P.; Li, Z.; Han, M.; Yu, J. Growth of Vertical Graphene Sheets on Silicon Nanoparticles Well-Dispersed on Graphite Particles for High-Performance Lithium-Ion Battery Anode. Small 2023, 20, 2307494. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, Y.; Wang, D.; Zhang, R.; Fan, H.; Feng, L.; Wen, G.; Qin, L.-C. A silicon/carbon/reduced-graphene composite of honeycomb structure for high-performance lithium-ion batteries. J. Alloys Compd. 2023, 944, 169185. [Google Scholar] [CrossRef]
- Li, L.; Zuo, Z.; Shang, H.; Wang, F.; Li, Y. In-situ constructing 3D graphdiyne as all-carbon binder for high-performance silicon anode. Nano Energy 2018, 53, 135–143. [Google Scholar] [CrossRef]
- Luo, P.; Zheng, C.; He, J.; Tu, X.; Sun, W.; Pan, H.; Zhou, Y.; Rui, X.; Zhang, B.; Huang, K. Structural Engineering in Graphite-Based Metal-Ion Batteries. Adv. Funct. Mater. 2022, 32, 2107277. [Google Scholar] [CrossRef]
- Chen, M.; Cao, W.; Wang, L.; Ma, X.; Han, K. Chessboard-Like Silicon/Graphite Anodes with High Cycling Stability toward Practical Lithium-Ion Batteries. ACS Appl. Energy Mater. 2021, 4, 775–783. [Google Scholar] [CrossRef]
- Müller, J.; Abdollahifar, M.; Doose, S.; Michalowski, P.; Wu, N.-L.; Kwade, A. Effects of carbon coating on calendered nano-silicon graphite composite anodes of LiB. J. Power Sources 2022, 548, 232000. [Google Scholar] [CrossRef]
- Sun, H.; Kong, X.; Park, H.; Liu, F.; Lee, Z.; Ding, F. Spiral Growth of Adlayer Graphene. Adv. Mater. 2022, 34, 2107587. [Google Scholar] [CrossRef]
- Xu, J.; Yin, Q.; Li, X.; Tan, X.; Liu, Q.; Lu, X.; Cao, B.; Yuan, X.; Li, Y.; Shen, L.; et al. Spheres of Graphene and Carbon Nanotubes Embedding Silicon as Mechanically Resilient Anodes for Lithium-Ion Batteries. Nano Lett. 2022, 22, 3054–3061. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cheng, Y.; Song, J.; Zhang, Y.; Shi, Q.; Wang, J.; Tian, F.; Yuan, S.; Su, Z.; Zhou, C.; et al. Functionalization-assistant ball milling towards Si/graphene anodes in high performance Li-ion batteries. Carbon 2021, 181, 300–309. [Google Scholar] [CrossRef]
- Fang, R.; Miao, C.; Mou, H.; Xiao, W. Facile synthesis of Si@TiO2@rGO composite with sandwich-like nanostructure as superior performance anodes for lithium ion batteries. J. Alloys Compd. 2020, 818, 152884. [Google Scholar] [CrossRef]
- Han, M.; Mu, Y.; Yuan, F.; Liang, J.; Jiang, T.; Bai, X.; Yu, J. Vertical graphene growth on uniformly dispersed sub-nanoscale SiOx/N-doped carbon composite microspheres with a 3D conductive network and an ultra-low volume deformation for fast and stable lithium-ion storage. J. Mater. Chem. A 2020, 8, 3822–3833. [Google Scholar] [CrossRef]
- Li, Y.; Yan, K.; Lee, H.-W.; Lu, Z.; Liu, N.; Cui, Y. Growth of conformal graphene cages on micrometre-sized silicon particles as stable battery anodes. Nat. Energy 2016, 1, 15029. [Google Scholar] [CrossRef]
- Jia, Z.; Zuo, Z.; Yi, Y.; Liu, H.; Li, D.; Li, Y.; Li, Y. Low temperature, atmospheric pressure for synthesis of a new carbon Ene-yne and application in Li storage. Nano Energy 2017, 33, 343–349. [Google Scholar] [CrossRef]
- Jia, Z.; Li, Y.; Zuo, Z.; Liu, H.; Huang, C.; Li, Y. Synthesis and Properties of 2D Carbon—Graphdiyne. Acc. Chem. Res. 2017, 50, 2470–2478. [Google Scholar] [CrossRef]
- Nazarian-Samani, M.; Nazarian-Samani, M.; Haghighat-Shishavan, S.; Kim, K.-B. Efficient stress alleviation and interface regulation in Cu4SiP8-CNT hybrid for ultra-durable Li and Na storage. Nano Energy 2021, 86, 106134. [Google Scholar] [CrossRef]
- Fan, X.; Cai, T.; Wang, S.; Yang, Z.; Zhang, W. Carbon Nanotube-Reinforced Dual Carbon Stress-Buffering for Highly Stable Silicon Anode Material in Lithium-Ion Battery. Small 2023, 19, 2300431. [Google Scholar] [CrossRef]
- Li, H.; Yao, B.; Li, M.; Zou, X.; Duan, R.; Li, H.; Jiang, Q.; Cao, G.; Li, J.; Yan, H.; et al. Three-Dimensional Carbon Nanotubes Buffering Interfacial Stress of the Silicon/Carbon Anodes for Long-Cycle Lithium Storage. ACS Appl. Mater. Interfaces 2024, 16, 53665–53674. [Google Scholar] [PubMed]
- Pei, Y.; Wang, Y.; Chang, A.-Y.; Liao, Y.; Zhang, S.; Wen, X.; Wang, S. Nanofiber-in-microfiber carbon/silicon composite anode with high silicon content for lithium-ion batteries. Carbon 2023, 203, 436–444. [Google Scholar] [CrossRef]
- Chen, S.; Qiu, L.; Cheng, H.-M. Carbon-Based Fibers for Advanced Electrochemical Energy Storage Devices. Chem. Rev. 2020, 120, 2811–2878. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-W.; Shim, H.-W.; Kim, J.-C.; Kim, D.-W. Uniform Si nanoparticle-embedded nitrogen-doped carbon nanofiber electrodes for lithium ion batteries. J. Alloys Compd. 2017, 728, 490–496. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef]
- Yan, Y.; Zhao, X.; Dou, H.; Wei, J.; Sun, Z.; He, Y.-S.; Dong, Q.; Xu, H.; Yang, X. MXene Frameworks Promote the Growth and Stability of LiF-Rich Solid–Electrolyte Interphases on Silicon Nanoparticle Bundles. ACS Appl. Mater. Interfaces 2020, 12, 18541–18550. [Google Scholar] [CrossRef]
- Li, X.; Huang, Z.; Shuck, C.E.; Liang, G.; Gogotsi, Y.; Zhi, C. MXene chemistry, electrochemistry and energy storage applications. Nat. Rev. Chem. 2022, 6, 389–404. [Google Scholar] [CrossRef]
- An, Y.; Tian, Y.; Zhang, Y.; Wei, C.; Tan, L.; Zhang, C.; Cui, N.; Xiong, S.; Feng, J.; Qian, Y. Two-Dimensional Silicon/Carbon from Commercial Alloy and CO2 for Lithium Storage and Flexible Ti3C2Tx MXene-Based Lithium–Metal Batteries. ACS Nano 2020, 14, 17574–17588. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, Y.; Cheng, Z.; Wang, X.; Huang, N.; Zhang, H. Ti3C2Tx MXene wrapped, carbon-coated porous Si sheets for improved lithium storage performance. Chin. Chem. Lett. 2024, 35, 108923. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, J.; Liu, J.; Feng, S.; Li, C.; Marsili, E.; Zhang, X. Silicon Nanospheres Supported on Conductive MXene Nanosheets as Anodes for Lithium-Ion Batteries. ACS Appl. Energy Mater. 2022, 6, 160–169. [Google Scholar] [CrossRef]
- Choi, S.-H.; Nam, G.; Chae, S.; Kim, D.; Kim, N.; Kim, W.S.; Ma, J.; Sung, J.; Han, S.M.; Ko, M.; et al. Robust Pitch on Silicon Nanolayer–Embedded Graphite for Suppressing Undesirable Volume Expansion. Adv. Energy Mater. 2019, 9, 1803121. [Google Scholar] [CrossRef]
- Zhang, Z.; Xi, F.; Ma, Q.; Wan, X.; Li, S.; Ma, W.; Chen, X.; Chen, Z.; Deng, R.; Ji, J.; et al. A nanosilver-actuated high-performance porous silicon anode from recycling of silicon waste. Mater. Today Nano 2022, 17, 100162. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, W.; Wang, D.; Wang, J.; Wang, C.; Xiong, Z.; Chen, F.-R.; Dong, H.; Xu, B.; Yan, X. Facile preparation of silicon/carbon composite with porous architecture for advanced lithium-ion battery anode. J. Electroanal. Chem. 2023, 937, 117427. [Google Scholar] [CrossRef]
- Nyamtara, K.J.; Song, J.K.; Karima, N.C.; Kim, S.H.; Nguyen, M.C.; Duong, T.P.M.; Lee, K.J.; Ahn, W. Two step pyrolysis synthesis method of graphite-enhanced Nano-Si/Pitch composite as long cycle life anode for lithium-ion batteries. J. Alloys Compd. 2024, 976, 173229. [Google Scholar] [CrossRef]
- Shao, D.; Tang, D.; Yang, J.; Li, Y.; Zhang, L. Nano-structured composite of Si/(S-doped-carbon nanowire network) as anode material for lithium-ion batteries. J. Power Sources 2015, 297, 344–350. [Google Scholar] [CrossRef]
- Cao, J.; Gao, J.; Wang, K.; Wu, Z.; Zhu, X.; Li, H.; Ling, M.; Liang, C.; Chen, J. Constructing globally consecutive 3D conductive network using P-doped biochar cotton fiber for superior performance of silicon-based anodes. J. Mater. Sci. Technol. 2024, 173, 181–191. [Google Scholar] [CrossRef]
- Shi, J.; Jiang, X.; Sun, J.; Ban, B.; Li, J.; Chen, J. A surface-engineering-assisted method to synthesize recycled silicon-based anodes with a uniform carbon shell-protective layer for lithium-ion batteries. J. Colloid Interface Sci. 2021, 588, 737–748. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and Its Derivative Materials: Synthesis and Promising Applications in Energy, Environmental, and Biomedical Fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef]
- Zhou, R.; Guo, H.; Yang, Y.; Wang, Z.; Li, X.; Zhou, Y. N-doped carbon layer derived from polydopamine to improve the electrochemical performance of spray-dried Si/graphite composite anode material for lithium ion batteries. J. Alloys Compd. 2016, 689, 130–137. [Google Scholar] [CrossRef]
- Tang, X.; Liu, D.; Wang, Y.-J.; Cui, L.; Ignaszak, A.; Yu, Y.; Zhang, J. Research advances in biomass-derived nanostructured carbons and their composite materials for electrochemical energy technologies. Prog. Mater. Sci. 2021, 118, 100770. [Google Scholar] [CrossRef]
- Wang, F.; Ouyang, D.; Zhou, Z.; Page, S.J.; Liu, D.; Zhao, X. Lignocellulosic biomass as sustainable feedstock and materials for power generation and energy storage. J. Energy Chem. 2021, 57, 247–280. [Google Scholar] [CrossRef]
- Lin, L.; Deng, B.; Sun, J.; Peng, H.; Liu, Z. Bridging the Gap between Reality and Ideal in Chemical Vapor Deposition Growth of Graphene. Chem. Rev. 2018, 118, 9281–9343. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Liu, S.; Chen, J.; Yu, Y.; Shang, Q.; Fakudze, S.; Liu, C.; Zhou, P.; Chu, Q. One-step synthesis of interface-coupled Si@SiOX@C from whole rice-husks for high-performance lithium storage. Electrochim. Acta 2022, 402, 139556. [Google Scholar] [CrossRef]
- Li, Y.; Tian, Y.; Duan, J.; Xiao, P.; Zhou, P.; Pang, L.; Li, Y. Multi-functional double carbon shells coated boron-doped porous Si as anode materials for high-performance lithium-ion batteries. Electrochim. Acta 2023, 462, 142712. [Google Scholar] [CrossRef]
- Li, J.; Rui, B.; Zhao, J.; He, R.; Liu, S.; Shi, W.; Wang, X.; Chang, L.; Cheng, Y.; Nie, P. Silicon particles confined in carbon nanotubes anode materials by green utilization of carbon dioxide in lithium-ion battery. J. Power Sources 2024, 597, 234131. [Google Scholar] [CrossRef]
- Mateev, V.; Ivanov, G.; Marinova, I. Modeling of Thermally Activated Fe2O3/Li2CO3 Electrochemical Cell. In Proceedings of the 2022 22nd International Symposium on Electrical Apparatus and Technologies (SIELA), Bourgas, Bulgaria, 1–4 June 2022; pp. 1–4. [Google Scholar]
- Chen, M.; Duan, P.; Zhong, Y.; Wu, Z.; Zhang, Z.; Wang, Y.; Guo, X.; Wang, X. Constructing a Sheet-Stacked Si/C Composite by Recycling Photovoltaic Si Waste for Li-Ion Batteries. Ind. Eng. Chem. Res. 2022, 61, 2809–2816. [Google Scholar] [CrossRef]
- Sui, X.; Huang, X.; Pu, H.; Wang, Y.; Chen, J. Tailoring MOF-derived porous carbon nanorods confined red phosphorous for superior potassium-ion storage. Nano Energy 2021, 83, 105797. [Google Scholar] [CrossRef]
- Wang, K.; Pei, S.; He, Z.; Huang, L.-a.; Zhu, S.; Guo, J.; Shao, H.; Wang, J. Synthesis of a novel porous silicon microsphere@carbon core-shell composite via in situ MOF coating for lithium ion battery anodes. Chem. Eng. J. 2019, 356, 272–281. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Yu, J.; Yuan, M.; Tan, Q.; Zhong, Z.; Su, F. High-performance Si-Containing anode materials in lithium-ion batteries: A superstructure of Si@Co–NC composite works effectively. Green Energy Environ. 2022, 7, 116–129. [Google Scholar] [CrossRef]
- Niu, X.; Wang, C.; Zhang, W.; Wang, D.; Shi, H.; Yu, L.; Wang, C.; Xiong, Z.; Ji, Z.; Gao, Y.; et al. A bimetallic ZIFs-triggered hierarchical carbon structure for stabilized silicon anode. Electrochim. Acta 2022, 403, 139671. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Ma, T.; Zhang, Y.; Huang, H. 2D Graphitic Carbon Nitride for Energy Conversion and Storage. Adv. Funct. Mater. 2021, 31, 2102540. [Google Scholar] [CrossRef]
- Wan, L.; Tang, Y.; Chen, L.; Wang, K.; Zhang, J.; Gao, Y.; Lee, J.Y.; Lu, T.; Xu, X.; Li, J.; et al. In-situ construction of g-C3N4/2CTx hybrid for superior lithium storage with significantly improved Coulombic efficiency and cycling stability. Chem. Eng. J. 2021, 410, 128349. [Google Scholar] [CrossRef]
- Zhong, Y.; Yu, B.; Xu, L.; Huang, Y.; Zheng, Y.; Li, J.; Huang, Z. g-C3N4 integrated silicon nanoparticle composite for high-performance Li-ion battery anodes. J. Mater. Sci. 2024, 59, 19915–19933. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, D.; Yuan, R.; Li, X.; Li, J.; Jiang, Z.; Li, A.; Chen, X.; Song, H. Simple Construction of Multistage Stable Silicon–Graphite Hybrid Granules for Lithium-Ion Batteries. Small 2023, 19, 2207167. [Google Scholar] [CrossRef]
- Fang, Y.; Lu, D.; Xie, J.; Guo, H.; Tian, J.; Sun, X.; Wang, D. Silicon-graphite composite anode materials with assembly structure by boron oxide auxiliary for high-performance lithium-ion batteries. Electrochim. Acta 2025, 146075. [Google Scholar] [CrossRef]
- Shi, J.; Li, R.; Li, J.; Liu, G. Surface-modification-assisted synthesis of in-situ graphene-doped carbon substrate coated silicon nanoparticles for boosting lithium storage performance. Powder Technol. 2023, 430, 118988. [Google Scholar] [CrossRef]
- Guo, X.; Yang, Z.; Wang, W.; Zhang, Y.; Yu, N.; Lu, C. Silicon/carbon nanotubes anode for lithium-ion batteries: Synthesis, interface and electrochemical performance. Surf. Interfaces 2024, 48, 104223. [Google Scholar] [CrossRef]
- Zhang, M.; Bai, N.; Lin, W.; Wang, H.; Li, J.; Ma, L.; Wang, X.; Zhang, D.; Cao, Z. Carbon-encapsulated silicon ordered nanofiber membranes as high-performance anode material for lithium-ion batteries. J. Alloys Compd. 2025, 1010, 177012. [Google Scholar] [CrossRef]
- Lin, J.; Wu, C.; Ling, W.; Liu, L.; Yang, X.; Zhou, N. Coil-like Si-based composites prepared by encapsulating self-healing liquid metal-coated nanosilicon via flexible MXene for high-performance lithium-ion battery anodes. J. Power Sources 2025, 629, 236067. [Google Scholar] [CrossRef]
- Huang, W.; Gao, J.; Miao, L.; Zhou, J.; Yang, G.; Liu, W.; Jiang, Y.; Qin, H.; Zhang, Z.; Lei, X.; et al. Si@Nitrogen-Doped Carbon Nanoparticles for Lithium-Ion Battery Anodes. ACS Appl. Nano Mater. 2024, 7, 10350–10360. [Google Scholar] [CrossRef]
- An, Y.; Tian, Y.; Liu, C.; Xiong, S.; Feng, J.; Qian, Y. One-Step, Vacuum-Assisted Construction of Micrometer-Sized Nanoporous Silicon Confined by Uniform Two-Dimensional N-Doped Carbon toward Advanced Li Ion and MXene-Based Li Metal Batteries. ACS Nano 2022, 16, 4560–4577. [Google Scholar] [CrossRef] [PubMed]
- Chae, S.; Kim, N.; Ma, J.; Cho, J.; Ko, M. One-to-One Comparison of Graphite-Blended Negative Electrodes Using Silicon Nanolayer-Embedded Graphite versus Commercial Benchmarking Materials for High-Energy Lithium-Ion Batteries. Adv. Energy Mater. 2017, 7, 1700071. [Google Scholar] [CrossRef]
- Zhu, S.; Zhou, J.; Guan, Y.; Cai, W.; Zhao, Y.; Zhu, Y.; Zhu, L.; Zhu, Y.; Qian, Y. Hierarchical Graphene-Scaffolded Silicon/Graphite Composites as High Performance Anodes for Lithium-Ion Batteries. Small 2018, 14, 1802457. [Google Scholar] [CrossRef] [PubMed]
- Abouali, S.; Garakani, M.A.; Silvestri, L.; Venezia, E.; Marasco, L.; Brescia, R.; Ansaldo, A.; Serri, M.; Panda, J.K.; Pugliese, G.; et al. From scaled-up production of silicon-graphene nanocomposite to the realization of an ultra-stable full-cell Li-ion battery. 2D Mater. 2021, 8, 035014. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).