Influence of Zr Addition on Structure and Performance of Rare Earth Mg-Based Alloys as Anodes in Ni/MH Battery
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials Preparation
2.2. Materials Characterization
2.3. Electrochemical Tests
3. Results and Discussion

| Samples | Phases | Space group (no.) | Phase abundance (wt%) | Lattice parameters (Å) | Cell volume (Å3) | Parameters of fit | |
|---|---|---|---|---|---|---|---|
| a | c | ||||||
| x = 0.05 | LaNi5 | P6/mmm (191) | 65.5 | 5.0301(5) | 3.9906(5) | 87.44(2) | Rwp = 2.7; Rp = 2.9 |
| LaNi3 | R-3m (166) | 24.1 | 5.0388(1) | 24.391(1) | 536.3(3) | ||
| ZrNi3 | P63/mmc (194) | 10.4 | 5.2805(3) | 4.2780(3) | 103.3(1) | ||
| x = 0.1 | LaNi5 | P6/mmm (191) | 49.1 | 5.0255(6) | 3.9862(5) | 87.19(2) | Rwp = 3.0; Rp = 3.4 |
| LaNi3 | R-3m (166) | 35.3 | 5.0484(1) | 24.601(1) | 543.0(3) | ||
| ZrNi3 | P63/mmc (194) | 15.6 | 5.3003(4) | 4.2805(6) | 104.1(2) | ||
| x = 0.2 | LaNi5 | P6/mmm (191) | 33.2 | 5.0232(6) | 3.9832(4) | 87.04(2) | Rwp = 4.8; Rp = 5.0 |
| LaNi3 | R-3m (166) | 40.6 | 5.0645(2) | 24.824(1) | 551.4(5) | ||
| ZrNi3 | P63/mmc (194) | 26.2 | 5.3166(3) | 4.2858(6) | 104.9(20) | ||
| x = 0.3 | LaNi5 | P6/mmm (191) | 23.5 | 5.0300(1) | 3.9872(8) | 87.36(4) | Rwp = 3.9; Rp = 4.3 |
| LaNi3 | R-3m (166) | 47.8 | 5.0797(2) | 25.087(1) | 560.6(4) | ||
| ZrNi3 | P63/mmc (194) | 28.7 | 5.4320(1) | 3.9806(1) | 101.7(5) | ||
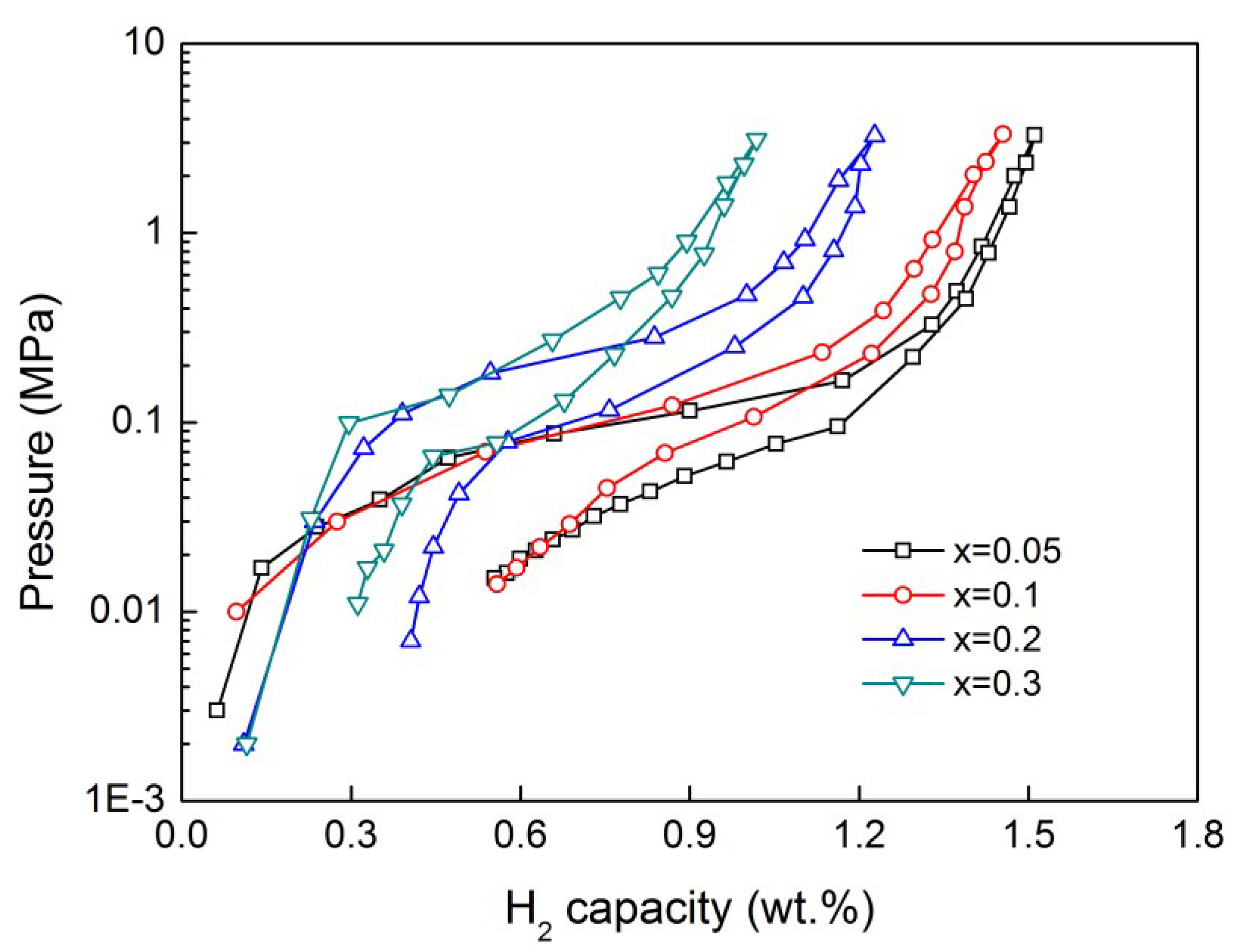

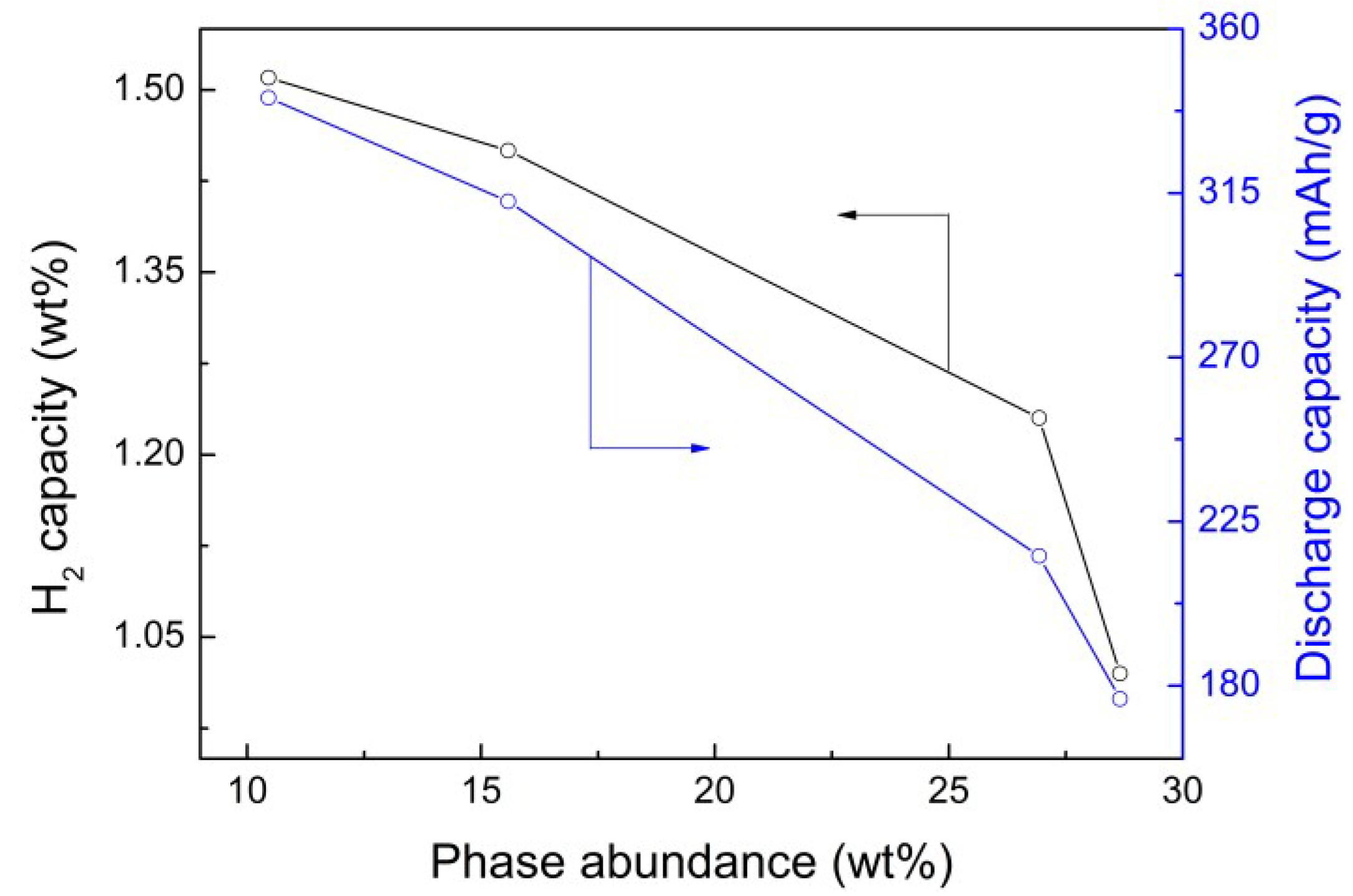
| Samples | H2 (wt.%) | Cmax (mAh/g) | Na | C60/Cmax (%) | HRD800 (%) |
|---|---|---|---|---|---|
| x = 0.05 | 1.51 ± 0.02 | 341.1 ± 0.5 | 1 | 53.9 ± 0.5 | 94.2 ± 0.8 |
| x = 0.1 | 1.45 ± 0.01 | 312.8 ± 0.8 | 1 | 55.2 ± 0.3 | 90.6 ± 0.6 |
| x = 0.2 | 1.23 ± 0.03 | 215.5 ± 0.6 | 3 | 60.8 ± 0.6 | 82.5 ± 0.3 |
| x = 0.3 | 1.02 ± 0.03 | 176.4 ± 0.4 | 2 | 58.4 ± 0.7 | 78.9 ± 0.4 |


| Samples | Polarization resistance, Rp (mΩ) | Exchange current density, I0 (mA/g) | Limiting current density, IL (mA/g) | Hydrogen diffusion coefficient, D (cm2/s) |
|---|---|---|---|---|
| x = 0.05 | 132.9 ± 1.0 | 196.5 ± 0.6 | 3432.1 ± 8.1 | (2.85 ± 0.04) × 10−10 |
| x = 0.1 | 141.7 ± 0.8 | 184.3 ± 0.8 | 2871.1 ± 5.4 | (2.62 ± 0.02) × 10−10 |
| x = 0.2 | 148.7 ± 0.4 | 175.6 ± 0.7 | 2802.4 ± 3.4 | (2.60 ± 0.01) × 10−10 |
| x = 0.3 | 80.4 ± 0.7 | 324.7 ± 0.9 | 2489.4 ± 9.4 | (2.39 ± 0.03) × 10−10 |

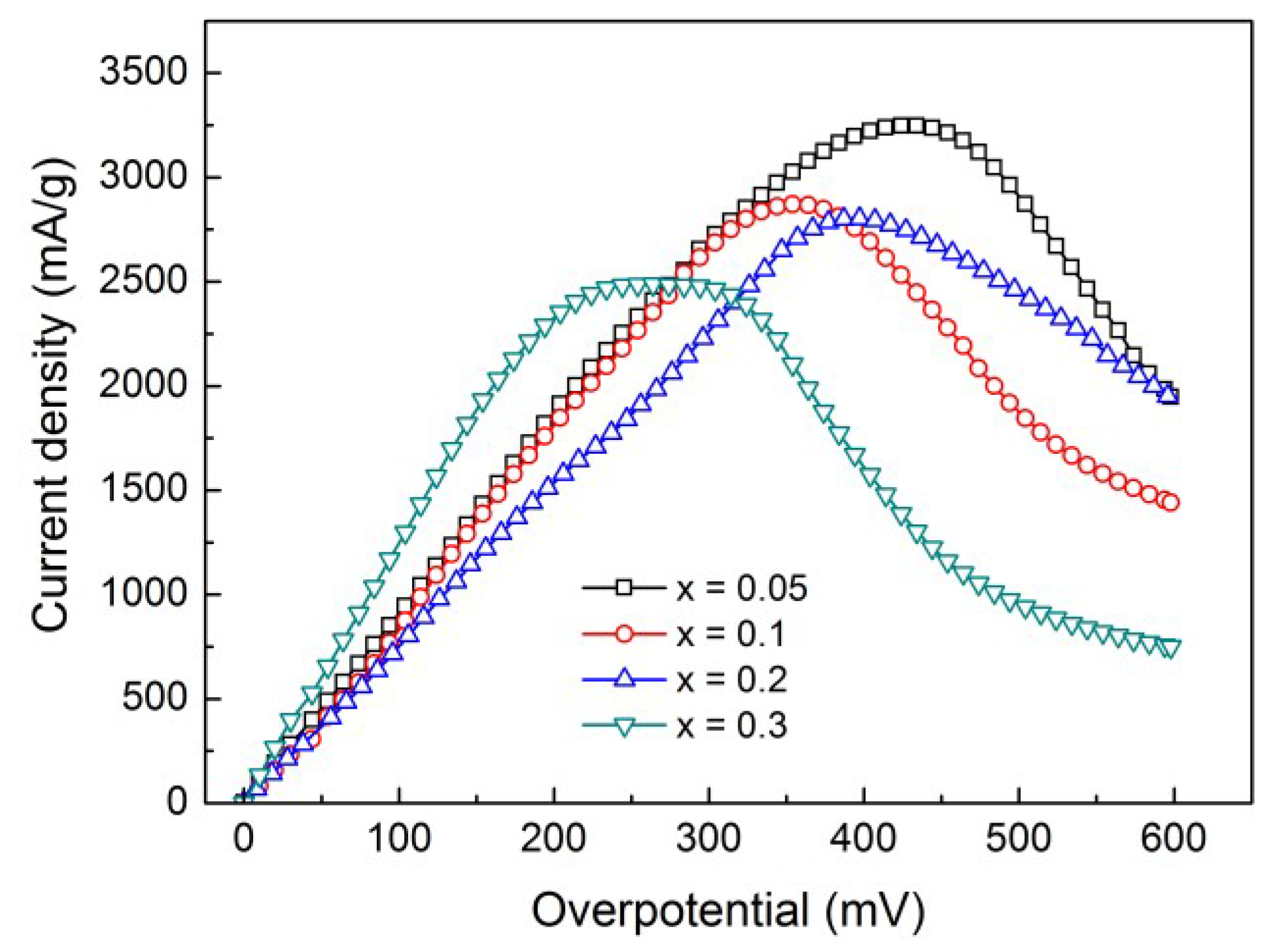
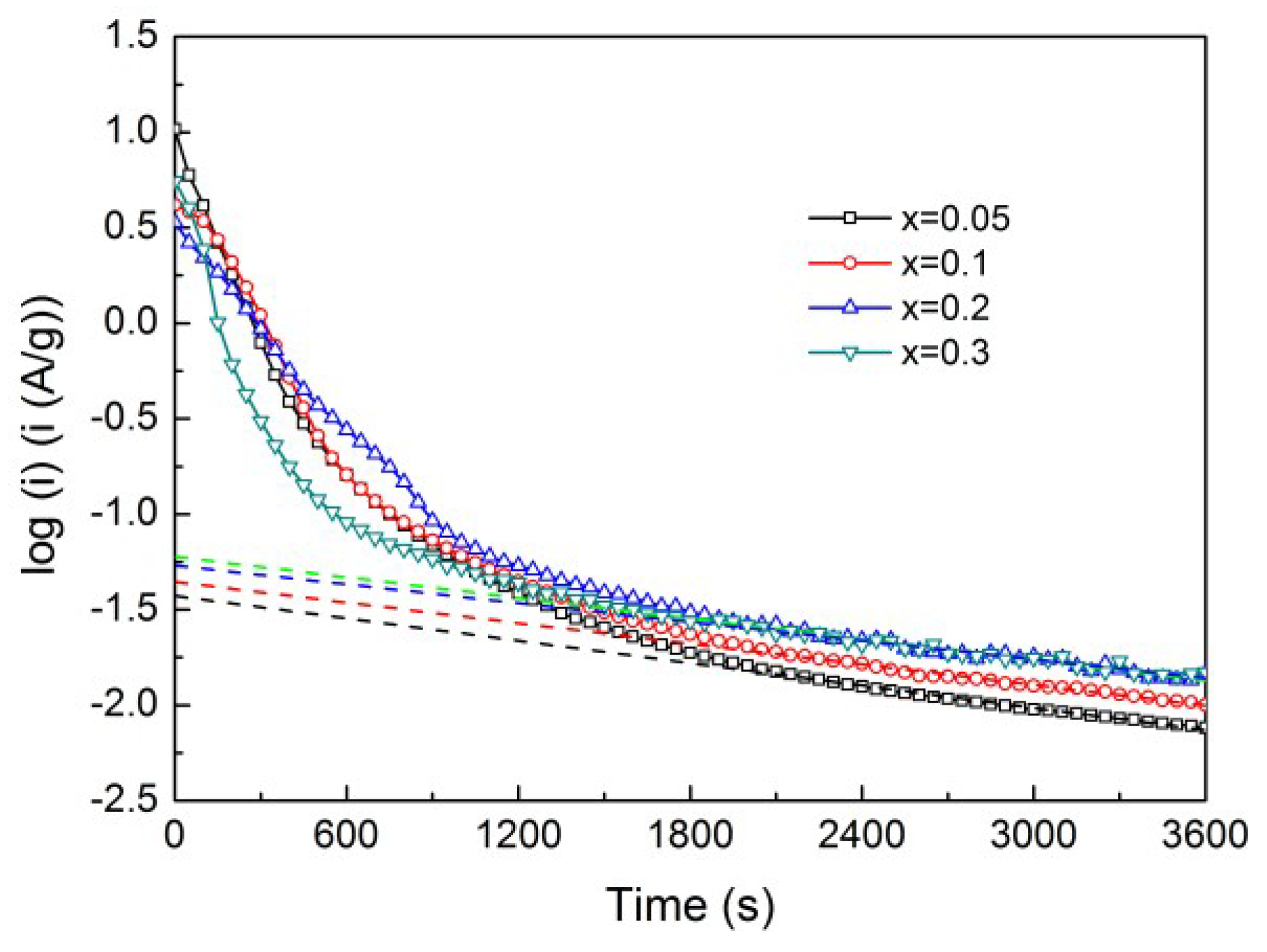
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Liu, Y.F.; Pan, H.G.; Gao, M.X.; Wang, Q.D. Advanced hydrogen storage alloys for Ni/MH rechargeable batteries. J. Mater. Chem. 2011, 21, 4743–4755. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Ma, L.Q. Recent progress in hydrogen storage alloys for nickel/metal hydride secondary batteries. Int. J. Hydrogen Energy 2009, 34, 4788–4796. [Google Scholar] [CrossRef]
- Li, W.; Du, Y.L. Effects of Y Substitution for La on the microstructure and electrochemical properties of LaNi3.55Mn0.4Al0.3Co0.75 hydrogen storage alloys. Mater. Trans. 2008, 49, 2229–2232. [Google Scholar] [CrossRef]
- Chu, H.L.; Zhang, Y.; Sun, L.X.; Qiu, S.J.; Qi, Y.N.; Xu, F.; Yuan, H.T. Structure and electrochemical properties of composite electrodes synthesized by mechanical milling Ni-free TiMn2-based alloy with La-based alloys. J. Alloys Compd. 2007, 446–447, 614–619. [Google Scholar] [CrossRef]
- Tsukahara, M.; Kamiya, T.; Takahashi, K.; Kawabata, A.; Sakurai, S.; Shi, J.; Takeshita, H.T.; Kuriyama, N.; Sakai, T. Hydrogen storage and electrode properties of V-based solid solution type alloys prepared by a thermic process. J. Electrochem. Soc. 2000, 147, 2941–2944. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, L.X.; Lei, Y.Q.; Wang, Q.D. The effect of partial substitution of Ti with Zr, Cr or V in the Mg35Ti10Ni55 electrode alloy on its electrochemical performance. Electrochim. Acta 2002, 47, 1739–1746. [Google Scholar] [CrossRef]
- Iwakura, C.; Inoue, H.; Furukawa, N.; Nohara, S. Effects of carbon crystallinity on hydriding-dehydriding and charge-discharge characteristics of MgNi alloy–carbon material composites. Mater. Trans. 2002, 43, 2706–2710. [Google Scholar] [CrossRef]
- Chen, J.; Kuriyama, N.; Takeshita, H.T.; Tanaka, H.; Sakai, T.; Haruta, M. Hydrogen storage alloys with PuNi3-type structure as metal hydride electrodes. Electrochem. Solid–State Lett. 2000, 3, 249–252. [Google Scholar] [CrossRef]
- Qiu, S.J.; Chu, H.L.; Zhang, J.; Zhang, Y.; Sun, L.X.; Xu, F.; Sun, D.L.; Ouyang, L.Z.; Zhu, M.; Grolier, J.P.E.; Frenkel, M. Effect of La partial substitution for Zr on the structural and electrochemical properties of Ti0.17Zr0.08–xLaxV0.35Cr0.1Ni0.3 (x = 0–0.04) electrode alloys. Int. J. Hydrogen Energy 2009, 34, 7246–7252. [Google Scholar] [CrossRef]
- Kadir, K.; Kuriyama, N.; Sakai, T.; Uehara, I.; Eriksson, L. Structural investigation and hydrogen capacity of CaMg2Ni9: A new phase in the AB2C9 system isostructural with LaMg2Ni9. J. Alloys Compd. 1999, 284, 145–154. [Google Scholar] [CrossRef]
- Kadir, K.; Sakai, T.; Uehara, I. Structural investigation and hydrogen storage capacity of LaMg2Ni9 and (La0.65Ca0.35)(Mg1.32Ca0.68)Ni9 of the AB2C9 type structure. J. Alloys Compd. 2000, 302, 112–117. [Google Scholar] [CrossRef]
- Kadir, K.; Sakai, T.; Uehara, I. Synthesis and structure determination of a new series of hydrogen storage alloys; RMg2Ni9 (R=La, Ce, Pr, Nd, Sm and Gd) built from MgNi2 Laves-type layers alternating with AB5 layers. J. Alloys Compd. 1997, 257, 115–121. [Google Scholar] [CrossRef]
- Kadir, K.; Sakai, T.; Uehara, I. Structural investigation and hydrogen capacity of YMg2Ni9 and (Y0.5Ca0.5)(MgCa)Ni9: New phases in the AB2C9 system isostructural with LaMg2Ni9. J. Alloys Compd. 1999, 287, 264–270. [Google Scholar] [CrossRef]
- Kohno, T.; Yoshida, H.; Kawashima, F.; Inaba, T.; Sakai, I.; Yamamoto, M.; Kanda, M. Hydrogen storage properties of new ternary system alloys: La2MgNi9, La5Mg2Ni23, La3MgNi14. J. Alloys Compd. 2000, 311, L5–L7. [Google Scholar]
- Liu, Y.F.; Cao, Y.H.; Huang, L.; Gao, M.X.; Pan, H.G. Rare earth–Mg–Ni-based hydrogen storage alloys as negative electrode materials for Ni/MH batteries. J. Alloys Compd. 2011, 509, 675–686. [Google Scholar] [CrossRef]
- Liu, Y.F.; Pan, H.G.; Gao, M.X.; Zhu, Y.F.; Lei, Y.Q.; Wang, Q.D. The effect of Mn substitution for Ni on the structural and electrochemical properties of La0.7Mg0.3Ni2.55−xCo0.45Mnx hydrogen storage electrode alloys. Int. J. Hydrogen Energy 2004, 29, 297–305. [Google Scholar] [CrossRef]
- Pan, H.G.; Liu, Y.F.; Gao, M.X.; Zhu, Y.F.; Lei, Y.Q.; Wang, Q.D. Electrochemical properties of the La0.7Mg0.3Ni2.65 − xMn0.1Co0.75Alx (x = 0–0.5 ) hydrogen storage alloy electrodes. J. Electrochem. Soc. 2005, 152, A326–A332. [Google Scholar] [CrossRef]
- Wu, J.M.; Li, J.; Zhang, W.P.; Muo, F.J.; Tai, L.C.; Xu, R.G. The study on the hydrogen storage properties of MmNi3.55Co0.75Mn0.7−xAlx compounds. J. Alloys Compd. 1997, 248, 180–184. [Google Scholar] [CrossRef]
- Notten, P.H.L.; Latroche, M.; Percheron-Guegan, A. The Influence of Mn on the crystallography and electrochemistry of nonstoichiometric AB5-type hydride forming compounds. J. Electrochem. Soc. 1999, 146, 3181–3189. [Google Scholar] [CrossRef]
- Chu, H.L.; Zhang, Y.; Qiu, S.J.; Qi, Y.N.; Sun, L.X.; Xu, F.; Wang, Q.; Dong, C. Electrochemical performances of cobalt-free La0.7Mg0.3Ni3.5−x(MnAl2)x (x = 0–0.20) hydrogen storage alloy electrodes. J. Alloys Compd. 2008, 457, 90–96. [Google Scholar] [CrossRef]
- Zhang, Y.; Lei, Y.Q.; Chen, L.X.; Yuan, J.; Zhang, Z.H.; Wang, Q.D. The effect of partial substitution of Zr for Ti on the electrochemical properties and surface passivation film of Mg35Ti10−xZrxNi55 (x = 1, 3, 5, 7, 9) electrode alloys. J. Alloys Compd. 2002, 337, 296–302. [Google Scholar] [CrossRef]
- Han, S.S.; Lee, Y.L.; Goo, N.H.; Jeong, W.T.; Lee, K.S. Improvement of electrode performances of Mg2Ni by mechanical alloying. J. Alloys Compd. 2002, 330, 841–845. [Google Scholar] [CrossRef]
- TOPAS V4: General Profile and Structure Analysis Software for Powder Diffraction Data. Bruker AXS: Karlsruhe, Germany, 2008.
- Msika, E.; Latroche, M.; Cuevas, F.; Percheron-Guégan, A. Zr-substitution in LaNi5-type hydride compound by room temperature ball milling. Mater. Sci. Eng. B 2004, 108, 91–95. [Google Scholar] [CrossRef]
- Pan, H.G.; Yue, Y.J.; Gao, M.X.; Wu, X.F.; Chen, N.; Lei, Y.Q.; Wang, Q.D. The effect of substitution of Zr for La on the electrochemical properties of La0.7−xZrxMg0.3Ni2.45Mn0.1Co0.75Al0.2 hydrogen storage electrode alloys. J. Alloys Compd. 2005, 397, 269–275. [Google Scholar] [CrossRef]
- Chu, H.L.; Zhang, Y.; Sun, L.X.; Tian, Q.F.; Xu, F.; Zhang, T.; Yuan, H.T. Structures and hydrogen storage properties of Mg45M5Co50 (M = Zr, Ni, Al) ternary alloys by mechanical alloying. Int. J. Electrochem. Sci. 2006, 1, 47–54. [Google Scholar]
- Seo, C.Y.; Choi, S.J.; Choi, J.; Park, C.N.; Lee, J.Y. Effect of V and Zr on the electrochemical properties of La-based AB5-type metal hydride electrodes. J. Alloys Compd. 2003, 351, 255–263. [Google Scholar] [CrossRef]
- Pan, H.G.; Liu, Y.F.; Gao, M.X.; Zhu, Y.F.; Lei, Y.Q. The structural and electrochemical properties of La0.7Mg0.3(Ni0.85Co0.15)x (x = 3.0–5.0) hydrogen storage alloys. Int. J. Hydrogen Energy 2003, 28, 1219–1228. [Google Scholar] [CrossRef]
- Chu, H.L.; Qiu, S.J.; Sun, L.X.; Zhang, Y.; Xu, F.; Jiang, T.; Li, W.X.; Zhu, M.; Hu, W.Y. The improved electrochemical properties of novel La–Mg–Ni-based hydrogen storage composites. Electrochim. Acta 2007, 52, 6700–6706. [Google Scholar] [CrossRef]
- Monnier, J.; Chen, H.; Joiret, S.; Bourgon, J.; Latroche, M. Identification of a new pseudo-binary hydroxide during calendar corrosion of (La, Mg)2Ni7-type hydrogen storage alloys for Nickel–Metal Hydride batteries. J. Power Sources 2014, 266, 162–169. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Pan, H.G.; Gao, M.X.; Ma, J.X.; Li, S.Q.; Wang, Q.D. The effect of Zr substitution for Ti on the microstructures and electrochemical properties of electrode alloys Ti1−xZrxV1.6Mn0.32Cr0.48Ni0.6. Int. J. Hydrogen Energy 2002, 27, 287–293. [Google Scholar] [CrossRef]
- Kuriyama, N.; Sakai, T.; Miyamura, H.; Uehara, I.; Ishikawa, H.; Iwasaki, T. Electrochemical impedance spectra and deterioration mechanism of metal hydride electrodes. J. Electrochem. Soc. 1992, 139, L72–L73. [Google Scholar] [CrossRef]
- Ratnakumar, B.V.; Witham, C.; Bowman, R.C., Jr.; Hightower, A.; Fultz, B. Electrochemical studies on LaNi5 − xSnx metal hydride alloys. J. Electrochem. Soc. 1996, 143, 2578–2584. [Google Scholar] [CrossRef]
- Notten, P.H.L.; Hokkeling, P. Double phase hydride forming compounds: A new class of highly electrocatalytic materials. J. Electrochem. Soc. 1991, 138, 1877–1885. [Google Scholar] [CrossRef]
- Niu, H.; Northwood, D.O. Enhanced electrochemical properties of ball-milled Mg2Ni electrodes. Int. J. Hydrogen Energy 2002, 27, 69–77. [Google Scholar] [CrossRef]
- Nishima, T.; Ura, H.; Uchida, I. Determination of chemical diffusion coefficients in metal hydride particles with a microelectrode technique. J. Electrochem. Soc. 1997, 144, 1273–1277. [Google Scholar] [CrossRef]
- Zheng, G.; Popov, B.N.; White, R.E. Electrochemical determination of the diffusion coefficient of hydrogen through an LaNi4.25Al0.75 electrode in alkaline aqueous solution. J. Electrochem. Soc. 1995, 142, 2695–2698. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, S.; Huang, J.; Chu, H.; Zou, Y.; Xiang, C.; Zhang, H.; Xu, F.; Sun, L.; Ouyang, L.; Zhou, H. Influence of Zr Addition on Structure and Performance of Rare Earth Mg-Based Alloys as Anodes in Ni/MH Battery. Metals 2015, 5, 565-577. https://doi.org/10.3390/met5020565
Qiu S, Huang J, Chu H, Zou Y, Xiang C, Zhang H, Xu F, Sun L, Ouyang L, Zhou H. Influence of Zr Addition on Structure and Performance of Rare Earth Mg-Based Alloys as Anodes in Ni/MH Battery. Metals. 2015; 5(2):565-577. https://doi.org/10.3390/met5020565
Chicago/Turabian StyleQiu, Shujun, Jianling Huang, Hailiang Chu, Yongjin Zou, Cuili Xiang, Huanzhi Zhang, Fen Xu, Lixian Sun, Liuzhang Ouyang, and Huaiying Zhou. 2015. "Influence of Zr Addition on Structure and Performance of Rare Earth Mg-Based Alloys as Anodes in Ni/MH Battery" Metals 5, no. 2: 565-577. https://doi.org/10.3390/met5020565






