Heavy Metal Behavior in Lichen-Mine Waste Interactions at an Abandoned Mine Site in Southwest Japan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area

2.2. Sampling Methods
2.3. Analytical Methods
2.4. Statistical Methods
3. Results
3.1. Microstructure of the Hyphae of Stereocaulon exutum Thallus and the Effects of the Lichen on Slag Weathering

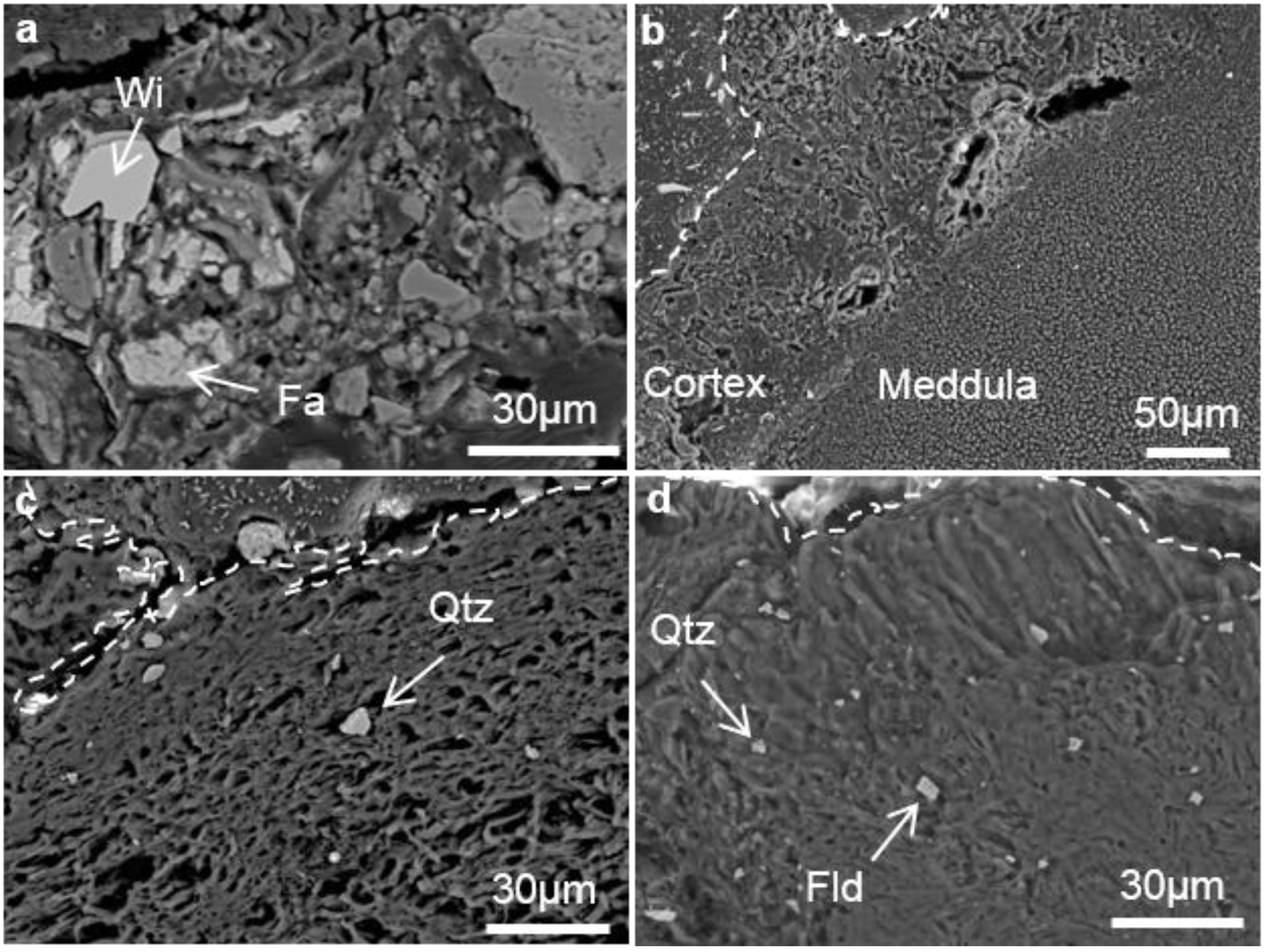
3.2. Heavy Metal Distribution in the Weathered Slag

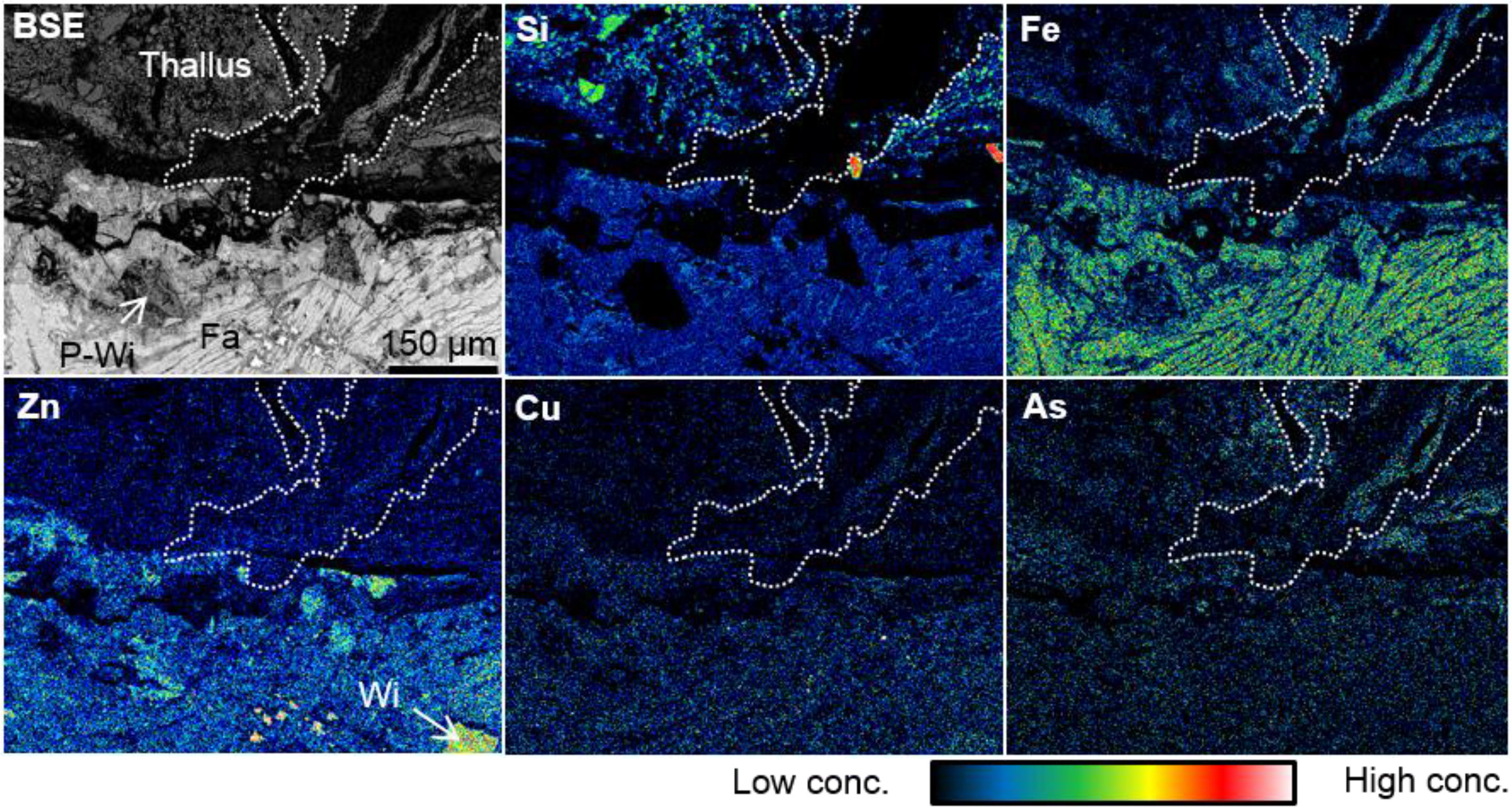
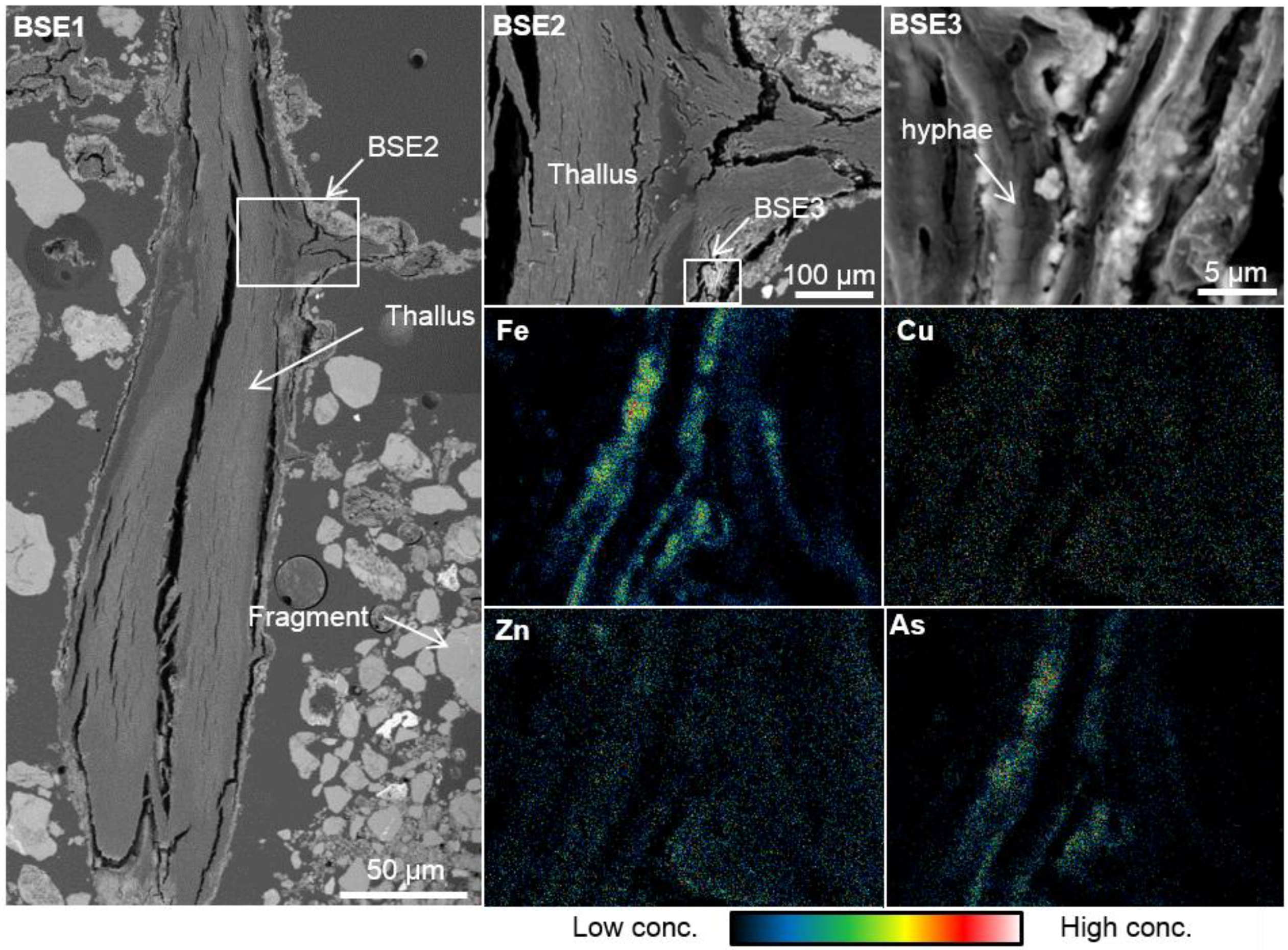
3.3. Elemental Distribution in the S. exutum Thallus


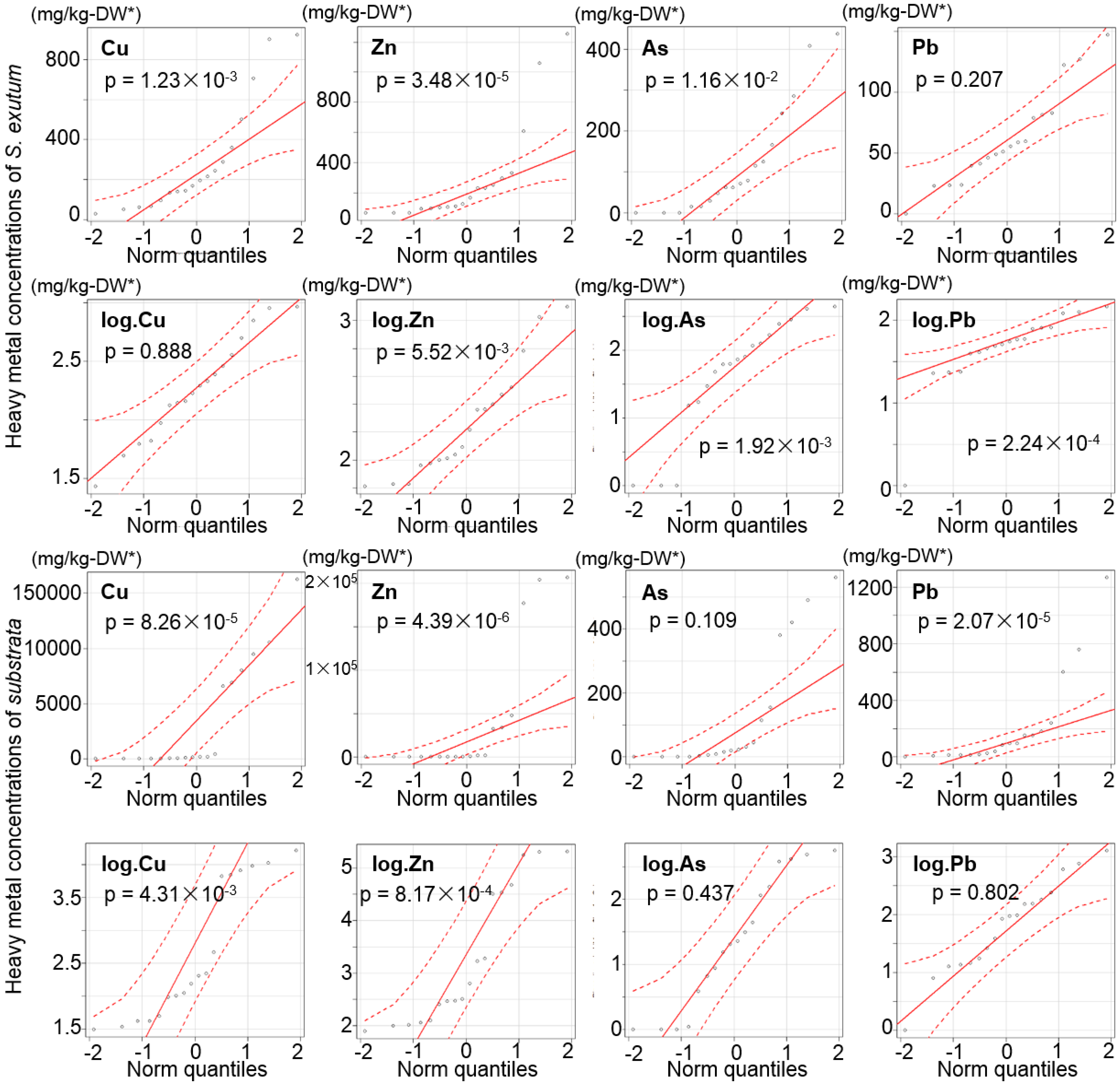
3.4. Heavy Metals and As Concentrations of Lichens and the Corresponding Substrata
| Elements | p-Values | |
|---|---|---|
| S. exutum | Substrata | |
| Cu | 1.23 × 10−3 | 8.26 × 10−5 |
| Zn | 3.48 × 10−5 | 4.39 × 10−6 |
| As | 1.16 × 10−2 | 0.109 |
| Pb | 0.207 | 2.07 × 10−5 |
| log.Cu | 0.888 | 4.31 × 10−3 |
| log.Zn | 5.52 × 10−3 | 8.17 × 10−4 |
| log.As | 1.92 × 10−3 | 0.437 |
| log.Pb | 2.24 × 10−4 | 0.802 |
| Elements | rs | p-Values |
|---|---|---|
| Cu | 0.618 | 7.43 × 10−3 |
| Zn | 0.829 | 1.28 × 10−5 |
| As | 0.421 | 0.119 |
| Pb | 0.336 | 0.188 |

4. Discussion
4.1. Effects of S. exutum on Heavy Metals and As Behavior in the Slag Weathering Process
4.2. The Accumulation and the Behavior of Heavy Metals in the Lichen Thallus
4.3. The Correlation of Cu and Zn Concentrations in the Lichen and Substrata
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Purvis, O.W. Chapter Three: Lichens and industrial pollution. In Ecology of Industrial Pollution; Batty, L.C., Hallberg, K.B., Eds.; Cambridge University Press: Cambridge, UK, 2010; pp. 41–69. [Google Scholar]
- Edwards, H.G.M.; Moody, C.D.; Villar, S.E.J.; Wynn-Williams, D.D. Raman spectroscopic detection of key biomarkers of cyanobacteria and lichen symbiosis in extreme Antarctic habitats: Evaluation for Mars Lander missions. Icarus 2005, 174, 560–571. [Google Scholar] [CrossRef]
- Bačkor, M.; Loppi, S. Interactions of lichens with heavy metals. Biol. Plant. 2009, 53, 214–222. [Google Scholar] [CrossRef]
- Adamo, P.; Violante, P. Weathering of rocks and neogenesis of minerals associated with lichen activity. Appl. Clay Sci. 2000, 16, 229–256. [Google Scholar] [CrossRef]
- Chen, J.; Blume, H.P.; Beyer, L. Weathering of rocks induced by lichen colonization—A review. Catena 2000, 39, 121–146. [Google Scholar] [CrossRef]
- Wilson, M.J.; Jones, D. Lichen weathering of minerals: Implications for pedogenesis. Geol. Soc. Lond. Spec. Publ. 2012, 11, 5–12. [Google Scholar] [CrossRef]
- Banfield, J.F.; Baker, W.W.; Welch, S.A.; Taunton, A. Biological impact on mineral dissolution: Application of the lichen model to understanding mineral weathering in the rhizosphere. Proc. Natl. Acad. Sci. USA 1999, 96, 3404–3411. [Google Scholar] [CrossRef] [PubMed]
- Favero-Longo, S.E.; Gazzano, C.; Girlanda, M.; Castelli, D.; Tretiach, M.; Baiocchi, C.; Piervittori, R. Physical and chemical deterioration of silicate and carbonate rocks by meristematic microcolonial fungi and endolothic lichens (Chaetothyriomycetidae). Geomicrobiol. J. 2011, 28, 732–744. [Google Scholar] [CrossRef]
- Conti, M.E.; Cecchetti, G. Biological monitoring: Lichens as bioindicators of air pollution assessment—A review. Environ. Pollut. 2001, 114, 471–492. [Google Scholar] [CrossRef]
- Golubev, A.V.; Golubeva, V.N.; Krylov, N.G.; Kuznetsova, V.F.; Mavrin, S.V.; Aleinikov, A.Y.; Hoppes, W.G.; Surano, K.A. On monitoring anthropogenic airborne uranium concentrations and 235U/238U isotopic ratio by Lichen—Bio-indicator technique. J. Environ. Radioact. 2005, 84, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Ohmura, Y.; Kawachi, M.; Ohtara, K.; Sugiyama, K. Long-term monitoring of Parmotrema tinctrumand qualitative changes of air pollution in Shimizu Ward, Shizuoka City, Japan. J. Jpn. Soc. Atmos. Environ. 2008, 43, 47–54. [Google Scholar]
- Pinho, P.; Augusto, S.; Martins-Loução, M.A.; Pereira, M.J.; Soarea, A.; Máguas, C.; Branquinho, C. Causes of change in nitrophytic and oligotrophic lichen species in a Mediterranean climate: Impact of land cover and atmospheric pollutants. Environ. Pollut. 2008, 154, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Guidotti, M.; Stella, D.; Dominici, C.; Blasi, G.; Owczarek, M.; Vitali, M.; Protano, C. Monitoring of traffic-related pollution in a province of central Italy with translocated lichen Pseudovernia furfuracea. Bulll. Environ. Contam. Toxicol. 2009, 83, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Čeburnis, D.; Steinnes, E. Conifer needles as biomonitors of atmospheric heavy metal deposition: Comparison with mosses and precipitation, role of the canopy. Atmos. Environ. 2000, 34, 4265–4271. [Google Scholar] [CrossRef]
- Loppi, S.; Pirintsos, S.A. Epiphytic lichens as sentinels for heavy metal pollution at forest ecosystems (central Italy). Environ. Pollut. 2003, 121, 327–332. [Google Scholar] [CrossRef]
- Nash, T.H. Sensitivity of lichens to sulfur dioxide. Am. Biol. Lichenol. Soc. 2012, 76, 333–339. [Google Scholar]
- Purvis, O.W.; Pawlik-Skowronska, B.; Cressey, G.; Jones, G.C.; Kearsley, A.; Spratt, J. Mineral phases and element composition of the copper hyperaccumulator lichens Lecanora polytropa. Mineral. Mag. 2008, 72, 607–616. [Google Scholar] [CrossRef]
- Purvis, O.W.; Bennett, J.P.; Spratt, J. Copper localization, elemental content, and thallus colour in the copper hyperaccumulator lichen Lecanora. sierrae from California. Lichenologist 2011, 43, 165–173. [Google Scholar] [CrossRef]
- Osyczka, P.; Rola, K. Response of the lichen Cladonia. rei Schaer. To strong heavy metal contamination of the substrate. Environ. Sci. Pollut. Res. Int. 2013, 20, 5076–5084. [Google Scholar] [CrossRef] [PubMed]
- Homepage of the Japan Meteorological Agency. Available online: http://www.data.jma.go.jp/gmd/risk/obsdl/index.php (accessed on 30 July 2015).
- Sueoka, Y.; Sakakibara, M. Primary phases and natural weathering of smelting slag at an abandoned mine site in southwest Japan. Minerals 2013, 3, 412–426. [Google Scholar] [CrossRef]
- Homepage of the Ministry of the environment, Japan. Available online: https://www.env.go.jp/kijun/dojou.html (accessed on 31 August 2015).
- Sakakibara, M.; Ohmori, Y.; Ha, N.T.H.; Sano, S.; Sera, K. Phytoremediation of heavy metal-contaminated water and sediment by Eleocharis. acicularis. Clean Soil Air Water 2011, 39, 735–741. [Google Scholar] [CrossRef]
- Imai, N.; Terashima, S.; Itoh, S.; Ando, A. Compilation of analytical data on nine GSJ geochemical reference samples, “Sedimentary Rock Series”. Geostand. Newsl. 1996, 20, 165–216. [Google Scholar] [CrossRef]
- Imai, N.; Terashima, S.; Itoh, S.; Ando, A. Compilation of analytical data for five GSJ geochemical reference samples: The “Instrumental Analysis Series”. Geostand. Newsl. 1998, 23, 223–250. [Google Scholar] [CrossRef]
- Sera, K.; Yanagisawa, T.; Tsunoda, H.; Futatsugawa, S.; Hatakeyama, S.; Saitoh, S.; Suzuki, S.; Orihara, H. Bio-PIXE at the Takizawa Facility (Bio-PIXE with a baby cyclotron). Int. J. PIXE 1992, 2, 325–330. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Friedmann, E.I. Endolithic microorganisms in the Antarctic cold desert. Science 1982, 215, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Sanders, W.B.; Ascaso, C.; Wierzchos, J. Physical interactions of two rhizomorph-forming lichens with their rock substrate. Bot. Acta 1994, 107, 432–439. [Google Scholar] [CrossRef]
- Wierzchos, J.; Ascaso, C. Application of back-scattered electron imaging to the study of the lichen-rock interface. J. Microsc. 1994, 175, 54–59. [Google Scholar] [CrossRef]
- Wierzchos, J.; Ascaso, C. Morphological and chemical features of bioweathered granitic biotite induced by lichen activity. Clays Clay Miner. 1996, 44, 652–657. [Google Scholar] [CrossRef]
- Creveld, M.C. Epilithic Lichen Communities in the Alpine Zone of Southern Norway; J. Gantner Verlag KG: Vaduz, Liechtenstein, 1981; pp. 48–55. [Google Scholar]
- Syers, J.K.; Iskandar, I.K. Pedogenetic significance of lichens. In The Lichens; Ahmadjian, V., Hale, M.E., Eds.; Academic Press: New York, NY, USA, 1973; pp. 225–248. [Google Scholar]
- Iskandar, I.K.; Syers, J.K. Solubility of lichen compounds in water: Pedogenetic implications. Lichenologist 1971, 5, 45–50. [Google Scholar] [CrossRef]
- Iskandar, I.K.; Syers, J.K. Metal-complex formation by lichen compounds. J. Soil Sci. 1972, 23, 255–265. [Google Scholar] [CrossRef]
- Ascaso, C.; Galvan, J. Studies on the pedogenetic action of lichen acids. Pedobiologia 1976, 16, 321–331. [Google Scholar]
- Jones, D.; Wilson, M.J. Chemical activity of lichens on mineral surfaces—A review. Int. Biodeterior. 1985, 21, 99–104. [Google Scholar]
- Wilson, M.J. Interactions between lichens and rocks. Cryptogam. Bot. 1995, 5, 299–305. [Google Scholar]
- Goyal, R.; Seaward, M.R. Metal uptake in terricolous lichens: Metal localization within the thallus. New Phytol. 1981, 89, 631–645. [Google Scholar] [CrossRef]
- Nieboer, E.; Ahmed, H.M.; Puckett, K.J.; Richardson, D.H.S. Heavy metal content of lichens in relation to distance from a nickel smelter in Sudbury, Ontario. Lichenologist 1972, 5, 292–304. [Google Scholar] [CrossRef]
- Nakajima, H.; Fujimoto, K.; Yoshitani, A.; Yamamoto, Y.; Sakurai, H.; Itoh, K. Effect of copper stress on cup lichens Cladonia. humilis and C. subconistea growing on copper-hyperaccumulating moss Scopelophila. cataractae. Ecotoxicol. Environ. Saf. 2012, 84, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, H.; Yamamoto, Y.; Yoshitani, A.; Itoh, K. Effect of metal stress on photosynthetic pigments in the Cu-hyperaccumulating lichens Cladonia. humilis and Stereocaulon. japonicum growing in Cu-polluted sites in Japan. Ecotoxicol. Environ. Saf. 2013, 97, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Nieboer, E.; Richardoson, D.H.S. Lichens as monitors of atmospheric depositions. In Atmospheric Pollutants in Natural Waters; Eisenreich, S.J., Ed.; Ann Arbor Science Publisher: Ann Arbor, MI, USA, 1981; pp. 339–388. [Google Scholar]
- Nieboer, E.; Richardoson, D.H.S.; Tomassini, F.D. Mineral uptake and release by lichens: An review. Bryologist 1978, 81, 226–246. [Google Scholar] [CrossRef]
- Nieboer, E.; Richardoson, D.H.S. The replacement of the nondescript term “heavy metals” by a biologically and chemically significant classification of mental ions. Environ. Pollut. 1980, 1, 3–26. [Google Scholar] [CrossRef]
- Brown, D.H.; Beckett, R.P. Uptake and effect of cations on lichen metabolism. Lichenologist 1984, 16, 173–188. [Google Scholar] [CrossRef]
- Shimada, T.; Shimizu, T.; Uehara, N. Aggregation property of thermo-sensitive copolymers having polyamino groups and carboxyl groups. Bunseki Kagaku 2002, 51, 689–695. [Google Scholar] [CrossRef]
- Sato, T.; Fukushi, K.; Yonada, T. Environmental behavior and management of hazardous inorganic anions in nature. J. MMIJ 2007, 123, 132–144. [Google Scholar] [CrossRef]
- Yaguchi, T. Classification and examination of fungi. Jpn. J. Food Microbiol. 2010, 27, 47–55. [Google Scholar] [CrossRef]
- Fahselt, D.; Madazia, S. Scanning electron microscopy of invasive fungi in lichens. Bryologist 2012, 104, 24–39. [Google Scholar] [CrossRef]
- Purvis, O.W. The occurrence of copper oxalate in lichens grown on copper sulphide-bearing rocks in Scandinavia. Lichenologist 1984, 16, 197–204. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sueoka, Y.; Sakakibara, M.; Sera, K. Heavy Metal Behavior in Lichen-Mine Waste Interactions at an Abandoned Mine Site in Southwest Japan. Metals 2015, 5, 1591-1608. https://doi.org/10.3390/met5031591
Sueoka Y, Sakakibara M, Sera K. Heavy Metal Behavior in Lichen-Mine Waste Interactions at an Abandoned Mine Site in Southwest Japan. Metals. 2015; 5(3):1591-1608. https://doi.org/10.3390/met5031591
Chicago/Turabian StyleSueoka, Yuri, Masayuki Sakakibara, and Koichiro Sera. 2015. "Heavy Metal Behavior in Lichen-Mine Waste Interactions at an Abandoned Mine Site in Southwest Japan" Metals 5, no. 3: 1591-1608. https://doi.org/10.3390/met5031591







