Improving Beneficiation of Copper and Iron from Copper Slag by Modifying the Molten Copper Slag
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Copper Slag
2.1.2. Flux
2.1.3. Compound Additive
2.2. Experimental Methods
2.2.1. Modification of Copper Slag
2.2.2. Flotation and Magnetic Separation
2.2.3. Mechanism of Modification
2.3. Evaluation Indexes
3. Results and Discussion
3.1. Thermodynamic Analysis
3.2. Modification of Molten Copper Slag
3.2.1. Reconstruction Phase of Copper Slag through Modifying
Effects of Binary Basicity on Recovery of Copper and Iron
Effects of Compound Additive Dosage on the Recovery of Copper and Iron
3.2.2. Heating Treatment System
Effects of Modification Temperature on the Recovery of Copper and Iron
Effects of Cooling Rate on the Recovery of Copper and Iron
Effects of the End Point Temperature on the Recovery of Copper and Iron
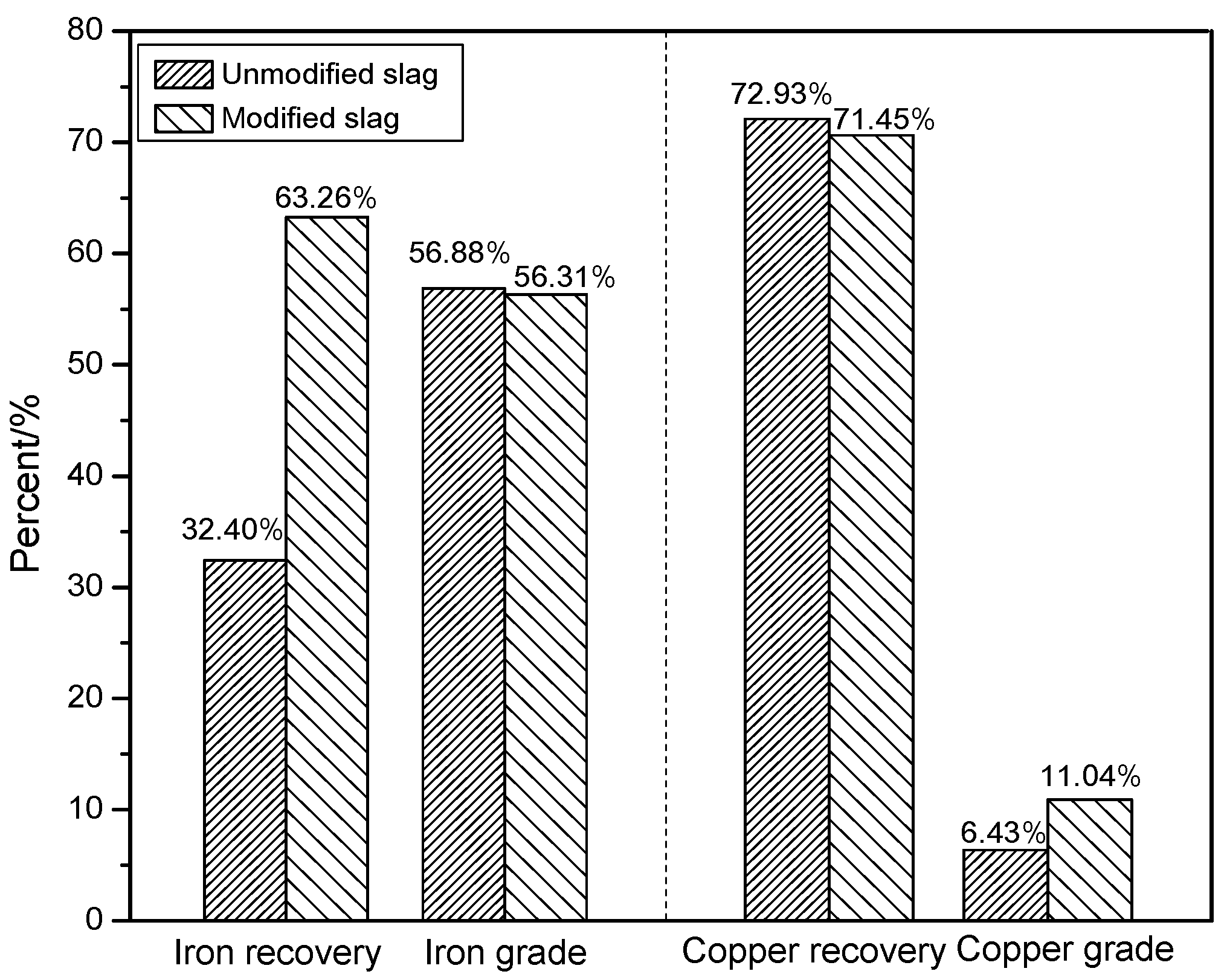
3.2.3. Comparison of Separation Indexes between Modified Slag and Unmodified Slag
3.3. Intensification Mechanisms of Modification of Copper Slag on Cu and Fe Separation
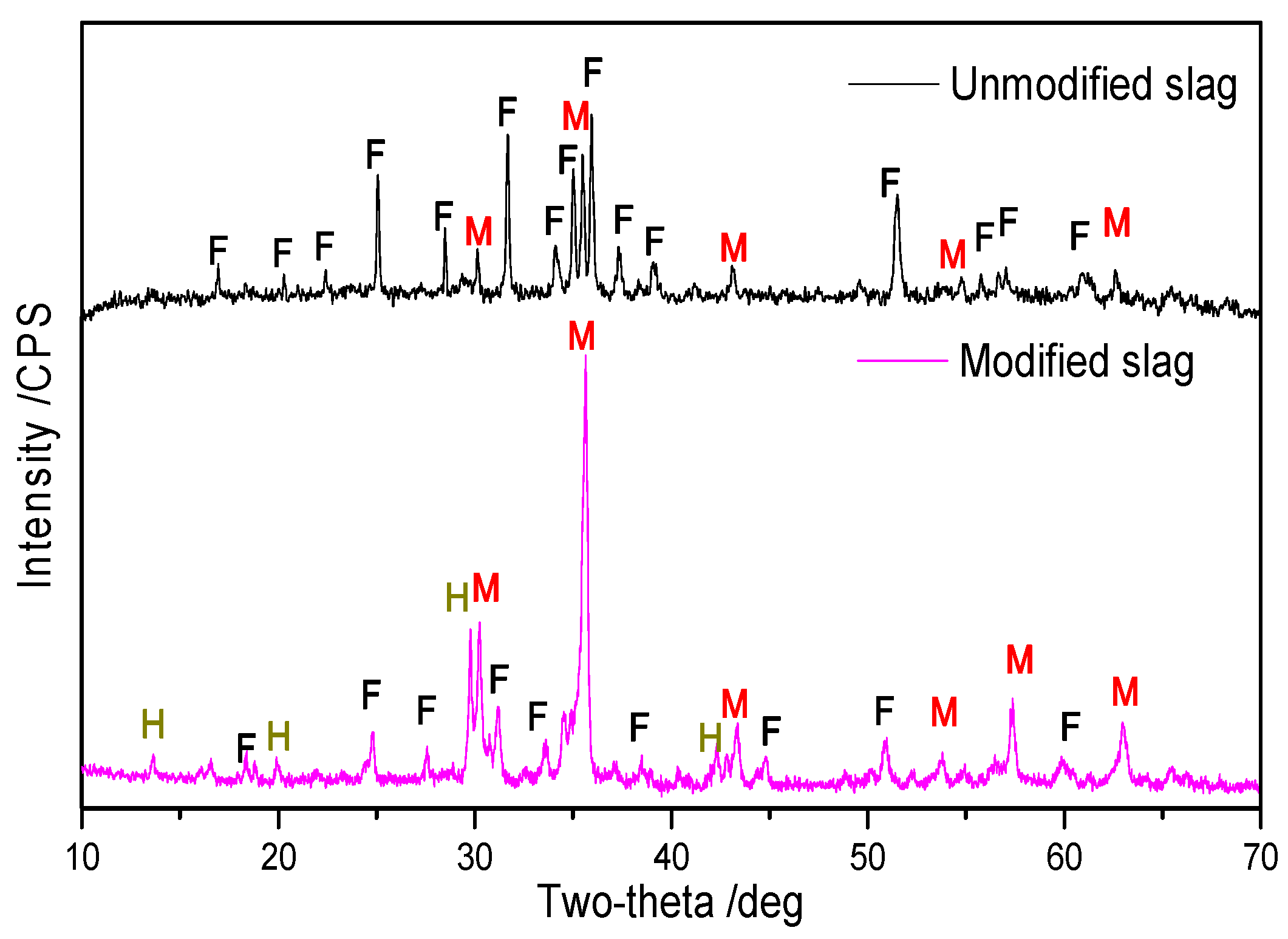
3.3.1. Phase Transformation by Modification
3.3.2. Microstructure Character of Copper Slag
4. Conclusions
- (1)
- Iron element in the copper slag mainly existed in the form of fayalite and magnetite, and the copper is present in sulfide form with fine size as well as being closely embedded in the fayalite matrix, revealing that traditional physical beneficiation process may result in poor valuable metal recovery.
- (2)
- The modification process for copper slag was conducted to improve the recovery of copper and iron with the optimized conditions at 0.45 binary basicity, 12% compound additive, modifying at 1350 °C for 120 min, and cooling at 1.5 °C/min down to 900 °C. The physical beneficiation studies showed that compared with unmodified slag, copper grade of rougher copper concentrate was increased from 6.43% to 11.04%; meanwhile, the copper recovery was kept about 72%, the corresponding iron recovery was increased significantly from 32.40% to 63.36%, and the total iron grade of magnetite concentrate was similar.
- (3)
- XRD analysis showed that the reconstruction of mineral phases for copper slag is effective because more fayalite was transformed to magnetite, which is beneficial to magnetic separation. Optical microscopy and ESEM-EDS analysis of modified slag indicated that valuable mineral (matte and magnetite) particles in the slag aggregate together and grow obviously, and the size is mostly over 50 μm, which is extremely favorable to the beneficiation of copper and iron.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Davenport, W.G.L.; King, M.; Schlesinger, M.; Biswas, A.K. Extractive Metallurgy of Copper; Pergamon Press: Oxford, UK, 2002. [Google Scholar]
- Gorai, B.; Jana, R.; Premchand, M. Characteristics and utilization of copper slag—A review. Resour. Conserv. Recycl. 2003, 39, 299–313. [Google Scholar] [CrossRef]
- Shen, H.; Forssberg, E. An overview of recovery of metals from slags. Waste Manag. 2003, 23, 933–949. [Google Scholar] [CrossRef]
- Shi, C.; Meyer, C.; Behnood, A. Utilization of copper slag in cement and concrete. Resour. Conserv. Recycl. 2008, 52, 1115–1120. [Google Scholar] [CrossRef]
- Alpa, I.; Deveci, H.; Sungun, H. Utilization of flotation wastes of copper slag as raw material in cement production. J. Hazard. Mater. 2008, 159, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Wang, H. Recovery of copper and iron in the converter slag from a copper smelter (in Chinese). J. Guangdong Nonferr. Met. 2003, 13, 83–88. [Google Scholar]
- Al-Jabri, K.; Taha, R.; Al-Ghassani, M. Use of copper slag and cement by-pass dust as cementitious materials. Cem. Concr. Aggreg. 2002, 24, 7–12. [Google Scholar]
- Antonijevica, M.M.; Dimitrijevic, M.D.; Stevanovic, Z.O.; Serbula, S.M.; Bogdanovic, G.D. Investigation of the possibility of copper recovery from the flotation tailings by acid leaching. J. Hazard. Mater. 2008, 158, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Rehani, S. Flotation of copper sulphide from copper smelter slag using multiple collectors and their mixtures. Int. J. Miner. Process. 2015, 143, 43–49. [Google Scholar] [CrossRef]
- Sarrafi, A.; Rahmati, B.; Hassani, H.R. Recovery of copper from reverberatory furnace slag by flotation. Miner. Eng. 2004, 17, 457–459. [Google Scholar] [CrossRef]
- Barnes, C.; Lumsdaine, J.; Hare, S. Copper converter slag treatment at Mount Isa Mines Limited, Mount Isa, Qld. AusIMM Proc. 1993, 298, 31–35. [Google Scholar]
- Bruckard, W.J.; Somerville, M.; Hao, F. The recovery of copper, by flotation, from calcium-ferrite-based slags made in continuous pilot plant smelting trials. Miner. Eng. 2004, 17, 495–504. [Google Scholar] [CrossRef]
- Mawejaa, K.; Mukongob, T.; Mutomboc, I. Cleaning of a copper matte smelting slag from a water-jacket furnace by direct reduction of heavy metals. J. Hazard. Mater. 2009, 164, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Altundogan, H.S.; Tumen, F. Metal recovery from copper converter slag by roasting with ferric sulphate. Hydrometallurgy 1997, 44, 261–267. [Google Scholar] [CrossRef]
- Urosevic, D.; Dimitrijevic, M.; Jankovic, Z.; Antic, D. Recovery of copper from copper slag and copper slag floatation tailings by oxidative leaching. Physicochem. Probl. Miner. Process. 2015, 51, 71–82. [Google Scholar]
- Muravyov, I.; Fomchenko, V.; Usoltsev, V.A.; Vasilyev, F.K. Leaching of copper and zinc from copper converter slag flotation tailings using H2SO4 and biologically generated Fe2(SO4)3. Hydrometallurgy 2012, 119, 40–46. [Google Scholar] [CrossRef]
- Zheng, J.; Xiao, K. The research on using dispersant agent to lron recovery in copper slag (in Chinese). Nonferr. Met. Sci. Eng. 2011, 6, 71–73. [Google Scholar]
- Yang, T.; Hu, J.; Wang, H. Concentration of Fe3O4 in roasted slag from copper impoverishment smelting (in Chinese). Chin. J. Process Eng. 2011, 11, 613–619. [Google Scholar]
- Yang, H.; Jing, L.; Dang, C. Iron recovery from copper-slag with lignite-based direct reduction followed by magnetic separation (in Chinese). Chin. J. Nonferr. Met. 2011, 21, 1165–1170. [Google Scholar]
- Yucel, O.; Sahin, F.; Sirin, B.; Addemir, O. A reduction study of copper slag in DC arc furnace. Scand. J. Metall. 1999, 28, 93–99. [Google Scholar]
- Zhou, X.L.; Zhu, D.Q.; Pan, J.; Wu, T.J. Utilization of waste copper slag to produce directly reduced Iron for weathering resistant steel. Int. ISIJ 2015, 55, 1347–1352. [Google Scholar]
- Kim, S.B.; Jo, S.K.; Shin, D.; Lee, J.C.; Jeong, S.B. A physico-chemical separation process for upgrading iron from waste copper slag. Int. J. Miner. Process. 2013, 124, 124–127. [Google Scholar] [CrossRef]
- Heo, J.H.; Kim, B.S.; Park, J.H. Effect of CaO addition on iron recovery from copper smelting slags by solid carbon. Metall. Mater. Trans. B 2013, 44, 1352–1363. [Google Scholar] [CrossRef]
- Durincka, D.; Engströmb, F.; Arnouta, S.; Heulensa, J.; Jonesa, P.T.; Björkmanb, B.; Blanpaina, B.; Wollants, P. Hot stage processing of metallurgical slags. Resour. Conserv. Recycl. 2008, 52, 1121–1131. [Google Scholar] [CrossRef]
- Fu, C. Nonferrous Metallurgy Principle; Metallurgical Industry Press: Beijing, China, 1984; pp. 1132–1145. [Google Scholar]
- Fu, J.; Jiang, T.; Zhu, D. Sintering and Pelletizing; Central South University of Technology Press: Changsha, China, 1996; pp. 100–142. [Google Scholar]
- Zhu, Z.Z.; He, J.Q. Metallurgy of Copper; Science Press: Sunyang, China, 2003; pp. 356–432. [Google Scholar]
- Zhang, L.N.; Zhang, L.; Wang, M.Y.; Che, Y.C.; Sui, Z.T. Thermodynamics of phase transformations in oxidation process of CaO-FeOx-SiO2 system with high iron content (in Chinese). Acta Phys. Sin. 2008, 24, 1540–1546. [Google Scholar]
- Chang, Y.A. Phase Relationships and Thermodynamics of the Ternary Copper-Iron-Sulfur System, Extractive Metallurgical of Copper; The Metallurgical Society of AIME: New York, NY, USA, 1976; pp. 367–397. [Google Scholar]
- German, R.M.; Suri, P.; Park, S.J. Review: Liquid phase sintering. J. Mater. Sci. 2009, 44, 1–39. [Google Scholar] [CrossRef]
- Konga, L.X.; Bai, J.; Li, W. The internal and external factor on coal ash slag viscosity at high temperatures, Part 1: Effect of cooling rate on slag viscosity, measured continuously. Fuel 2015, 158, 968–975. [Google Scholar] [CrossRef]












| Element | TFe | FeO | SiO2 | CaO | MgO | Al2O3 | Cu | Pb | S | P | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Percentage | 39.10 | 42.85 | 31.11 | 2.44 | 1.79 | 2.29 | 0.80 | 0.13 | 0.45 | 0.10 | 0.88 |
| Mineral | Ferric Sulfate | Magnetite | Iron Sulfide | Hematite | Fayalite | Fetotal |
|---|---|---|---|---|---|---|
| Content | 0.41 | 12.85 | 0.01 | 3.75 | 22.08 | 39.10 |
| Fraction | 1.05 | 32.86 | 0.03 | 9.59 | 56.47 | 100.00 |
| Mineral | Copper Oxide | Metallic Copper | Copper Sulfide | Combined Copper Oxide | Cutotal |
|---|---|---|---|---|---|
| Content | 0.02 | 0.18 | 0.46 | 0.14 | 0.80 |
| Fraction | 2.50 | 22.50 | 57.50 | 17.50 | 100.00 |
| Element | TFe | SiO2 | CaO | MgO | Al2O3 | MnO | Cu | S |
|---|---|---|---|---|---|---|---|---|
| Percentage | 52.44 | 2.09 | 0.77 | 0.68 | 1.63 | 9.3 | 1.23 | 12.13 |
| Modification Temperature/°C | 1250 | 1300 | 1350 | 1400 |
|---|---|---|---|---|
| Equilibrium constant | 1.91 × 104 | 1.33 × 104 | 0.94 × 104 | 0.69 × 104 |
| Mineral | FeS | Cu2S | Cu | Cu2S-FeS (Matte) |
|---|---|---|---|---|
| temperature | 1190 | 1126 | 1083 | 950–1100 |
| Element | TFe | SiO2 | CaO | MgO | Al2O3 | S | P | Cu | Pb | Zn |
|---|---|---|---|---|---|---|---|---|---|---|
| Percentage | 56.31 | 7.22 | 2.59 | 0.64 | 2.75 | 0.03 | 0.04 | 0.15 | 0.03 | 0.03 |
| Minerals | Grinding Size/(−0.074 mm %) | ||||||
|---|---|---|---|---|---|---|---|
| 53 | 58 | 64 | 70 | 76 | 82 | 85 | |
| Copper matte | 76.34 | 84.34 | 88.65 | 93.21 | 95.68 | 97.12 | 98.03 |
| Magnetic | 89.63 | 93.23 | 94.56 | 95.78 | 96.82 | 97.51 | 98.32 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Z.; Zhu, D.; Pan, J.; Wu, T.; Zhang, F. Improving Beneficiation of Copper and Iron from Copper Slag by Modifying the Molten Copper Slag. Metals 2016, 6, 86. https://doi.org/10.3390/met6040086
Guo Z, Zhu D, Pan J, Wu T, Zhang F. Improving Beneficiation of Copper and Iron from Copper Slag by Modifying the Molten Copper Slag. Metals. 2016; 6(4):86. https://doi.org/10.3390/met6040086
Chicago/Turabian StyleGuo, Zhengqi, Deqing Zhu, Jian Pan, Tengjiao Wu, and Feng Zhang. 2016. "Improving Beneficiation of Copper and Iron from Copper Slag by Modifying the Molten Copper Slag" Metals 6, no. 4: 86. https://doi.org/10.3390/met6040086
APA StyleGuo, Z., Zhu, D., Pan, J., Wu, T., & Zhang, F. (2016). Improving Beneficiation of Copper and Iron from Copper Slag by Modifying the Molten Copper Slag. Metals, 6(4), 86. https://doi.org/10.3390/met6040086





