The Effects of Corrosive Media on Fatigue Performance of Structural Aluminum Alloys
Abstract
:1. Introduction
2. The Experimental Method
2.1. Materials and Specimens
2.2. Testing Procedures
3. Results and Discussions
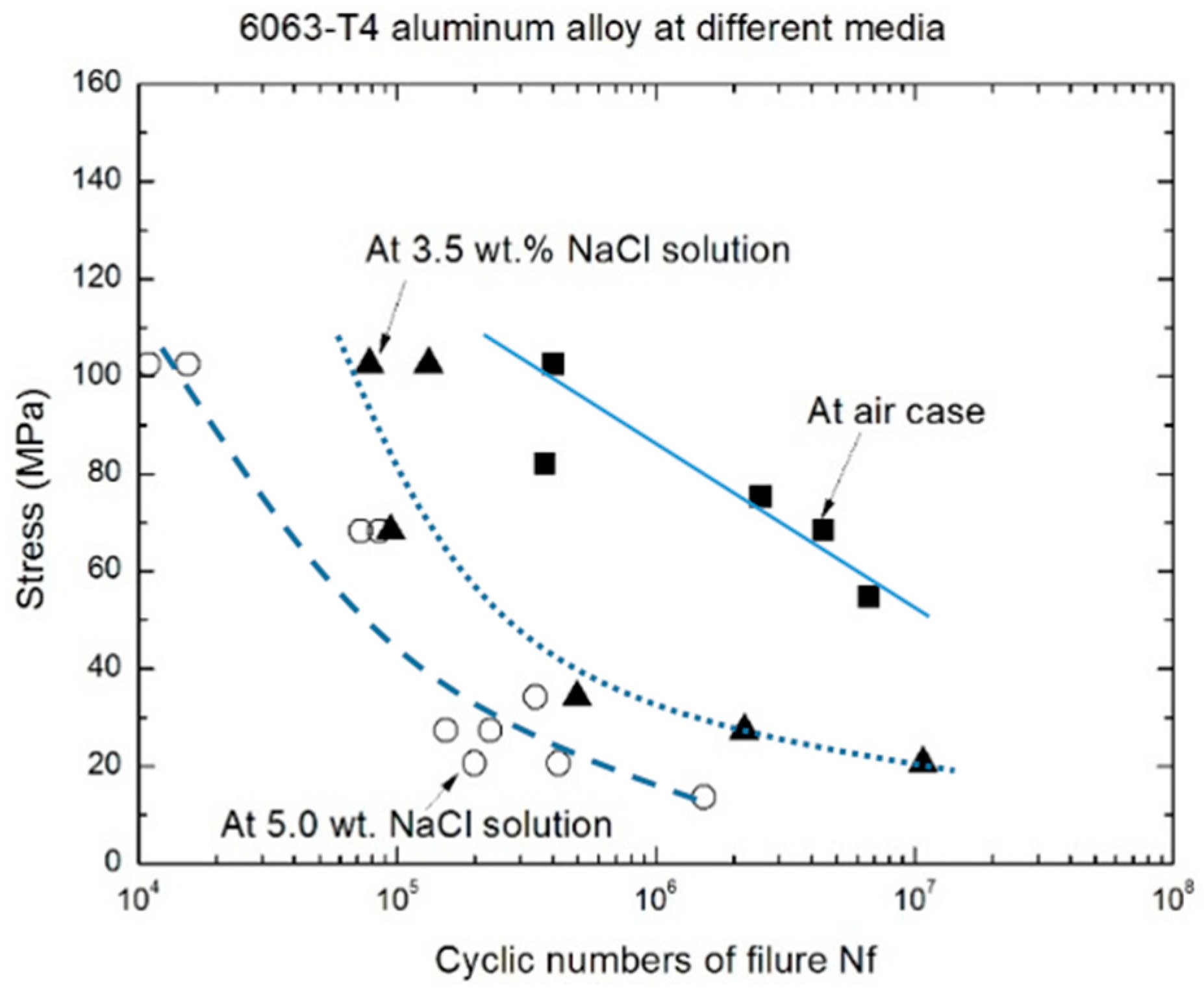
3.1. Fatigue Life Characteristics
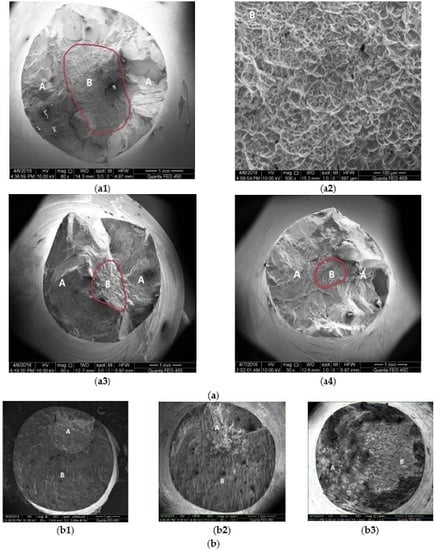
3.2. Fatigue Fracture Characteristics
4. Conclusions
- The effect of corrosive media is much stronger on the fatigue life of high strength aluminum alloys (such as AA7475-T7351 and AA7075-T651) than that of high ductility aluminum alloys (such as AA2024-CZ and AA2024-T4). For example, when the stress ratio is 0.5, the corrosion fatigue lives of AA7475-T7351 and AA7075-T651 at 5.0 wt. % NaCl aqueous solution are approximately the same values of about 2.5 × 104, and the corrosion fatigue lives of AA2024-CZ and AA2024-T4 at 5.0 wt. % NaCl aqueous solution are 2 × 105, 6 × 105, respectively.
- With increasing of cycles to failure, the effect of mechanical properties of materials on the corrosion fatigue performance becomes relatively weak. For example, when the stress ratio is 0.2, the corrosion fatigue lives of AA7475-T7351 and AA7075-T651 at 5.0 wt. % NaCl aqueous solution are approximately the same values of about 6.5 × 105, and the corrosion fatigue lives of AA2024-CZ and AA2024-T4 at 5.0 wt. % NaCl aqueous solution are 1.6 × 106, 3 × 106, respectively.
- When the dropping corrosive liquid rate is less than 1.6 mL/min, the effect of dropping liquid rate on the fatigue performance of AA2024-T4 aluminum alloy cannot be ignored. This is because the fatigue life at corrosive NaCl liquid rate with 1.6 mL/min is slightly smaller than that at corrosive NaCl liquid rate with 2.5 mL/min.
- For the AA2024-CZ aluminum alloy, the crack initiation and propagation life under stress corrosion mode is much shorter than that under mechanical fatigue mode after prior-corrosion at the same stress level, in which in the former, the coupling effect of corrosion media and stress is stronger than that in the latter, even if there are some corrosion pits including the maximum depth of corrosion pit of about 50–60 μm.
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviation
| α | the stress concentration factor (1.08) |
| E | Young’s modulus |
| HCF | high-cyclic fatigue |
| L | geometry sizes of sample |
| LCH | low-cyclic fatigue |
| g | acceleration of gravity (9.8 m/s2) |
| R | stress ratio |
| Re | surface roughness |
| Nf | number of cycles to failure |
| σ | engineering stress amplitude (MPa) |
| σ0.2 | the material’s offset yield strength |
| σb | the tensile strength |
| δ | the percentage elongation |
References
- Yi, D.Q.; Zhou, M.Z.; Liu, H.Q.; Wang, B.; Yang, S. Effect of Temperature and Corrosive Environment on Cyclic Fatigue and Final Fracture Behavior of 2524 Aluminum Alloy. Int. J. Soc. Mater. Eng. Resour. 2010, 17, 58–63. [Google Scholar] [CrossRef]
- Wang, R. A fracture model of corrosion fatigue crack propagation of aluminum alloys based on the materials elements fracture ahead of a crack tip. Int. J. Fatigue 2008, 30, 1376–1386. [Google Scholar] [CrossRef]
- Na, K.H.; Pyun, S.I. Comparison of susceptibility to pitting corrosion of AA2024-T4, AA7075-T651 and AA7475-T761 aluminium alloys in neutral chloride solutions using electrochemical noise analysis. Corros. Sci. 2007, 50, 248–258. [Google Scholar] [CrossRef]
- Warner, J.S. Inhibition of Environmental Fatigue Crack Propagation in Age-Hardenable Aluminum Alloys; The School of Engineering and Applied Science University of Virginia: Charlottesville, VA, USA, 2010. [Google Scholar]
- Murakami, Y.; Kanezaki, T.; Mine, Y.; Metall, S. Effect of Humidity on Fatigue Strength of Age-Hardened Al Alloy under Rotating Bending. Mater. Trans. A 2008, 39, 1327–1339. [Google Scholar] [CrossRef]
- Wang, X.S.; Li, X.D.; Yang, H.H.; Kawagoishi, N.; Pan, P. Environment-induced fatigue cracking behavior of aluminum alloys and modification methods. Corros. Rev. 2015, 33, 119–137. [Google Scholar] [CrossRef]
- Frulla, G.; Avalle, G.; Sapienza, V. Preliminary evaluation of the fatigue behavior of aluminum alloy in corrosive environment. Aircr. Eng. Aerosp. Technol. 2015, 87, 165–171. [Google Scholar]
- Takahashi, H.; Kasahara, K.; Fujiwara, K.; Seo, M. The cathodic polarization of aluminum covered with anodic oxide-films in a neutral borate solution—I. The mechanism of rectification. Corros. Sci. 1994, 36, 677–688. [Google Scholar] [CrossRef]
- Li, X.D.; Wang, X.S.; Ren, H.H.; Chen, Y.L.; Mu, Z.T. Effect of prior corrosion state on the fatigue small cracking behavior of 6151-T6 aluminum alloy. Corros. Sci. 2012, 55, 26–33. [Google Scholar] [CrossRef]
- Stanzl, S.E.; Mayer, H.R.; Tschegg, E.K. The influence of air humidity on near-threshold fatigue crack growth of 2024-T3 aluminum alloy. Mater. Sci. Eng. A 1991, 147, 45–54. [Google Scholar] [CrossRef]
- Wasekar, N.P.; Jyothirmayi, A.; Sundarajian, G. Influence of pre-corrosion on the high cycle fatigue behavior of microarc oxidation coated 6061-T6 aluminum alloy. Int. J. Fatigue 2011, 33, 1268–1276. [Google Scholar] [CrossRef]
- Wang, X.S.; Guo, X.W.; Li, X.D.; Ge, D.Y. Improvement on the fatigue performance of 2024-T4 alloy by synergistic coating technology. Materials 2014, 7, 3533–3546. [Google Scholar] [CrossRef]
- Ishihara, S.; Saka, S.; Nan, Z.Y.; Goshima, T.; Sunada, S. Prediction of corrosion fatigue lives of aluminum alloy on the basis of corrosion pit growth law. Fatigue Fract. Eng. Mater. Struct. 2006, 29, 472–480. [Google Scholar] [CrossRef]
- Yang, H.H.; Wang, Y.L.; Wang, X.S.; Pan, P.; Jia, D.W. Synergistic effect of environmental media and stress on the fatigue fracture behavior of aluminum alloys. Fatigue Fract. Eng. Mater. Struct. 2016. [Google Scholar] [CrossRef]
- Magnin, T. Recent advances for corrosion fatigue mechanisms. ISIJ Int. 1995, 35, 223–233. [Google Scholar] [CrossRef]
- Maitra, S.; English, G.C. Environmental Factors Affecting Localized Corrosion of 7075-T7351 Aluminum Alloy Plate. Metall. Trans. A 1982, 13, 161–166. [Google Scholar] [CrossRef]
- Ricker, R.E.; Duquette, D.J. The role of hydrogen in corrosion fatigue of high purity Al-Zn-Mg exposed to water vapor. Metall. Trans. A 1988, 19, 1775–1783. [Google Scholar] [CrossRef]
- Standard Practice for Presentation of Constant Amplitude Fatigue Test Results for Metallic Materials; ASTM International: West Conshohocken, PA, USA, 2004. [CrossRef]
- Chang, H.; Han, E.H.; Wang, J.Q.; Ke, W. Effect of cathodic polarization on corrosion fatigue life of the LY12CZ aluminum alloy. Acta Metall. 2005, 41, 556–560. (In Chinese) [Google Scholar]
- Hall, M.M., Jr. Effect of cyclic crack opening displacement rate on corrosion fatigue crack velocity and fracture mode transitions for Al-Zn-Mg-Cu alloys. Corros. Sci. 2014, 81, 132–143. [Google Scholar] [CrossRef]
- Wu, X.R. Handbook of Mechanical Properties; Aviation Industry Press: Beijing, China, 1997. [Google Scholar]
- Wang, X.S.; Kawagoishi, N. Effect of specimen configuration and loading types on fatigue life of an annealed 0.42% carbon steels. Key Eng. Mater. 2010, 417, 121–124. [Google Scholar]
- Jones, K.; Hoeppner, D.W. Prior corrosion and fatigue of 2024-T3 aluminum alloy. Corros. Sci. 2006, 48, 3109–3122. [Google Scholar] [CrossRef]
- Sundararajan, G.; Wasekar, N.P.; Ravi, N. The influence of the coating technique on the high cycle fatigue life of alumina coated Al 6061 alloy. Trans. Indian Inst. Met. 2010, 63, 203–208. [Google Scholar] [CrossRef]
- Nickel, D.; Dietrich, D.; Mehner, T.; Frint, P.; Spieler, D.; Lampke, T. Effect of Strain Localization on Pitting Corrosion of an AlMgSi0.5 Alloy. Metals 2015, 5, 172–191. [Google Scholar] [CrossRef]








| Al Alloys | E (GPa) | σ0.2 (MPa) | σb (MPa) | δ (%) |
|---|---|---|---|---|
| AA7475-T7351 | 70.5 | 434 | 503 | 10.2 |
| AA7075-T651 | 72.0 | 505 | 570 | 11.0 |
| AA2024-CZ | 71.0 | 260 | 290 | 17.0 |
| AA2024-T4 | 71.0 | 325 | 470 | 20.0 |
| AA6063-T4 | 69.0 | 90 | 170 | 22.0 |
| Al Alloys | Zn | Mg | Cu | Mn | Cr | Ti | Fe | Si | Al |
|---|---|---|---|---|---|---|---|---|---|
| AA7475-T7351 | 5.89 | 2.48 | 1.59 | <0.01 | 0.22 | 0.02 | 0.06 | <0.03 | Bal. |
| AA7075-T651 | 5.60 | 2.50 | 1.60 | 0.32 | 0.40 | 0.06 | 0.50 | 0.40 | Bal. |
| AA2024-CZ | 0.07 | 1.49 | 4.36 | 0.46 | <0.01 | 0.01 | 0.25 | 0.14 | Bal. |
| AA2024-T4 | 0.25 | 1.60 | 4.50 | 0.80 | 0.10 | <0.01 | 0.50 | 0.50 | Bal. |
| AA6063-T4 | 0.10 | 0.70 | 0.10 | 0.10 | 0.10 | 0.10 | 0.35 | 0.40 | Bal. |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Wang, Y.; Wang, X.; Pan, P.; Jia, D. The Effects of Corrosive Media on Fatigue Performance of Structural Aluminum Alloys. Metals 2016, 6, 160. https://doi.org/10.3390/met6070160
Yang H, Wang Y, Wang X, Pan P, Jia D. The Effects of Corrosive Media on Fatigue Performance of Structural Aluminum Alloys. Metals. 2016; 6(7):160. https://doi.org/10.3390/met6070160
Chicago/Turabian StyleYang, Huihui, Yanling Wang, Xishu Wang, Pan Pan, and Dawei Jia. 2016. "The Effects of Corrosive Media on Fatigue Performance of Structural Aluminum Alloys" Metals 6, no. 7: 160. https://doi.org/10.3390/met6070160
APA StyleYang, H., Wang, Y., Wang, X., Pan, P., & Jia, D. (2016). The Effects of Corrosive Media on Fatigue Performance of Structural Aluminum Alloys. Metals, 6(7), 160. https://doi.org/10.3390/met6070160