Diatoms and Their Capability for Heavy Metal Removal by Cationic Exchange
Abstract
:1. Introduction
2. Methodology
3. Results and Discussion
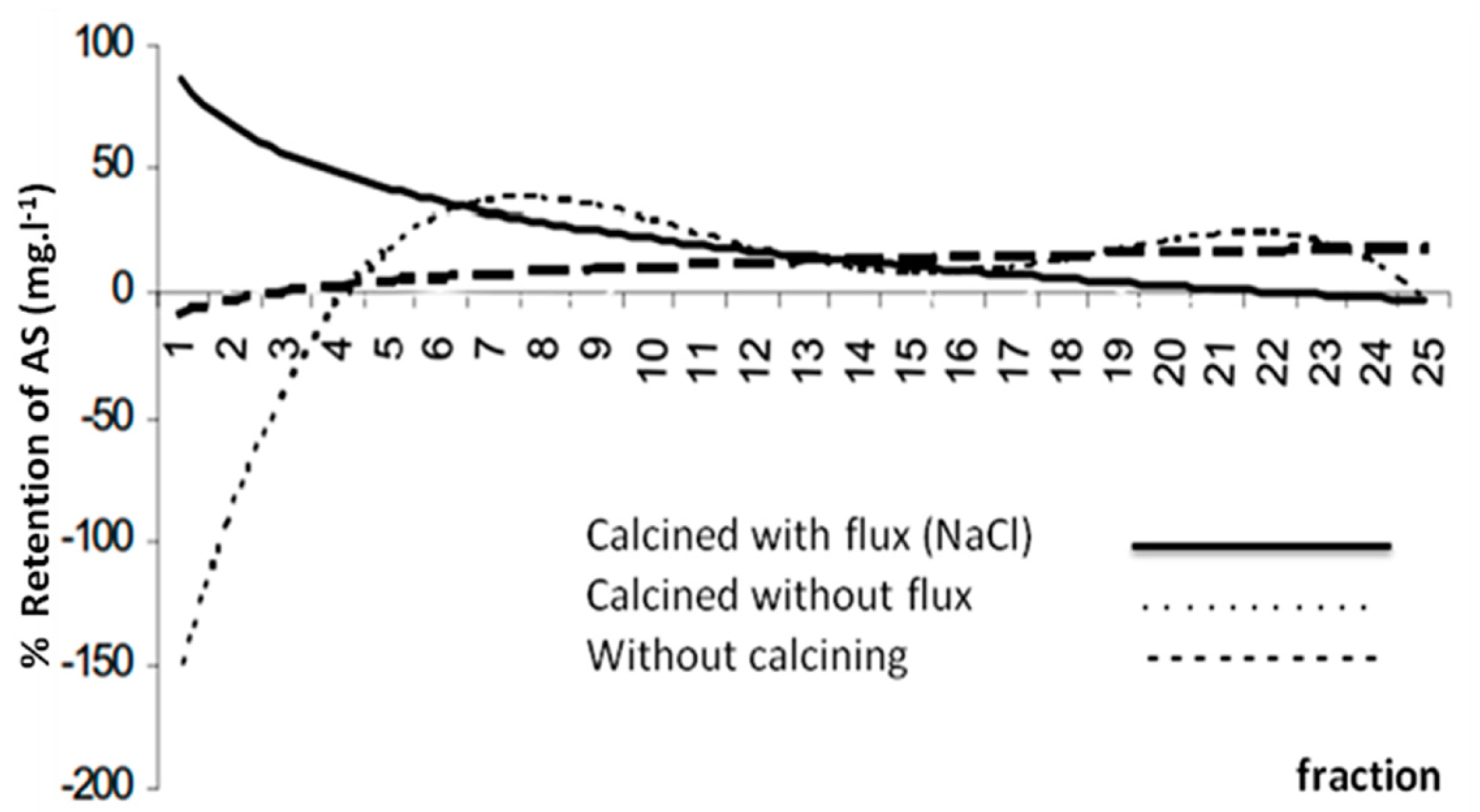
4. Exchange of Metals
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kadey, F.L. Diatomite. In Industrial Minerals & Rocks, 4th ed.; Society for Mining, Metallurgy & Exploration: New York, NY, USA, 1975; pp. 101–115. [Google Scholar]
- Avila, J.H.; Moreno, F.P.; Espinoza, I.B.; Rodríguez, E.S. Caracterización De Diatomitas De Hidalgo, México. Posible Uso Como Adsorbente En La Disminución De Arsénico En Agua Potable; XL Congreso Mexicano de Química: Guadalajara, Jalisco, México, 2005; pp. 38–42. (In Spanish) [Google Scholar]
- Martínez, M.; Duro, L.; Rovira, M.; De Pablo, J. Sorption of cadmium and nickel (II) on a natural zeolite rich in clinoptilolite. In Natural Microporous Materials in Environmental Technology; Springer: Dordrecht, The Netherlands, 1999; pp. 327–334. [Google Scholar]
- Merchán, W.N.; Melo, S.G.; Sánchez, S.M. Mineralogía y geoquímica de diatomitas (Boyacá, Colombia). Geol. Colomb. 2007, 32, 77. [Google Scholar]
- Boulet, P.; Greenwell, H.C.; Stackhouse, S.; Coveney, P.V. Recent advances in understanding the structure and reactivity of clays using electronic structure calculations. J. Mol. Struct. TEOCHEM 2006, 762, 33–48. [Google Scholar] [CrossRef]
- Bickmore, B.R.; Rosso, K.M.; Nagy, K.L.; Cygan, R.T.; Tadanier, C.J. Ab initio determination of edge surface structures for dioctahedral 2:1 phyllosilicates: Implications for acid-base reactivity. Clay Clay Miner. 2003, 51, 359–371. [Google Scholar] [CrossRef]
- Churakov, S.V. Ab initio study of sorption on pyrophillite: Structure and acidity of the edge sites. J. Phys. Chem. B 2006, 110, 4135–4146. [Google Scholar] [CrossRef] [PubMed]
- Churakov, S.V. Structure and dynamics of the water films confined between edges of pyrophyllite: A first principle study. Geochim. Cosmochim. Acta 2007, 71, 1130–1144. [Google Scholar] [CrossRef]
- Stackhouse, S.; Coveney, P.V.; Sandre, E. Plane-wave density functional theoric study of formation of clay-polymer nanocomposite materials by self-catalyzed in situ intercalative polymerization. J. Am. Chem. Soc. 2001, 123, 11764. [Google Scholar] [CrossRef] [PubMed]
- Briones-Jurado, C.; Agacino-Valdés, E. Brönsted sites on acid-treated montmorillonite: A theorical study with probe molecules. J. Phys. Chem. A 2009, 113, 8994–9001. [Google Scholar] [CrossRef] [PubMed]
- Suter, J.L.; Kabalan, L.; Khader, M.; Coveney, P.V. Ab initio molecular dynamics study of the interlayer and micropore structure of aqueous montmorillonite clays. Geochim. Cosmoschim. Acta 2015, 169, 17–29. [Google Scholar] [CrossRef]
- Awal, M.R.; Urata, S.; Jyo, A.; Tamada, M.; Katakai, A. Arsenate removal from water by a weak-base anion exchange fibrous adsorbent. Water Res. 2008, 42, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Awal, M.R.; Jyo, A. Rapid column-mode removal of arsenate from water by crosslinked poly (allylamine) resin. Water Res. 2009, 43, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Awal, M.R.; Jyo, A. Assessing of phosphorous removal by polymeric anion exchange. Desalination 2011, 281, 111–117. [Google Scholar] [CrossRef]
- Awal, M.R.; Jyo, A.; Ihara, T.; Seko, N.; Tamada, M.; Lim, K.T. Enhanced trace phosphate removal from water by zirconium (IV) loaded fibrous adsorbent. Water Res. 2011, 45, 4592–4600. [Google Scholar] [CrossRef] [PubMed]
- Awal, M.R. Assessing of lead (III) capturing from contamined wastewater using ligand doped conjugate adsorbent. Chem. Eng. J. 2006, 289, 65–73. [Google Scholar] [CrossRef]
- Awal, M.R.; Hasan, M.M. A novel fine-tuning mesoporous adsorbent for simultaneous lead (II) detection and removal from wastewater. Sens. Actuators B Chem. 2014, 202, 395–403. [Google Scholar] [CrossRef]
- Awal, M.R.; Hasan, M.M.; Shahat, A. Functionalized novel mesoporous adsorbent for selective lead (II) ions monitoring and removal from wastewater. Sens. Actuators B Chem. 2014, 203, 854–863. [Google Scholar] [CrossRef]
- Awal, M.R.; Hasan, M.M. Novel conjugate adsorbent for visual detection and removal of toxic lead (II) ions from water. Microporous Mesoporous Mater. 2014, 196, 261–269. [Google Scholar] [CrossRef]
- Yuan, P; Wu, D.Q.; He, H.P.; Lin, Z.Y. The hydroxyl species and acid sites on diatomite surface: A combined IR and Raman study. Appl. Surf. Sci. 2004, 227, 30–39. [Google Scholar] [CrossRef]
- Murphy, V.; Tofail, S.A.M.; Highes, H.; McLoughlin, P. A novel study of hexavalent chromium detoxification by selected seaweed species using SEM-EDX and XPS analysis. Chem. Eng. J. 2009, 148, 425–433. [Google Scholar] [CrossRef]
- Murphy, V.; Hughes, H.; McLoughlin, P. Cu (II) binding by dried biomass of red, green and brown macroalgae. Water Res. 2007, 41, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Sheng, P.X.; Ting, Y.P.; Chen, J.P.; Hong, L. Sorption of lead, copper, cadmium, zinc and nickel by marine algal biomass: Characterization of biosorptive capacity and investigation of mechanisms. J. Colloid Interface Sci. 2004, 275, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, C.; Campos-García, J.; Devars, S.; Gutiérrez-Corona, F.; Loza-Tavera, H.; Torres-Guzmán, J.C.; Moreno-Sánchez, R. Interactions of chromium with microorganism and plants. FEMS Microbiol. Rev. 2001, 25, 335–347. [Google Scholar] [CrossRef] [PubMed]
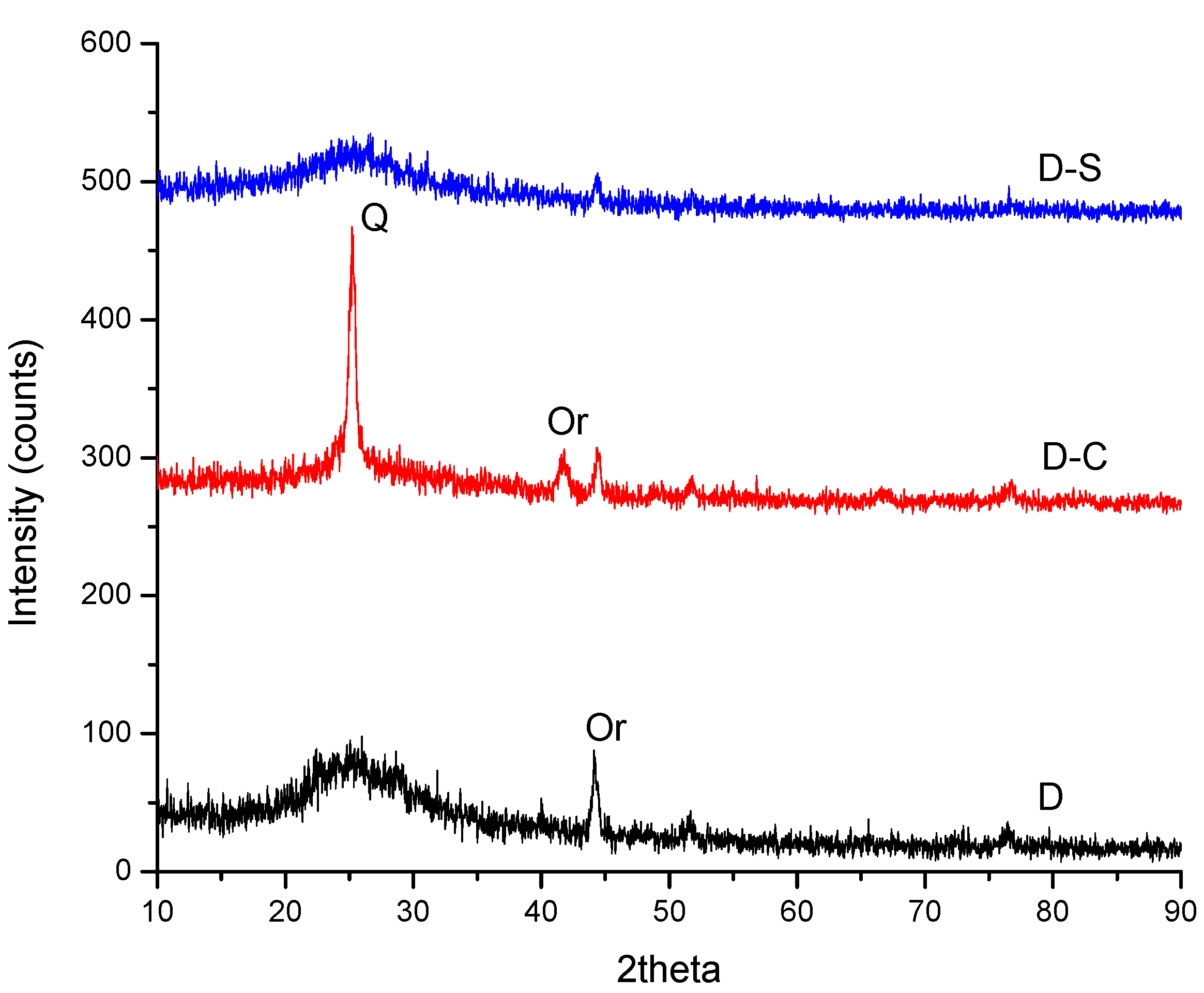
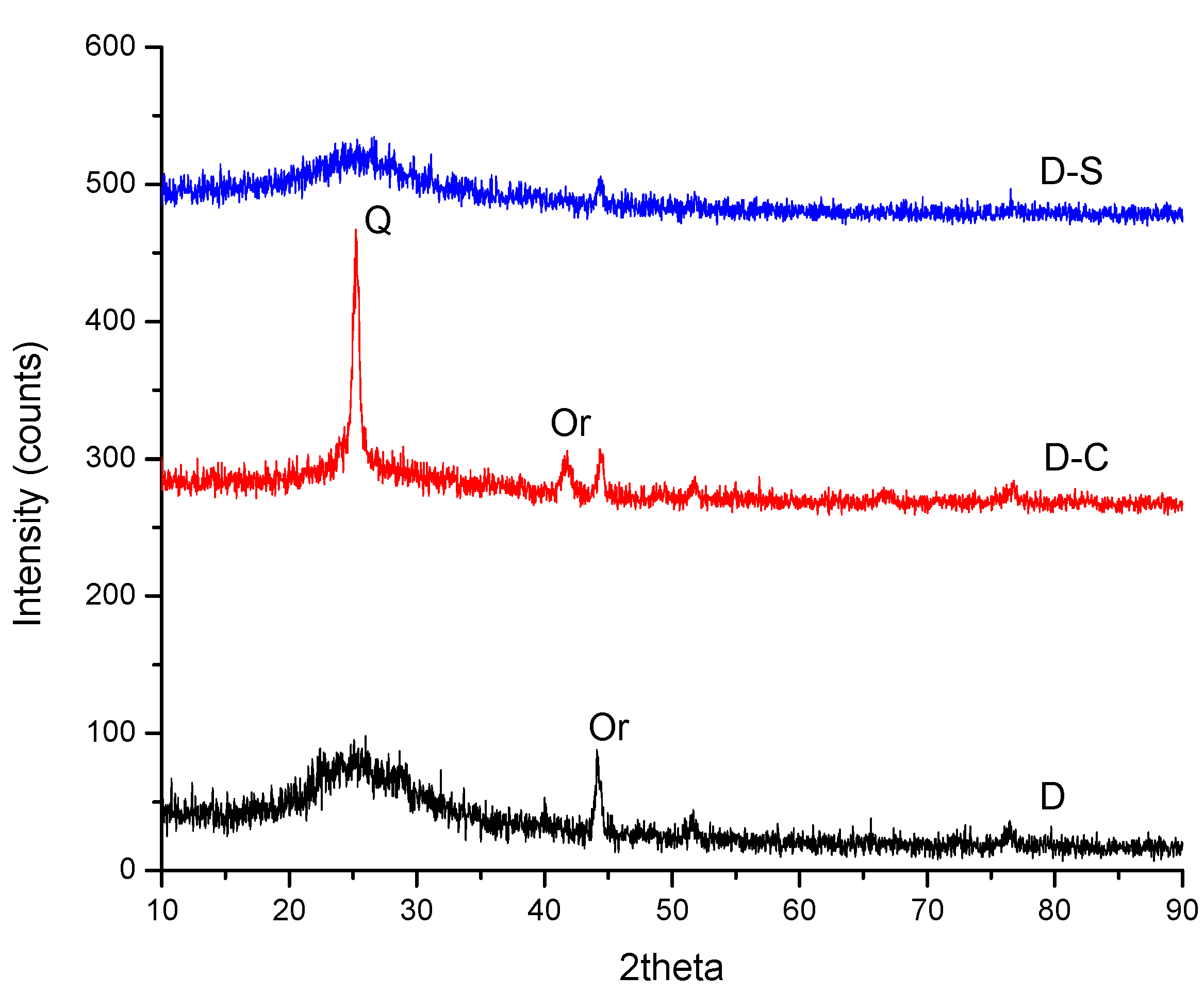

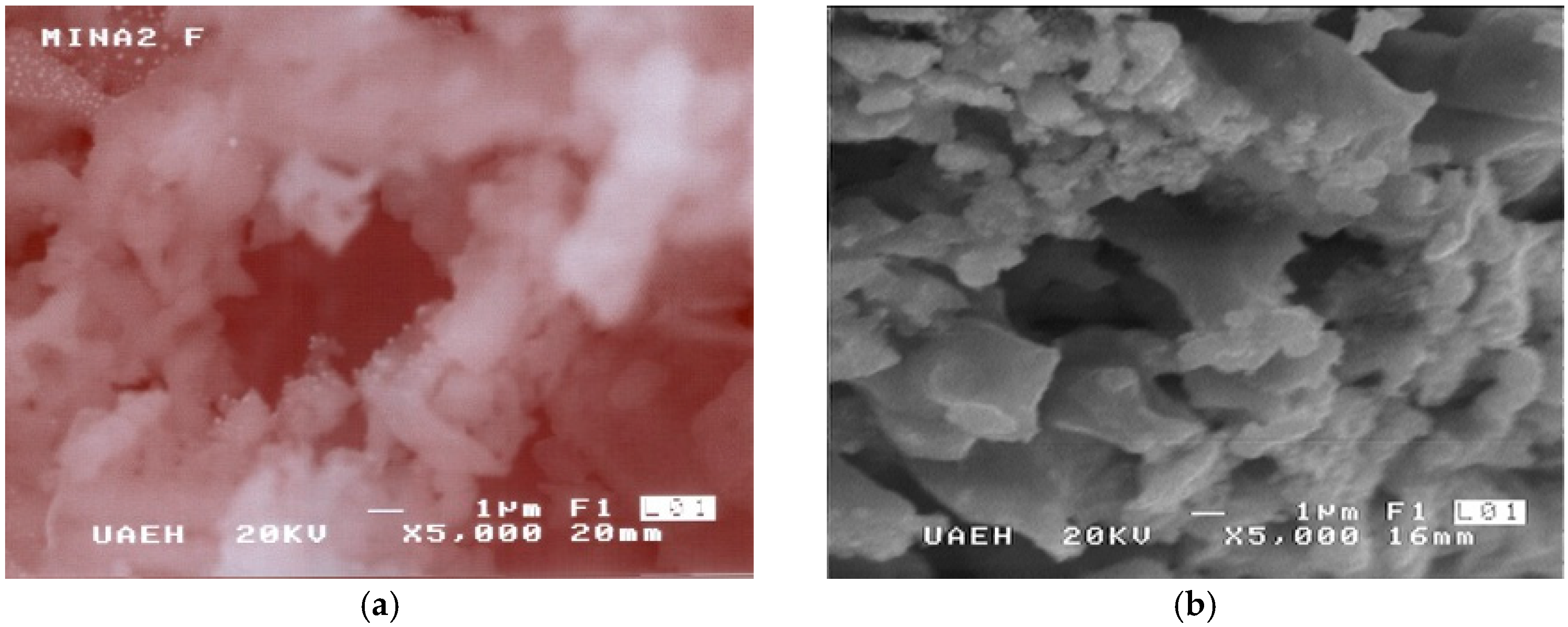
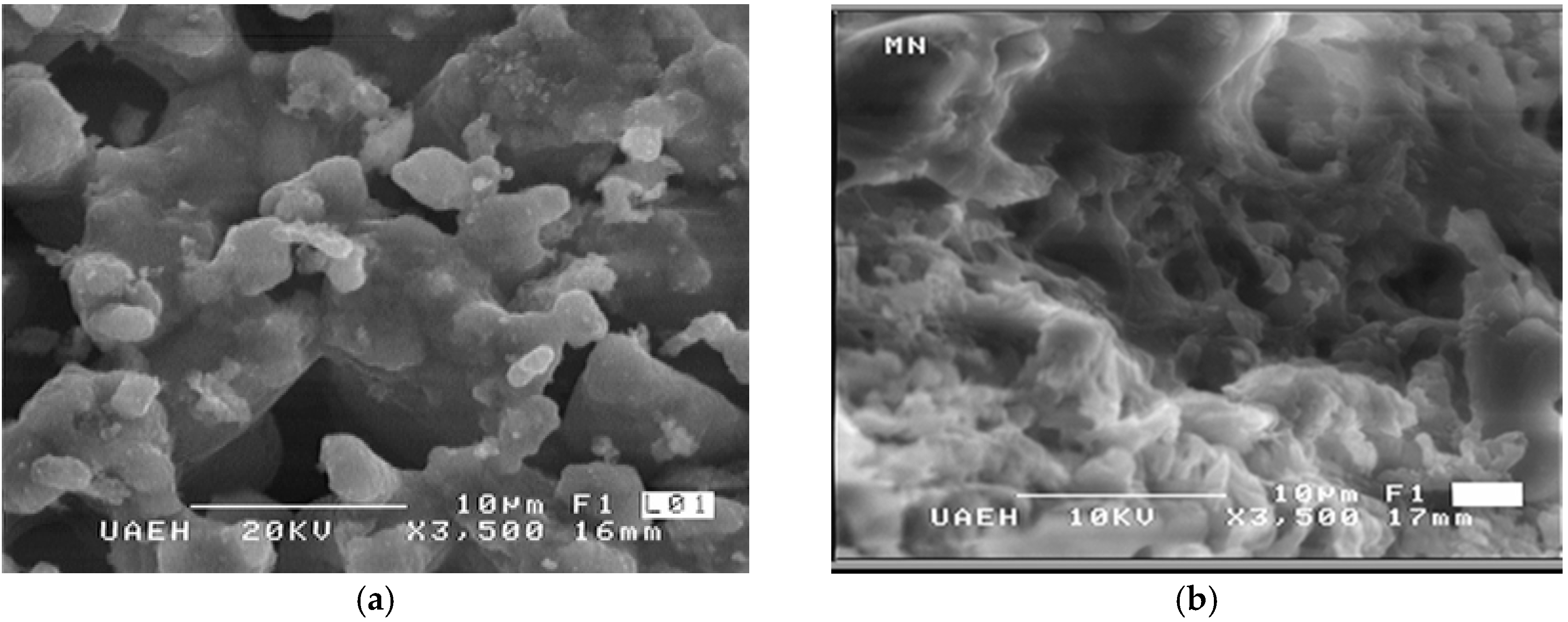
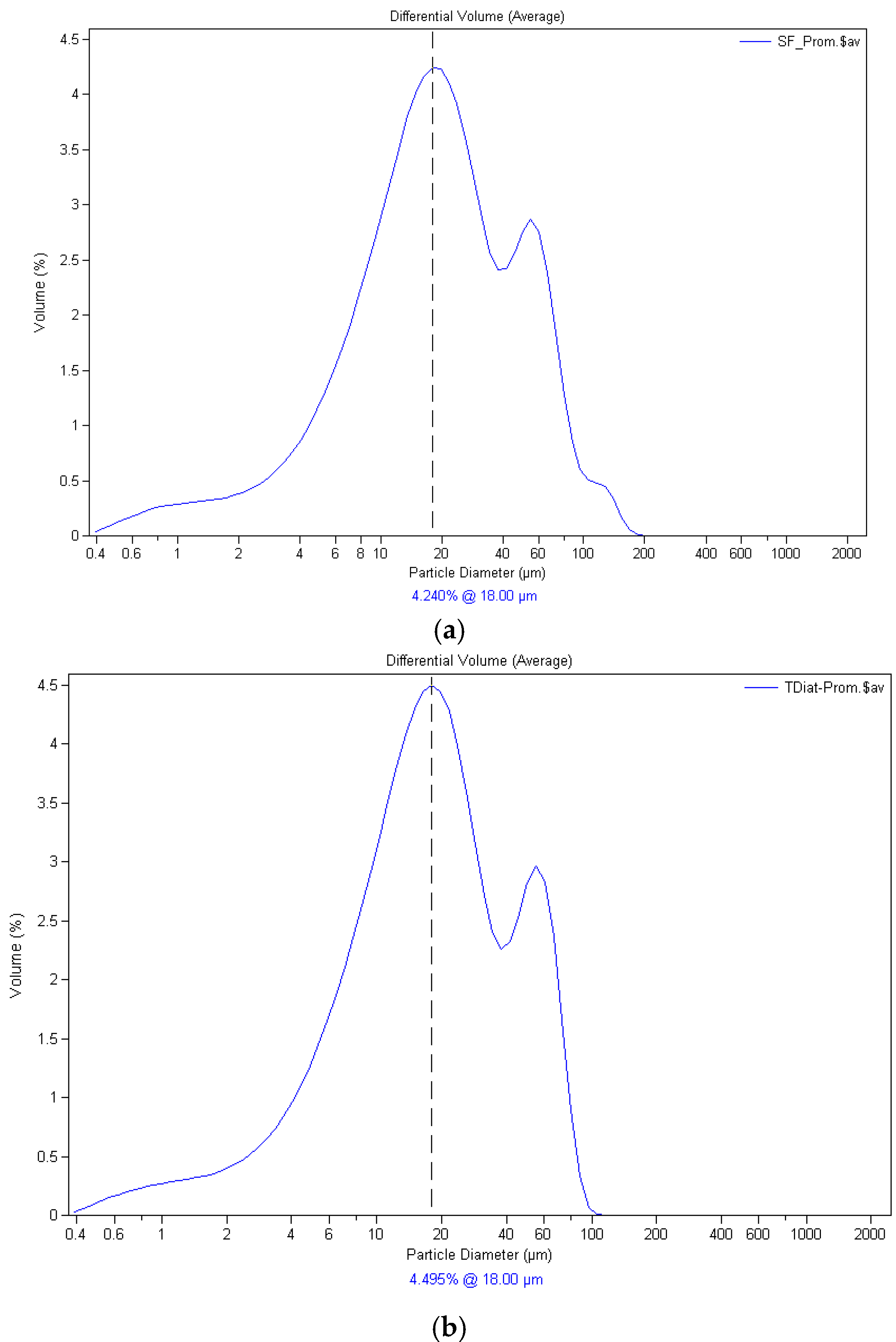

| Origin | Mineral Species |
|---|---|
| Jalisco | Silica, Albite, Muscovite, Potassium Feldspar, Mogonita, and Andesite |
| Hidalgo | Silica, Albite, Muscovite, Potassium Feldspar, Mogonita, and Andesite |
| Origin | Treatment | Element % wt | |||||||
|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | K2O | Na2O | CaO | FeO | MgO | TiO | ||
| Hidalgo | Natural | 70.0 | 11.63 | 2.41 | 6.10 | 0.85 | 1.95 | 1.79 | 0.50 |
| Calcined without flux | 81.50 | 12.00 | 2.10 | 1.45 | 0.10 | 1.44 | 0.48 | 0 | |
| Calcined with flux | 82.00 | 11.15 | 1.96 | 2.82 | 0.13 | 1.646 | 0.51 | 0 | |
| Jalisco | Natural | 93.58 | 3.03 | 0.92 | 0.24 | 0.11 | 1.81 | 0.40 | 0 |
| Calcined without flux | 96.39 | 3.25 | 0.05 | 0.23 | 0.0 | 0 | 0.08 | 0 | |
| Calcined with flux | 94.49 | 2.90 | 0.7 | 1.75 | 0.07 | 0 | 0.09 | 0 | |
| Property | Sample from Hidalgo | Sample from Jalisco | ||||
|---|---|---|---|---|---|---|
| Diatom Calcined with Flux | Diatom Calcined without Flux | Natural Diatom | Diatom Calcined with Flux | Diatom Calcined without Flux | Natural Diatom | |
| Real density (g/cm3) | 2.37 | 2.22 | 2.15 | 2.35 | 2.20 | 2.13 |
| Global density (g/cm3) | 0.50 | 0.52 | 0.61 | 0.52 | 0.54 | 0.62 |
| Total porosity (%) | 89.95 | 81.5 | 77.2 | 89.59 | 79.85 | 71.1 |
| Specific surface (m2/g) | 16.391 | 17.74 | 22.53 | 16.59 | 17.94 | 22.07 |
| Compressive strength (kg/cm2) | 17.7 | 16.49 | 15.00 | 18.3 | 17.51 | 17.00 |
| Color | White | Rose | Cream-Grey | White | Rose | Cream-Grey |
| pH | 10 | 7 | 7 | 10 | 8 | 7 |
| Average pore size (µm) | 7 | 3 | 1.5 | 6.7 | 4.6 | 1.8 |
| Metal | Initial Concentration in Solution (±0.004 mg/L) | Final Concentration (in Solution) after Filtering with Diatom from Hidalgo (±0.004 mg/L) | Final Concentration (in Solution) after Filtering with Diatom from Jalisco (±0.004 mg/L) | Efficiency of Exchange of Diatom from Hidalgo (%) | Efficiency of Exchange of Diatom from Jalisco (%) |
|---|---|---|---|---|---|
| As3+ | 5.27 | 0. 175 | 0.185 | 96.67 | 96.48 |
| Ag+ | 4.28 | 0.063 | 0.066 | 98.52 | 98.46. |
| Ni2+ | 3.95 | 0.188 | 0.180 | 95.24 | 95.44 |
| Cr6+ | 4.09 | 3.790 | 3.710 | 7.33 | 9.29 |
| Pb2+ | 4.081 | 0.048 | 0.049 | 98.82 | 98.80 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Ávila, J.; Salinas-Rodríguez, E.; Cerecedo-Sáenz, E.; Reyes-Valderrama, M.I.; Arenas-Flores, A.; Román-Gutiérrez, A.D.; Rodríguez-Lugo, V. Diatoms and Their Capability for Heavy Metal Removal by Cationic Exchange. Metals 2017, 7, 169. https://doi.org/10.3390/met7050169
Hernández-Ávila J, Salinas-Rodríguez E, Cerecedo-Sáenz E, Reyes-Valderrama MI, Arenas-Flores A, Román-Gutiérrez AD, Rodríguez-Lugo V. Diatoms and Their Capability for Heavy Metal Removal by Cationic Exchange. Metals. 2017; 7(5):169. https://doi.org/10.3390/met7050169
Chicago/Turabian StyleHernández-Ávila, Juan, Eleazar Salinas-Rodríguez, Eduardo Cerecedo-Sáenz, Ma. Isabel Reyes-Valderrama, Alberto Arenas-Flores, Alma Delia Román-Gutiérrez, and Ventura Rodríguez-Lugo. 2017. "Diatoms and Their Capability for Heavy Metal Removal by Cationic Exchange" Metals 7, no. 5: 169. https://doi.org/10.3390/met7050169
APA StyleHernández-Ávila, J., Salinas-Rodríguez, E., Cerecedo-Sáenz, E., Reyes-Valderrama, M. I., Arenas-Flores, A., Román-Gutiérrez, A. D., & Rodríguez-Lugo, V. (2017). Diatoms and Their Capability for Heavy Metal Removal by Cationic Exchange. Metals, 7(5), 169. https://doi.org/10.3390/met7050169






