Abstract
A new method for the separation, pre-concentration and accurate determination of trace amounts of Pb and Cd in water samples using Amberlite XAD-16 resin functionalized with a new chelating ligand, 3-(2-hydroxyphenyl)-1H-1,2,4-triazole-5(4H)-thione (HTT), Amberlite XAD-16-HTT and inductively coupled plasma atomic emission spectrometry (ICP-AES) is reported in the present study. Fourier transform infrared (FTIR) spectroscopy was used to characterize the chelating resin. The effects of analytical parameters such as the pH of the medium, amount of adsorbent, type and volume of eluent, flow rate of the sample solution, volume of the sample solution, and matrix interference on the retention of metal ions were investigated. Also, 1 M HNO3 was used for the elution of the sorbed metals, and ICP-AES was used for the analysis of elutes offline. The results indicate that pH 5 is the optimum pH for the sorption of Pb and Cd ions. The limit of detection was found to be 0.16 and 0.22 μg/L for Pb and Cd, respectively, by applying a pre-concentration factor of 50. The method was validated using the international water reference material (NIST 1643e). The developed enrichment method has a high selectivity, sensitivity, and reproducibility; this method was successfully applied for the determination of Pb and Cd in surface water samples collected in Nellore District, Penner River belt as well as Bay of Bengal, Andhra Pradesh, India.
1. Introduction
The pollutants in ground and surface waters need to be regularly monitored to meet legislative requirements [1]. Many organic and inorganic toxic compounds have been designated as priority pollutants, and their accurate measurement is necessary to ensure drinking water quality. Often, toxic metals enter the human body via the food chain and drinking water, causing health problems [2]. Although Pb and Cd are natural trace components of the aquatic environment, an excessive intake of these metals is detrimental to human health. High levels of Cd affects the functioning of the kidney and liver, whereas Pb could damage the testes, the brain and other vital body organs through oxidative stress [3]. Many people are known to have suffered from Itai-Itai syndrome by consuming rice contaminated with Cd [4]. An accurate determination of heavy metals such as Pb and Cd in water samples at trace levels is therefore of paramount importance to monitor drinking water quality.
Recent advances in the development of analytical techniques have progressively led to an improved and accurate determination of very low concentrations of metals in aqueous environments [5,6]. Such techniques include graphite furnace atomic absorption spectrometry (GFAAS) [7], flame-atomic absorption spectrometry (FAAS) [8,9,10], inductively coupled plasma mass spectrometry (ICP-MS) [11,12], and inductively coupled plasma atomic emission spectrometry (ICP-AES), which are routinely used for the quantification of trace metals in environmental and food samples [13,14,15]. Most techniques require appropriate analytical separation and pre-concentration of the metals of interest. These methods include liquid–liquid extraction [16], co-precipitation [17], ion exchange techniques [18], and solid-phase extraction (SPE) [19,20]. Amongst these, SPE is often preferred owing to its simplicity and ease of application. It has the following advantages: (i) simple and fast operation, (ii) low-cost, (iii) high pre-concentration factor, (iv) rapid separation of phases, (v) choice of solid phase and (vi) flexibility of use with different detection techniques [21,22,23,24]. The use of solid supports functionalized with ligands, such as R-nitroso-α-naphthol [25], 1-(2-pyridilazo)-2-naphthol [26], piperidinedithiocarbamate [27], o-aminobenzoicacid [28], 2-(methylthio)aniline, and 2-aminothiophenol [29,30], has been suggested in the literature. The complexation characteristics of the reagent and the sorption behavior of metal ions is influenced by the donor (soft or hard atom), and the metal atom in addition to the size of the formed chelate ring and the number of donor atom binding sites on the reagent. There is an increasing interest in the development of stable, high capacity and low-cost functionalized resins to improve the efficacy of analytical techniques for determining the concentration of toxic elements in an aqueous environment.
In the present study, a novel, facile yet sensitive analytical method has been developed for the pre-concentration and separation of Pb and Cd in an aqueous environment. The column was loaded with functionalized Amberlite XAD-16 by the covalent coupling of a synthesized ligand, 3-(2-hydroxyphenyl)-1H-1,2,4-triazole-5(4H)-thione (HTT), with the polymer backbone using an azo spacer. Then, 10 mL of 1 M HNO3 was used for eluting the sorbed metals, which were then analyzed by ICP-AES. The experimental conditions were optimized to ensure high extraction efficiency. The pre-concentration factor was found to be ~50 for a 500 mL sample solution.
2. Materials and Methods
2.1. Apparatus
For the analysis of metals, i.e., Pb and Cd, an ICP-AES (Varian, Liberty Series II, Mulgrave, Australia) was used keeping incident power at 1.1 kW, plasma gas flow at 15 L/min, auxiliary gas flow at 1.5 L/min, photomultiplier tube voltage at 700 V, observation height at 14 mm, pump rate at 15 rpm, and assigning the sample uptake time of 25 s. The wavelengths used were 220.382 and 226.502 nm for Pb and Cd, respectively. For characterizing both the functionalized resin and the new chelating compound, Fourier transform infrared (FTIR) spectrometer (Thermo-Nicolet FTIR, IR-200, Madison, WI, USA) was used. The solution pH was measured with an ELICO (Li-129, Hyderabad, India) pH meter.
2.2. Chemicals and Solutions
Doubly-distilled water was used for the preparation of aqueous solutions, while analytical-grade chemicals were used without further purification. All reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Amberlite XAD-16 resin (Sigma-Aldrich) was purchased with a surface area of 900 m2/g, pore diameter of 100 Å, particle mesh size of 20–60 and pore volume 1.82 mL/g were used as the resin. Before use, the resin beads were sequentially treated with 4 M HCl and 2 M NaOH solutions to remove both acidic as well as basic impurities. Water was then used for washing until neutral pH was obtained. The purified resin was filtered, washed with ethanol, and vacuum dried prior to use. A mixed standard solution (100 μg/mL) was prepared by diluting Pb and Cd stock solutions (1000 μg/mL). For preparing working standards, the mixed standard solutions were appropriately diluted with double-distilled water.
2.3. Synthesis of 3-(2-hydroxyphenyl)-1H-1,2,4-triazole-5(4H)-thione (HTT)
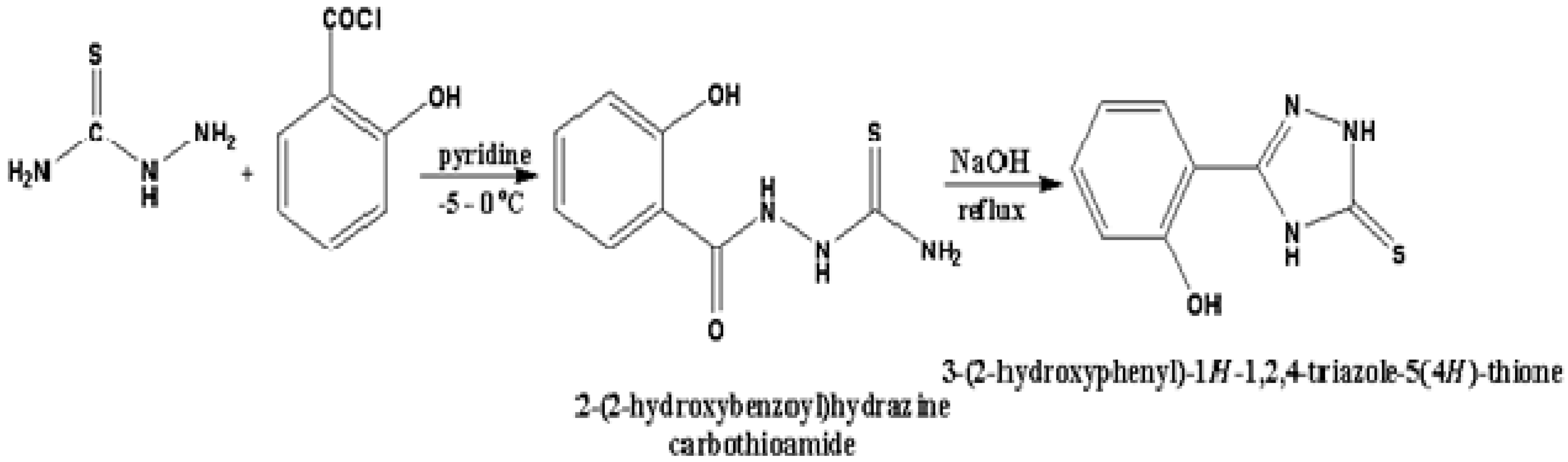
Under constant stirring in the temperature range of −5 to 0 °C, 2-hydroxybenzoyl chloride (170 mmol) was added drop wise to a solution containing thiosemicarbazide (230 mmol) in absolute pyridine (10 mL). The reaction mixture was then stirred for 3 h at room temperature before being poured into H2O, yielding 47 g precipitate of HTT (blue color), which was refluxed in 100 mL of 2NNaOH solution for 3 h and then cooled to room temperature. The precipitate was filtered and recrystallized in ethanol. The synthesis of HTT is shown in Scheme 1. The yield was 82%. The IR spectrum of HTT was recorded using a KBr pellet. IR (νmax, cm−1): 3396 (N–H), 3158 (O–H), 2976 (Ar–H), 1656 (C=N), 1229 (C=S), and 1107 (C–N). 1H NMR (DMSO-d6): δ 11.43 (1H, s, NH), 9.89 (1H, s, OH), 7.34–7.31 (m, 2H, Ar), 6.83–6.81 (d, 2H, Ar), 3.86 (1H, s, NH). 13C NMR (DMSO-d6): δ 177.5, 156.3, 139.4, 131.1, 126.6, 120.2, 119.2, 115.9. APCI-MS (m/z) 194 [M + H]. Anal. calcd for C8H7N3OS: C, 49.73; H, 3.65; N, 21.75; Found: C, 49.68; H, 3.61; N, 20.71.
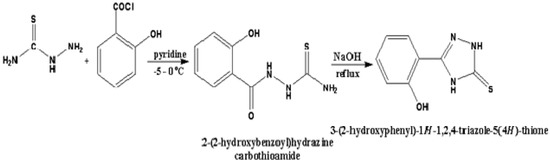
Scheme 1.
Schematic representation of synthesis of 3-(2-hydroxyphenyl)-1H-1,2,4-triazole-5(4H)-thione (HTT).
2.4. Functionalization of Amberlite XAD-16 with HTT
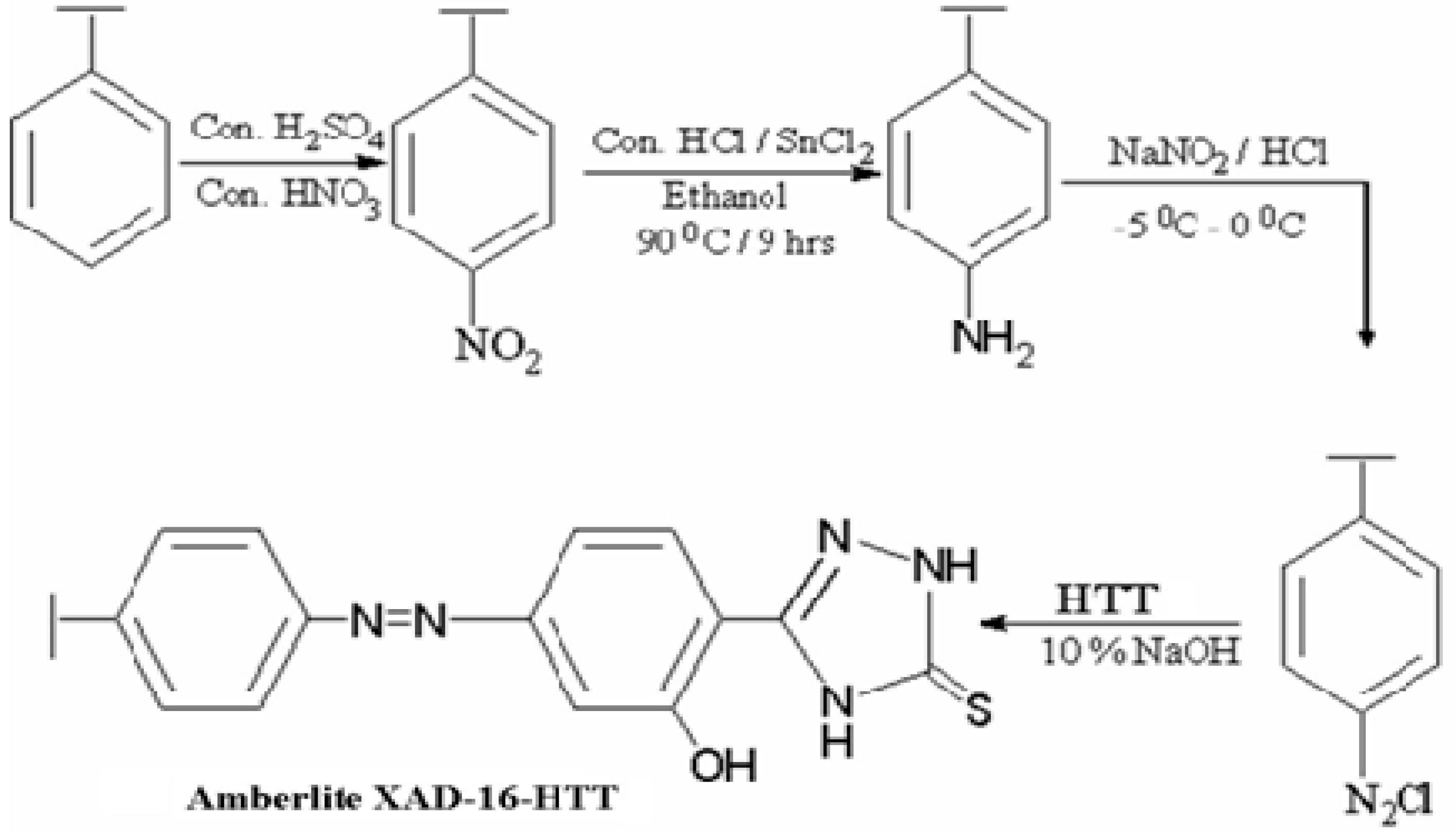
The resin was functionalized using the procedure reported in literature [20]. A nitrating mixture containing conc. HNO3 and conc. H2SO4 (10:25 mL v/v) was preparedin an ice bath. Then, a 5 g sample of Amberlite XAD-16 resin was slowly added to the mixture over a period of 30 min with stirring. The mixture was then heated to 60 °C in a temperature-controlled water bath and kept at this temperature for 1 h with stirring before being poured into ice-water. The nitrated resin was filtered and washed with water until the effluents yielded neutral pH. Then, it was reduced with 40 g SnCl2 in the presence of 50 mL ethanol and 45 mL HCl by refluxing at 90 °C for 9 h. The final product was filtered and washed sequentially using distilled water and 2 M NaOH to remove any impurity.
Then, 100 mL of 2 M HCl was used to treat the amino polymer (45 g) for 30 min, which was then washed to remove excess HCl before suspending in 250 mL of ice-cold water. The resin was next diazotized with an equimolar mixture of NaNO2 and 1 M HCl by adding 1 mL each time and testing the reaction mixture with starch-iodide paper. This was continued until a permanent blue color was obtained. To avoid temperature-induced degradation, the diazotized Amberlite XAD-16 was filtered at −5 to 0 °C and then treated with HTT (3 g in a mixture of 25 mL of 4% NaOH and 100 mL water) for 24 h in the temperature range of 0–5 °C. This produced dark brownish crimson colored beads, which were filtered and washed using 4 M HCl acid and double-distilled water before subjecting to vacuum-drying. Steps involved in the synthesis Amberlite XAD-16-HTT are depicted in Scheme 2.
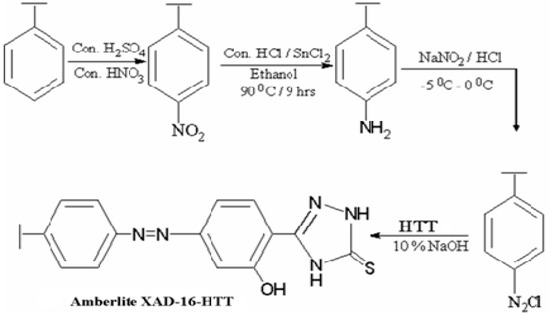
Scheme 2.
Functionalization of Amberlite XAD-16 with HTT.
2.5. Sample Collection
Five liters of surface water were obtained from Penner River belt as well as Bay of Bengal, Nellore District, Andhra Pradesh, India, filtered using membrane filters (0.45 μm) and kept at 4 °C until analysis.
2.6. Solid-Phase Extraction (SPE)Procedure for the Pre-Concentration and Determination of Metal Ions
Amberlite XAD-16 (500 mg) was functionalized with HTT by stirring in double-distilled water; it was poured into a 100 mm long glass column (10 mm i.d.) equipped with a disc and a stopcock at the bottom. To prevent the loss of resin beads during the sample loading, a small amount of glass wool was placed on the disk. Then, 2 M HCl was used to treat the column bed before washing with double-distilled water to remove any acid stain. An aliquot of the 500 mL of sample solution containing 50 μg/mL of Pb or Cd at pH 5 was flowed through the column before washing with double-distilled water. To strip bound metal ions from the packed bed of resins, 10 mL of 1 M HNO3 was used. The metal ion concentration in theelutes were determined by ICP-AES.
3. Results
3.1. Fourier Transform Infrared (FTIR) Spectral Analysis of HTT and Amberlite XAD-16-HTT
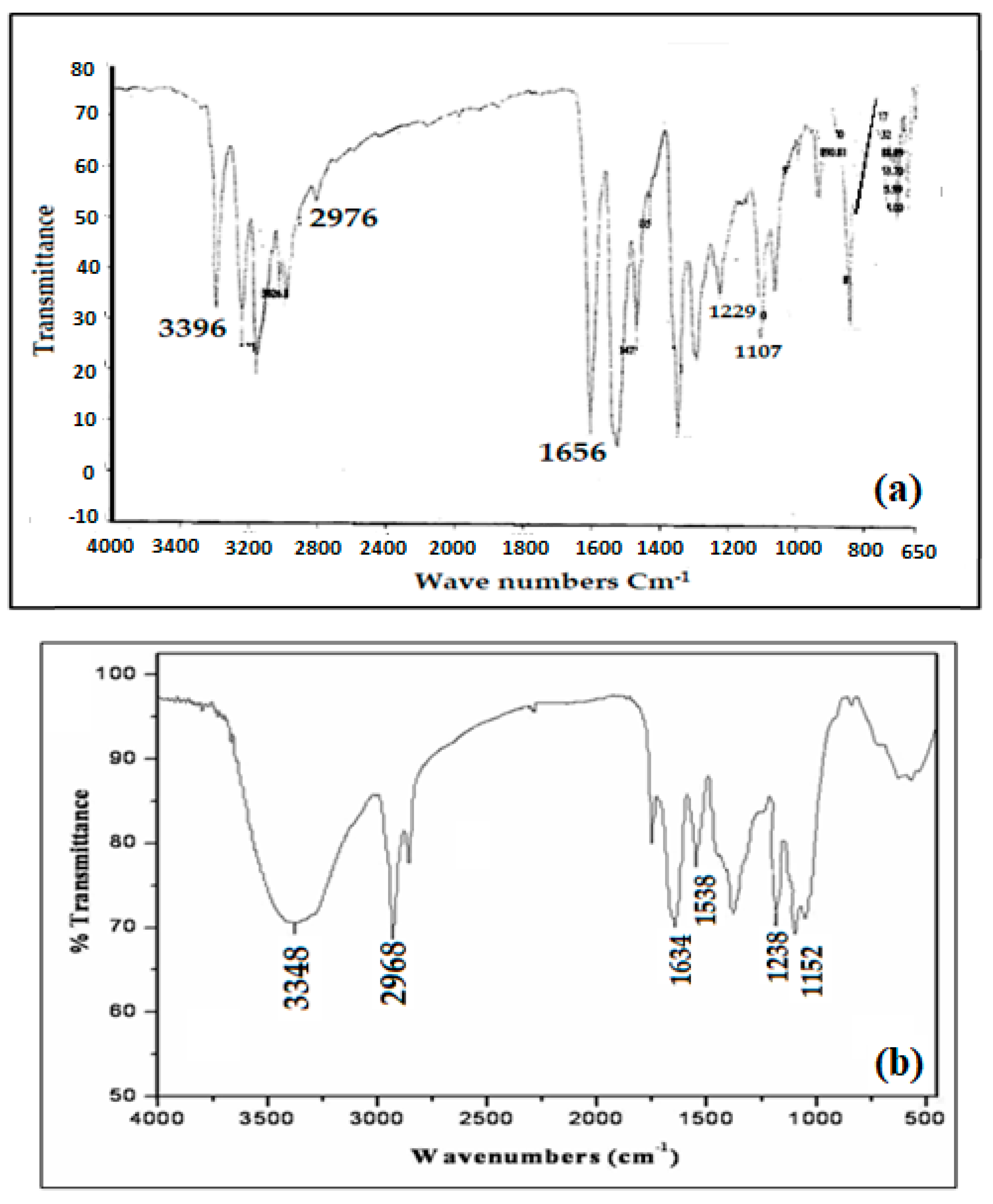
The structure of the functionalized Amberlite XAD-16-HTT was confirmed by FTIR analysis as shown in Figure 1a,b. The FTIR spectrum was recorded using a KBr pellet containing HTT and functionalized Amberlite XAD-16-HTT. The FTIR spectrum of HTT in Figure 1a showed bands at 3396, 2976, 1656, 1229, and 1107 cm−1 that can be attributed to N–H, Ar–H, C=S, C=N, and C–N stretching, respectively. On the other hand, in the FTIR spectrum of functionalized Amberlite XAD-16-HTT (Figure 1b), new additional bands of the modified resin appear at 3348, 2968, 1634, 1538, 1238, and 1152 cm−1, which can be attributed to N–H, Ar–H, C=S, N=N, C=N, and C–N stretching, respectively. Similarities between ligand (HTT) spectrum and that of modified resin confirm the functionalization of HTT onto Amberlite XAD-16.
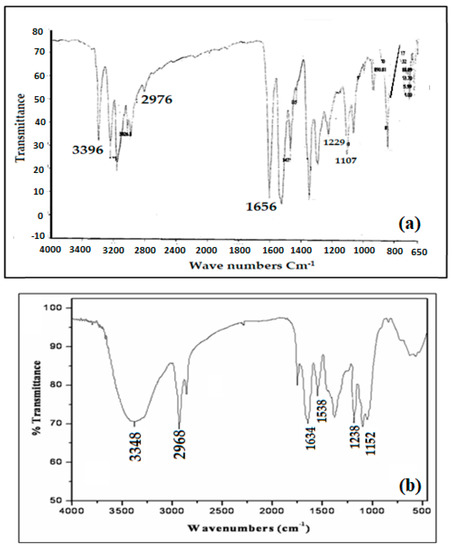
Figure 1.
Fourier transform infrared (FTIR) spectra of HTT (a,b) functionalized Amberlite XAD-16-HTT.
3.2. Effect of pH
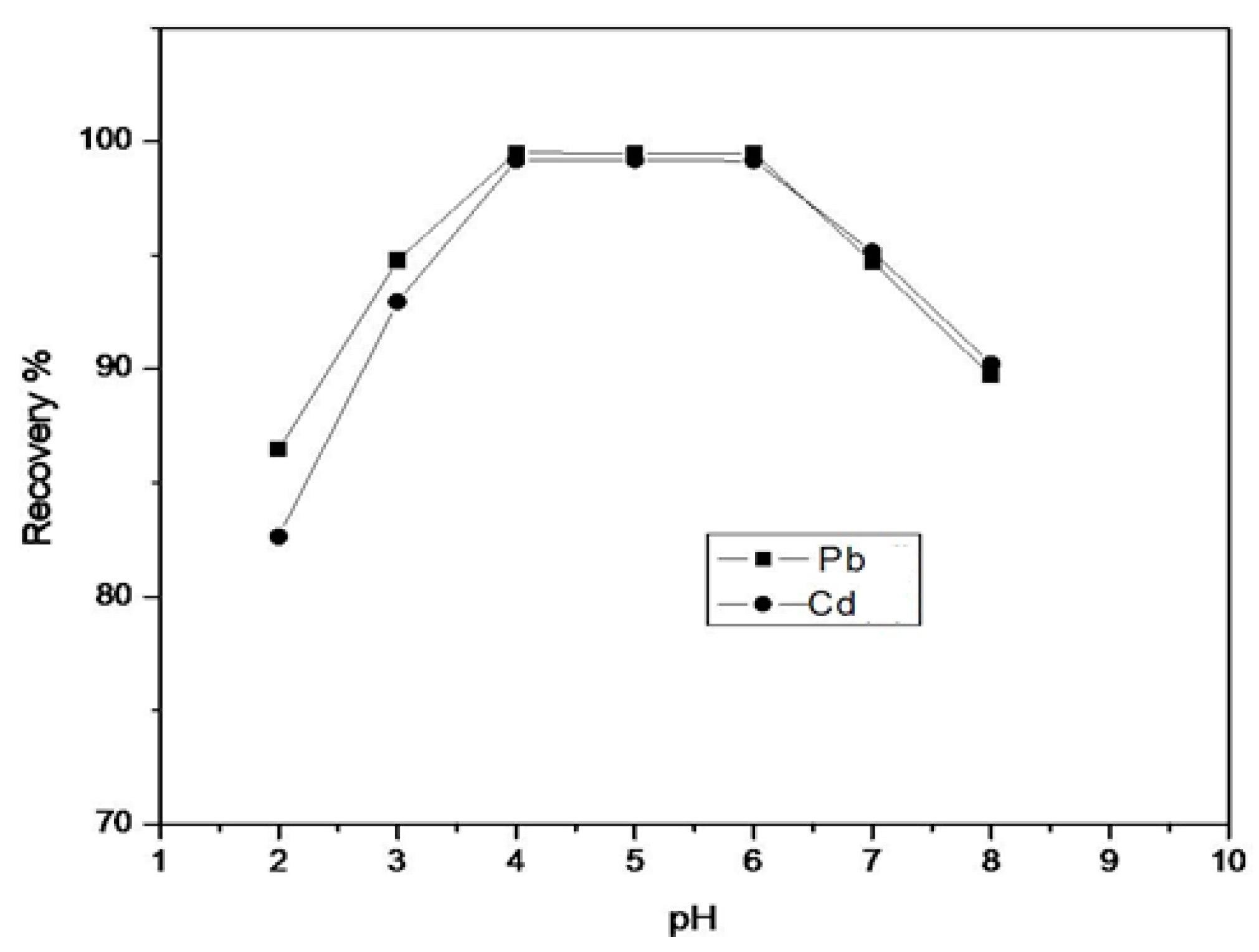
In SPE studies, the effect of pH on the uptake of Pb and Cd onto the resin functionalized with HTT was investigated using the column procedure. This consisted of placing 500 mg of functionalized resin in a column, through which 10 mL of the metal ion solution containing 5 µg/mL of mixed Pb and Cd was passed at a flow-rate of 2.5 mL/min. The pH of the aqueous solutionvaried from 2 to 8 using appropriate buffers. The experiments were carried out in triplicate. Figure 2 shows the data for the metal ion recovery. For maximum recovery, the optimum pH was found to be in the range of 4–6. It is evident from the figure that the highest recovery is achieved at pH 5. Hence, this was taken as the optimum pH for the SPE of metal ions.
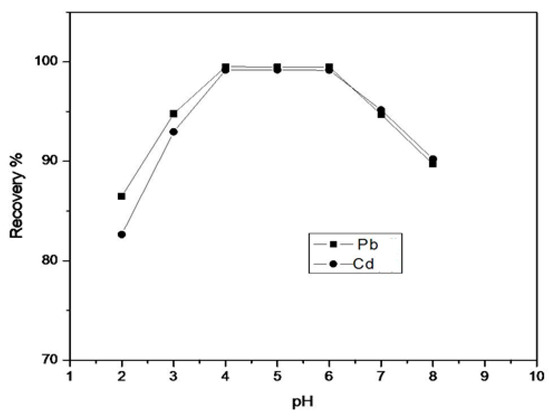
Figure 2.
Effect of pH on the percent recovery of metal ions.
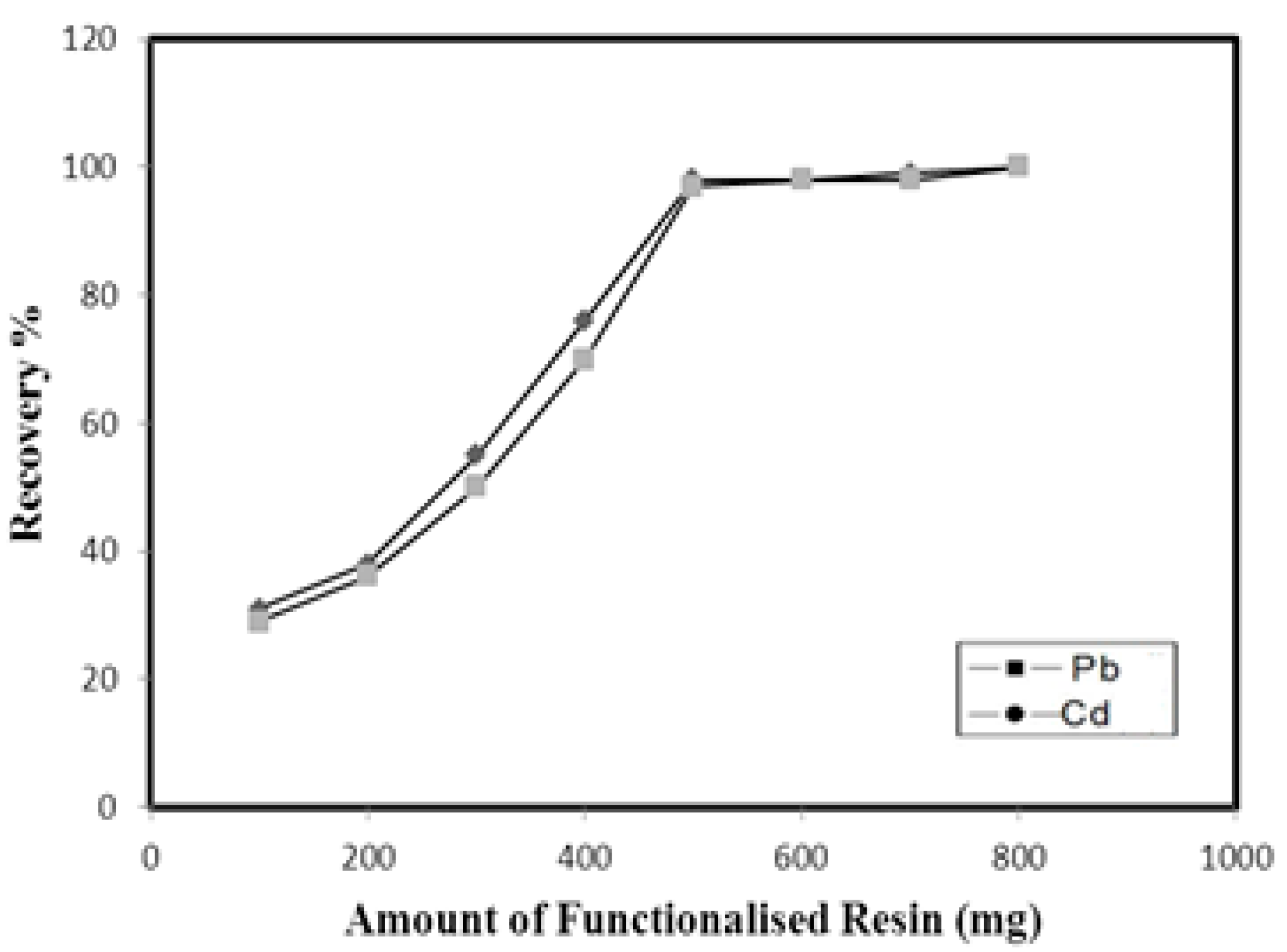
3.3. Effect of the Amount of Resin on the Pre-Concentration of Metal Ions
For the maximum recovery of metals in environmental samples, a sufficient amount of resin is needed to ensure the total retention of metals onto its surface. The amount of Amberlite XAD-16-HTT resin required for the quantitative retention of Pb and Cd was studied by varying the amount of Amberlite XAD-16-HTT resin (100–800 mg) filled in the column. The maximum recoveries of all the analytes were achieved when 500 mg of resin was used in the column. Therefore, 500 mg of Amberlite XAD-16-HTT resin was used for subsequent experiments. The results are shown in Figure 3.
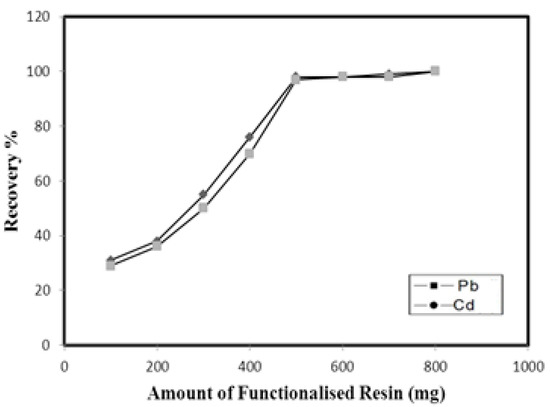
Figure 3.
Effect of amount of functionalized resin on recovery of metal ions.
3.4. Effect of Flow Rate
The effect of the flow rate on the metal ion recovery was studied by varying it between 1–5 mL/min. The optimum flow rate for the quantitative sorption of metal ions on the resin was 2.5 mL/min, since the recovery of metal ions decreased at higher flow-rates. This decrease in the recovery can be attributed to the low residence time, resulting in insufficient contact of metal ions with the functionalized resin. Lower flow rates (<1 mL/min) were not studied to avoid long analysis times. The flow rate of the sample solution was therefore kept at 2.5 mL/min.
3.5. Eluent Selection and Concentration Optimization
Various acids were screened to choose the best eluent for the desorption of metal ions from Amberlite XAD-16-HTT. Eluents such as 1 M HCl and 1 M HNO3were used for desorbing the adsorbed Pb and Cd ions. For each sample, the recovery percentages of Pb and Cd ions were computed. Results are presented in Table 1 for different concentration of acids. Clearly, 1 M HNO3 solution is the most suitable eluent, which was therefore selected for the desorption of metal ions.

Table 1.
Recovery of trace metal ions, Pb and Cd using various eluents.
3.6. Effect of Sample Volume
We also investigated the effect of sample volume on the elution of Pb and Cd by choosing different volumes of water in the range 100–1000 mL (Amberlite XAD-16-HTT resin 500 mg). Results are shown in Table 2. Clearly, recoveries were rather comparable up to 500 mL of aqueous phase (pre-concentration factor ~50) for both cases. Higher sample volumes (>500 mL), however, led to a decrease in the recovery. Hence, a sample volume of 500 mL was used for all further studies.

Table 2.
Effect of sample volume on recovery of metal ions.
3.7. Sorption Capacity
We analyzed the sorption capacity of Amberlite XAD-16-HTT resin using the batch method. 1 g resin was saturated with a mixed Pb and Cd solution of 50 μg/mL concentration by equilibrating using a mechanical shaker. The solid was then filtered and subsequently washed with distilled water. The metal concentrations in the supernatant liquid were determined by ICP-AES. By applying material balance, the mass of metals sorbed onto Amberlite XAD-16-HTT resin wascomputed. The sorption capacities of resin for Pd and Cd were 5.46 and 5.21 mg/g, respectively.
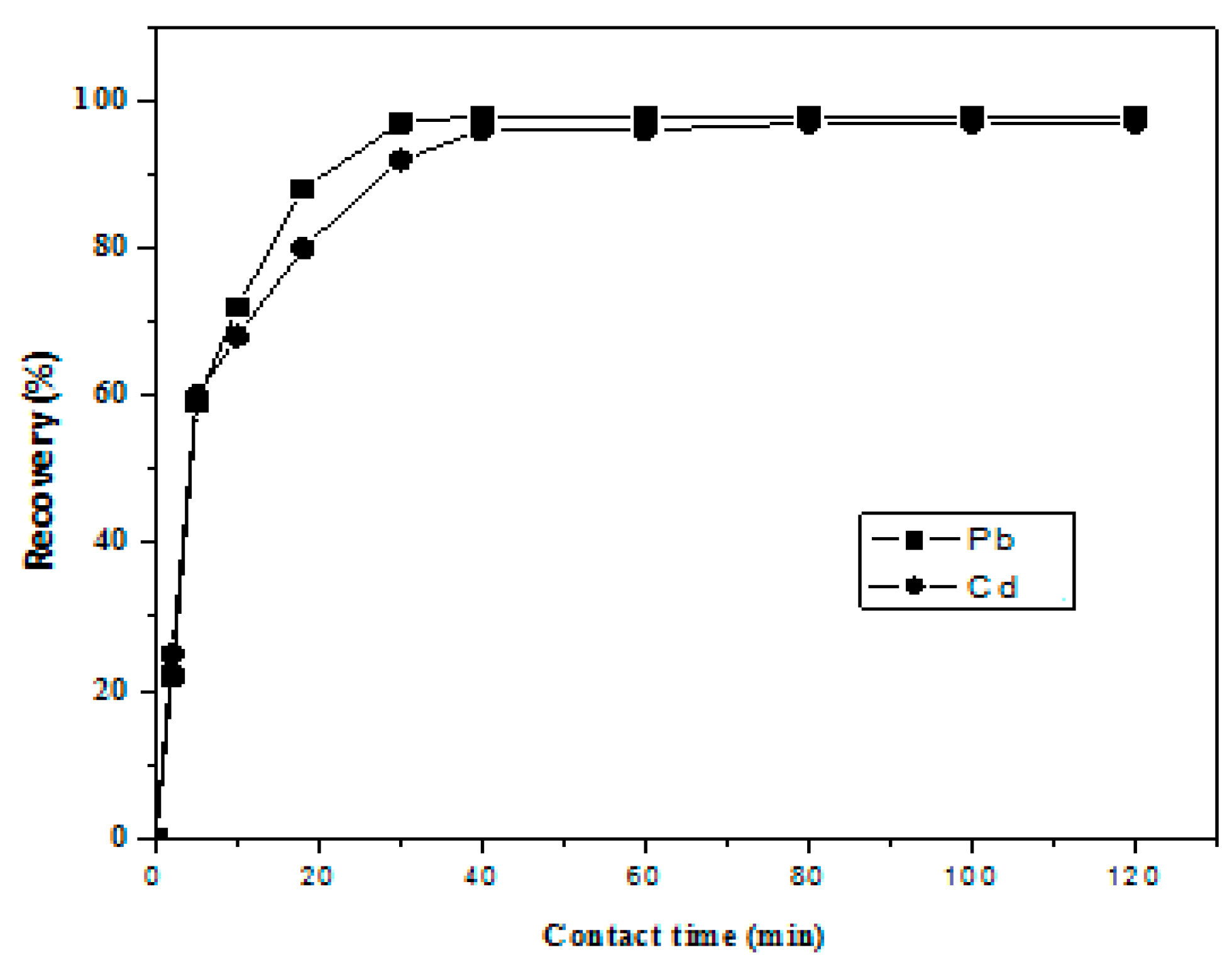
3.8. Sorption Kinetics
Sorption kinetics is of crucial importance since it yields information about the sorption rate of metals onto reagent loaded resin during the dynamic column mode analysis of water, so we employed a batch method to study the uptake rate of metal ions on Amberlite XAD-16-HTT. At 30 ± 0.1 °C, 50 µg/mL of individual metal ion solutions wasadded to 1 g mass of resin, the resulting mixture was stirred for 2, 5, 10, 20, 30, 40, 50, 60, 90, and 120 min. Then, 1 M HNO3 was used to elute metal ions from the resin surface for analysis using ICP-AES. The results, presented in Figure 4, were used to determine the loading half-time (t1/2), which is the time required to reach 50% sorption of total metals present in the solution. Its value was less than 5 min for both cases; 4.4 and 4.1 min for Pb and Cd, respectively [20]. These results clearly indicate the rapid sorption kinetics in the present case.
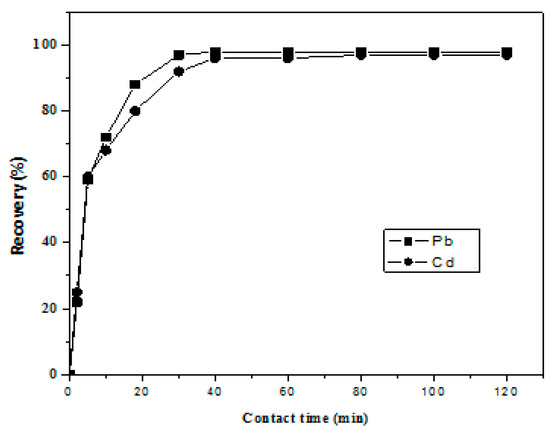
Figure 4.
Kinetics of sorption Pb and Cd ions on Amberlite XAD-16-HTT resin.
3.9. Effect of Matrix Ions
We also examined the effect of matrix ions on the pre-concentration of analytes using optimized conditions. The tolerance limit of matrix ions was taken as the value that causedless than 5% error in the reading. It is evident from results presented in Table 3 that the common cations and anions present in natural water do not affect the pre-concentration of Pb and Cd. However, the presence of chelators in the water can affect the accuracy of measurement due to the formation of metal-chelating agent complex [31].

Table 3.
Tolerance limits of matrix ions for Pb and Cd determination (n = 5).
3.10. Resin Stability and Reusability
We examined the stability of the present resin (Amberlite XAD-16-HTT) using 1–6 M HNO3 by shaking it for 4 h with acid solutions of varying concentrations before filtering. The treated resin was washed to remove acid and air-dried, and its sorption capacity was determined. The difference between the sorption capacity of the acid-treated resin with that of the untreated resin was less than 2.8%. Clearly, the effectiveness of the present resin is not compromised even with highly concentrated acids (as high as 6 M). Moreover, multiple sorption-desorption batch cycles using the same resin was also carried out to examine its reusability. Even several sorption–desorption cycles hardly affected its sorption capacity. A capacity degradation of less than 1.4% was observed after 10 cycles.
3.10.1. Accuracy of the Method
The accuracy of the present technique was assessed by analyzing the metal ions that were present in NIST 1643e Water and the results, shown in Table 4, clearly show good agreement with the certified values. It also confirms that the procedure developed for the determination of metal ions is free of interferences.

Table 4.
Recovery of Pb and Cd from standard reference materials after pre-concentration on Amberlite XAD-16-HTT resin.
3.10.2. Detection of Trace Metal Ions in Water Samples
We also collected natural water samples from Pennar River (Nellore district, AP, India) and Bay of Bengal, Indian Ocean to determine the Pb and Cd ions concentration using the present analytical technique. The results shown in Table 5 indicate that the concentrations of Pb and Cd are in the ranges of 7.40–15.48 and 0.74–7.14 μg/L, respectively.

Table 5.
Determination of Pb and Cd ions in different water samples (n = 5).
3.10.3. Analytical Features
The recovery and precision of the proposed technique were determined by carrying out successive retention and elution cycles under optimum conditions, which were comprised of using 500 mL of a sample solution containing 50 μg/mL of Pb and Cd. The results are presented in Table 6. Recoveries were more than 99% for both cases while the standard deviation was 1.8% and 2.3% for lead and cadmium, respectively. On the other hand, the detection limits (LOD), which is the concentration equivalent to three times the standard deviation of the reagent blank (1 M HNO3) of 10 measurements, were found to be 0.16 and 0.22 μg/L for Pb and Cd, respectively.

Table 6.
Key analytical parameters of Pb and Cd.
3.10.4. Comparison with Other Methods
A comparison of the proposed method with other SPE methods is summarized in Table 7. The proposed SPE method developed using a new analytical reagent without any carrier element has relatively high pre-concentration factors, lowRSDs (Relative Standard Deviation), and low (Detection Limit) DLs compared to other methods reported in the literature.

Table 7.
Comparative data from some recent studies on off-line solid-phase extraction (SPE) systems based on the use of Amberlite resins.
4. Conclusions
The present SPE method, based on the functionalized Amberlite XAD-16-HTT, is applicable for the separation and pre-concentration of Pb and Cd in water samples for their analysis by ICP-AES. The optimum experimental conditions, e.g., the effect of pH, sample volume, flow rate, eluent concentration, sorption capacity, sorption kinetics etc., were investigated. Both metal ions were effectively retained by the functionalized resin at pH 5, and desorbed using 10 mL of 1 M HNO3. The functionalized chelating resin Amberlite XAD-16-HTT has the following advantages: stable in an acidic medium, good resin-loading capacity, very short loading half-time (t1/2), and faster exchange kinetics during the metal ion uptake. The reusability of the resin was tested in more than 10 cycles without affecting its sorption capacity. The developed SPE method has the advantage of a high enrichment factor in addition to a high tolerance limits for foreign ions and low detection limits for both Pb and Cd. This proposed method exhibits high tolerance limits for matrix ions and is effective for the pre-concentration and determination of Pb and Cd ions in water samples.
Acknowledgments
The authors from King Saud Universityappreciates the support from the Deanship of Scientific Research at the King Saud University for theResearch Group, RGP-1437-003.
Author Contributions
Nadavala Siva Kumar, Vummiti Dharmendra, Vudagandla Sreenivasulu and Mohammad Asif conceived, designed and supervised the experiments and put together the initial draft of the paper; Vummiti Dharmendra performed the experiments; Ahmed A. Ibrahim and Vysetti Balaram carried out the literature review; Mohammad Asif and Nadavala Siva Kumar analyzed the data, finalized the paper and Nadavala Siva Kumar was the main corresponding author during the review process.
Conflicts of Interest
The authors declare no conflict of interest.
References
- US Environmental Protection Agency. SW846 On-Line, Methods for Chemical Analysis of Water and Wastes. Available online: https://www.epa.gov/hw#table (accessed on 19 January 2017).
- Balaram, V. Recent advances in the determination of elemental impurities in pharmaceuticals—Status, challenges and moving frontiers. Trends Anal. Chem. 2016, 80, 83–95. [Google Scholar] [CrossRef]
- Murata, I.; Hirono, T.; Saeki, Y.; Nakagawa, S. Cadmium enteropathy, renal osteomalacia (“ItaiItai” disease in Japan). Bull.Soc. Int. Chir. 1970, 29, 34–42. [Google Scholar] [PubMed]
- Spencer, D.W. Ocean disposal reconsidered. Oceanus 1990, 33, 4–12. [Google Scholar]
- Pereira, M.D.; Arruda, M.A.Z. Trends in preconcentration procedures for metal determination using atomic spectrometry techniques. Microchim. Acta 2003, 141, 115–131. [Google Scholar] [CrossRef]
- Ghaedi, M.; Fathi, M.R.; Shokrollahi, A.; Shajarat, F. Highly selective and sensitive preconcentration of mercury ion and determination by cold vapor atomic absorption spectroscopy. Anal. Lett. 2006, 39, 1171–1185. [Google Scholar] [CrossRef]
- Manjusha, R.; Dash, K.; Karunasagar, D. UV-photolysis assisted digestion of food samples for the determination of selenium by electrothermal atomic absorption spectrometry (ETAAS). Food Chem. 2007, 105, 260–265. [Google Scholar] [CrossRef]
- Mashhadizadeh, M.H.; Pesteh, M.; Talakesh, M.; Sheikhshoaie, I.; Ardakani, M.M.; Karimi, M.A. Solid phase extraction of copper(II) by sorption on octadecyl silica membrane disk modified with a new Schiff base and determination with atomic absorption spectrometry. Spectrochim. Acta Part B 2008, 63, 885–888. [Google Scholar] [CrossRef]
- Lemos, V.A.; DaSilva, D.G.; DeCarvalho, A.L.; Santana, D.D.; Novaes, G.D.; DosPassos, A.S. Synthesis of amberlite XAD-2-PC resin for preconcentration and determination of trace elements in food samples by flame atomic absorption spectrometry. Microchem. J. 2006, 84, 14–21. [Google Scholar] [CrossRef]
- Da-Col, J.A.; Domene, S.M.A.; Pereira-Filho, E.R. Fast determination of Cd, Fe, Pb, and Zn in food using AAS. Food Anal. Methods 2009, 2, 110–115. [Google Scholar] [CrossRef]
- Hammer, D.; Nicolas, M.; Andrey, D. Improved chromium determination in various food matrices using dynamic reaction cell ICP-MS. Atom.Spectrosc. 2005, 26, 203–208. [Google Scholar]
- Nardi, E.P.; Evangelista, F.S.; Tormen, L.; Pierre, T.D.S.; Curtius, A.J.; De-Souza, S.S.; Barbosa, F. The use of inductively coupled plasma mass spectrometry (ICP-MS) for the determination of toxic and essential elements in different types of food samples. Food Chem. 2009, 112, 727–732. [Google Scholar] [CrossRef]
- He, Q.; Chang, X.J.; Huang, X.P.; Hu, Z. Determination of trace elements in food samples by ICP-AES after preconcentration with p-toluenesulfonylamide immobilized on silica gel and nanometer SiO2. Microchim. Acta 2008, 160, 147–152. [Google Scholar] [CrossRef]
- Balaram, V.; Anjaiah, K.V.; Reddy, M.R.P. A comparative study on the trace and rare earth element analysis of an Indian polymetallic nodule reference sample by inductively coupled plasma atomic emission spectrometry and inductively coupled plasma mass spectrometry. Analyst 1995, 120, 1401–1406. [Google Scholar] [CrossRef]
- Balaram, V. Developments and trends in inductively coupled plasma mass spectrometry and its influence on the recent advances in trace metal analysis. Curr. Sci. 1995, 69, 640–649. [Google Scholar]
- Malvanker, P.L.; Shinde, V.M. Ion-pair extraction and determination of copper(II) and zinc(II) in environmental and pharmaceutical samples. Analyst 1991, 116, 1081–1084. [Google Scholar] [CrossRef]
- Divrikli, U.; Elci, L. Determination of some trace metals in water and sediment samples by flame atomic absorption spectrometry after coprecipitation with cerium(IV) hydroxide. Anal. Chim. Acta 2002, 452, 231–235. [Google Scholar] [CrossRef]
- Dumont, J.; Cote, M.; Hubert, J. Preconcentration of trace elements in aluminum alloys using a chelating ion-exchanger and determination by ICP-AES. Appl. Spectrosc. 1989, 43, 1132–1135. [Google Scholar] [CrossRef]
- Naresh Kumar, B.; Harinath, Y.; Seshaiah, K. Separation and preconcentration of Cd(II), Cu(II), Ni(II), and Pb(II) in water and food Samples using Amberlite XAD-2 functionalized with 3-(2-Nitrophenyl)-1H-1,2,4-triazole-5(4H)-thione and determination by Inductively Coupled Plasma–Atomic Emission Spectrometry. J. Agric. Food Chem. 2011, 59, 11352–11358. [Google Scholar]
- Suneetha, Y.; Naresh Kumar, B.; Harinath, Y.; Reddy, D.H.K.; Seshaiah, K. Functionalization of cross linked chitosan with 2-aminopyridine-3-carboxylic acid for solid phase extraction of cadmium and zinc ions and their determination by atomic absorption spectrometry. Microchim. Acta 2012, 176, 169–176. [Google Scholar] [CrossRef]
- Kumar, B.N.; Harinath, Y.; Sathyanarayana, B.; Suneeta, Y.; Seshaiah, K. Solid phase extraction of trace metals in water and leafy vegetables using a resin functionalized with potassium 2-benzoylhydrazinecarbodithioate and determination by ICP–AES. Int. J. Environ. Anal. Chem. 2012, 92, 1341–1351. [Google Scholar] [CrossRef]
- Tsogas, G.Z.; Giokas, D.L.; Vlessidis, A.G. Graphite furnace and hydride generation atomic absorption spectrometric determination of cadmium, lead, and tin traces in natural surface waters: Study of preconcentration technique performance. J. Hazard. Mater. 2009, 163, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Sabarudin, A.; Noguchi, O.; Oshima, M.; Higuchi, K.; Motomizu, S. Application of chitosan functionalized with 3,4-dihydroxy benzoic acid moiety for on-line preconcentration and determination of trace elements in water samples. Microchim. Acta 2007, 159, 341–348. [Google Scholar] [CrossRef]
- Gentscheva, G.; Tzvetkova, P.; Vassileva, P.; Lakov, L.; Peshev, O.; Ivanova, E. Analytical characterization of a silica gel sorbent with thioetheric sites. Microchim. Acta 2006, 156, 303–306. [Google Scholar] [CrossRef]
- Lemos, V.A.; Baliza, P.X.; Santos, J.S. Synthesis of α-Nitroso-β-Naphthol modified Amberlite XAD-2 resin and its application in on-line solid phase extraction system for Cobalt pre-concentration. Sep. Sci. Technol. 2004, 39, 3317–3330. [Google Scholar] [CrossRef]
- Narin, I.; Soylak, M.; Kayakirilmaz, K.; Elci, L.; Dogan, M. Preparation of chelating resin by immobilizing 1-(2-pyridylazo) 2-naphtol on Amberlite XAD-16 and its application of solid phase extraction of Ni(II), Cd(II), Co(II), Cu(II), Pb(II), and Cr(III) in natural water samples. Anal. Lett. 2003, 36, 641–658. [Google Scholar] [CrossRef]
- Ramesh, A.; Mohan, K.R.; Seshaiah, K. Preconcentration of trace metals on Amberlite XAD-4 resin coated with dithiocarbamates and determination by inductively coupled plasma-atomic emission spectrometry in saline matrices. Talanta 2002, 57, 243–252. [Google Scholar] [CrossRef]
- Cuekicu, S.D.; Filik, H.; Apak, R. Use of an o-aminobenzoic acid-functionalized XAD-4 copolymer resin for the separation and preconcentration of heavy metal (II) ions. Anal. Chim. Acta 2004, 505, 15–24. [Google Scholar]
- Guo, Y.; Din, B.; Liu, Y.; Chang, X.; Meng, S.; Tian, M. Preconcentration of trace metals with 2-(methylthio)aniline-functionalized XAD-2 and their determination by flame atomic absorption spectrometry. Anal. Chim. Acta 2004, 504, 319–324. [Google Scholar] [CrossRef]
- Lemos, V.A.; Baliza, P.X. Amberlite XAD-2 functionalized with 2-aminothiophenol as a new sorbent for on-line preconcentration of cadmium and copper. Talanta 2005, 67, 564–570. [Google Scholar] [CrossRef] [PubMed]
- North, A.E.; Sarpong-Kumankomah, S.; Bellavie, A.R.; White, W.M.; Gailer, J. Environmentally relevant concentrations of aminopolycarboxylate chelating agents mobilize Cd from humic acid. J. Environ. Sci. 2017, 57, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Narin, I.; Soylak, M.; Elci, L.; Dogan, M. Separation and enrichment of chromium, copper, nickel and lead in surface seawater samples on a column filled with Amberlite XAD-2000. Anal. Lett. 2004, 34, 1935–1947. [Google Scholar] [CrossRef]
- Uzun, A.; Soylak, M.; Elci, L. Preconcentration and separation with Amberlite XAD-4 resin; determination of Cu, Fe, Pb, Ni, Cd and Bi at trace levels in waste water samples by flame atomic absorption spectrometry. Talanta 2001, 54, 197–202. [Google Scholar] [CrossRef]
- Rao, G.P.C.; Seshaiah, K.; Rao, Y.K.; Wang, M.C. Solid phase extraction of Cd, Cu, and Ni from leafy vegetables and plant leaves using Amberlite XAD-2 functionalized with 2-Hydroxy-acetophenone-thiosemicarbazone (HAPTSC) and determination by inductively coupled plasma atomic emission spectroscopy. J. Agric. Food Chem. 2006, 54, 2868–2872. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).