Recovery of Iron from Pyrolusite Leaching Slag by a Lab-Scale Circulation Process of Oxalic Acid Leaching and Ultraviolet Irradiation
Abstract
:1. Introduction
2. Materials and Methods
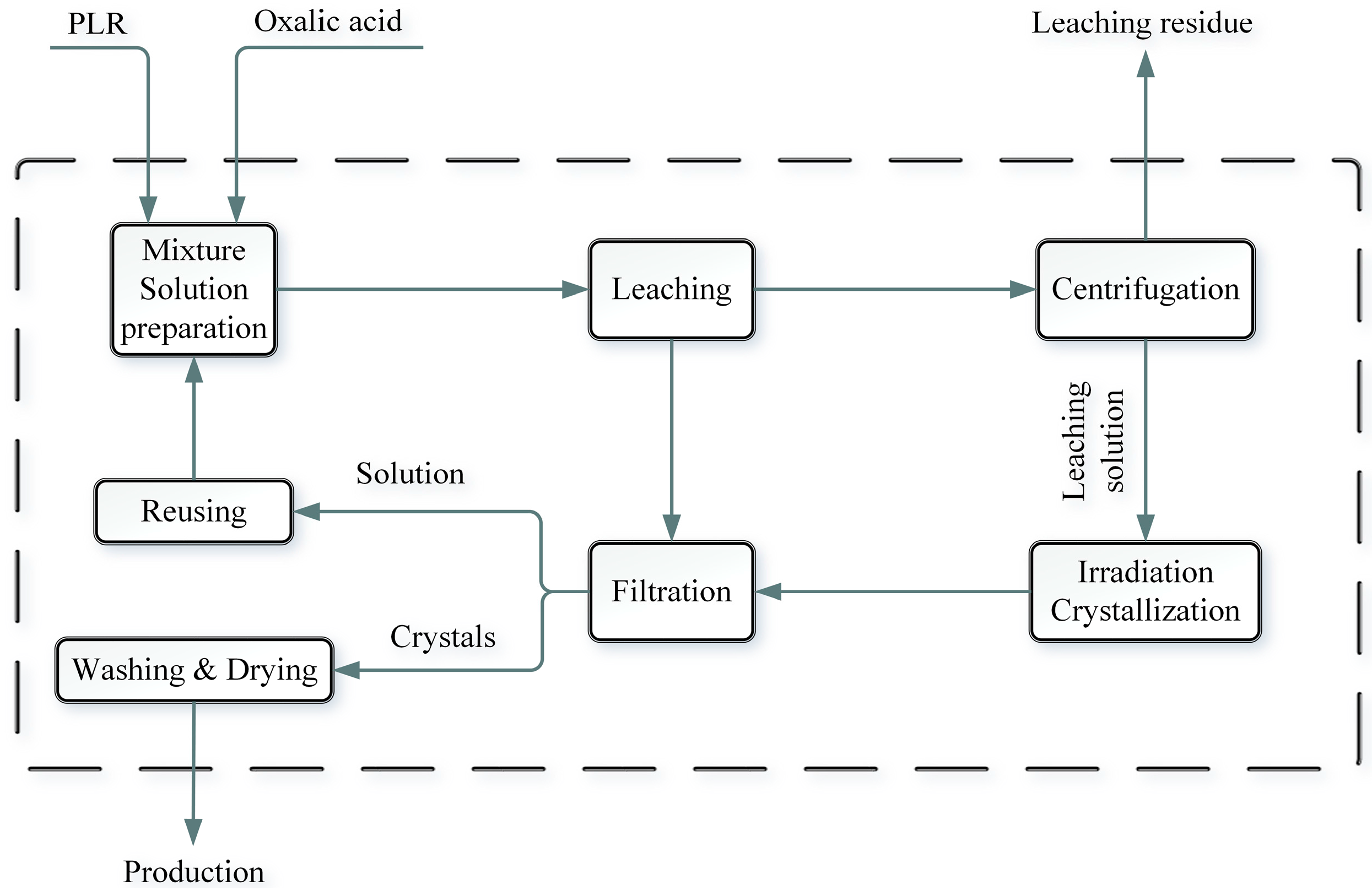
2.1. Experimental Flow
2.1.1. Leaching of Slag with Oxalic Acid
2.1.2. Irradiation Experiments
2.2. Materials
2.3. Operation Parameters
2.4. Sample Analysis
3. Results
3.1. Analysis of Slag
3.2. Leaching of Slag with Oxalic Acid
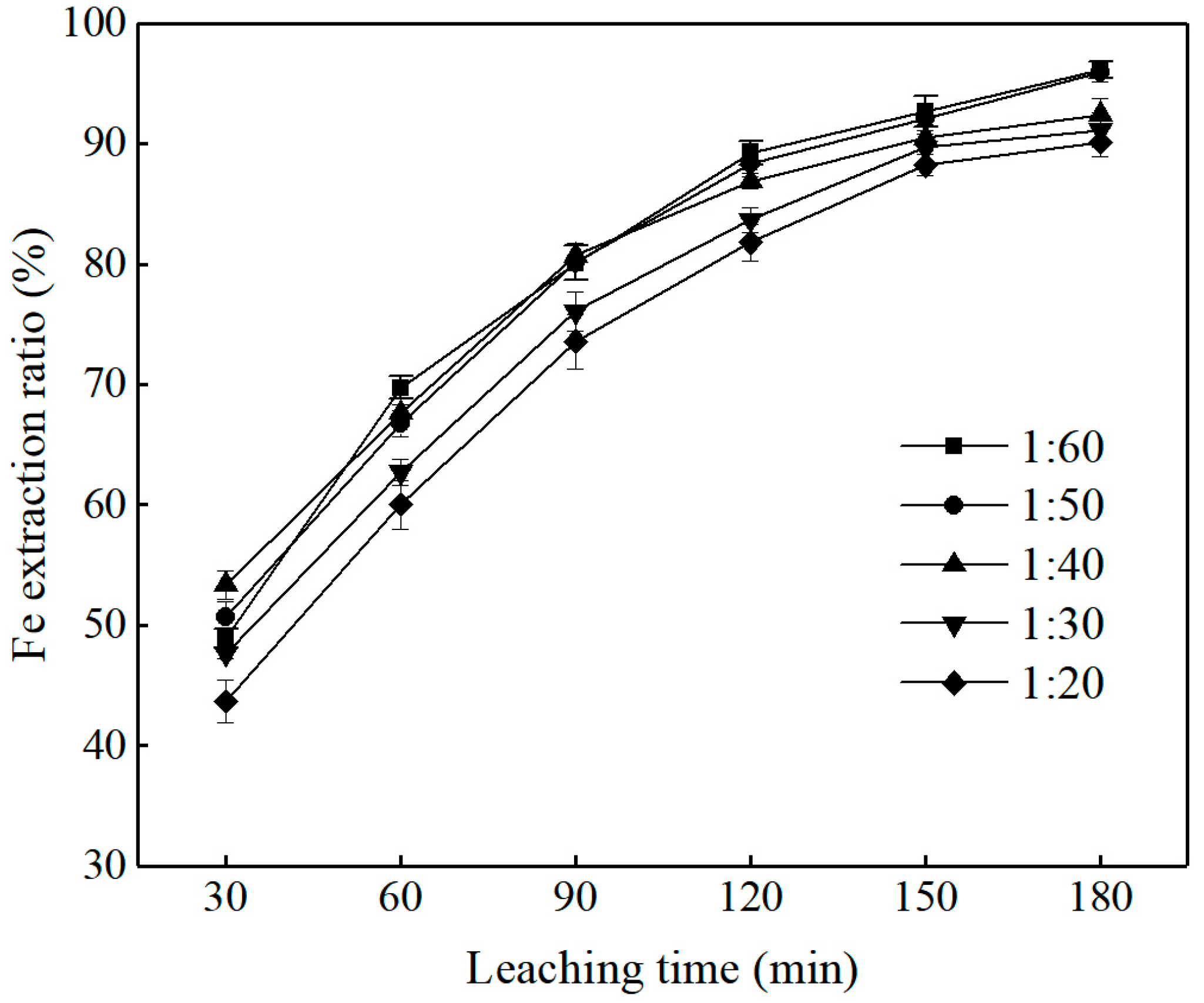
3.2.1. Effect of Solid/Liquid(S/L) Ratio
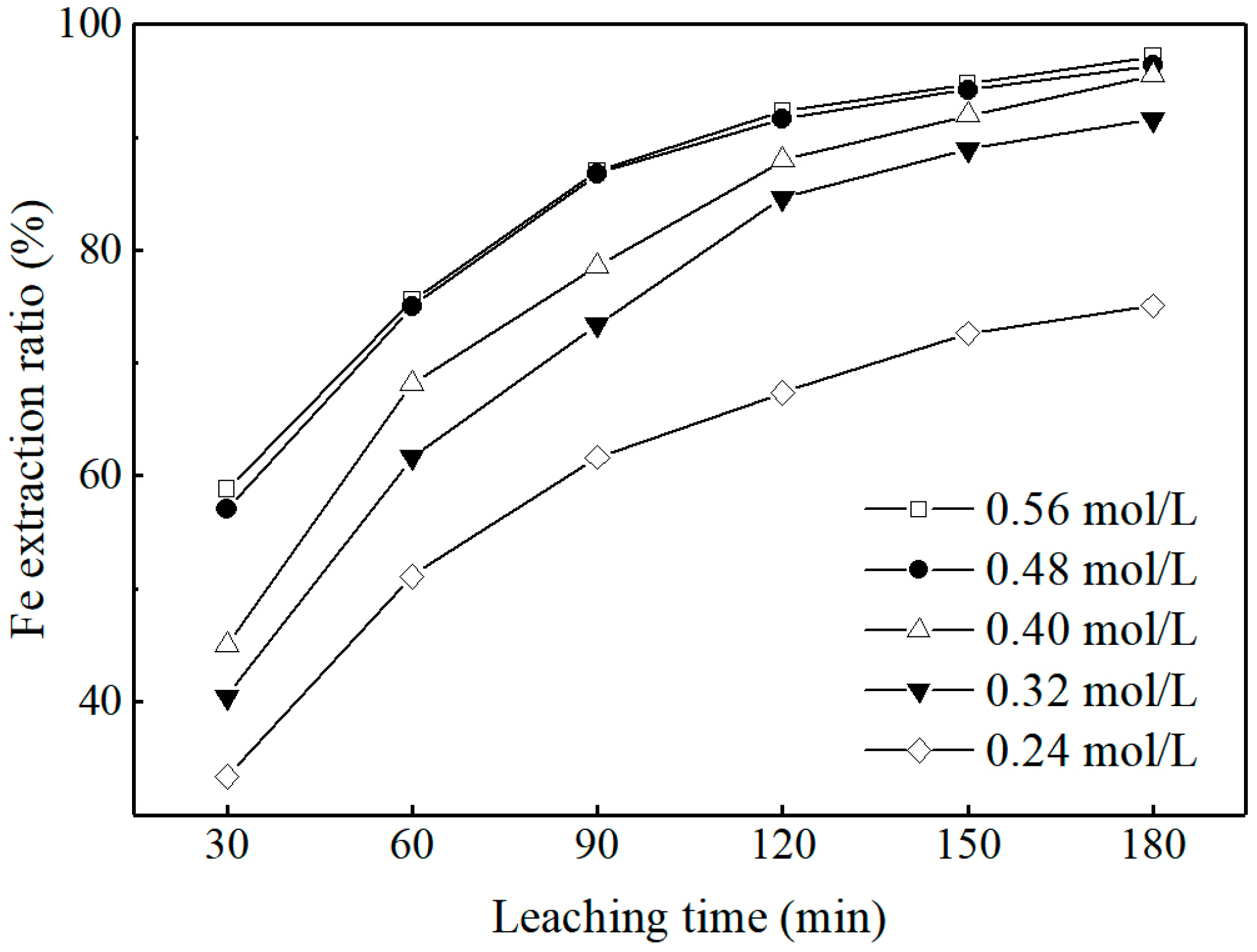
3.2.2. Effect of Oxalic Acid Concentration
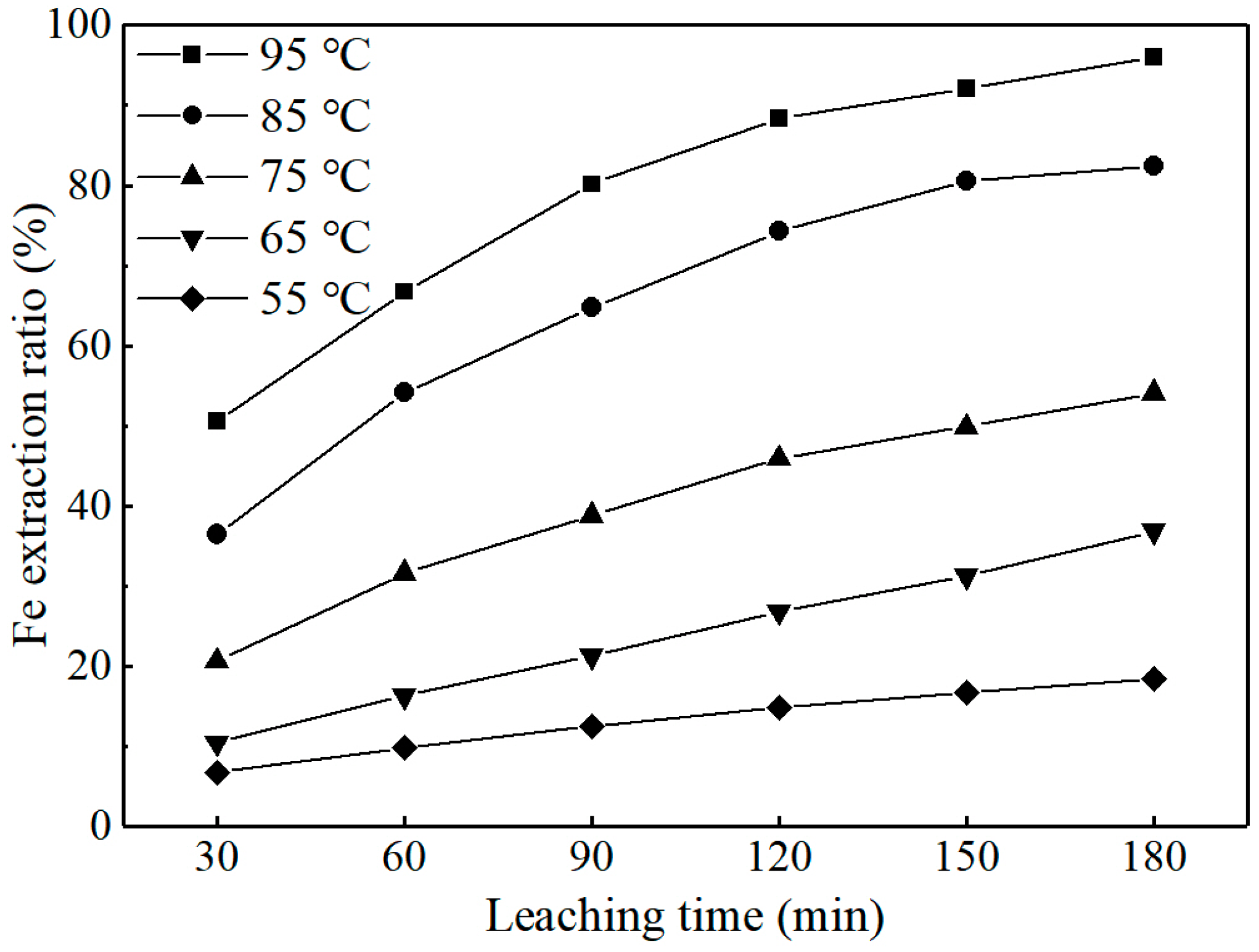
3.2.3. Effect of Leaching Temperature
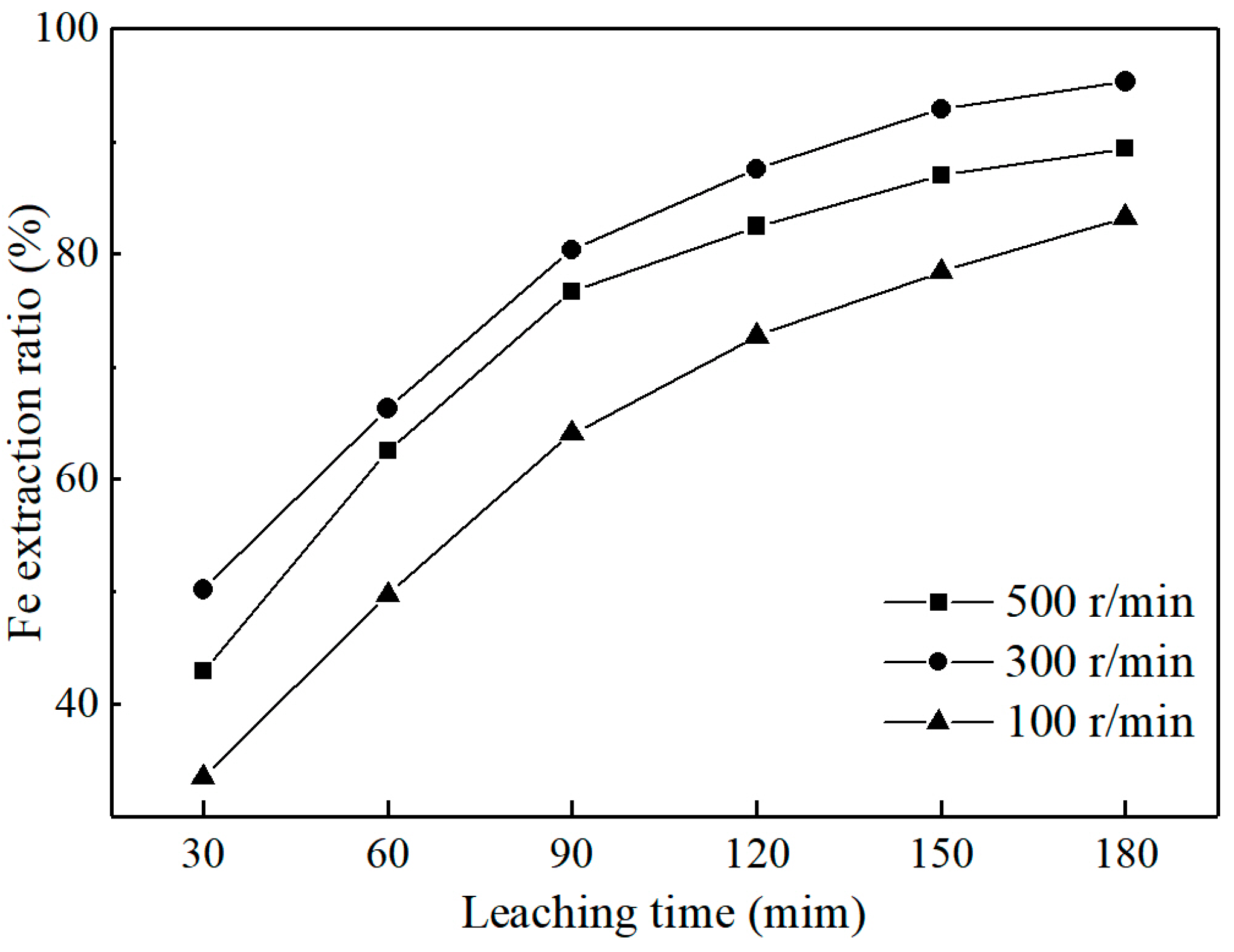
3.2.4. Effect of Stirring Rate
3.3. Irradiation of [Fe(C2O4)n](3−2n)+ Solution with Ultraviolet Light
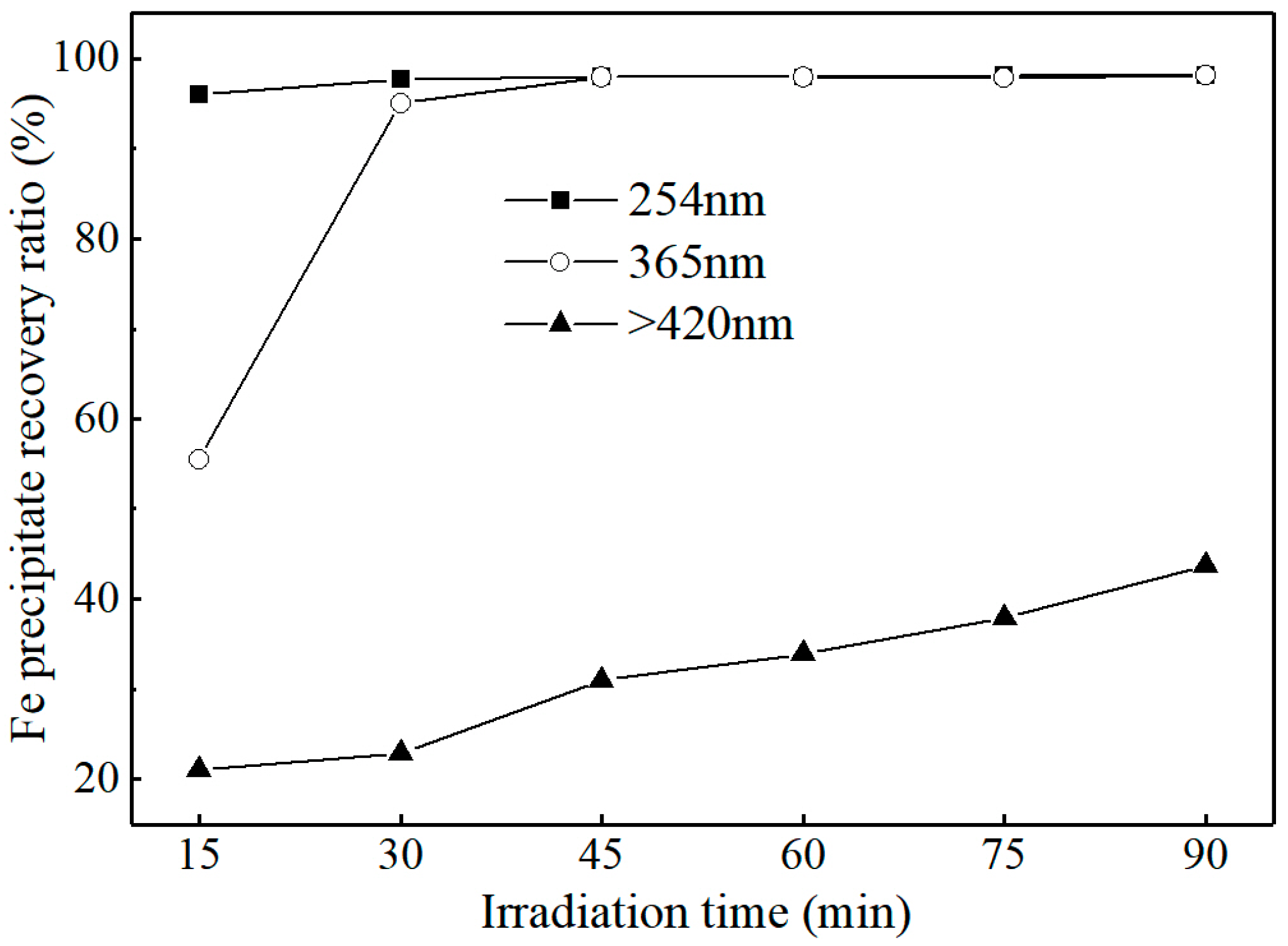
3.3.1. Effect of Irradiation Wavelengths
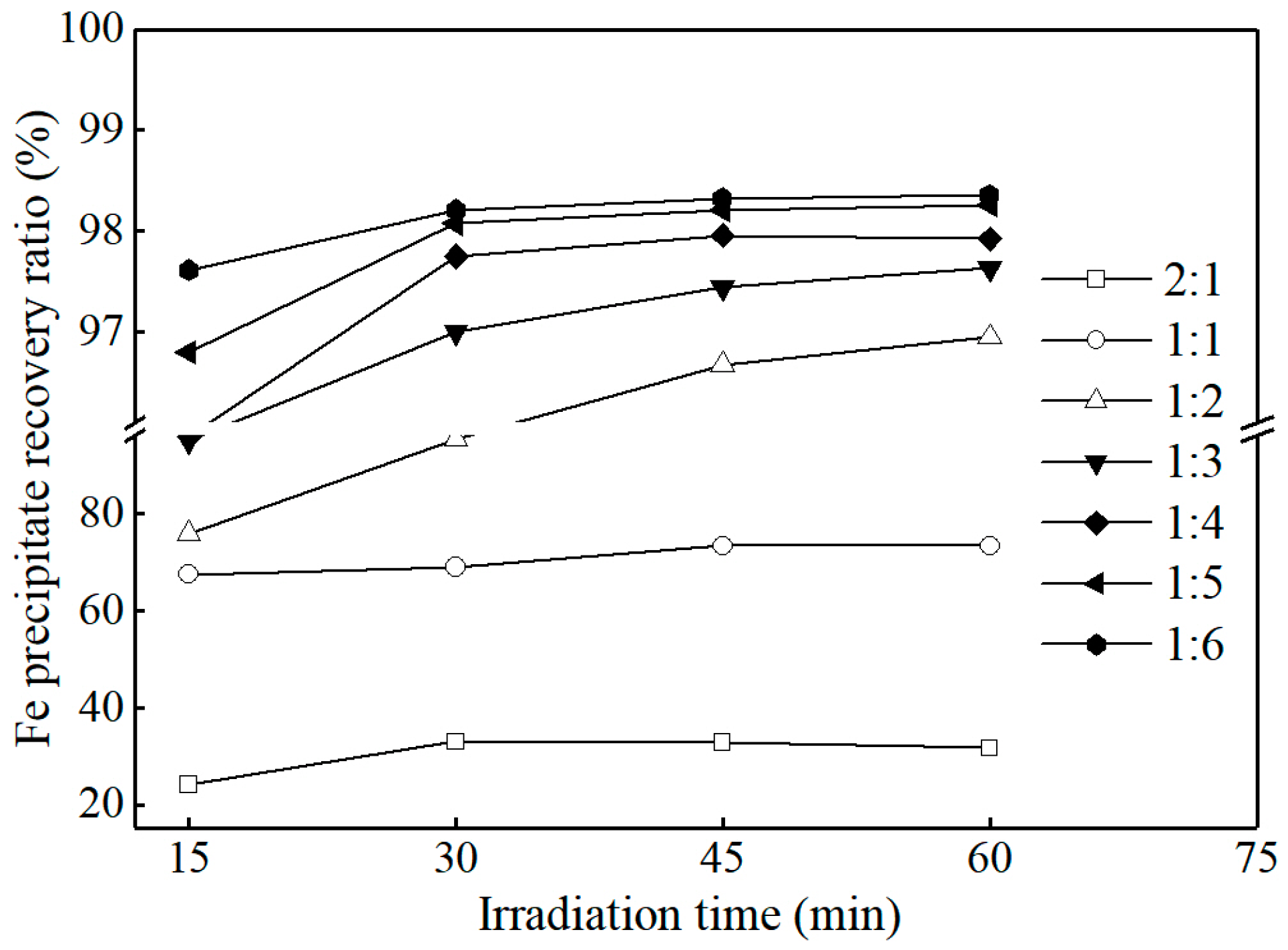
3.3.2. Effect of Iron and Oxalic Acid
3.4. Performance of the Combined Process
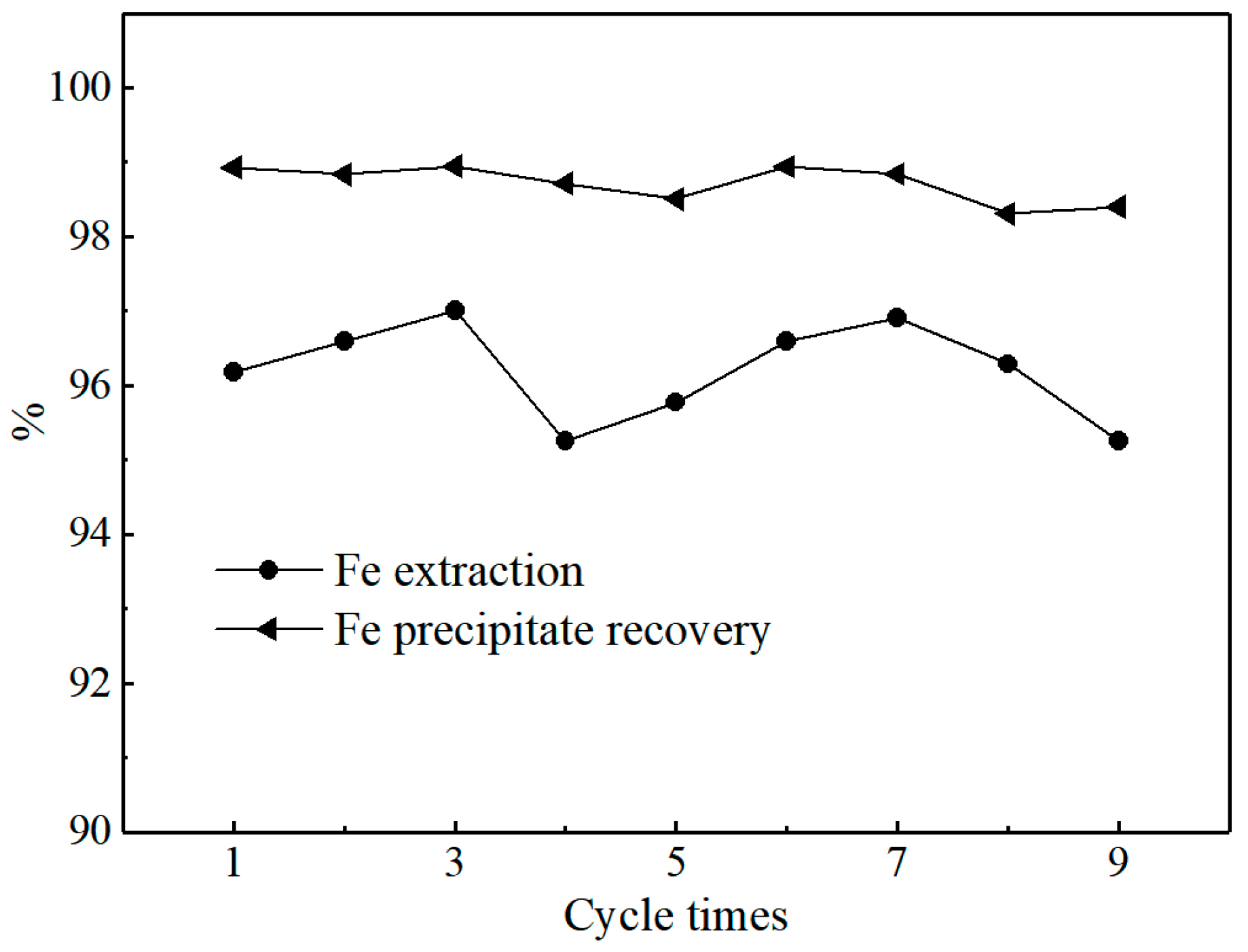
3.4.1. Stability of Cycle Running Test
3.4.2. Analysis of Precipitation
3.4.3. Material Balance
4. Conclusions
- (1)
- In the leaching experiment, the Fe extraction ratio of 95.89% is reached in the optimal condition of S/L ratio at 1:50, oxalic acid concentration at 0.40 mol/L, leaching temperature at 95 °C, and stirring rate at 300 r/min within 3 h.
- (2)
- In the irradiation experiment, 97.75% of iron in leaching solution was precipitated by 30 min irradiation at 254 nm ultraviolet light and Fe/oxalic acid molar ratio at 1:4.
- (3)
- In the test of 9 continuous cycles, average leaching and precipitation ratio of iron are 96.21% and 98.71%, respectively. The ferrous oxalate with grade of 99.32% was produced from pyrolusite leaching slag by the combined process of oxalic acid leaching and ultraviolet irradiation.
- (4)
- Material balance indicated that 95.17% of iron is recovered in the form of FeC2O4·2H2O and 58.52% of oxalic acid is recycled. 17.61% of oxalic acid was lost in irradiation process, used as reductant to reduce Fe3+ to Fe2+, and decomposed into CO2.
- (5)
- The technology realized the recovery of iron from pyrolusite leaching slag by a lab-scale circulation process of oxalic acid leaching and ultraviolet irradiation, which has extensive applied foreground contributing to its advantages of stable performance, high iron recovery efficiency, and sustainable development.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, J.; Zhou, G. A review of manganese ore resources in China and its processing technology process. China’s Manganese Ind. 2006, 24, 1–5. [Google Scholar]
- Bilinski, H.; Kwokal, Ž.; Branica, M. Formation of some manganese minerals from ferromanganese factory waste disposed in the Krka River Estuary. Water Res. 1996, 30, 495–500. [Google Scholar] [CrossRef]
- Senanayake, G. A mixed surface reaction kinetic model for the reductive leaching of manganese dioxide with acidic sulfur dioxide. Hydrometallurgy 2004, 73, 215–224. [Google Scholar] [CrossRef]
- Sahoo, R.N.; Naik, P.K.; Das, S.C. Leaching of manganese from low-grade manganese ore using oxalic acid as reductant in sulphuric acid solution. Hydrometallurgy 2001, 62, 157–163. [Google Scholar] [CrossRef]
- You, Z.; Li, G.; Zhang, Y.; Peng, Z.; Jiang, T. Extraction of manganese from iron rich MnO2 ores via selective sulfation roasting with SO2 followed by water leaching. Hydrometallurgy 2015, 156, 225–231. [Google Scholar] [CrossRef]
- Qiu, C.; Zhou, Z.; Li, M. Research on preparation of unburned bricks using tailings of desulfurization of pyrolusite slurry. J. Chengdu Univ. Sci. Ed. 2014, 33, 398–401. [Google Scholar]
- Naik, P.K.; Sukla, L.B.; Das, S.C. Aqueous SO2 leaching studies on Nishikhal manganese ore through factorial experiment. Hydrometallurgy 2000, 54, 217–228. [Google Scholar] [CrossRef]
- Naik, P.K.; Nathsarma, K.C.; Das, S.C.; Misra, V.N. Leaching of low grade Joda manganese ore with sulphur dioxide in aqueous medium. Miner. Process. Extr. Metall. 2013, 112, 131–134. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, S.; Wang, X.; Chen, B.; Yang, H.; Wang, S.; Luo, P. Recovery of iron from chromium vanadium-bearing titanomagnetite concentrate by direct reduction. JOM 2016, 68, 2698–2703. [Google Scholar] [CrossRef]
- Li, M.; Peng, B.; Chai, L.; Peng, N.; Yan, H.; Hou, D. Recovery of iron from zinc leaching residue by selective reduction roasting with carbon. J. Hazard. Mater. 2012, 237, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Jiao, R.; Xing, P.; Wang, C.; Ma, B.; Chen, Y.-Q. Recovery of iron from copper tailings via low-temperature direct reduction and magnetic separation: Process optimization and mineralogical study. Int. J. Miner. Metall. Mater. 2017, 24, 974–982. [Google Scholar] [CrossRef]
- Tuncuk, A.; Akcil, A. Iron removal in production of purified quartz by hydrometallurgical process. Int. J. Miner. Process. 2016, 153, 44–50. [Google Scholar] [CrossRef]
- Ma, B.; Wang, C.; Yang, W.; Yang, B.; Zhang, Y. Selective pressure leaching of Fe (II)-rich limonitic laterite ores from Indonesia using nitric acid. Miner. Eng. 2013, 45, 151–158. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, X.; Wang, M.; Wang, H.; Xian, P. Recovery of iron from red mud by selective leach with oxalic acid. Hydrometallurgy 2015, 157, 239–245. [Google Scholar] [CrossRef]
- Taxiarchou, M.; Panias, D.; Douni, I.; Paspaliaris, I.; Kontopoulos, A. Removal of iron from silica sand by leaching with oxalic acid. Hydrometallurgy 1997, 46, 215–227. [Google Scholar] [CrossRef]
- Veglió, F.; Passariello, B.; Barbaro, M.; Plescia, P.; Marabini, A.M. Drum leaching tests in iron removal from quartz using oxalic and sulphuric acids. Int. J. Miner. Process. 1998, 54, 183–200. [Google Scholar] [CrossRef]
- Sobianowska-Turek, A.; Szczepaniak, W.; Maciejewski, P.; Gawlik-Kobylińska, M. Recovery of zinc and manganese, and other metals (Fe, Cu, Ni, Co, Cd, Cr, Na, K) from Zn-MnO2 and Zn-C waste batteries: Hydroxyl and carbonate co-precipitation from solution after reducing acidic leaching with use of oxalic acid. J. Power Sources 2016, 325, 220–228. [Google Scholar] [CrossRef]
- Ma, Y.; Du, X.; Shen, Y.; Li, G.; Li, M. Crystallization and beneficiation of magnetite for iron recycling from nickel slags by oxidation-magnetic separation. Metals 2017, 7, 321. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, H.; Xue, X.; Yuan, S. Separation and recovery of iron and rare earth from bayan obo tailings by magnetizing roasting and (NH4)2SO4 activation roasting. Metals 2017, 7, 195. [Google Scholar] [CrossRef]
- Ambikadevi, V.R.; Lalithambika, M. Effect of organic acids on ferric iron removal from iron-stained kaolinite. Appl. Clay Sci. 2000, 16, 133–145. [Google Scholar] [CrossRef]
- Lahiri, A. Influence of ascorbate and oxalic acid for the removal of iron and alkali from alkali roasted ilmenite to produce synthetic rutile. Ind. Eng. Chem. Res. 2010, 49, 8847–8851. [Google Scholar] [CrossRef]
- Peng, A.; He, Z.; Yu, C.; Xiao, W.; Zhuang, X.; Liu, Y. Research on preparation of battery-grade ferrous oxalate from by-product ferrous sulfate. Inorg. Chem. Ind. 2012, 44, 60–62. [Google Scholar]
- Heelan, J.; Gratz, E.; Zheng, Z.; Wang, Q.; Chen, M.; Apelian, D.; Wang, Y. Current and prospective Li-Ion battery recycling and recovery processes. JOM 2016, 68, 2632–2638. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, S.; Zhu, Z. Photochemical properties and application of ferric-oxalate complexes. Jiangxi Chem. Ind. 2008, 26–29. [Google Scholar]
- Yu, Z.; Shi, Z.; Chen, Y.; Niu, Y.; Wang, Y.; Wan, P. Red-mud treatment using oxalic acid by UV irradiation assistance. Trans. Nonferrous Met. Soc. China 2012, 22, 456–460. [Google Scholar] [CrossRef]
- Sun, W.; Su, S.; Wang, Q.; Ding, S. Lab-scale circulation process of electrolytic manganese production with low-grade pyrolusite leaching by SO2. Hydrometallurgy 2013, 133, 118–125. [Google Scholar] [CrossRef]
- Panias, D.; Taxiarchou, M.; Paspaliaris, I.; Kontopoulos, A. Mechanisms of dissolution of iron oxides in aqueous oxalic acid solutions. Hydrometallurgy 1996, 42, 257–265. [Google Scholar] [CrossRef]
- Zuo, Y.; Hoigne, J. Formation of hydrogen-peroxide and depletion of oxalic-acid in atmospheric water by photolysis of iron(III) oxalato complexes. Environ. Sci. Technol. 1992, 26, 1014–1022. [Google Scholar] [CrossRef]
- Mazurek, K. Recovery of vanadium, potassium and iron from a spent vanadium catalyst by oxalic acid solution leaching, precipitation and ion exchange processes. Hydrometallurgy 2013, 134–135, 26–31. [Google Scholar] [CrossRef]
- Jia, S.; Cai, C. Process Design for Unit Operations of Chemical Engineering; Tianjin University: Tianjin, China, 2011; ISBN 978-7-5618-4112-9. [Google Scholar]
- Vincze, L.; Papp, S. Individual quantum yields of Fe3+OXn2−Hm+ complexes in aqueous acidic solutions (OX2− ≡ C2O42−, n = 1–3, m = 0,1). J. Photochem. 1987, 36, 289–296. [Google Scholar] [CrossRef]
- Zhang, K.; Yang, X.; Wu, J.; Huang, X.; Yao, Y. Optimization of the process parameters for the synthesis process of battery-grade ferrous oxalate by response surface method. Nano 2016, 11, 1650123. [Google Scholar] [CrossRef]

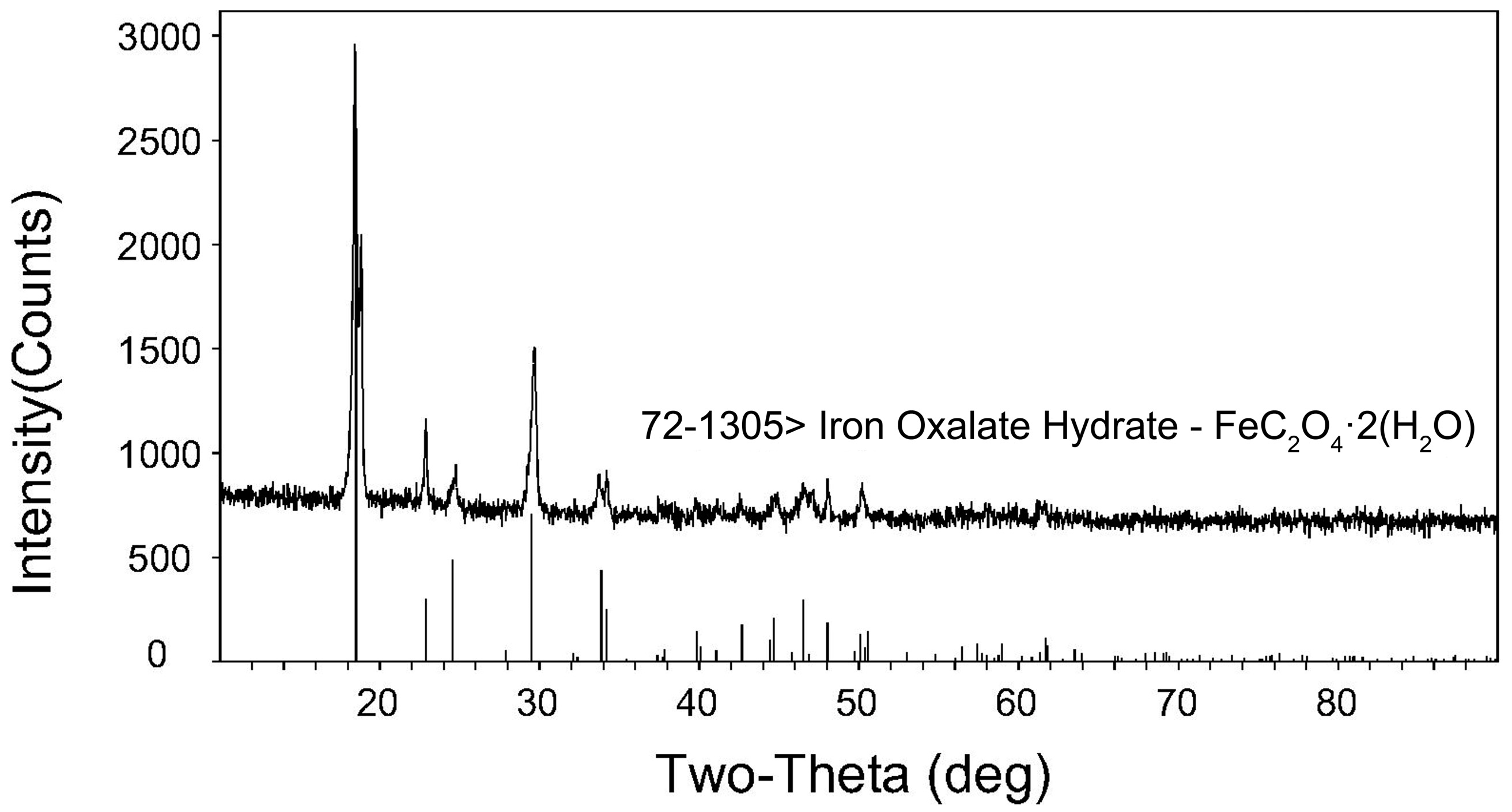
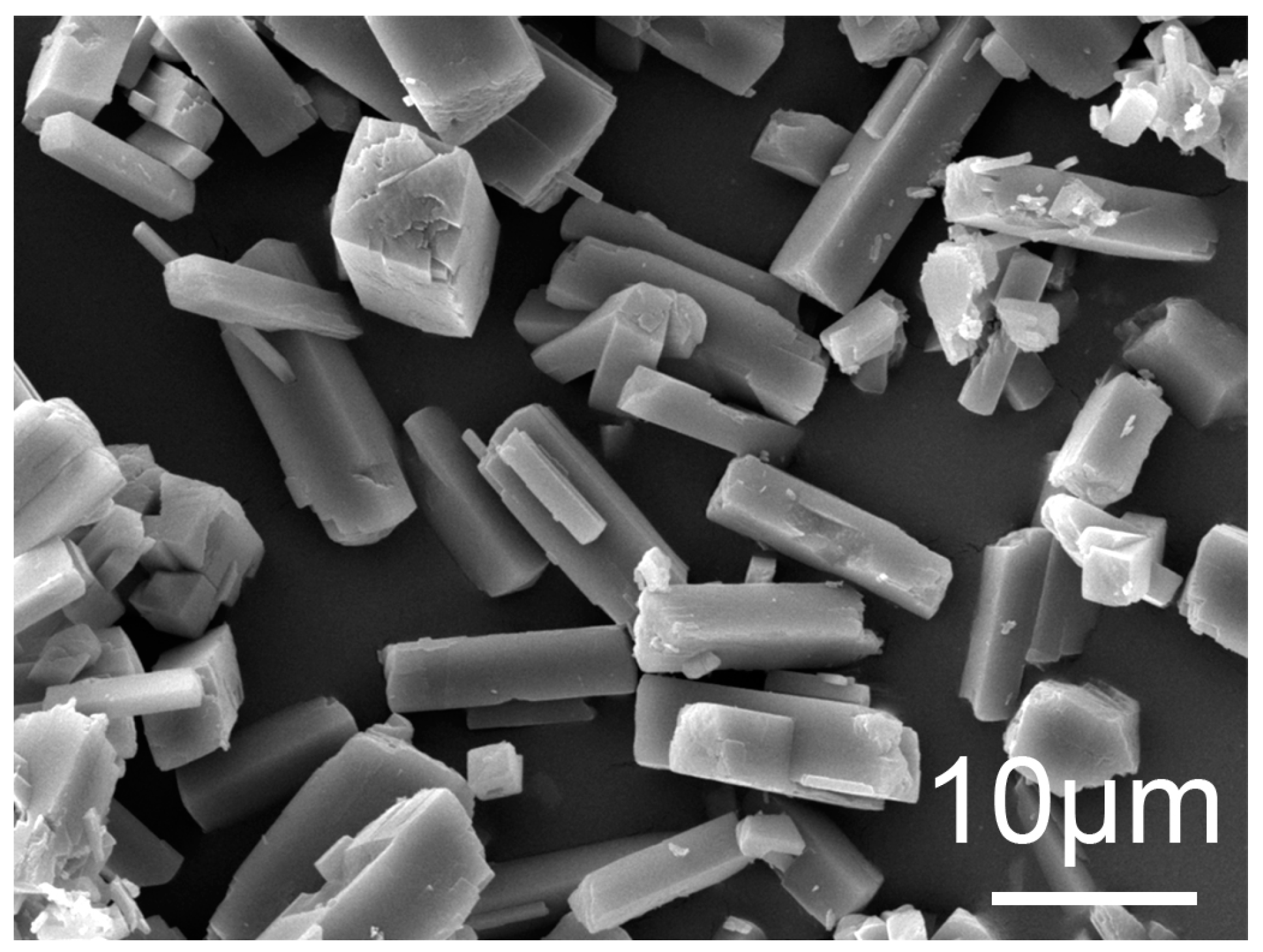
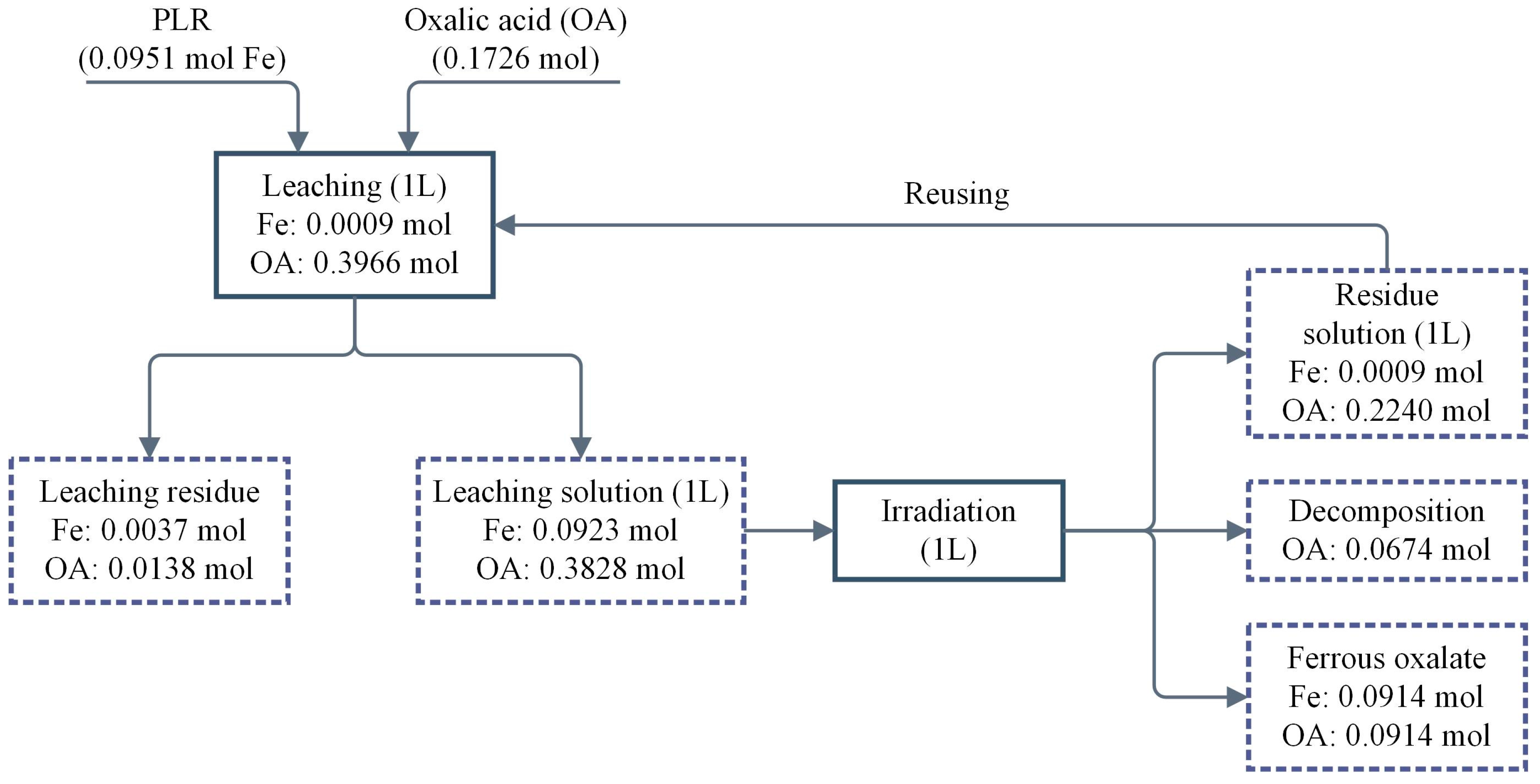
| Fe | Mn | Al | Ba | K | Ti | Mg | Ca |
|---|---|---|---|---|---|---|---|
| 26.62 | 2.6 | 1.75 | 0.49 | 0.26 | 0.09 | 0.09 | 0.08 |
| Zn | V | Ni | Cr | Cu | Pb | Sr | Others |
| 0.044 | 0.035 | 0.03 | 0.028 | 0.02 | 0.016 | 0.014 | 67.833 |
| Process | Condition | Value |
|---|---|---|
| Leaching | Stirring rate | 300 r/min |
| Leaching time | 3 h | |
| S/L ratio | 1:50 | |
| Temperature | 95 °C | |
| Oxalic acid concentration | 0.40 mol/L | |
| Irradiation | Wavelengths | 254 nm |
| Irradiation time | 1 h |
| FeC2O4·2H2O | Water | Cu | Cr | Ni | Zn | Al |
|---|---|---|---|---|---|---|
| 99.32 | 0.06 | 5.47 × 10−4 | 2.15 × 10−4 | 1.29 × 10−4 | 7.47 × 10−4 | 7.73 × 10−4 |
| Project | Leaching Process | Irradiation Process | Total |
|---|---|---|---|
| Fe | 96.11 | 99.02 | 95.17 |
| Oxalic Acid | 96.52 | 58.52 | 56.48 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, B.; Wang, B.; Su, S.; Ding, S.; Sun, W. Recovery of Iron from Pyrolusite Leaching Slag by a Lab-Scale Circulation Process of Oxalic Acid Leaching and Ultraviolet Irradiation. Metals 2018, 8, 8. https://doi.org/10.3390/met8010008
Deng B, Wang B, Su S, Ding S, Sun W. Recovery of Iron from Pyrolusite Leaching Slag by a Lab-Scale Circulation Process of Oxalic Acid Leaching and Ultraviolet Irradiation. Metals. 2018; 8(1):8. https://doi.org/10.3390/met8010008
Chicago/Turabian StyleDeng, Biao, Bozhi Wang, Shijun Su, Sanglan Ding, and Weiyi Sun. 2018. "Recovery of Iron from Pyrolusite Leaching Slag by a Lab-Scale Circulation Process of Oxalic Acid Leaching and Ultraviolet Irradiation" Metals 8, no. 1: 8. https://doi.org/10.3390/met8010008
APA StyleDeng, B., Wang, B., Su, S., Ding, S., & Sun, W. (2018). Recovery of Iron from Pyrolusite Leaching Slag by a Lab-Scale Circulation Process of Oxalic Acid Leaching and Ultraviolet Irradiation. Metals, 8(1), 8. https://doi.org/10.3390/met8010008




