Reduction Kinetics of Hematite Powder in Hydrogen Atmosphere at Moderate Temperatures
Abstract
:1. Introduction
2. Experiment
3. Results and Discussion
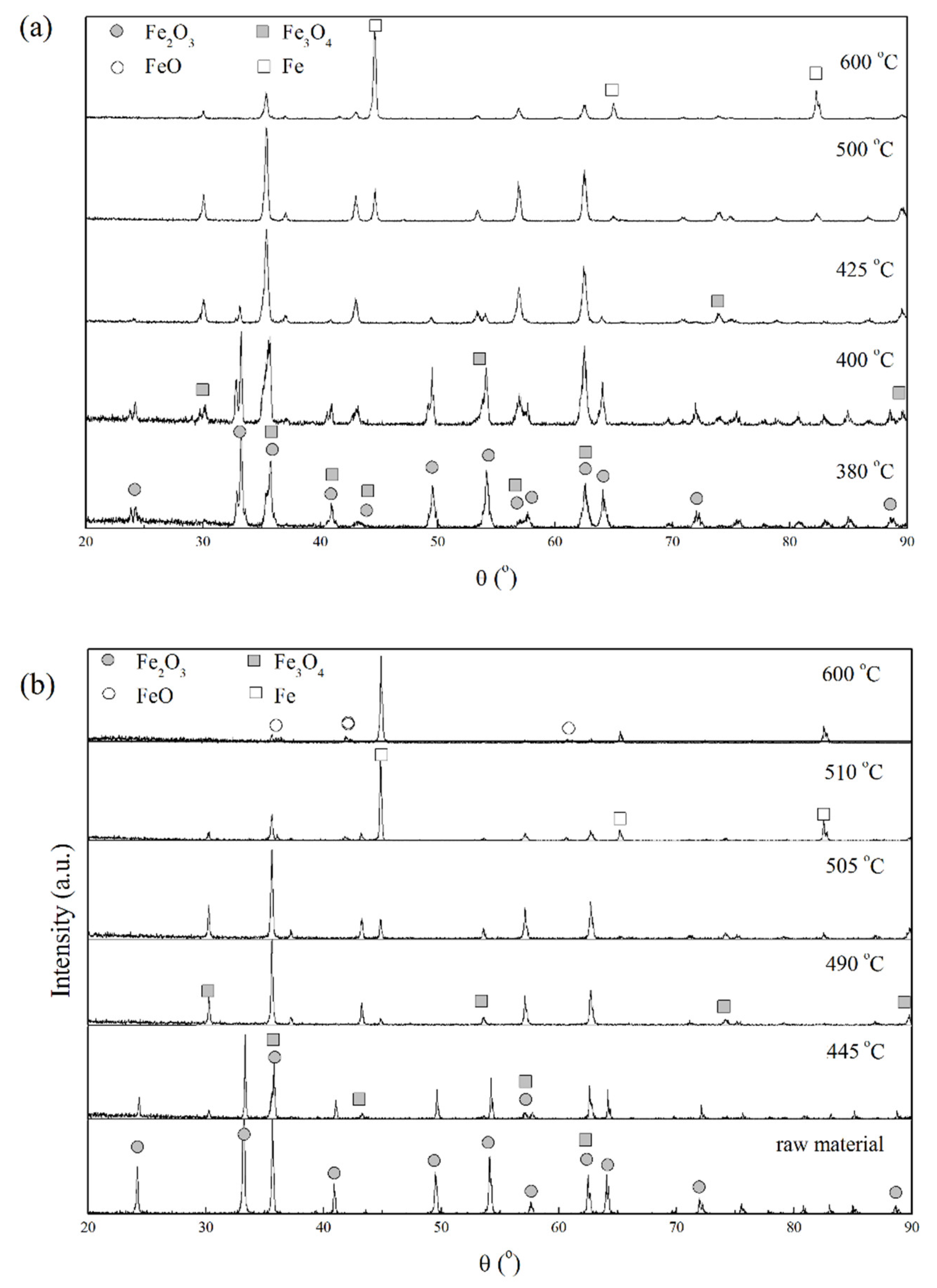
3.1. Phase Transformation
3.2. Morphology
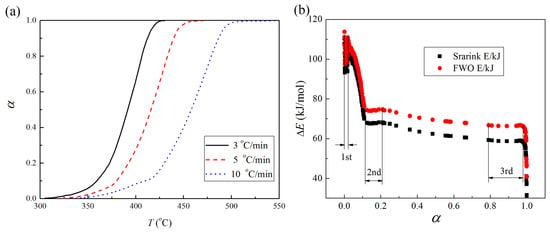
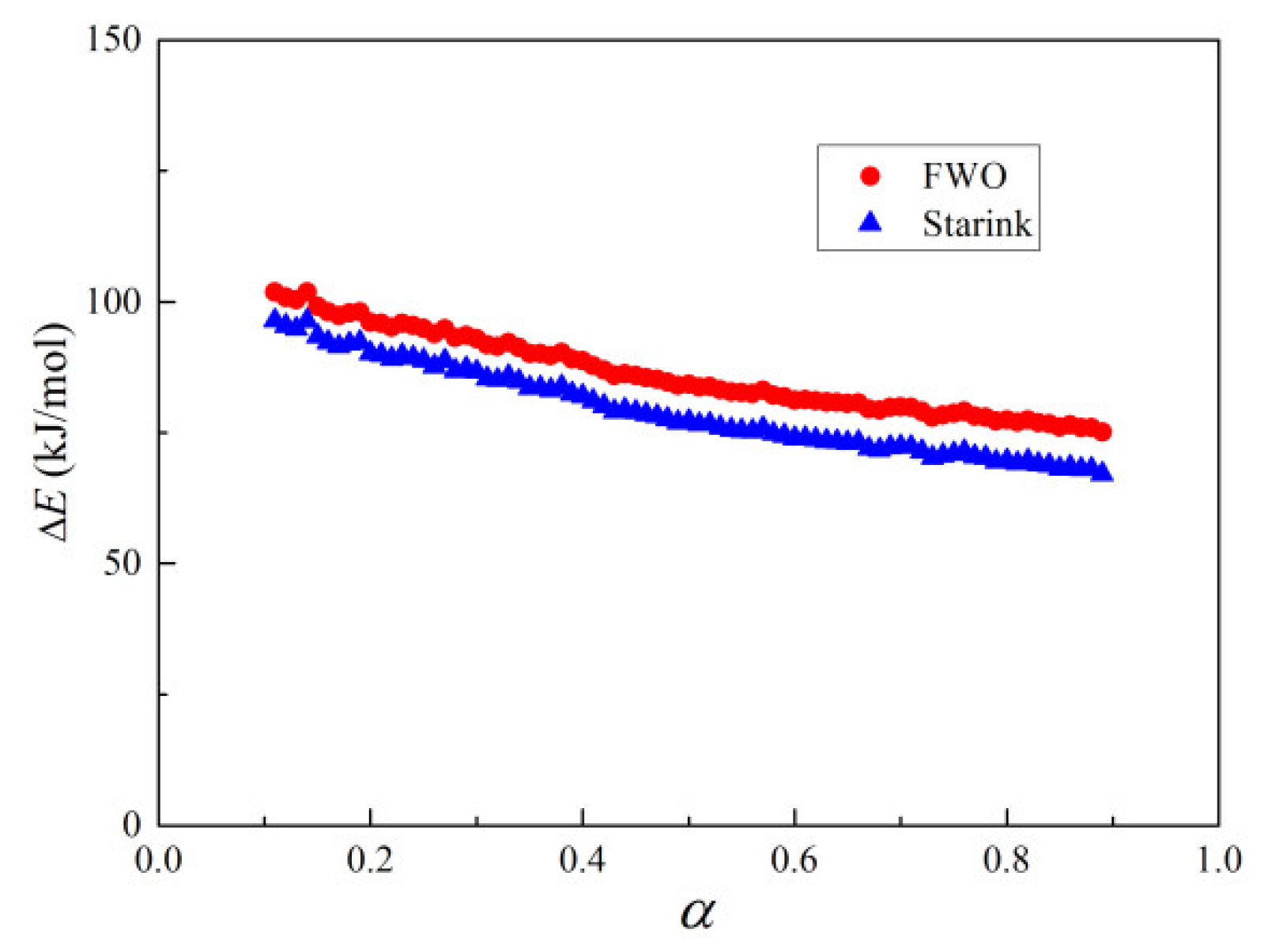
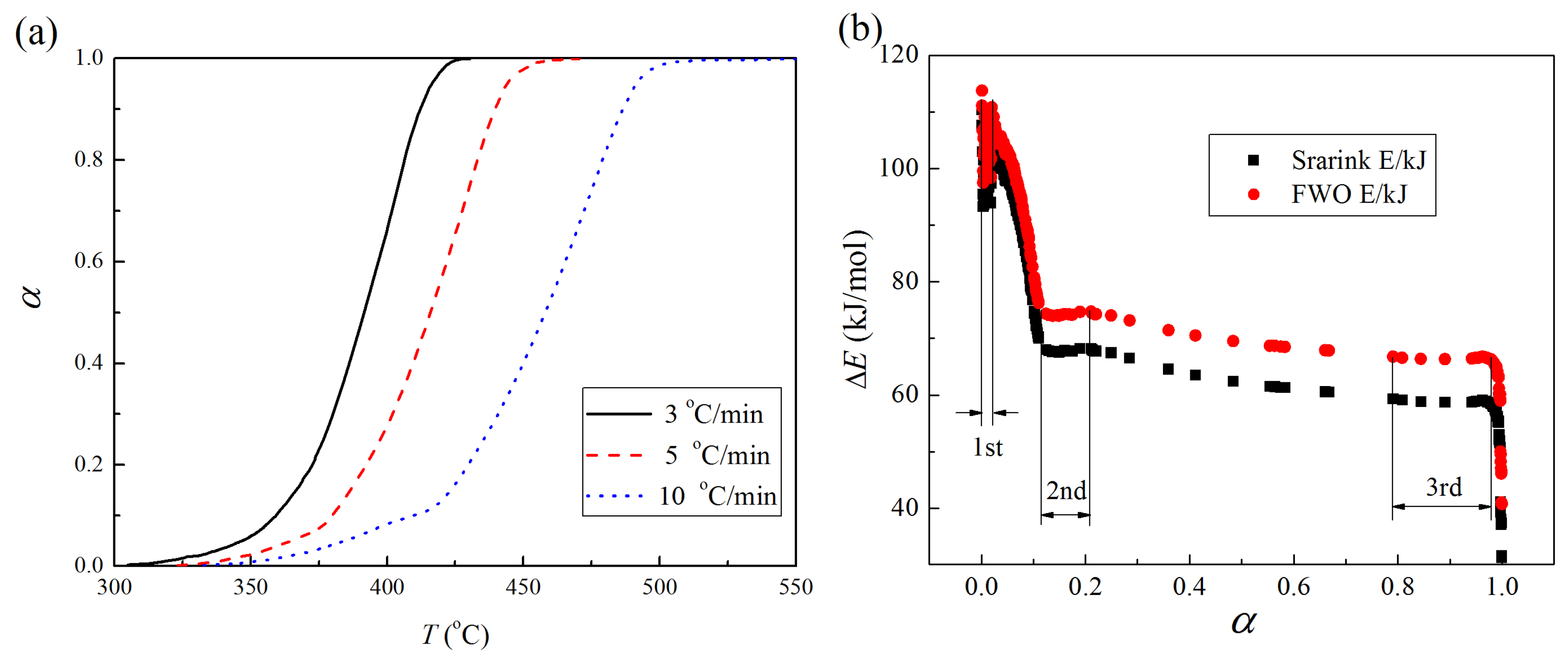
3.3. Kinetics
3.3.1. Annealed Hematite
3.3.2. Hematite without Heat Treatment
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- International Energy Agency. Tracking Industrial Energy Efficiency and CO2 Emissions; Media, Stedi: Paris, France, 2007; p. 24. [Google Scholar]
- Wang, X.; Lin, B. How to reduce CO2 emissions in China’s iron and steel industry. Renew. Sustain. Energy Rev. 2016, 57, 1496–1505. [Google Scholar] [CrossRef]
- Guo, D.; Hu, M.; Pu, C.; Xiao, B.; Hu, Z.; Liu, S.; Wang, X.; Zhu, X. Kinetics and mechanisms of direct reduction of iron ore-biomass composite pellets with hydrogen gas. Int. J. Hydrogen Energy 2015, 40, 4733–4740. [Google Scholar] [CrossRef]
- Hughes, R.; Kam, E.; Mogadam-Zadeh, H. The reduction of iron ores by hydrogen and carbon monoxide and their mixtures. Thermochim. Acta 1982, 59, 361–377. [Google Scholar] [CrossRef]
- Da Costa, A.R.; Wagner, D.; Patisson, F. Modelling a new, low CO2 emissions, hydrogen steelmaking process. J. Clean. Prod. 2013, 46, 27–35. [Google Scholar] [CrossRef]
- Sohn, H.Y. Suspension Hydrogen Reduction of Iron Ore Concentrate; University of Utah: Salt Lake City, UT, USA, 2008. [Google Scholar]
- Sohn, H. Suspension ironmaking technology with greatly reduced energy requirement and CO2 emissions. Steel Times Int. 2007, 31, 68. [Google Scholar]
- Sohn, H.; Moo, E.C.; Zhang, Y.; Ramos, J.E. Suspension reduction technology for ironmaking with low CO2 emission and energy requirement. In Proceedings of the Iron and Steel Technology Conference, St. Louis, MO, USA, 4–7 May 2009. [Google Scholar]
- Sohn, H.Y.; Mohassab, Y. Development of a Novel Flash Ironmaking Technology with Greatly Reduced Energy Consumption and CO2 Emissions. J. Sustain. Metall. 2016, 2, 216–227. [Google Scholar] [CrossRef]
- Elzohiery, M.; Sohn, H.Y.; Mohassab, Y. Kinetics of Hydrogen Reduction of Magnetite Concentrate Particles in Solid State Relevant to Flash Ironmaking. Steel Res. Int. 2017, 88, 1600133. [Google Scholar] [CrossRef]
- Fan, D.-Q.; Sohn, H.Y.; Mohassab, Y.; Elzohiery, M. Computational Fluid Dynamics Simulation of the Hydrogen Reduction of Magnetite Concentrate in a Laboratory Flash Reactor. Metall. Mater. Trans. B 2016, 47, 3489–3500. [Google Scholar] [CrossRef]
- Olsson, O. Low-Emission Steel Production: Decarbonising Heavy Industry. Available online: https://www.sei.org/perspectives/low-emission-steel-production-hybrit/ (accessed on 11 April 2018).
- Turkdogan, E.; Vinters, J. Gaseous reduction of iron oxides: Part I. Reduction of hematite in hydrogen. Metall. Mater. Trans. B 1971, 2, 3175–3188. [Google Scholar] [CrossRef]
- Park, E.; Ostrovski, O. Reduction of titania-ferrous ore by hydrogen. ISIJ Int. 2004, 44, 999–1005. [Google Scholar] [CrossRef]
- Dang, J.; Chou, K.C.; Hu, X.J.; Zhang, G.H. Reduction kinetics of metal oxides by hydrogen. Steel Res. Int. 2013, 84, 526–533. [Google Scholar] [CrossRef]
- Fruehan, R.; Li, Y.; Brabie, L.; Kim, E.J. Final stage of reduction of iron ores by hydrogen. Scand. J. Metall. 2005, 34, 205–212. [Google Scholar] [CrossRef]
- Habermann, A.; Winter, F.; Hofbauer, H.; Zirngast, J.; Schenk, J.L. An experimental study on the kinetics of fluidized bed iron ore reduction. ISIJ Int. 2000, 40, 935–942. [Google Scholar] [CrossRef]
- Pineau, A.; Kanari, N.; Gaballah, I. Kinetics of reduction of iron oxides by H2: Part I: Low temperature reduction of hematite. Thermochim. Acta 2006, 447, 89–100. [Google Scholar] [CrossRef]
- Pineau, A.; Kanari, N.; Gaballah, I. Kinetics of reduction of iron oxides by H2: Part II. Low temperature reduction of magnetite. Thermochim. Acta 2007, 456, 75–88. [Google Scholar] [CrossRef]
- Piotrowski, K.; Mondal, K.; Wiltowski, T.; Dydo, P.; Rizeg, G. Topochemical approach of kinetics of the reduction of hematite to wüstite. Chem. Eng. J. 2007, 131, 73–82. [Google Scholar] [CrossRef]
- Dang, J.; Hu, X.J.; Zhang, G.H.; Hou, X.M.; Yang, X.B.; Chou, K.C. Kinetics of reduction of titano-magnetite powder by H2. High Temp. Mater. Proc. 2013, 32, 229–236. [Google Scholar] [CrossRef]
- Munteanu, G.; Ilieva, L.; Andreeva, D. TPR data regarding the effect of sulfur on the reducibility of α-Fe2O3. Thermochim. Acta 1999, 329, 157–162. [Google Scholar] [CrossRef]
- Jozwiak, W.K.; Kaczmarek, E.; Maniecki, T.P.; Ignaczak, W.; Maniukiewicz, W. Reduction behavior of iron oxides in hydrogen and carbon monoxide atmospheres. Appl. Catal. A 2007, 326, 17–27. [Google Scholar] [CrossRef]
- Sastri, M.V.C.; Viswanath, R.P.; Viswanathan, B. Studies on the reduction of iron oxide with hydrogen. Int. J. Hydrogen Energy 1982, 7, 951–955. [Google Scholar] [CrossRef]
- Chung, F.H. Quantitative interpretation of X-ray diffraction patterns of mixtures. I. Matrix-flushing method for quantitative multicomponent analysis. J. Appl. Crystallogr. 1974, 7, 519–525. [Google Scholar] [CrossRef] [Green Version]
- Snyder, R.L. The use of reference intensity ratios in X-ray quantitative analysis. Powder Diffr. 1992, 7, 186–193. [Google Scholar] [CrossRef]
- ICDD. JCPDS PDF-2 Database; ICDD: Newtown Square, PA, USA, 2001; Volume 51. [Google Scholar]
- Stølen, S.; Glöckner, R.; Grønvold, F. Nearly stoichiometric iron monoxide formed as a metastable intermediate in a two-stage disproportionation of quenched wüstite. Thermodynamic and kinetic aspects. Thermochim. Acta 1995, 256, 91–106. [Google Scholar] [CrossRef]
- Khader, M.M.; El-Anadouli, B.E.; El-Nagar, E.; Ateya, B.G. Kinetics of the reduction of Fe2O3 with hydrogen. J. Solid State Chem. 1991, 93, 283–290. [Google Scholar] [CrossRef]
- Emel’yanov, D.; Korolev, K.; Mikhailenko, M.; Knot’ko, A.; Oleinikov, N.; Tret’yakov, Y.D.; Boldyrev, V. Mechanochemical Synthesis of Wüstite, Fe1–xO, in High-Energy Apparatuses. Inorg. Mater. 2004, 40, 632–635. [Google Scholar] [CrossRef]
- Chen, Z.; Chou, K.-C.; Morita, K. Mechanism of Metastable Wüstite Formation in the Reduction Process of Iron Oxide below 570 °C. Mater. Trans. 2016, 57, 1660–1663. [Google Scholar] [CrossRef]
- Hayes, P. Analysis of Product Morphologies and Reaction Mechanisms on Gaseous Reduction of Iron Oxides. Steel Res. Int. 2011, 82, 480–493. [Google Scholar] [CrossRef]
- Baguley, P.; John, D.H.S.; Hayes, P. The conditions for the formation of lath and porous magnetite on reduction of hematite in H2/H2O gas mixtures. Metall. Mater. Trans. B 1983, 14, 513–514. [Google Scholar] [CrossRef]
- Rau, M.-F.; Rieck, D.; Evans, J.W. Investigation of iron oxide reduction by TEM. Metall. Trans. B 1987, 18, 257–278. [Google Scholar] [CrossRef]
- Weiss, B.; Sturn, J.; Voglsam, S.; Strobl, S.; Mali, H.; Winter, F.; Schenk, J. Experimental and Morphological Investigations of the Reduction from Coarse Hematite to Magnetite and Wüstite under Fluidized Bed Conditions. Steel Res. Int. 2010, 81, 93–99. [Google Scholar] [CrossRef]
- Ettabirou, M.; Dupré, B.; Gleitzer, C. Nucleation and early growth of magnetite on synthetic and natural hematite crystals. React. Solids 1986, 1, 329–343. [Google Scholar] [CrossRef]
- Gleitzer, C. Some remarkable features in the reduction of iron oxides. Solid State Ionics 1990, 38, 133–141. [Google Scholar] [CrossRef]
- Holme, B. Morphology and Crystallographic Relationships in Reduced Magnetite: A Comprehensive Structural Study of the Porous Iron Ammonia Synthesis Catalyst. J. Catal. 1997, 167, 12–24. [Google Scholar] [CrossRef]
- Flynn, J.H.; Wall, L.A. A quick, direct method for the determination of activation energy from thermogravimetric data. J. Polym. Sci. Part B Polym. Lett. 1966, 4, 323–328. [Google Scholar] [CrossRef]
- Ozawa, T. Estimation of activation energy by isoconversion methods. Thermochim. Acta 1992, 203, 159–165. [Google Scholar] [CrossRef]
- Starink, M.J. The determination of activation energy from linear heating rate experiments: A comparison of the accuracy of isoconversion methods. Thermochim. Acta 2003, 404, 163–176. [Google Scholar] [CrossRef]
- Pourghahramani, P.; Forssberg, E. Reduction kinetics of mechanically activated hematite concentrate with hydrogen gas using nonisothermal methods. Thermochim. Acta 2007, 454, 69–77. [Google Scholar] [CrossRef]



| Ea (kJ/mol) | 1st Stage | 2nd Stage | 3rd Stage |
|---|---|---|---|
| FWO | 105.0 | 74.3 | 66.6 |
| Starink | 100.8 | 67.8 | 58.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Dang, J.; Hu, X.; Yan, H. Reduction Kinetics of Hematite Powder in Hydrogen Atmosphere at Moderate Temperatures. Metals 2018, 8, 751. https://doi.org/10.3390/met8100751
Chen Z, Dang J, Hu X, Yan H. Reduction Kinetics of Hematite Powder in Hydrogen Atmosphere at Moderate Temperatures. Metals. 2018; 8(10):751. https://doi.org/10.3390/met8100751
Chicago/Turabian StyleChen, Zhiyuan, Jie Dang, Xiaojun Hu, and Hongyan Yan. 2018. "Reduction Kinetics of Hematite Powder in Hydrogen Atmosphere at Moderate Temperatures" Metals 8, no. 10: 751. https://doi.org/10.3390/met8100751
APA StyleChen, Z., Dang, J., Hu, X., & Yan, H. (2018). Reduction Kinetics of Hematite Powder in Hydrogen Atmosphere at Moderate Temperatures. Metals, 8(10), 751. https://doi.org/10.3390/met8100751